Abstract
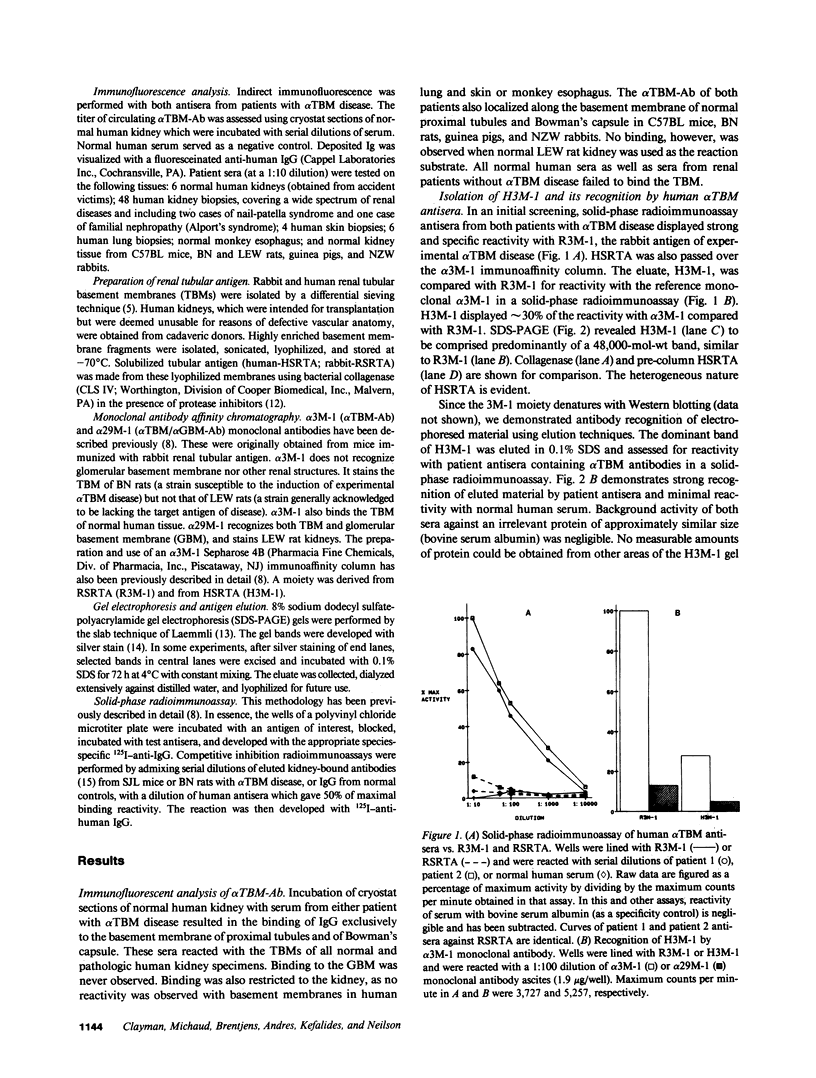
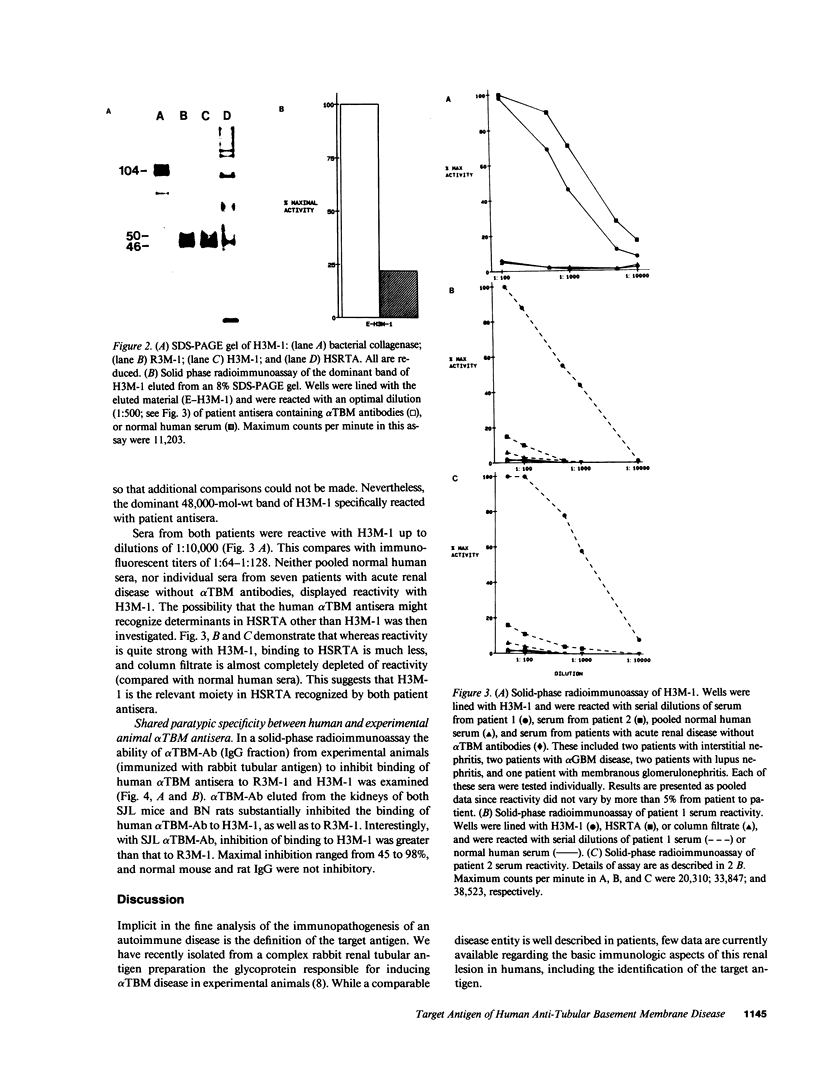
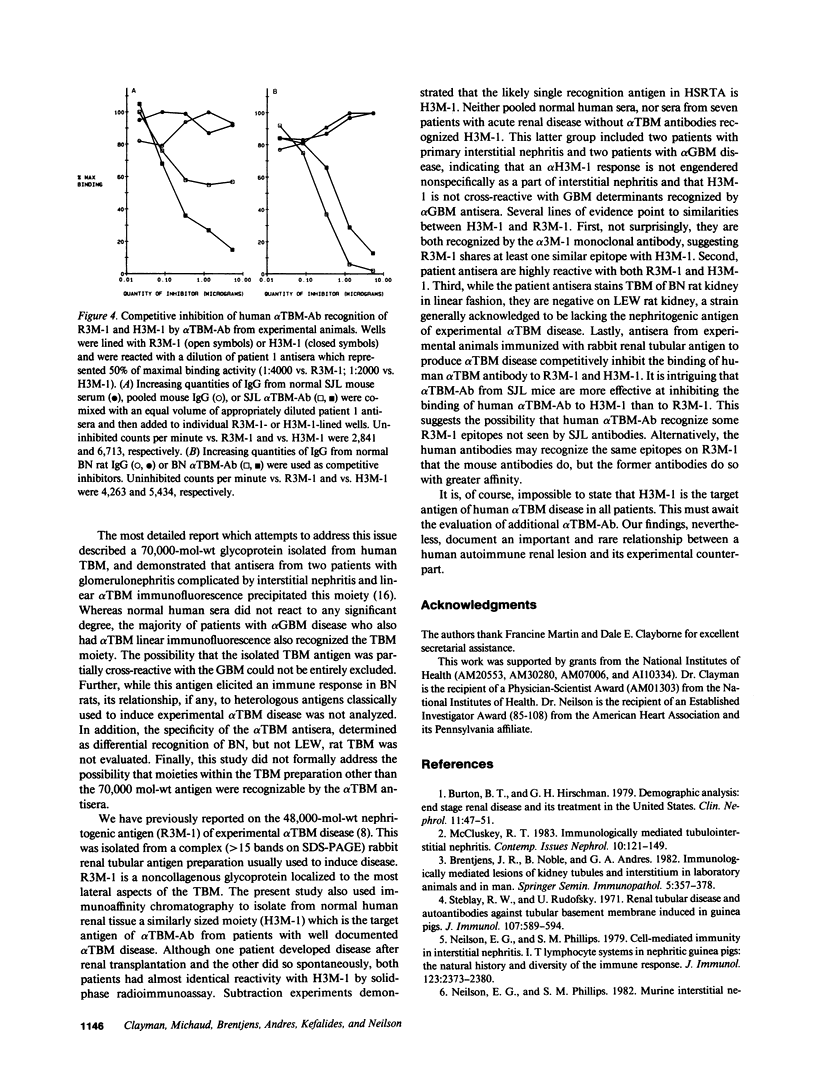
Using a monoclonal anti-tubular basement membrane antibody (alpha TBM-Ab) affinity column, we isolated from collagenase-solubilized human renal tissue (HSRTA) a predominantly 48,000-mol-wt moiety (H3M-1) which is selectively recognized by antisera from two patients with alpha TBM-Ab-associated interstitial nephritis (alpha TBM disease). Whereas both antisera had alpha TBM-Ab titers of 1:64-1:128 by immunofluorescence on tissue sections, their reactivity with H3M-1 in a solid-phase radioimmunoassay was demonstrable at dilutions up to 1:10,000. While these sera displayed some reactivity with pre-column HSRTA, this was markedly less than with H3M-1. HSRTA depleted of H3M-1 by passage over the alpha TBM-Ab affinity column was almost completely depleted of reactivity. Neither pooled normal human sera nor sera from patients with a variety of renal lesions not associated with alpha TBM-Ab (including interstitial nephritis and antiglomerular basement membrane disease) were reactive with H3M-1. Both patient antisera containing alpha TBM-Ab were also highly reactive with R3M-1, the 48,000-mol-wt rabbit glycoprotein antigen of experimental alpha TBM disease. Furthermore, a competitive inhibition radioimmunoassay revealed that alpha TBM-Ab from rodents with experimental alpha TBM disease could inhibit 45-98% of the R3M-1 binding reactivity of patient antisera and 85% of the H3M-1 binding reactivity of patient antisera, thus suggesting paratypic cross-reactivity. We conclude, therefore, that tubular basement membrane target epitopes and their paratypic recognition are highly conserved among mammals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergstein J., Litman N. Interstitial nephritis with anti-tubular-basement-membrane antibody. N Engl J Med. 1975 Apr 24;292(17):875–878. doi: 10.1056/NEJM197504242921701. [DOI] [PubMed] [Google Scholar]
- Border W. A., Lehman D. H., Egan J. D., Sass H. J., Glode J. E., Wilson C. B. Antitubular basement-membrane antibodies in methicillin-associated interstitial nephritis. N Engl J Med. 1974 Aug 22;291(8):381–384. doi: 10.1056/NEJM197408222910803. [DOI] [PubMed] [Google Scholar]
- Brentjens J. R., Noble B., Andres G. A. Immunologically mediated lesions of kidney tubules and interstitium in laboratory animals and in man. Springer Semin Immunopathol. 1982;5(3):357–378. doi: 10.1007/BF01892093. [DOI] [PubMed] [Google Scholar]
- Burton B. T., Hirschman G. H. Demographic analysis: end-stage renal disease and its treatment in the United States. Clin Nephrol. 1979 Feb;11(2):47–51. [PubMed] [Google Scholar]
- Clayman M. D., Martinez-Hernandez A., Michaud L., Alper R., Mann R., Kefalides N. A., Neilson E. G. Isolation and characterization of the nephritogenic antigen producing anti-tubular basement membrane disease. J Exp Med. 1985 Feb 1;161(2):290–305. doi: 10.1084/jem.161.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graindorge P. P., Mahieu P. R. Radioimmunologic method for detection of antitubular basement membrane antibodies. Kidney Int. 1978 Dec;14(6):594–606. doi: 10.1038/ki.1978.168. [DOI] [PubMed] [Google Scholar]
- Klassen J., Kano K., Milgrom F., Menno A. B., Anthone S., Anthone R., Sepulveda M., Elwood C. M., Andres G. A. Tubular lesions produced by autoantibodies to tubular basement membrane in human renal allografts. Int Arch Allergy Appl Immunol. 1973;45(5):675–689. doi: 10.1159/000231067. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Neilson E. G., Gasser D. L., McCafferty E., Zakheim B., Phillips S. M. Polymorphism of genes involved in anti-tubular basement membrane disease in rats. Immunogenetics. 1983;17(1):55–65. doi: 10.1007/BF00364289. [DOI] [PubMed] [Google Scholar]
- Neilson E. G., McCafferty E., Phillips S. M., Clayman M. D., Kelly C. J. Antiidiotypic immunity in interstitial nephritis. II. Rats developing anti-tubular basement membrane disease fail to make an antiidiotypic regulatory response: the modulatory role of an RT7.1+, OX8- suppressor T cell mechanism. J Exp Med. 1984 Apr 1;159(4):1009–1026. doi: 10.1084/jem.159.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson E. G., Phillips S. M. Cell-mediated immunity in interstitial nephritis. I. T lymphocyte systems in nephritic guinea pigs: the natural history and diversity of the immune response. J Immunol. 1979 Nov;123(5):2373–2380. [PubMed] [Google Scholar]
- Neilson E. G., Phillips S. M. Murine interstitial nephritis. I. Analysis of disease susceptibility and its relationship of pleiomorphic gene products defining both immune-response genes and a restrictive requirement for cytotoxic T cells at H-2K. J Exp Med. 1982 Apr 1;155(4):1075–1085. doi: 10.1084/jem.155.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steblay R. W., Rudofsky U. Renal tubular disease and autoantibodies against tubular basement membrane induced in guinea pigs. J Immunol. 1971 Aug;107(2):589–594. [PubMed] [Google Scholar]