Abstract
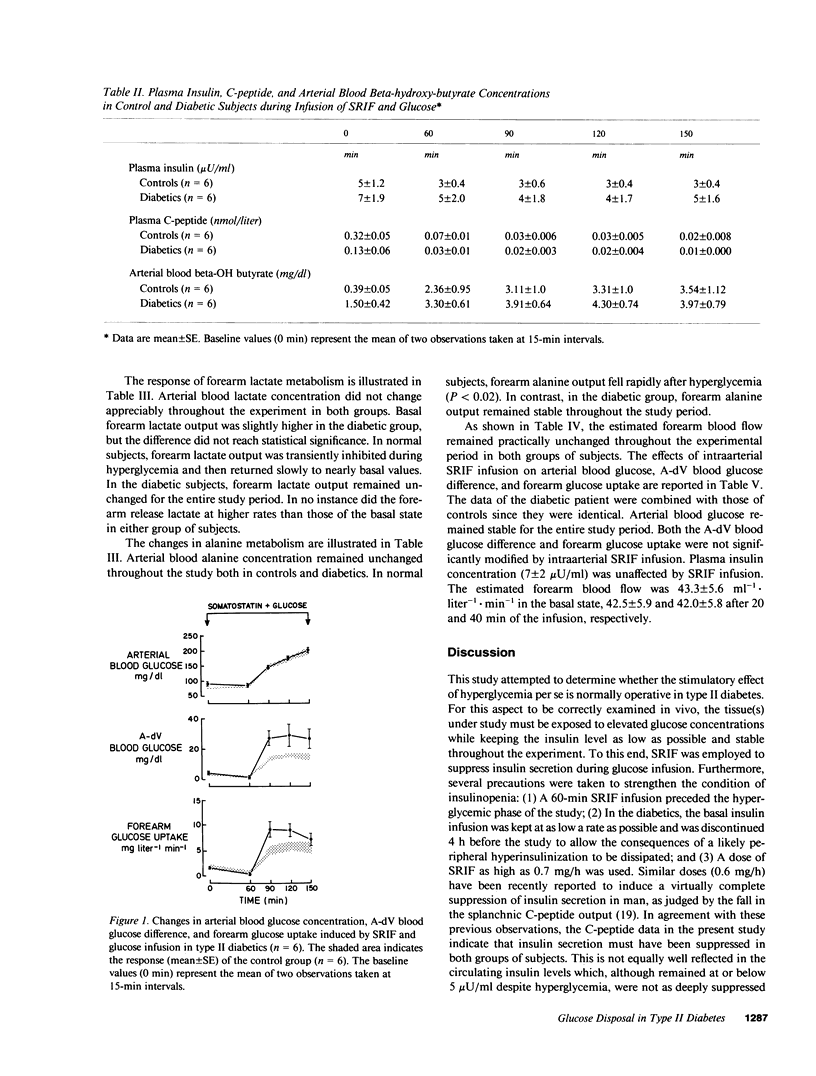
The effect of hyperglycemia per se on glucose uptake by muscle tissue was quantitated in six controls and six type II diabetics by the forearm technique, under conditions of insulin deficiency induced by somatostatin (SRIF) infusion (0.7 mg/h). Blood glucose concentration was clamped at its basal value during the first 60 min of SRIF infusion and then raised to approximately 200 mg/dl by a variable glucose infusion. Plasma insulin levels remained at or below 5 microU/ml during SRIF infusion, including the hyperglycemic period. No appreciable difference between controls and diabetics was present in the basal state as to forearm glucose metabolism. After 60 min of SRIF infusion and euglycemia, forearm glucose uptake fell consistently from 2.1 +/- 0.7 mg X liter-1 X min-1 to 1.0 +/- 0.6 (P less than 0.05) and from 1.7 +/- .2 to 0.4 +/- 0.3 (P less than 0.02) in the control and diabetic groups, respectively. The subsequent induction of hyperglycemia caused a marked increase in both the arterial-deep venous blood glucose difference (P less than 0.02-0.01) and forearm glucose uptake (P less than 0.01-0.005). However, the response in the diabetic group was significantly greater than that observed in controls. The incremental area of forearm glucose uptake was 276 +/- 31 mg X liter-1 X 90 min and 532 +/- 81 in the control and diabetic groups, respectively (P less than 0.02). In the basal state, the forearm released lactate and alanine both in controls and diabetic subjects at comparable rates. No increment was observed after hyperglycemia, despite the elevated rates of glucose uptake. It is concluded that (1) hyperglycemia per se stimulates forearm glucose disposal to a greater extent in type II diabetics than in normal subjects; and (2) the resulting increment of glucose disposal does not accelerate the forearm release of three carbon compounds. The data support the hypothesis that hyperglycemia per se may play a compensatory role for the defective glucose disposal in type II diabetes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES R., ZIERLER K. L., ANDERSON H. M., STAINSBY W. N., CADER G., GHRAYYIB A. S., LILIENTHAL J. L., Jr Measurement of blood flow and volume in the forearm of man; with notes on the theory of indicator-dilution and on production of turbulence, hemolysis, and vasodilatation by intra-vascular injection. J Clin Invest. 1954 Apr;33(4):482–504. doi: 10.1172/JCI102919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTERFIELD W. J., HOLLING H. E. Peripheral glucose metabolism in fasting control subjects and diabetic patients. Clin Sci. 1959 May;18:147–174. [PubMed] [Google Scholar]
- Björntorp P., Sjöström L. Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism. 1978 Dec;27(12 Suppl 2):1853–1865. doi: 10.1016/s0026-0495(78)80004-3. [DOI] [PubMed] [Google Scholar]
- Buschiazzo H., Exton J. H., Park C. R. Effects of glucose on glycogen synthetase, phosphorylase, and glycogen deposition in the perfused rat liver. Proc Natl Acad Sci U S A. 1970 Feb;65(2):383–387. doi: 10.1073/pnas.65.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The origin of alanine produced in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3677–3684. [PubMed] [Google Scholar]
- Cherrington A. D., Caldwell M. D., Dietz M. R., Exton J. H., Crofford O. B. The effect of somatostatin on glucose uptake and production by rat tissues in vitro. Diabetes. 1977 Aug;26(8):740–748. doi: 10.2337/diab.26.8.740. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Chiasson J. L., Liljenquist J. E., Jennings A. S., Keller U., Lacy W. W. The role of insulin and glucagon in the regulation of basal glucose production in the postabsorptive dog. J Clin Invest. 1976 Dec;58(6):1407–1418. doi: 10.1172/JCI108596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Felber J. P., Thiébaud D., Maeder E., Jéquier E., Hendler R., DeFronzo R. A. Effect of somatostatin-induced insulinopenia on glucose oxidation in man. Diabetologia. 1983 Oct;25(4):325–330. doi: 10.1007/BF00253195. [DOI] [PubMed] [Google Scholar]
- Fineberg S. E., Schneider S. H. Studies on the relationship between insulin concentrations and insulin action. Diabetes Care. 1982 May-Jun;5(3):292–299. doi: 10.2337/diacare.5.3.292. [DOI] [PubMed] [Google Scholar]
- Ginsberg H., Kimmerling G., Olefsky J. M., Reaven G. M. Demonstration of insulin resistance in untreated adult onset diabetic subjects with fasting hyperglycemia. J Clin Invest. 1975 Mar;55(3):454–461. doi: 10.1172/JCI107951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. S., Scarlett J. A., Griffin J., Olefsky J. M., Kolterman O. G. In vivo deactivation of peripheral, hepatic, and pancreatic insulin action in man. Diabetes. 1982 Oct;31(10):929–936. doi: 10.2337/diab.31.10.929. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of human C-peptide in serum. Diabetologia. 1975 Dec;11(6):541–548. doi: 10.1007/BF01222104. [DOI] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Holmes P. A., Mansour T. E. Glucose as a regulator of glycogen phosphorylase in rat diaphragm. I. Effect of glucose and related compounds on phosphorylase and glycogen levels. Biochim Biophys Acta. 1968 Mar 11;156(2):266–274. doi: 10.1016/0304-4165(68)90255-9. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R., Khairallah E. A., Goldberg A. L. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem. 1974 Dec 10;249(23):7623–7629. [PubMed] [Google Scholar]
- Reaven G. M., Bernstein R., Davis B., Olefsky J. M. Nonketotic diabetes mellitus: insulin deficiency or insulin resistance? Am J Med. 1976 Jan;60(1):80–88. doi: 10.1016/0002-9343(76)90536-2. [DOI] [PubMed] [Google Scholar]
- Revers R. R., Fink R., Griffin J., Olefsky J. M., Kolterman O. G. Influence of hyperglycemia on insulin's in vivo effects in type II diabetes. J Clin Invest. 1984 Mar;73(3):664–672. doi: 10.1172/JCI111258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Gerich J. E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979 Jul;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Mechanism and significance of insulin resistance in non-insulin-dependent diabetes mellitus. Diabetes. 1981 Dec;30(12):990–995. doi: 10.2337/diab.30.12.990. [DOI] [PubMed] [Google Scholar]
- Saccà L., Cicala M., Trimarco B., Ungaro B., Vigorito C. Differential effects of insulin on splanchnic and peripheral glucose disposal after an intravenous glucose load in man. J Clin Invest. 1982 Jul;70(1):117–126. doi: 10.1172/JCI110583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccà L., Orofino G., Petrone A., Vigorito C. Direct assessment of splanchnic uptake and metabolic effects of human and porcine insulin. J Clin Endocrinol Metab. 1984 Aug;59(2):191–196. doi: 10.1210/jcem-59-2-191. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Kramer K. J., Tobin J. D., Insel P. A., Liljenquist J. E., Berman M., Andres R. A model of the kinetics of insulin in man. J Clin Invest. 1974 May;53(5):1481–1492. doi: 10.1172/JCI107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud D., Jacot E., DeFronzo R. A., Maeder E., Jequier E., Felber J. P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982 Nov;31(11):957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. EFFECT OF VERY SMALL CONCENTRATIONS OF INSULIN ON FOREARM METABOLISM. PERSISTENCE OF ITS ACTION ON POTASSIUM AND FREE FATTY ACIDS WITHOUT ITS EFFECT ON GLUCOSE. J Clin Invest. 1964 May;43:950–962. doi: 10.1172/JCI104981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. ROLES OF INSULIN AND GROWTH HORMONE, BASED ON STUDIES OF FOREARM METABOLISM IN MAN. Medicine (Baltimore) 1963 Nov;42:385–402. doi: 10.1097/00005792-196311000-00002. [DOI] [PubMed] [Google Scholar]


