Abstract
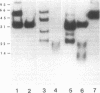
A partial gene product was identified in a pedigree with hemophilia B due to a partial deletion of the Factor IX gene (Chen, S.-H.,S. Yoshitake, P.F. Chance, G.L. Bray, A.R. Thompson, C.R. Scott, and K. Kurachi, 1985, J. Clin. Invest., 76:2161-2164). Levels of this mutant protein in plasma of affected family members studied ranged from 24 to 36 ng/ml (0.6-0.9 U/dl or percent of normal) by a solid-phase immunoassay which is sensitive and specific for the calcium-dependent conformation of human Factor IX. No Factor IX antigen could be detected in patients' plasmas by a non-calcium-requiring monoclonal anti-Factor IX antibody (less than 2 ng/ml). The unconcentrated urine from the five affected family members and four obligate heterozygotes the five affected family members and four obligate heterozygotes tested contained calcium-dependent Factor IX antigen levels ranging from 64 to 160 ng/ml (1.6-4.0 U/dl) and from 10 to 68 ng/ml (0.25-1.7 U/dl), respectively. Of nine normal volunteers screened, three had detectable calcium-dependent antigen in unconcentrated first morning-voided urines with 9.6-16.8 ng/ml (0.24-0.42 U/dl), while the remaining six had detectable urinary antigen only after a 10-fold concentration. Abnormal and normal urinary Factor IX antigen species were concentrated, immunoaffinity purified, electrophoresed, immunoblotted, and distinguished by autoradiography after incubation with 125I-polyclonal calcium-requiring anti-Factor IX. After reducing purified or concentrated samples, a single abnormal 36,000-mol-wt band was identified in the urines from the four affected family members and four obligate heterozygotes tested. Electrophoresis of the reduced urinary Factor IX antigen from the one normal subject tested showed a broad 15,000-20,000-mol-wt band. This normal band was smaller than the species in patients' urines, and was seen as a minor component in the samples from the heterozygotes. No abnormal antigen could be detected in urine from the two other female family members tested. Thus, abnormal urinary Factor IX antigen represents a marker for the presence of the hemophilic Factor IX gene in this family.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezeaud A., Guillin M. C. Quantitation of prothrombin activation products in human urine. Br J Haematol. 1984 Dec;58(4):597–606. doi: 10.1111/j.1365-2141.1984.tb06106.x. [DOI] [PubMed] [Google Scholar]
- Bray G. L., Weinmann A. F., Thompson A. R. Calcium-specific immunoassays for factor IX: reduced levels of antigen in patients with vitamin K disorders. J Lab Clin Med. 1986 Mar;107(3):269–278. [PubMed] [Google Scholar]
- Briët E., Reisner H. M., Roberts H. R. Inhibitors in Christmas disease. Prog Clin Biol Res. 1984;150:123–139. [PubMed] [Google Scholar]
- Chen S. H., Yoshitake S., Chance P. F., Bray G. L., Thompson A. R., Scott C. R., Kurachi K. An intragenic deletion of the factor IX gene in a family with hemophilia B. J Clin Invest. 1985 Dec;76(6):2161–2164. doi: 10.1172/JCI112222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli F., Choo K. H., Rees D. J., Boyd Y., Rizza C. R., Brownlee G. G. Gene deletions in patients with haemophilia B and anti-factor IX antibodies. Nature. 1983 May 12;303(5913):181–182. doi: 10.1038/303181a0. [DOI] [PubMed] [Google Scholar]
- Kasper C. K. Measurement of factor VIII inhibitors. Prog Clin Biol Res. 1984;150:87–98. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Liebman H. A., Limentani S. A., Furie B. C., Furie B. Immunoaffinity purification of factor IX (Christmas factor) by using conformation-specific antibodies directed against the factor IX-metal complex. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3879–3883. doi: 10.1073/pnas.82.11.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCTOR R. R., RAPAPORT S. I. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol. 1961 Sep;36:212–219. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- Peake I. R., Furlong B. L., Bloom A. L. Carrier detection by direct gene analysis in a family with haemophilia B (factor IX deficiency). Lancet. 1984 Feb 4;1(8371):242–243. doi: 10.1016/s0140-6736(84)90123-5. [DOI] [PubMed] [Google Scholar]
- Roberts H. R., Cromartie R. Overview of inhibitors to factor VIII and IX. Prog Clin Biol Res. 1984;150:1–18. [PubMed] [Google Scholar]
- Smith K. J. Monoclonal antibodies to coagulation factor IX define a high-frequency polymorphism by immunoassays. Am J Hum Genet. 1985 Jul;37(4):668–679. [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Thompson A. R. Labeled factor IX kinetics in patients with hemophilia-B. Blood. 1981 Sep;58(3):625–629. [PubMed] [Google Scholar]
- Thompson A. R. Factor IX and prothrombin in amniotic fluid and fetal plasma: constraints on prenatal diagnosis of hemophilia B and evidence of proteolysis. Blood. 1984 Oct;64(4):867–874. [PubMed] [Google Scholar]
- Thompson A. R. Factor IX antigen by radioimmunoassay. Abnormal factor IX protein in patients on warfarin therapy and with hemophilia B. J Clin Invest. 1977 May;59(5):900–910. doi: 10.1172/JCI108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. R. Factor XII and other hemostatic protein abnormalities in nephrotic syndrome patients. Thromb Haemost. 1982 Aug 24;48(1):27–32. [PubMed] [Google Scholar]
- Thompson A. R. Monoclonal antibody to an epitope on the heavy chain of factor IX missing in three hemophilia-B patients. Blood. 1983 Nov;62(5):1027–1034. [PubMed] [Google Scholar]
- Thompson A. R. Structure, function, and molecular defects of factor IX. Blood. 1986 Mar;67(3):565–572. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yoshitake S., Schach B. G., Foster D. C., Davie E. W., Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry. 1985 Jul 2;24(14):3736–3750. doi: 10.1021/bi00335a049. [DOI] [PubMed] [Google Scholar]