Abstract
Background
Prior economic analysis that compared the 12-gene assay to published patterns of care predicted the assay would improve outcomes while lowering medical costs for stage II, T3, mismatch-repair-proficient (MMR-P) colon cancer patients. This study assessed the validity of those findings with real-world adjuvant chemotherapy (aCT) recommendations from the US third-party payer perspective.
Methods
Costs and quality-adjusted life-years (QALYs) were estimated for stage II, T3, MMR-P colon cancer patients using guideline-compliant, state-transition probability estimation methods in a Markov model. A study of 141 patients from 17 sites in the Mayo Clinic Cancer Research Consortium provided aCT recommendations before and after knowledge of the 12-gene assay results. Progression and adverse events data with aCT regimens were based on published literature. Drug and administration costs for aCT were obtained from 2014 Medicare Fee Schedule. Sensitivity analyses evaluated the drivers and robustness of the primary outcomes.
Results
After receiving the 12-gene assay results, physician recommendations in favor of aCT decreased 22 %; fluoropyrimidine monotherapy and FOLFOX recommendations each declined 11 %. Average per-patient drugs, administration, and adverse events costs decreased $US2,339, $US733, and $US3,211, respectively. Average total direct medical costs decreased $US991. Average patient well-being improved by 0.114 QALYs. Savings are expected to persist even if the cost of oxaliplatin drops by >75 % due to generic substitution.
Conclusions
This study provides evidence that real-world changes in aCT recommendations due to the 12-gene assay are likely to reduce direct medical costs and improve well-being for stage II, T3, MMR-P colon cancer patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s40273-014-0207-1) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| The 12-gene assay provides additional recurrence risk information that influences physicians’ adjuvant chemotherapy recommendations in real-world clinical settings for stage II, T3, mismatch-repair-proficient (MMR-P) colon cancer patients. |
| Recommendation changes due to the 12-gene assay are likely to lead to both savings in direct medical costs and increased quality-adjusted survival. |
| Patients with lower risk of recurrence whose adjuvant treatment recommendations are changed away from adjuvant chemotherapy with the 12-gene assay avoid costly adverse events. |
Introduction
Colon cancer is the third most commonly diagnosed cancer in the US, and the third leading cause of cancer deaths [1]. The American Cancer Society projects 96,800 new colon cancer cases in 2014 [2]. Of patients with incident colon cancer, 20 % are expected to be diagnosed with stage II, T3 disease, characterized by a tumor that has spread into the outermost layers of the colon (T3), with no regional lymph node metastases (N0) or distant metastases (M0) [3–5]. These patients have a 67 % overall survival rate and an 88 % relative survival rate at 5 years [6].
Patients with stage II colon cancer are recommended to be treated with surgical tumor resection and optional adjuvant chemotherapy (aCT) [7]. Adjuvant treatment with 5-fluorouracil and leucovorin chemotherapy (5-FU/LV) has been found to improve survival compared with surgery alone for stage III colon cancer patients, but the recurrence risk reduction benefit among stage II colon cancer patients was not significant and remains uncertain [8]. The National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) guidelines indicate that aCT should not be routinely used for all eligible patients with stage II colon cancer, as only approximately one-fourth of those patients are expected to have a recurrence within 5 years [7, 9]. Instead, the adjuvant treatment decision-making process should take into account recurrence-risk factors such as bowel perforation or obstruction, poorly differentiated tumor, surgical margins, number of lymph nodes examined, and DNA mismatch-repair status.
A patient with stage II, T3, mismatch-repair-proficient (MMR-P), colon cancer who chooses aCT has several chemotherapy regimen options according to clinical guidelines, including 5-FU/LV, capecitabine, or combination chemotherapy with oxaliplatin (e.g., FOLFOX: 5-FU/LV and oxaliplatin) [7, 9]. Capecitabine was demonstrated to be equivalent to 5-FU/LV among stage III colon cancer patients [10]. FOLFOX has been shown to provide greater benefit than 5-FU/LV for stage III colon cancer patients; however, benefit was uncertain for stage II patients [11, 12]. Furthermore, the addition of oxaliplatin carried greater risks of acute and possibly irreversible adverse events [11, 12]. Guidelines indicate that FOLFOX may be considered for high-risk, stage II colon cancer, and is only appropriate if the recurrence risk reduction provided by the addition of oxaliplatin offsets the greater risks of associated adverse events.
The 12-gene assay (Oncotype DX® Colon Cancer Assay, Genomic Health, Inc., Redwood City, CA USA) is a tumor-tissue gene-expression assay that has been clinically validated in stage II colon cancer patients. It has been shown to predict patients’ risks of recurrence to inform treatment decisions for patients with T3, MMR-P tumors where conventional measures are not informative [13, 14]. In the clinical validation study using tumor blocks from the QUASAR (Quick and Simple and Reliable) randomized clinical trial, the 3-year risk of recurrence among patients with stage II, T3, MMR-P colon cancer who were treated with surgery alone was 16 % [13]. Despite this, without the 12-gene assay, 52 % of patients with stage II, T3, MMR-P colon cancer were recommended chemotherapy according to the recently completed clinical decision impact study [15].
Previous economic analyses comparing modelled aCT decisions using the 12-gene assay to published patterns of care from the NCCN Colon/Rectum Cancer Outcomes Database predicted the assay would improve outcomes while lowering medical costs [16]. Since then, the oxaliplatin drug cost has decreased following the patent expiry, and 4-year follow-up data showing long-term adverse events with FOLFOX have been reported from the MOSAIC (Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer) randomized clinical trial. Most importantly, the recently completed clinical decision impact study conducted across 17 sites in the Mayo Clinic Cancer Research Consortium (MCCRC) has shown that the 12-gene assay affects physicians’ aCT recommendations in the real world, reducing recommendations for aCT whether with fluoropyrimidine monotherapy or with combination chemotherapy with oxaliplatin [15]. This study assessed the validity of the results of the previous economic analysis using updated costs and real adjuvant therapy recommendations for patients with stage II, T3, MMR-P colon cancer before and after the 12-gene assay results were available.
Methods
Patient Population and Clinical Decision Impact
A clinical decision impact study assessed the effects of the 12-gene assay on physicians’ aCT recommendations for patients with stage II, T3, MMR-P colon cancer in clinical practice [15]. A total of 141 patients were enrolled across 17 sites in the MCCRC. All patients had undergone surgery and were eligible for aCT. The following data were extracted from this study for each of the enrolled patients: age, gender, 12-gene assay result (on a continuous scale of 0 for low to 100 for high), and aCT recommendations before and after the 12-gene assay results were available. Median patient age was 64 years (range 27–87); 58 (41 %) patients were female. The median 12-gene assay result was 25 (range 2–52). The continuous 12-gene assay results can be categorized into risk groups based on pre-specified cut-offs, which were provided to physicians in the results reports (a sample physician report is provided in the Electronic Supplementary Material [ESM] online resource 1). Using these criteria, 101 (72 %) patients were ‘low risk’ (result <30), 33 (23 %) were ‘intermediate risk’ (result 30–40), and seven (5 %) were ‘high risk’ (result ≥41). This economic analysis did not consider the risk categories, but used each patient’s individual continuous 12-gene assay result on the 0–100 scale.
Participating physicians in the MCCRC study recorded aCT recommendations before and after the 12-gene assay result was available. Treatment recommendations were recorded as one of three options. From least to most intense, the treatment options were (1) observation, (2) fluoropyrimidine monotherapy (5-FU/LV or capecitabine), or (3) combination chemotherapy with oxaliplatin. A change from option 1 to any other option, or from option 2 to option 3, was considered an increase in recommended treatment intensity; a change from option 3 to any other option, or from option 2 to option 1, was considered a decrease. Among patients recommended fluoropyrimidine monotherapy (option 2), 50 % were expected to receive the 5-FU/LV aCT regimen, and 50 % the single-agent oral capecitabine aCT regimen, based on the results of a survey of oncologists’ preferred aCT regimens for treatment of stage II colon cancer patients [17]. All patients recommended to combination chemotherapy with oxaliplatin (option 3) were expected to receive FOLFOX, specifically the mFOLFOX6 regimen, based on the survey of oncologists and the NCCN guidelines [7, 17]. After the 12-gene assay results were available, physician-recommended treatment intensity increased for 16 patients (11 %), and decreased for 47 (33 %) patients (Table 1). Recommendations in favor of aCT decreased 22 % (from 52 to 30 %); recommendations in favor of fluoropyrimidine monotherapy or FOLFOX regimens each decreased by approximately 11 %.
Table 1.
Adjuvant chemotherapy recommendation changes with the 12-gene assay
| Pre-assay recommendations | Post-assay recommendations, n (%) | Total | ||
|---|---|---|---|---|
| Observation | Fluoropyrimidine monotherapya | FOLFOXb | ||
| Observation | 54 (38.3) | 8 (5.7)* | 6 (4.3)* | 68 (48.2) |
| Fluoropyrimidine monotherapya | 24 (17.0)** | 8 (5.7) | 2 (1.4)* | 34 (24.1) |
| FOLFOXb | 21 (14.9)** | 2 (1.4)** | 16 (11.3) | 39 (27.7) |
| Total | 99 (70.2) | 18 (12.8) | 24 (17.0) | 141 (100.0) |
Clinical utility data were extracted from Srivastava et al. [15]
5-FU/LV 5-fluorouracil and leucovorin, FOLFOX 5-FU/LV and oxaliplatin
a5-FU/LV or capecitabine
bCombination chemotherapy with oxaliplatin
* Increased recommended treatment intensity
** Decreased recommended treatment intensity
Analytical Framework
The cost effectiveness of the 12-gene assay was evaluated from the US third-party payer perspective using a decision analytic framework according to international guidelines published jointly by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and the Society of Medical Decision Making, and the ISPOR Consolidated Health Economic Evaluation Reporting Standards (Fig. 1a, checklist in ESM 2) [18–25]. Each patient’s quality-adjusted survival and medical resource use were assessed over a lifetime using an annual cycle length given treatment recommendations with and without the 12-gene assay. Health states considered were ‘no recurrence’, ‘recurrence’, and ‘death’ (Fig. 1b). Direct medical costs included in the analysis were those for the 12-gene assay, aCT drugs and administration, management of aCT-related adverse events, monitoring, metastasis, and mortality.
Fig. 1.
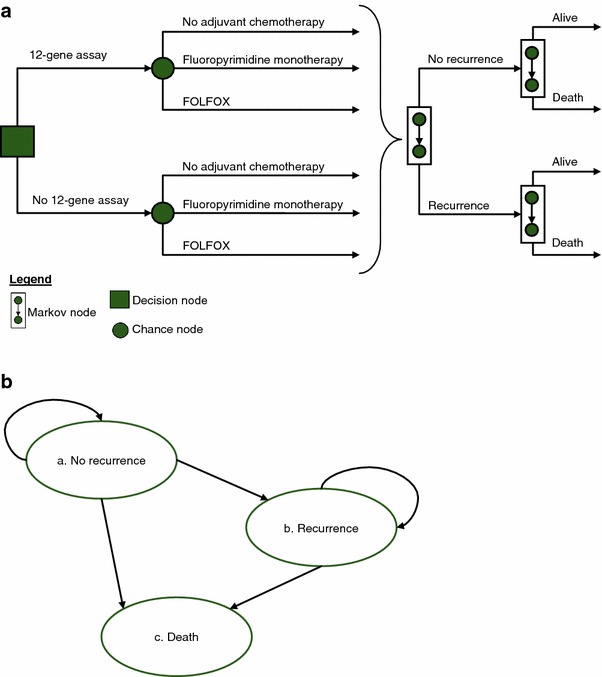
Analytic framework. a Decision analytic framework. In each annual cycle, a patient may transition to another health state due to recurrence or death. The level of 3-year recurrence risk is based on each patient’s 12-gene assay result. b Health state transitions. Transitions between health states may occur in the direction of the arrows. Transition probabilities based on data from the QUASAR randomized clinical trial and clinical validation study and whether or not the patient chose to undergo adjuvant chemotherapy (with usual care or also having available 12-gene assay results). FOLFOX 5-fluorouracil and leucovorin, and oxaliplatin, QUASAR Quick and Simple and Reliable
Each of the 141 patients in the MCCRC real-world clinical decision impact study was considered individually in the analysis. Transition probabilities were assigned based on each patient’s continuous 12-gene assay score, age, gender, and physician’s aCT recommendations. The patient’s baseline risk of recurrence was based on his or her continuous 12-gene assay score. Mortality rates with and without metastases were based on the patient’s age and gender. The risk of adverse events and the reduction in recurrence risk with aCT were based on the treatments recommended to that patient by his or her physician before and after the 12-gene assay results were made available. Outcomes were averaged across all patients before and after the 12-gene assay to determine the assay’s effects.
Data Sources
Data were extracted from US Government agencies’ databases and publications, and peer-reviewed literature. The PubMed and the Tufts Cost-Effectiveness Analysis (CEA) registry databases were searched for articles published between 1966 and August 2012 on recurrence and mortality risks, costs of treatment, adverse events, metastasis, and mortality, and patients’ quality of life. Newer data were preferred to better reflect clinical practice, and longer-term data were preferred for adverse events and quality of life to capture the effects of chronic adverse events.
Transition Probabilities
The QUASAR clinical trial randomized patients with stage II colon cancer treated with surgery to either observation or aCT with 5-FU/LV [8]. A study using tumor blocks from these patients validated the ability of the 12-gene assay to predict the likelihood of recurrence [13]. Using the 3-year recurrence risk without aCT by 12-gene assay result observed among patients with T3, MMR-P tumors in the clinical validation trial (Fig. 2) [26], a constant annual recurrence rate was calculated for each patient based on his or her continuous 12-gene assay result; the annual rate was applied over the patient’s lifetime.
Fig. 2.
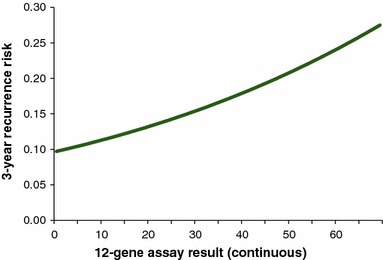
Three-year recurrence risk by 12-gene assay result for stage II, T3, MMR-P colon cancer patients. Data from the QUASAR clinical validation trial was extracted from Kerr et al. [26]. MMR-P DNA mismatch repair proficient, QUASAR Quick and Simple and Reliable
The recurrence-reduction benefit with aCT in the stage II colon cancer population is uncertain [8, 11, 12]. However, the NCCN guidelines recommend, based on available evidence, that aCT be considered for patients with stage II colon cancer who are considered at high risk for recurrence after surgery [7]. Since the 12-gene assay reduces aCT use, this analysis conservatively assumed that aCT does provide benefit in the stage II colon cancer treatment setting, even though the recurrence-risk-reduction results of randomized clinical trials did not reach significance.
Relative reductions in recurrence risk specific to each aCT regimen were extracted from randomized clinical trials. The relative risk reduction with fluoropyrimidine monotherapy (5-FU/LV or capecitabine) compared with observation for stage II colon cancer patients was extracted from the QUASAR randomized clinical trial (Table 2) [8]. In the absence of randomized clinical trial data on capecitabine aCT in the stage II colon cancer population, the relative risk reduction with capecitabine compared with observation was assumed to be equivalent to the relative risk reduction with 5-FU/LV in accordance with the NCCN guidelines’ interpretation of the X-ACT (Xeloda in Adjuvant Colon Cancer Therapy) randomized clinical trial of stage III colon cancer patients [7, 10]. The relative risk reduction with FOLFOX compared with fluoropyrimidine monotherapy for stage II colon cancer patients was extracted from the MOSAIC and the NSABP (National Surgical Adjuvant Breast and Bowel Project) C-07 randomized clinical trials [11, 12].
Table 2.
Input parameter values and ranges
| Input parameter | Value | Sensitivity analysis range | |||
|---|---|---|---|---|---|
| Low | High | Reason | Distribution | ||
| Change in aCT use due to 12-gene assay [15] (%) | |||||
| Any aCT (fluoropyrimidine monotherapy or FOLFOX) | −22 | −11.0 | −32.0 | 95 % CI | Normal |
| % of recommendations for aCT that are for FOLFOX | 3.7 | 2.8 | 4.6 | ±25 % | Normal |
| Relative risk reduction | |||||
| Fluoropyrimidine monotherapy vs. surgery only [8] | 18 | 0 | 37 | No benefit, 95 % CI | Log normal |
| FOLFOX vs. fluoropyrimidine monotherapy [11, 12] | 12 | 0 | 35 | No benefit, 95 % CI | Log normal |
| Costs ($US) | |||||
| 12-gene assay | 3,640 | ||||
| Fluoropyrimidine monotherapy | |||||
| Drugs [7, 32] | 17,756 | 13,317 | 22,195 | ±25 % | Normal, ≥0 % |
| Administration [31, 34] | 2,060 | 1,545 | 2,575 | ±25 % | Normal, ≥0 % |
| Adverse events [11, 38, 39] | 6,186 | 4,639 | 7,732 | ±25 % | Normal, ≥0 % |
| FOLFOX | |||||
| Drugs [7, 32] | 3,050 | 763 | 3,813 | −75 %, +25 % | Normal, ≥0 % |
| Administration [31, 34] | 4,696 | 3,522 | 5,870 | ±25 % | Normal, ≥0 % |
| Adverse events [11, 38, 39] | 23,589 | 17,692 | 29,486 | ±25 % | Normal, ≥0 % |
| Long-term costs | |||||
| Metastasis (total from diagnosis to death) [42] | 152,251 | 114,188 | 190,313 | ±25 % | Normal, ≥0 % |
| No metastasis [40] | |||||
| Monitoring (annual) | 2,792 | 2,094 | 3,491 | ±25 % | Normal, ≥0 % |
| Non-cancer-related death (12 months prior to death) | 13,775 | 10,331 | 17,219 | ±25 % | Normal, ≥0 % |
| Quality of life [11, 12, 43–47] | |||||
| Utilitiesa | |||||
| No aCT or recurrence | 0.87 | 0.65 | 1.00 | −25 %, perfect health | Beta |
| Recurrence | 0.42 | 0.32 | 0.53 | ±25 % | Beta |
| QALY decrements with adjuvant chemotherapyb | |||||
| Fluoropyrimidine monotherapy | 1.0 | 0.75 | 1.25 | ±25 % | Normal |
| FOLFOX | 1.1 | 0.82 | 1.37 | ±25 % | Normal |
| Annual mortality rate after recurrence [28] (%) | |||||
| Females | |||||
| <45 years old | 39 | 29 | 48 | ±25 % | Normal, ≥0 % |
| 45–54 years old | 43 | 32 | 53 | ±25 % | Normal, ≥0 % |
| >54 years old | 70 | 52 | 87 | ±25 % | Normal, ≥0 % |
| Males | |||||
| <45 years old | 44 | 33 | 55 | ±25 % | Normal, ≥0 % |
| 45–54 years old | 45 | 34 | 56 | ±25 % | Normal, ≥0 % |
| >54 years old | 60 | 45 | 75 | ±25 % | Normal, ≥0 % |
| Other assumptions | |||||
| Drug cost over average sales price | 6 | 4.5 | 7.5 | ±25 % | Normal, ≥0 % |
| Time preference discount [30] | 3 | 1 | 5 | ISPOR guidelines | Normal, ≥0 % |
All costs reported in $US, year 2014 values
aCT adjuvant chemotherapy, CI confidence interval, FOLFOX 5-fluorouracil and leucovorin, and oxaliplatin, ISPOR International Society for Pharmacoeconomics and Outcomes Research, QALY quality-adjusted life-year
aOn a 0–1 scale where 0 is death and 1 is best attainable health
bOne-time decrement to quality-adjusted survival
The clinical validation trial demonstrated that the 12-gene assay result was not significantly associated with the relative reduction in recurrence risk with fluoropyrimidine monotherapy [13]. Therefore, in this analysis, the same relative risk reductions specific to fluoropyrimidine monotherapy or FOLFOX were applied for all patients recommended to receive each aCT, regardless of their 12-gene assay results. This also implied that the absolute reductions in recurrence risk with either aCT increased with higher baseline recurrence risks (i.e., with higher 12-gene assay results).
Annual mortality rates due to causes other than colon cancer were age and gender dependent, and were based on vital statistics for the US reported by the Centers for Disease Control and Prevention (Table 2) [27]. Mortality rates after recurrence were also age and gender dependent based on an analysis of patients with metastatic colon cancer in the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) database [28].
Costs
All costs were standardized to $US, year 2014 values. Costs reported in literature in earlier currencies were inflated to 2014 values using consumer indices for medical care published by the US Department of Labor, Bureau of Labor Statistics [29]. A 3 % time preference discount rate was applied to future costs and benefits as recommended in the Good Research Practices for Cost-Effectiveness Analysis Alongside Clinical Trials report by the ISPOR [30].
The list price of the 12-gene assay was $US3,640 (Table 2); actual costs to payers may vary. Drug dosages and administration schedules for fluoropyrimidine monotherapy and FOLFOX were based on the NCCN guidelines for colon cancer and the published recommendations of a certified professional medical reimbursement coder [7, 31]. Drug costs were from the average sales prices most recently published by the Centers of Medicare and Medicaid Services (CMS) (April–June 2014) to ensure that the analysis accounted for the effect of the oxaliplatin patent expiration on price [32]. CMS reimbursement for drugs is 6 % over the average sales price [33]. Administration costs are from the 2014 CMS Physician Fee Schedule [34]. Treatment compliance was estimated to be 95 % for all regimens based on studies of therapy completion and number of cycles completed by stage III colon cancer patients who begin aCT [35–37]. In total, the aCT-related cost for fluoropyrimidine monotherapy was $US26,002 per patient treated, compared with $US31,335 for FOLFOX (Table 2). The drug cost for fluoropyrimidine monotherapy ($US17,756) was higher than that for FOLFOX ($US3,050) due to the high cost of drugs for capecitabine aCT ($US34,579).
Published randomized clinical trials and analyses of medical costs and claims provided aCT-related adverse event incidences and management costs [11, 12, 38, 39]. Adverse event management costs were $US17,403 higher with FOLFOX than with fluoropyrimidine monotherapy ($US23,589 vs. 6,186) due to higher risks associated with FOLFOX for serious adverse events including peripheral sensory neuropathy (PSN) during and up to 4 years after treatment. PSN management cost $US3,542 in the first 6 months and $US3,244 annually afterwards for patients whose symptoms do not remit [38]. Patients who did not receive aCT avoided these risks and costs. Additional details on adverse event costs are available in ESM 3.
Long-term costs for monitoring, metastasis, and death were from analyses of patient claims databases. The annual cost of monitoring and the cost of the last year of life prior to a non-cancer-related death for patients diagnosed with stage II colon cancer were extracted from an analysis of direct medical costs for these patients compared with matched non-cancer controls in the SEER-Medicare database [40]. The total cost of metastasis—from diagnosis until death, and including treatment with biologics—was derived from a published analysis of the paid amounts of adjudicated claims for metastatic colorectal cancer patients in the Thomson Reuters MarketScan® Commercial Claims and Encounter Database and the Medicare Supplemental Coordination of Benefits Database, and adjusted for the decreased cost of FOLFOX with generic oxaliplatin [17, 34, 41, 42].
Quality of Life
Patient benefit was evaluated through change in quality-adjusted survival. This was calculated as the sum of health-utility scores multiplied by years spent in each health state over a lifetime, less the disutility associated with aCT treatment. Each health state (no recurrence, recurrence, death) was assigned a health-utility score, ranging from 0 (death) to 1 (best attainable health), extracted from a published time trade-off survey of colon cancer patients in the US (Table 2) [43].
The effect of aCT treatment on quality of life was incorporated as a one-time decrement in the year treatment was administered. This decrement with aCT was derived from a time trade-off survey study by investigators in Australia of 100 patients with stage II or III colon cancer who had completed 5-FU/LV (83 %) or FOLFOX (17 %) aCT within the previous 3–60 months [44]. The additional survival necessary to make aCT worthwhile to these patients was approximately 14.0 months on average (12.2 quality-adjusted months, or 1.0 quality-adjusted life-year [QALY], given a 0.87 utility of remission).
Quality of life has not been studied for aCT regimens independently. The time trade-off study of aCT [44], along with the difference between reported adverse event rates in randomized clinical trials of these aCT regimens [11, 12], and published disutilities related to adverse events [43, 45–47], were used to calculate individual decrements for each aCT regimen. To estimate the decrement to quality of life with fluoropyrimidine monotherapy, capecitabine was again considered equivalent to 5-FU/LV [7, 10]. Published disutilities assessed using time trade-off methods were preferred for consistency, but were not always available in literature; other studies addressing the quality-of-life impact of adverse events included qualitative interviews, prior CEA assumptions, and standard gamble studies. Additional details on the decrements to quality of life with fluoropyrimidine monotherapy treatment compared with FOLFOX treatment are available in ESM 3.
Sensitivity Analyses
One-way and probabilistic sensitivity analyses assessed the robustness of the results. Each parameter was assigned a distribution based on its specific type and a range based on (1) the MCCRC decision impact study if applicable, (2) 95 % confidence intervals (CIs) for parameters extracted from published literature if reported, or (3) guideline or broad ±25 % ranges otherwise (Table 2) [48, 49]. The drug costs for FOLFOX were varied across a larger range (−75 %, +25 %) due to the recent oxaliplatin patent expiry [50, 51].
One-way sensitivity analyses varied each parameter separately across its individual range to determine the parameters whose ranges most changed the primary endpoints. Probabilistic sensitivity analyses assessed robustness using 1,000 second-order Monte Carlo simulations. The probabilities of cost savings and cost effectiveness were evaluated for different cost-effectiveness thresholds. These thresholds represented different valuations for a gain (willingness to pay [WTP]) or loss (willingness to accept [WTA]) of a year of quality-adjusted survival [49]. Though the exact relationship between WTP and WTA is unclear, it has been shown that people require more savings to accept a loss in quality-adjusted survival than they are willing to pay for a gain of equal magnitude (i.e., WTA > WTP) [52]. The probability of cost effectiveness was evaluated given three different relationships between WTP and WTA. In the first, the value of a year of quality-adjusted survival does not change depending on whether it is gained or lost (WTA = WTP). In the second, society requires savings equal to four times the value of a gain in quality-adjusted survival to accept a loss of identical magnitude (WTA = 4 × WTP). In the third, society is unwilling to accept making patients worse off, regardless of the magnitude of cost savings (WTA = infinity).
Results
Main Analysis
The decrease in recommendations toward aCT with the addition of the 12-gene assay improved quality-adjusted survival by 0.114 years per patient on average (Table 3). The reduction in adverse events translated to an average gain of 0.230 years in quality-adjusted survival per patient. This was offset by a smaller decrease associated with recurrence due to the shift toward less intense treatments with smaller recurrence risk-reduction benefits (e.g., observation instead of aCT, particularly FOLFOX).
Table 3.
Impact of the 12-gene assay per patient on average
| Description | Before 12-gene assay | After 12-gene assay | Difference |
|---|---|---|---|
| Use of adjuvant chemotherapy (%) | |||
| Fluoropyrimidine monotherapy | 24.1 | 12.8 | −11.3 |
| FOLFOX | 27.7 | 17.0 | −10.6 |
| Quality-adjusted survival | |||
| aCT related | −0.545 | −0.315 | 0.230 |
| Recurrence related | 8.546 | 8.430 | −0.117 |
| Total | 8.001 | 8.115 | 0.114 |
| Costs ($US) | |||
| 12-gene assay | 3,640 | 3,640 | |
| Acute aCT related | |||
| Drugs | 5,125 | 2,786 | −2,339 |
| Administration | 1,796 | 1,062 | −733 |
| Adverse events | 8,016 | 4,805 | −3,211 |
| Long term | 89,829 | 91,482 | 1,653 |
| Total | 104,767 | 103,775 | −991 |
All costs reported in $US, year 2014 values
aCT adjuvant chemotherapy, FOLFOX 5-fluorouracil and leucovorin, and oxaliplatin
With the 12-gene assay, lifetime direct medical costs decreased $US991 per patient (Table 3). The per-patient cost of drugs, administration, and adverse event management decreased by $US2,339, $US733, and $US3,211, respectively. These offset the increases in long-term costs for monitoring, metastasis, and/or death ($US1,653) and the cost of the 12-gene assay ($US3,640), leading to overall savings.
Most of the patient benefits and savings with the 12-gene assay were accrued in the first year with the decreased likelihood of aCT. Some additional benefit was accrued in following years as patients who avoided FOLFOX also avoided the risks of chronic adverse events, which have been documented at 4-years follow-up after treatment. At 5 years after diagnosis, the quality-adjusted survival increases and cost savings associated with avoiding aCT were fully realized, but some of the offsets due to recurrence occur after 5 years, and were not yet accrued. At a time horizon of 5 years, the 12-gene assay increased quality-adjusted survival by 0.214 QALYs, and reduced costs by $US1,424.
Sensitivity Analyses
The one-way sensitivity analysis showed quality-adjusted survival improvements with the 12-gene assay across all input ranges (Fig. 3a). The parameters whose variation most affected quality-adjusted survival were the (1) benefit of fluoropyrimidine monotherapy over surgery alone, (2) benefit of FOLFOX over fluoropyrimidine monotherapy, and (3) time preference discount rate.
Fig. 3.
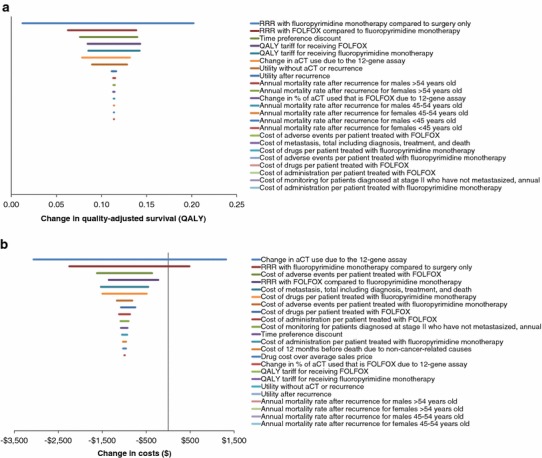
One-way sensitivity analyses. a Quality-adjusted survival impact of the 12-gene assay. b Cost impact of the 12-gene assay. Inputs listed from top to bottom in order of magnitude of influence on outcome. Horizontal lines show range of outcome across range of input. aCT adjuvant chemotherapy, FOLFOX 5-fluorouracil and leucovorin, and oxaliplatin, QALY quality-adjusted life-year, RRR relative risk reduction
The effect of the 12-gene assay on direct medical costs changed the most when the following parameters were varied: (1) change due to the 12-gene assay in the proportion of patients recommended toward aCT, (2) benefit of fluoropyrimidine monotherapy over surgery alone, and (3) cost to manage adverse events associated with FOLFOX (Fig. 3b). Cost savings are expected if recommendations in favor of aCT decrease by more than 17 %. If recommendations toward aCT decreased by 11 % (clinical decision impact study demonstrated a 22 % decrease [95 % CI 11–32]), the 12-gene assay was cost effective at $US16,782 per QALY gained.
The probabilistic sensitivity analysis showed a 76.1 % probability of cost savings and quality-adjusted survival improvement with the 12-gene assay (quadrant IV in Fig. 4a). Quality-adjusted survival gains were shown in 96.1 % of scenarios; therefore, the relationship between WTA and WTP did not affect the probability of cost effectiveness by more than 3.9 %. At a cost-effectiveness threshold of $US50,000 per 1 QALY gain, the probability of cost effectiveness was at least 95.5 % (Fig. 4b).
Fig. 4.
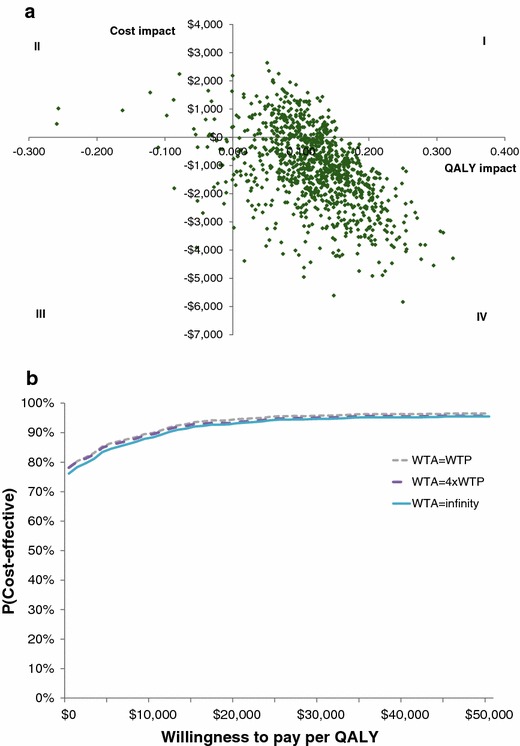
Probabilistic sensitivity analyses. a Cost-effectiveness plane. b Probability of cost effectiveness by threshold. 1,000 simulations were performed. WTP willingness to pay per additional QALY gained, QALY quality-adjusted life-year, WTA willingness to accept cost savings per QALY lost
Discussion
A previous study investigated the effect of the 12-gene assay on patient outcomes and costs by comparing the assay with patterns of aCT use published by the NCCN [16]. This new analysis built upon previous work by incorporating real-world clinical treatment recommendations and by considering different types of aCT (fluoropyrimidine monotherapy, FOLFOX) as different treatment options. Each option was associated with a different relative recurrence risk reduction, and different costs for drugs, administration, and adverse event management. In considering different types of aCT separately, this analysis also incorporated recently published findings from the MOSAIC study, which reported the prevalence of long-term PSN observed 48 months after FOLFOX treatment [11]. The analysis also accounted for the dramatically decreased cost of oxaliplatin after the recent patent expiry [50, 51]. In total, use of the 12-gene assay increased average quality-adjusted survival by 0.114 years and lowered direct medical costs by $US991 per patient.
Drug and administration costs decreased with the use of the 12-gene assay, but the largest magnitude of savings was due to the reduction in adverse events. The cost savings with the 12-gene assay were in large part due to changes in physicians’ recommendations, which redirected patients away from the increased risks of serious, costly, and possibly chronic adverse events with aCT, particularly FOLFOX. The cost to manage adverse events was nearly four times higher with FOLFOX than with fluoropyrimidine monotherapy. Randomized clinical trials of FOLFOX and 5-FU/LV have shown higher rates of acute adverse events and chronic PSN, persisting even 48 months after treatment with FOLFOX [12]. For a FOLFOX-treated patient who experienced chronic PSN unremitting 4 years after aCT, PSN management costs would total $US16,517 [38]. The ability of the 12-gene assay to change physicians’ aCT recommendations not only away from aCT in general, but away from FOLFOX specifically, contributed to the cost-saving result. This was reinforced by the one-way sensitivity analysis, which highlighted the cost of adverse event management with FOLFOX as one of the parameters whose variation most changed the magnitude of the cost savings with the 12-gene assay.
Sensitivity analyses demonstrated the robustness of the quality-adjusted survival benefit and cost-saving results. The recent oxaliplatin patent expiry led to large decreases in drug cost; the payment allowance limit published by CMS for 0.5 mg of oxaliplatin decreased from $US9.245 to $US0.535 in 1 year (October 2012 to October 2013) [50, 51]. This analysis used data from the most recent file (effective April–June 2014), in which the payment allowance limit for 0.5 mg of oxaliplatin was $US0.564. Savings with the 12-gene assay were not sensitive to the cost of drugs for FOLFOX, which ranked eighth in effect on the cost impact in the one-way sensitivity analysis. When the cost of all chemotherapy drugs for the FOLFOX regimen was decreased by 75 % to $US763, the analysis showed $US748 savings per patient on average. Savings are expected to persist even if the decreasing trend in oxaliplatin drug cost continues. Probabilistic sensitivity analyses showed that the probability of both cost savings and increased quality-adjusted survival with the 12-gene assay was 76.1 %.
The results of this analysis of the aCT recommendations for stage II, T3, MMR-P colon cancer patients treated across 17 sites in the MCCRC were similar to those of a previous analysis comparing the 12-gene assay with NCCN-published treatment patterns. The prior study predicted aCT use would decrease by 17 % [16]. In real-world clinical settings, a 22 % decrease in recommendations toward aCT was observed after the 12-gene assay results were available [15]. This analysis also differed from the prior study by using data on aCT-related quality-of-life decrement from a larger survey specific to this target population (patients with non-metastatic colon cancer treated with aCT) [44]. The prior study used utilities data for any chemotherapy, elicited from a smaller survey of community members and patients with stage II–IV colorectal cancer, and did not include methods to allow for valuing health states worse than death [43]. In this analysis, the larger quality-of-life decrement with aCT and the greater decrease in aCT use compared with the prior study led to a greater quality-adjusted survival improvement (0.114 QALYs here vs. 0.032 previously predicted). Despite the larger decrease in aCT use, cost savings in this analysis persisted but were smaller in magnitude ($US991 here vs. $US3,237 [$US2,971 in 2011 values] previously predicted), in large part due to the lower drug cost of newly generic oxaliplatin. Both analyses indicated that the quality-adjusted survival improvement and cost savings were robust. This analysis, which incorporated data on the effect of the 12-gene assay on clinical aCT recommendations in real-world clinical settings, confirmed the cost-saving result and showed patient benefits greater than previously projected.
Interpretation of these results is limited by the availability of data on patients’ preferences for aCT treatment, especially with newer agents. In a survey of 100 stage II or III colon cancer patients treated with adjuvant 5-FU/LV or FOLFOX, the quality-of-life decrement with any aCT regimen was found to be greater than 1 QALY on average [44]. However, the quality-of-life effects of specific aCT regimens have not been directly surveyed. The differences in adverse event risks with different aCT regimens suggest that there may be large differences in effects on quality of life of these regimens. This analysis estimated the difference between the quality-of-life effects of fluoropyrimidine monotherapy and FOLFOX based on the differences in adverse events incidence in randomized clinical trials. Patients’ preferences may be influenced by additional factors that were not captured here. Quality-adjusted survival benefits with the 12-gene assay persisted across the ranges of all parameters in the one-way sensitivity analysis, indicating that the findings were robust. Even so, given the large decrement in quality of life associated with aCT, and the differences in adverse event risks and costs between the fluoropyrimidine monotherapy regimens and FOLFOX, it could be helpful to better understand the patient experience with aCT to improve adjuvant treatment decision making for patients with colon cancer.
Guidelines from NCCN recommend that aCT for stage II colon cancer should be reserved for patients considered at high risk of recurrence. Distinguishing between patients at low risk of recurrence and those at high risk is uncertain despite the use of current clinicopathological factors (e.g., tumor size, histopathology). Consequently, some patients receive aCT when the benefits are likely negligible. The 12-gene assay has more discriminating ability than traditional risk factors for identifying patients with sufficiently low risk to avoid aCT [13, 14]. Prior analysis of treatment patterns showed the 12-gene assay was likely to lead to quality-adjusted survival improvements and cost savings compared with NCCN-published treatment patterns [16]. The recently published decision impact study revealed that physicians are comfortable foregoing chemotherapy for patients if the 12-gene assay reveals low risk, even if those patients would be considered at intermediate risk with traditional clinicopathological factors alone. This analysis demonstrated that the physicians’ use of the 12-gene assay spared patients from unnecessary toxicity risks, increased quality-adjusted survival, and was cost saving.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by Genomic Health, Inc. (Redwood City, CA, USA). Cedar Associates received grant funding for the design and conduct of the study, and for preparation of the manuscript. The authors would like to acknowledge Dr. Calvin Chao, Dr. Haluk Tezcan, and Ms. Margarita Lopatin, employed at Genomic Health, for providing data on the risk of recurrence by the continuous scale result of the 12-gene assay, and suggestions to the analysis.
Author contributions
JH and TY contributed to the cost-effectiveness analysis design, constructed the Markov analysis, and drafted the manuscript. SA, RB, LR, GSrivastava, GSoori, SD, RM, JK, GK, and MM participated in the collection and interpretation of clinical data, and contributed to the clinical validation of the cost-effectiveness analysis. Corresponding author JH is the guarantor for the overall content.
Conflict of interest
Genomic Health, Inc. (Redwood City, CA, USA) sponsored the study. JH and TY are employees of Cedar Associates, LLC, an independent research consulting firm retained by the sponsor. SA, RB, LR, GSrivastava, GSoori, SD, RM, JK, GK, and MM have no conflicts to declare.
References
- 1.Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Colorectal cancer facts and figures 2014–2016. American Cancer Society, Atlanta; 2014. http://www.cancer.org/acs/groups/content/documents/document/acspc-042280.pdf. Accessed April 11 2014.
- 3.American Joint Committee on Cancer. AJCC cancer staging manual, 7th edition: colon and rectum cancer staging. http://www.cancerstaging.org/staging/posters/colon8.5x11.pdf. Accessed November 29 2011.
- 4.National Cancer Institute. Surveillance epidemiology and end results SEER*Stat Software Version 8.1.5. 2014. http://seer.cancer.gov/seerstat/. Accessed June 18 2014.
- 5.United States Census Bureau. National population projections: middle series. 2012. http://www.census.gov/population/projections/data/national/2012/downloadablefiles.html. Accessed June 18 2014.
- 6.Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28(2):264–271. doi: 10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: colon cancer version 3.2013. 2013. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed June 26 2013.
- 8.Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 9.Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 10.Twelves C, Wong A, Nowacki MP, Abt M, Burris H, 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352(26):2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 11.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 12.Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29(28):3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray RG, Quirke P, Handley K, Lopatin M, Magill L, Baehner FL, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol. 2011;29(35):4611–4619. doi: 10.1200/JCO.2010.32.8732. [DOI] [PubMed] [Google Scholar]
- 14.Venook AP, Niedzwiecki D, Lopatin M, Ye X, Lee M, Friedman PN, et al. Biologic determinants of tumor recurrence in stage II colon cancer: validation study of the 12-gene recurrence score in cancer and leukemia group B (CALGB) 9581. J Clin Oncol. 2013;31(14):1775–1781. doi: 10.1200/JCO.2012.45.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava G, Renfro LA, Behrens RJ, Lopatin M, Chao C, Soori GS, et al. Prospective multicenter study of the impact of oncotype DX colon cancer assay results on treatment recommendations in stage II colon cancer patients. Oncologist. 2014;19(5):492–497. doi: 10.1634/theoncologist.2013-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornberger J, Lyman GH, Chien R, Meropol NJ. A multigene prognostic assay for selection of adjuvant chemotherapy in patients with T3, stage II colon cancer: impact on quality-adjusted life expectancy and costs. Value Health. 2012;15(8):1014–1021. doi: 10.1016/j.jval.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Interactive Clinical Intelligence. OncoReport: Medical Oncology, 3rd Trimester. 2010.
- 18.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM modeling good research practices task force–6. Value Health. 2012;15(6):835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices–overview: a report of the ISPOR-SMDM modeling good research practices task force–1. Value Health. 2012;15(6):796–803. doi: 10.1016/j.jval.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force–7. Value Health. 2012;15(6):843–850. doi: 10.1016/j.jval.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS)-explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Moller J. Modeling using discrete event simulation: a report of the ISPOR-SMDM modeling good research practices task force–4. Value Health. 2012;15(6):821–827. doi: 10.1016/j.jval.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM modeling good research practices task force–5. Value Health. 2012;15(6):828–834. doi: 10.1016/j.jval.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M. Conceptualizing a model: a report of the ISPOR-SMDM modeling good research practices task force–2. Value Health. 2012;15(6):804–811. doi: 10.1016/j.jval.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force–3. Value Health. 2012;15(6):812–820. doi: 10.1016/j.jval.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Kerr D, Gray R, Quirke P, Watson D, Yothers G, Lavery I, et al. A quantitative multi-gene RT-PCR assay for prediction of recurrence in stage II colon cancer: selection of the genes in 4 large studies and results of the independent, prospectively-designed QUASAR validation study. J Clin Oncol. 2009;27(15s):suppl; abstr 4000.
- 27.Arias E. National Vital Statistics Reports Volume 61, Number 3: United States Life Tables, 2008. 2012. http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_03.pdf. Accessed November 8 2012. [PubMed]
- 28.Hendifar A, Yang D, Lenz F, Lurje G, Pohl A, Lenz C, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009;15(20):6391–6397. doi: 10.1158/1078-0432.CCR-09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States Department of Labor, Bureau of Labor Statistics. Consumer price index—all urban consumers, medical care. 2014. http://www.bls.gov/data/. Accessed April 29 2014.
- 30.Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA task force report. Value Health. 2005;8(5):521–533. doi: 10.1111/j.1524-4733.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 31.Parman C. Coding: the 7 deadly sins of infusion center documentation. In: Oncology Issues. 2010. http://www.accc-cancer.org/education/pdf/FILN/FILN-4-The-7-Deadly-Sins-of-Infusion-Center-Documentation.pdf.
- 32.Centers for Medicare and Medicaid Services. Payment Allowance Limits for Medicare Part B Drugs, Effective April 1, 2014 through June 30, 2014. 2014. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2014ASPFiles.html. Accessed April 29 2014.
- 33.Centers for Medicare and Medicaid Services. Medicare part B drug average sales price. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/. Accessed June 30 2014.
- 34.Centers for Medicare and Medicaid Services. Physician fee schedule. 2014. http://www.cms.gov/apps/physician-fee-schedule/. Accessed April 29 2014.
- 35.Chapuis PH, Bokey EL, Clarke S, Beale P, Dent OF. Adjuvant chemotherapy for stage C colonic cancer in a multidisciplinary setting. ANZ J Surg. 2009;79(10):685–692. doi: 10.1111/j.1445-2197.2009.05052.x. [DOI] [PubMed] [Google Scholar]
- 36.Dobie SA, Baldwin LM, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98(9):610–619. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibbs P, McLaughlin S, Skinner I, Jones I, Hayes I, Chapman M, et al. Re: completion of therapy by medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98(21):1582. doi: 10.1093/jnci/djj416. [DOI] [PubMed] [Google Scholar]
- 38.Callaghan B, McCammon R, Kerber K, Xu X, Langa KM, Feldman E. Tests and expenditures in the initial evaluation of peripheral neuropathy. Arch Intern Med. 2012;172(2):127–132. doi: 10.1001/archinternmed.2011.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu E, Schulman KL, Zelt S, Song X. Costs associated with complications are lower with capecitabine than with 5-fluorouracil in patients with colorectal cancer. Cancer. 2009;115(7):1412–1423. doi: 10.1002/cncr.24131. [DOI] [PubMed] [Google Scholar]
- 40.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Habbema JD, Kuipers EJ. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst. 2009;101(20):1412–1422. doi: 10.1093/jnci/djp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Medicare and Medicaid Services. Payment allowance limits for medicare part B drugs, effective January 1, 2008 through March 31, 2008. 2008. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/01a_2008aspfiles.html. Accessed April 29 2014.
- 42.Song X, Zhao Z, Barber B, Gregory C, Cao Z, Gao S. Cost of illness in patients with metastatic colorectal cancer. J Med Econ. 2011;14(1):1–9. doi: 10.3111/13696998.2010.536870. [DOI] [PubMed] [Google Scholar]
- 43.Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preference values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391–400. doi: 10.1007/s11136-010-9589-5. [DOI] [PubMed] [Google Scholar]
- 44.Blinman P, Duric V, Nowak AK, Beale P, Clarke S, Briscoe K, et al. Adjuvant chemotherapy for early colon cancer: what survival benefits make it worthwhile? Eur J Cancer. 2010;46(10):1800–1807. doi: 10.1016/j.ejca.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 45.Aballea S, Chancellor JV, Raikou M, Drummond MF, Weinstein MC, Jourdan S, et al. Cost-effectiveness analysis of oxaliplatin compared with 5-fluorouracil/leucovorin in adjuvant treatment of stage III colon cancer in the US. Cancer. 2007;109(6):1082–1089. doi: 10.1002/cncr.22512. [DOI] [PubMed] [Google Scholar]
- 46.Cook J, Richardson J, Street A. A cost utility analysis of treatment options for gallstone disease: methodological issues and results. Health Econ. 1994;3(3):157–168. doi: 10.1002/hec.4730030305. [DOI] [PubMed] [Google Scholar]
- 47.Grunberg SM, Srivastava A, Grunberg KJ, Weeks J. Intensity of chemotherapy-induced emesis and overall survival as determinants of a global utility score. Support Care Cancer. 2002;10(8):624–629. doi: 10.1007/s00520-002-0381-0. [DOI] [PubMed] [Google Scholar]
- 48.Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Handbooks in health economic evaluation series. Oxford: Oxford University Press; 2006. [Google Scholar]
- 49.Severens JL, Brunenberg DE, Fenwick EA, O’Brien B, Joore MA. Cost-effectiveness acceptability curves and a reluctance to lose. Pharmacoeconomics. 2005;23(12):1207–1214. doi: 10.2165/00019053-200523120-00005. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Medicare and Medicaid Services. Payment allowance limits for medicare part B drugs, effective October 1, 2012 through December 31, 2012. 2012. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2012ASPFiles.html. Accessed November 8 2012.
- 51.Centers for Medicare and Medicaid Services. Payment allowance limits for medicare part B drugs, effective October 1, 2013 through December 31, 2013. 2013. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2013ASPFiles.html. Accessed October 9 2013.
- 52.O’Brien BJ, Gertsen K, Willan AR, Faulkner LA. Is there a kink in consumers’ threshold value for cost-effectiveness in health care? Health Econ. 2002;11(2):175–180. doi: 10.1002/hec.655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


