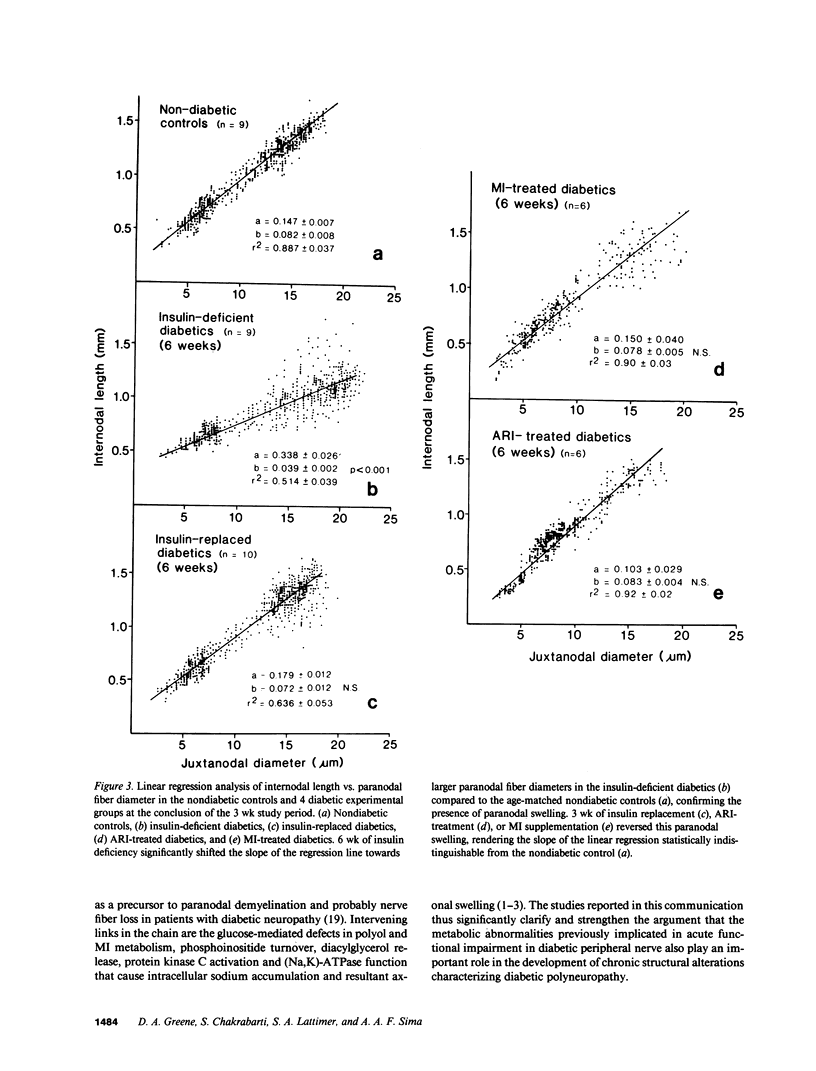
Abstract
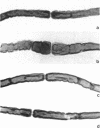
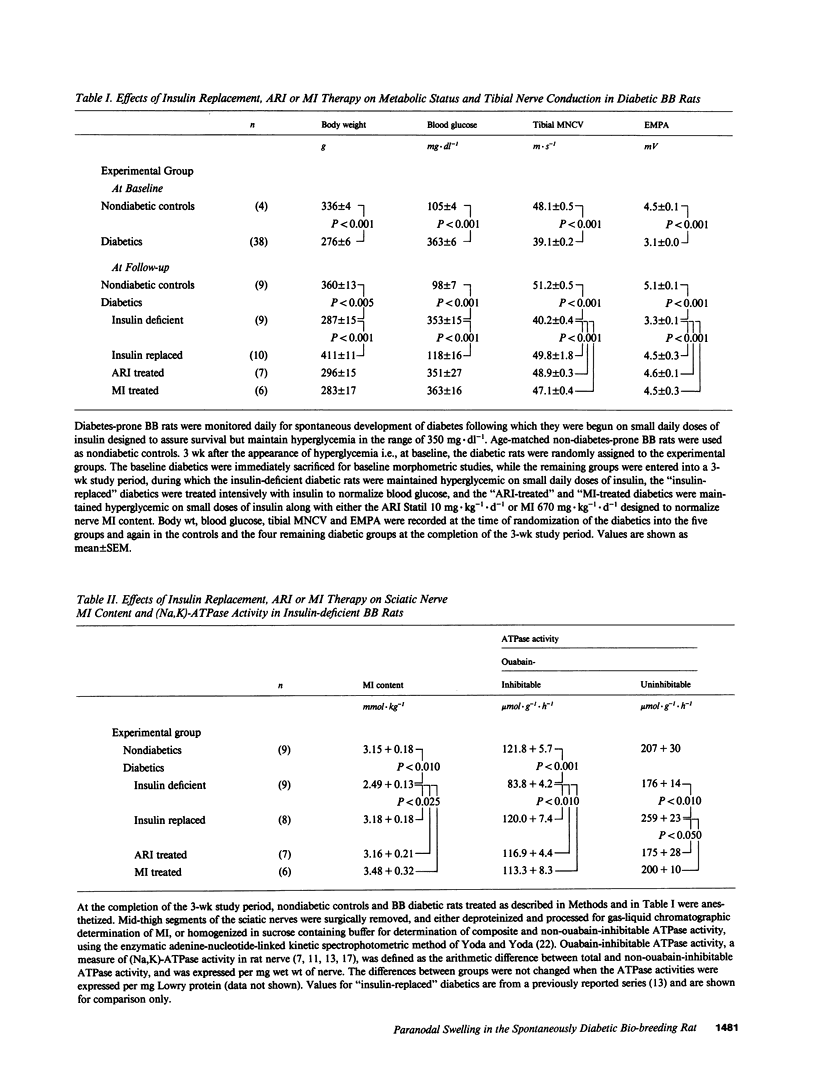
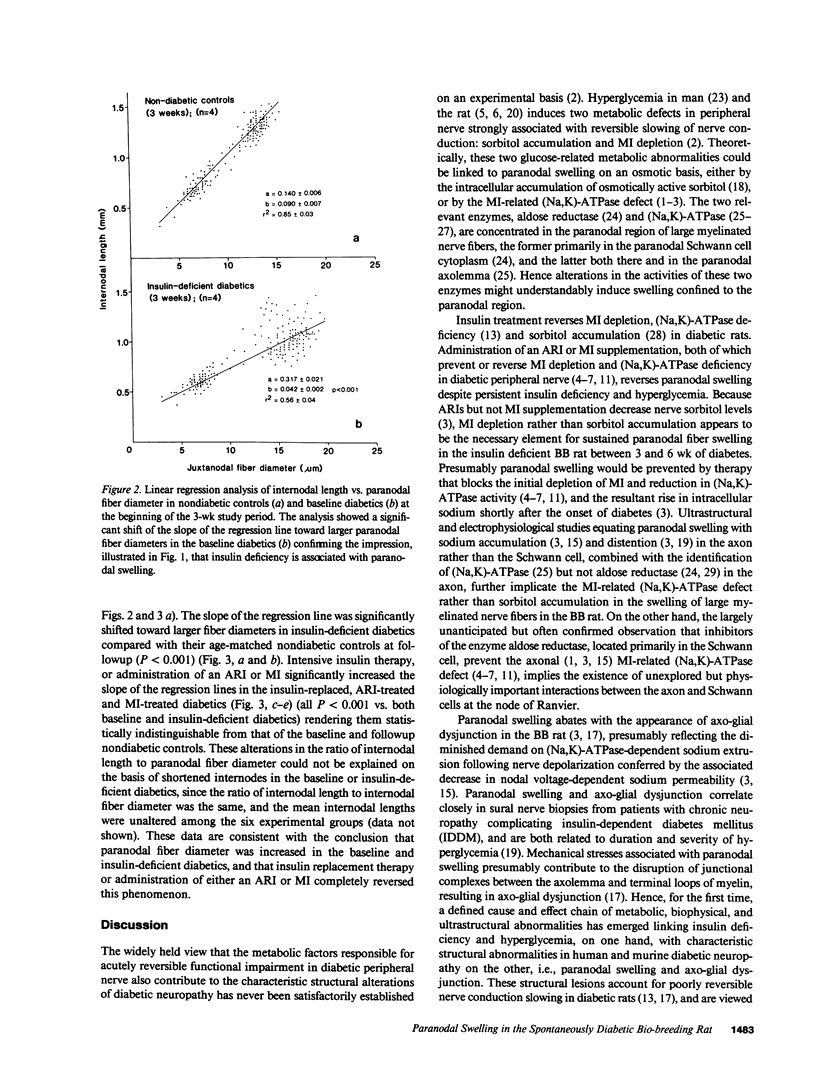
Axo-glial dysjunction refers to the disruption of important junctional complexes that anchor terminal loops of myelin to the paranodal axolemma in diabetic human and animal peripheral nerve. Neither axo-glial dysjunction nor the preceeding acute localized paranodal swelling has been specifically attributed to discrete metabolic consequences of insulin deficiency or hyperglycemia. Two metabolic sequelae of hyperglycemia in diabetic nerve, sorbitol accumulation via aldose reductase, and (Na,K)-ATPase deficiency related to myo-inositol depletion, were explored as possible underlying causes of acute paranodal swelling in the spontaneously diabetic bio-breeding rat. 3 wk of insulin replacement, or therapy with an aldose reductase inhibitor or myo-inositol completely reversed paranodal swelling in sural nerve fibers after 3 wk of untreated insulin deficiency. These observations suggest that insulin deficiency and hyperglycemia cause reversible paranodal swelling, and ultimately poorly reversible axo-glial dysjunction, via the myo-inositol-related (Na,K)-ATPase defect rather than by the osmotic effects of sorbitol accumulation within nerve fibers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brismar T. Electrophysiology and morphology of myelinated nerve fibers. IV. Nodal function of pathological nerve fibers. Experientia. 1983 Sep 15;39(9):946–953. doi: 10.1007/BF01989759. [DOI] [PubMed] [Google Scholar]
- Brismar T., Sima A. A. Changes in nodal function in nerve fibres of the spontaneously diabetic BB-Wistar rat: potential clamp analysis. Acta Physiol Scand. 1981 Dec;113(4):499–506. doi: 10.1111/j.1748-1716.1981.tb06928.x. [DOI] [PubMed] [Google Scholar]
- Das P. K., Bray G. M., Aguayo A. J., Rasminsky M. Diminished ouabain-sensitive, sodium-potassium ATPase activity in sciatic nerves of rats with streptozotocin-induced diabetes. Exp Neurol. 1976 Oct;53(1):285–288. doi: 10.1016/0014-4886(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Finegold D., Lattimer S. A., Nolle S., Bernstein M., Greene D. A. Polyol pathway activity and myo-inositol metabolism. A suggested relationship in the pathogenesis of diabetic neuropathy. Diabetes. 1983 Nov;32(11):988–992. doi: 10.2337/diab.32.11.988. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973 Apr 19;288(16):831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- Gillon K. R., Hawthorne J. N. Sorbitol, inositol and nerve conduction in diabetes. Life Sci. 1983 Apr 25;32(17):1943–1947. doi: 10.1016/0024-3205(83)90045-0. [DOI] [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Action of sorbinil in diabetic peripheral nerve. Relationship of polyol (sorbitol) pathway inhibition to a myo-inositol-mediated defect in sodium-potassium ATPase activity. Diabetes. 1984 Aug;33(8):712–716. doi: 10.2337/diab.33.8.712. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Impaired energy utilization and Na-K-ATPase in diabetic peripheral nerve. Am J Physiol. 1984 Apr;246(4 Pt 1):E311–E318. doi: 10.1152/ajpendo.1984.246.4.E311. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Impaired rat sciatic nerve sodium-potassium adenosine triphosphatase in acute streptozocin diabetes and its correction by dietary myo-inositol supplementation. J Clin Invest. 1983 Sep;72(3):1058–1063. doi: 10.1172/JCI111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Protein kinase C agonists acutely normalize decreased ouabain-inhibitable respiration in diabetic rabbit nerve. Implications for (Na,K)-ATPase regulation and diabetic complications. Diabetes. 1986 Feb;35(2):242–245. doi: 10.2337/diab.35.2.242. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Sodium- and energy-dependent uptake of myo-inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J Clin Invest. 1982 Nov;70(5):1009–1018. doi: 10.1172/JCI110688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S., Ulbrecht J., Carroll P. Glucose-induced alterations in nerve metabolism: current perspective on the pathogenesis of diabetic neuropathy and future directions for research and therapy. Diabetes Care. 1985 May-Jun;8(3):290–299. doi: 10.2337/diacare.8.3.290. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Yagihashi S., Lattimer S. A., Sima A. A. Nerve Na+-K+-ATPase, conduction, and myo-inositol in the insulin-deficient BB rat. Am J Physiol. 1984 Oct;247(4 Pt 1):E534–E539. doi: 10.1152/ajpendo.1984.247.4.E534. [DOI] [PubMed] [Google Scholar]
- Ludvigson M. A., Sorenson R. L. Immunohistochemical localization of aldose reductase. I. Enzyme purification and antibody preparation--localization in peripheral nerve, artery, and testis. Diabetes. 1980 Jun;29(6):438–449. doi: 10.2337/diab.29.6.438. [DOI] [PubMed] [Google Scholar]
- Mayer J. H., Tomlinson D. R. Prevention of defects of axonal transport and nerve conduction velocity by oral administration of myo-inositol or an aldose reductase inhibitor in streptozotocin-diabetic rats. Diabetologia. 1983 Nov;25(5):433–438. doi: 10.1007/BF00282524. [DOI] [PubMed] [Google Scholar]
- Mayhew J. A., Gillon K. R., Hawthorne J. N. Free and lipid inositol, sorbitol and sugars in sciatic nerve obtained post-mortem from diabetic patients and control subjects. Diabetologia. 1983 Jan;24(1):13–15. doi: 10.1007/BF00275940. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Ernst S. A., Siegel G. J., Agranoff B. W. Immunocytochemical localization of (Na+, K+)-ATPase in the goldfish optic nerve. J Neurochem. 1981 Jan;36(1):107–115. doi: 10.1111/j.1471-4159.1981.tb02384.x. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Brismar T. Reversible diabetic nerve dysfunction: structural correlates to electrophysiological abnormalities. Ann Neurol. 1985 Jul;18(1):21–29. doi: 10.1002/ana.410180105. [DOI] [PubMed] [Google Scholar]
- Sima A. A. Can the BB-rat help to unravel diabetic neuropathy? Neuropathol Appl Neurobiol. 1985 Jul-Aug;11(4):253–264. doi: 10.1111/j.1365-2990.1985.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Hay K. Functional aspects and pathogenetic considerations of the neuropathy in the spontaneously diabetic BB-Wistar rat. Neuropathol Appl Neurobiol. 1981 Sep-Oct;7(5):341–350. doi: 10.1111/j.1365-2990.1981.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Lattimer S. A., Yagihashi S., Greene D. A. Axo-glial dysjunction. A novel structural lesion that accounts for poorly reversible slowing of nerve conduction in the spontaneously diabetic bio-breeding rat. J Clin Invest. 1986 Feb;77(2):474–484. doi: 10.1172/JCI112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. A., Winegrad A. I., Martin D. B. Significance of tissue myo-inositol concentrations in metabolic regulation in nerve. Science. 1982 Aug 27;217(4562):848–851. doi: 10.1126/science.6285474. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Sherman W. R., Kurien M. M., Moonsammy G. I., Wisgerhof M. Polyol accumulations in nervous tissue of rats with experimental diabetes and galactosaemia. J Neurochem. 1967 Nov;14(11):1057–1066. doi: 10.1111/j.1471-4159.1967.tb09516.x. [DOI] [PubMed] [Google Scholar]
- Vorbrodt A. W., Lossinsky A. S., Wisniewski H. M. Cytochemical localization of ouabain-sensitive, K+-dependent p-nitro-phenylphosphatase (transport ATPase) in the mouse central and peripheral nervous systems. Brain Res. 1982 Jul 15;243(2):225–234. doi: 10.1016/0006-8993(82)90245-1. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Jean D. H., Whitaker J. N., McLaughlin B. J., Albers R. W. Immunocytochemical localization of the sodium, potassium activated ATPase in knifefish brain. J Neurocytol. 1977 Oct;6(5):571–581. doi: 10.1007/BF01205220. [DOI] [PubMed] [Google Scholar]
- Yoda A., Yoda S. A new simple preparation method for NaK-ATPase-rich membrane fragments. Anal Biochem. 1981 Jan 1;110(1):82–88. doi: 10.1016/0003-2697(81)90115-9. [DOI] [PubMed] [Google Scholar]