Abstract
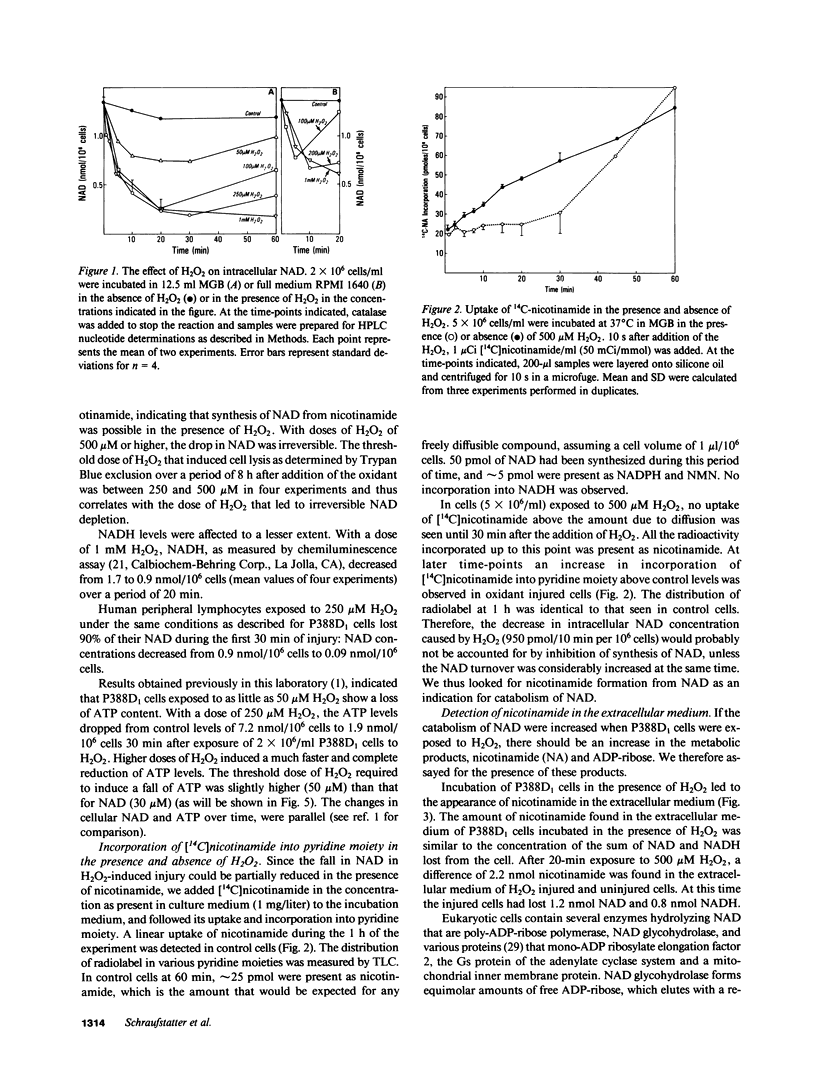
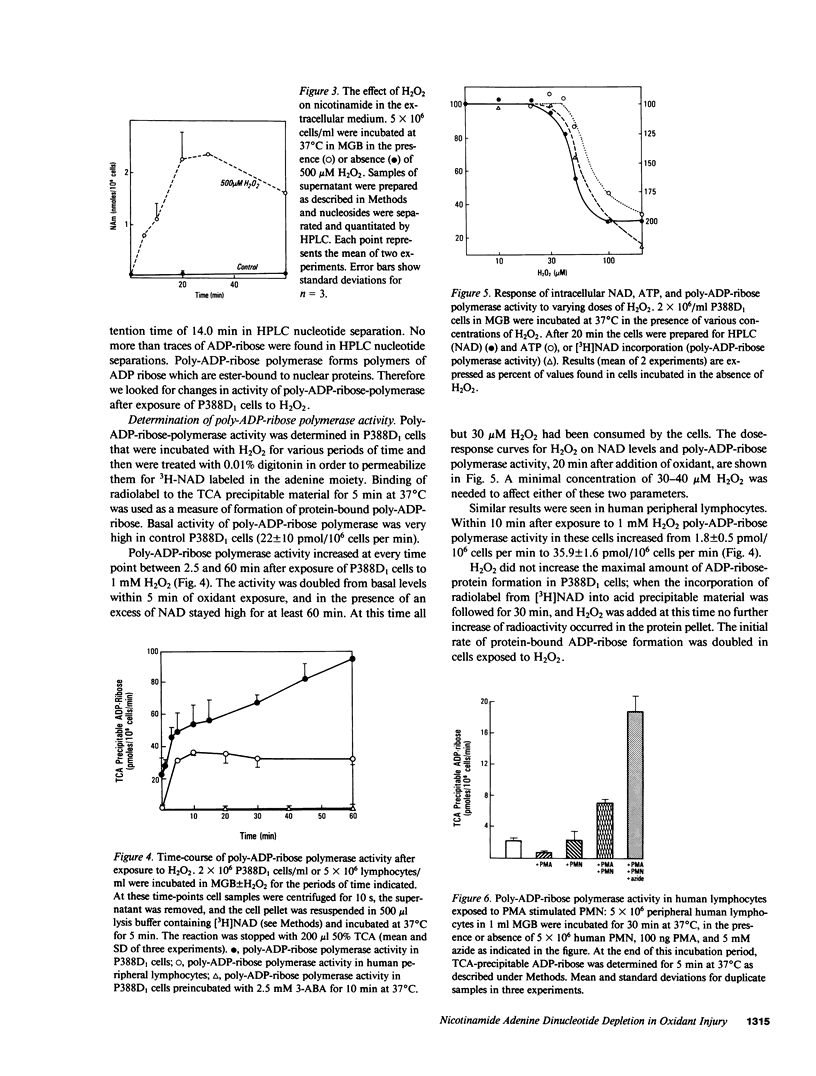
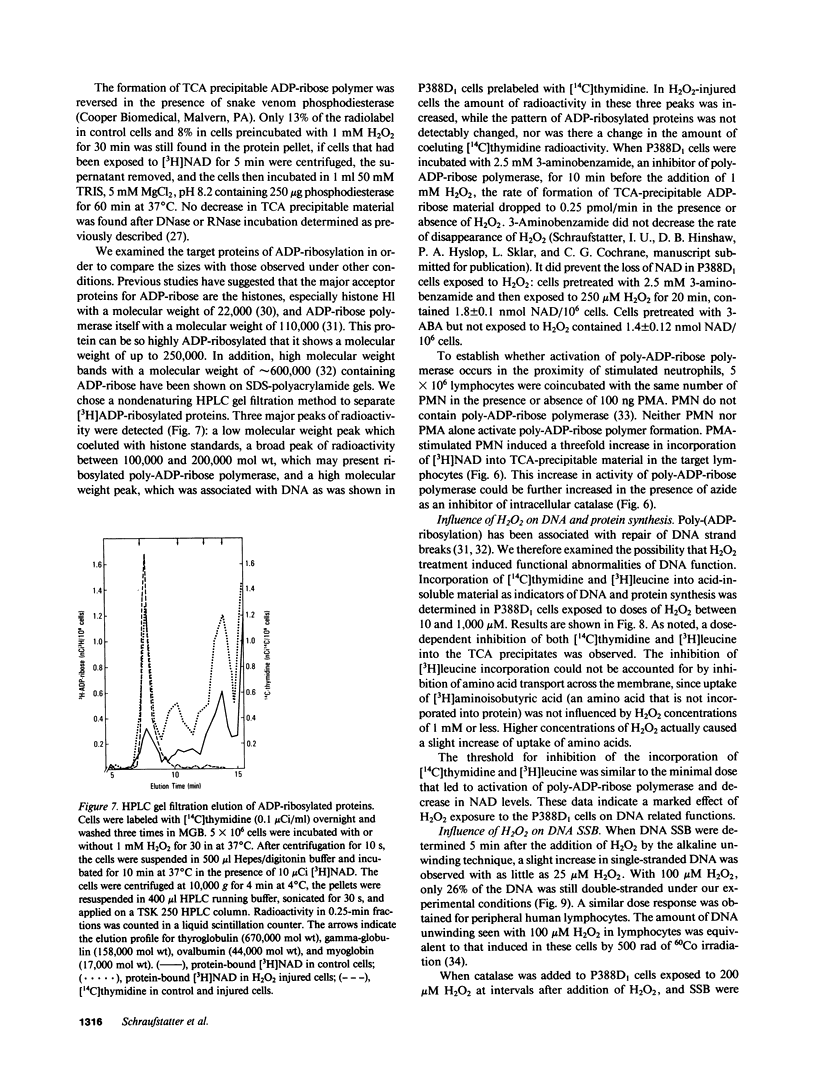
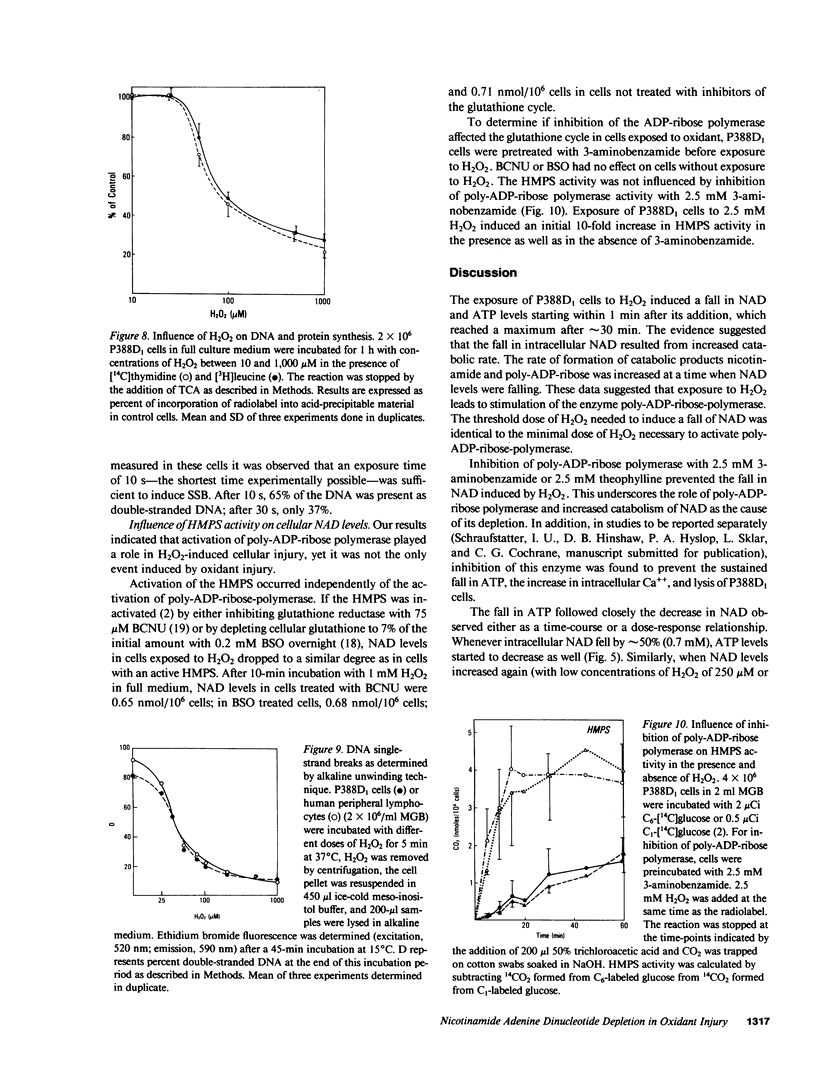
To determine the biochemical basis of the oxidant-induced injury of cells, we have studied early changes after exposure of P388D1 murine macrophages to hydrogen peroxide. Total intracellular NAD+ levels in P388D1 cells decreased with H2O2 concentrations of 40 microM or higher. Doses of H2O2 between 0.1 and 2.5 mM led to an 80% depletion of NAD within 20 min. With doses of H2O2 of 250 microM or lower, the fall in NAD and, as shown previously, ATP, was reversible. Higher doses of H2O2 that cause ultimate lysis of the cells, induced an irreversible depletion of NAD and ATP. Poly-ADP-ribose polymerase, a nuclear enzyme associated with DNA damage and repair, which catalyzes conversion of NAD to nicotinamide and protein-bound poly-ADP-ribose, was activated by exposure of the cells to concentrations of 40 microM H2O2 or higher. Activation of poly-ADP-ribose polymerase was also observed in peripheral lymphocytes incubated in the presence of phorbol myristate acetate-stimulated polymorphonuclear neutrophils. Examination of the possibility that DNA alteration was involved was performed by measurement of thymidine incorporation and determination of DNA single-strand breaks (SSB) in cells exposed to H2O2. H2O2 at 40 microM or higher inhibited DNA synthesis, and induced SSB within less than 30 s. These results suggest that DNA damage induced within seconds after addition of oxidant may lead to stimulation of poly-ADP-ribose polymerase, and a consequent fall in NAD. Excessive stimulation of poly-ADP-ribose polymerase leads to a fall in NAD sufficient to interfere with ATP synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R., Lawrence S. D., Sattler G. L., Pitot H. C. ADP-ribosyltransferase activity in cultured hepatocytes. Interactions with DNA repair. J Biol Chem. 1982 May 25;257(10):5528–5535. [PubMed] [Google Scholar]
- BAKER N., WILSON L. Inhibition of tumor glycolysis by hydrogen peroxide formed from autoxidation of unsaturated fatty acids. Biochem Biophys Res Commun. 1963 Apr 2;11:60–64. doi: 10.1016/0006-291x(63)90028-7. [DOI] [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Berger N. A., Adams J. W., Sikorski G. W., Petzold S. J., Shearer W. T. Synthesis of DNA and poly(adenosine diphosphate ribose) in normal and chronic lymphocytic leukemia lymphocytes. J Clin Invest. 1978 Jul;62(1):111–118. doi: 10.1172/JCI109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N. A. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985 Jan;101(1):4–15. [PubMed] [Google Scholar]
- Berger N. A., Sikorski G. W., Petzold S. J., Kurohara K. K. Association of poly(adenosine diphosphoribose) synthesis with DNA damage and repair in normal human lymphocytes. J Clin Invest. 1979 Jun;63(6):1164–1171. doi: 10.1172/JCI109410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow R. L., Tzeng D. Y., Williams L. V., Baehner R. L. The comparative responses of human polymorphonuclear leukocytes obtained by counterflow centrifugal elutriation and Ficoll-Hypaque density centrifugation. I. Resting volume, stimulus-induced superoxide production, and primary and specific granule release. J Lab Clin Med. 1983 Nov;102(5):732–742. [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Brigelius R. Glutathione oxidation and activation of pentose phosphate cycle during hydroperoxide metabolism. A comparison of livers from fed and fasted rats. Hoppe Seylers Z Physiol Chem. 1983 Aug;364(8):989–996. doi: 10.1515/bchm2.1983.364.2.989. [DOI] [PubMed] [Google Scholar]
- Butt T. R., Smulson M. Relationship between nicotinamide adenine dinucleotide concentration and in vitro synthesis of poly(adenosine diphosphate ribose) on purified nucleosomes. Biochemistry. 1980 Nov 11;19(23):5235–5242. doi: 10.1021/bi00564a013. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Fiskum G., Craig S. W., Decker G. L., Lehninger A. L. The cytoskeleton of digitonin-treated rat hepatocytes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer H., Ahmad T. Severe generalized glutathione reductase deficiency after antitumor chemotherapy with BCNU" [1,3-bis(chloroethyl)-1-nitrosourea]. J Lab Clin Med. 1977 May;89(5):1080–1091. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. T., Jr Thin-layer chromatographic separation of intermediates of the pyridine nucleotide cycle. Anal Biochem. 1983 Apr 1;130(1):185–188. doi: 10.1016/0003-2697(83)90667-x. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Robkin L. Hydrogen peroxide lowers ATP levels in platelets without altering adenyalte energy charge and platelet function. J Biol Chem. 1977 Mar 10;252(5):1752–1757. [PubMed] [Google Scholar]
- Holtlund J., Kristensen T., Ostvold A. C., Laland S. G. ADP-ribosylation in permeable HeLa S3 cells. Eur J Biochem. 1983 Jan 17;130(1):47–51. doi: 10.1111/j.1432-1033.1983.tb07115.x. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P., Williams G. T. Role of DNA breaks and ADP-ribosyl transferase activity in eukaryotic differentiation demonstrated in human lymphocytes. Nature. 1982 Nov 25;300(5890):368–370. doi: 10.1038/300368a0. [DOI] [PubMed] [Google Scholar]
- Kawaichi M., Ueda K., Hayaishi O. Multiple autopoly(ADP-ribosyl)ation of rat liver poly(ADP-ribose) synthetase. Mode of modification and properties of automodified synthetase. J Biol Chem. 1981 Sep 25;256(18):9483–9489. [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Lewis J. G., Adams D. O. Induction of 5,6-ring-saturated thymine bases in NIH-3T3 cells by phorbol ester-stimulated macrophages: role of reactive oxygen intermediates. Cancer Res. 1985 Mar;45(3):1270–1275. [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxide-induced loss of pyridine nucleotides and release of calcium from rat liver mitochondria. J Biol Chem. 1980 Oct 10;255(19):9325–9330. [PubMed] [Google Scholar]
- Malik N., Miwa M., Sugimura T., Thraves P., Smulson M. Immunoaffinity fractionation of the poly(ADP-ribosyl)ated domains of chromatin. Proc Natl Acad Sci U S A. 1983 May;80(9):2554–2558. doi: 10.1073/pnas.80.9.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S. S., Raivio K. O., Seegmiller J. E. Adenine nucleotide degradation during energy depletion in human lymphoblasts. Adenosine accumulation and adenylate energy charge correlation. J Biol Chem. 1979 Sep 25;254(18):8956–8962. [PubMed] [Google Scholar]
- McCreanor G. M., Bender D. A. The role of catabolism in controlling tissue concentrations of nicotinamide nucleotide coenzymes. Biochim Biophys Acta. 1983 Sep 13;759(3):222–228. doi: 10.1016/0304-4165(83)90316-1. [DOI] [PubMed] [Google Scholar]
- McWilliams R. S., Cross W. G., Kaplan J. G., Birnboim H. C. Rapid rejoining of DNA strand breaks in resting human lymphocytes after irradiation by low doses of 60Co gamma rays or 14.6-MeV neutrons. Radiat Res. 1983 Jun;94(3):499–507. [PubMed] [Google Scholar]
- Morioka K., Tanaka K., Ono T. Acceptors of poly(ADP-ribosylation) in differentiation inducer-treated and untreated Friend erythroleukemia cells. Biochim Biophys Acta. 1982 Dec 31;699(3):255–263. doi: 10.1016/0167-4781(82)90115-4. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nduka N., Skidmore C. J., Shall S. The enhancement of cytotoxicity of N-methyl-N-nitrosourea and of gamma-radiation by inhibitors of poly(ADP-ribose) polymerase. Eur J Biochem. 1980 Apr;105(3):525–530. doi: 10.1111/j.1432-1033.1980.tb04528.x. [DOI] [PubMed] [Google Scholar]
- Paine A. J., Allen C. M., Durkacz B. W., Shall S. Evidence that poly(ADP-ribose) polymerase is involved in the loss of NAD from cultured rat liver cells. Biochem J. 1982 Feb 15;202(2):551–553. doi: 10.1042/bj2020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogolotti A. L., Jr, Santi D. V. High-pressure liquid chromatography--ultraviolet analysis of intracellular nucleotides. Anal Biochem. 1982 Nov 1;126(2):335–345. doi: 10.1016/0003-2697(82)90524-3. [DOI] [PubMed] [Google Scholar]
- ROITT I. M. The inhibition of carbohydrate metabolism in ascites-tumour cells by ethyleneimines. Biochem J. 1956 Jun;63(2):300–307. doi: 10.1042/bj0630300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin P. W., Jacobson M. K., Mitchell V. R., Busbee D. L. Reduction of nicotinamide adenine dinucleotide levels by ultimate carcinogens in human lymphocytes. Cancer Res. 1980 Jun;40(6):1803–1807. [PubMed] [Google Scholar]
- Rubin R., Farber J. L. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984 Feb 1;228(2):450–459. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Schraufstätter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Glutathione cycle activity and pyridine nucleotide levels in oxidant-induced injury of cells. J Clin Invest. 1985 Sep;76(3):1131–1139. doi: 10.1172/JCI112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto S., Carrera C. J., Kubota M., Wasson D. B., Carson D. A. Mechanism of deoxyadenosine and 2-chlorodeoxyadenosine toxicity to nondividing human lymphocytes. J Clin Invest. 1985 Feb;75(2):377–383. doi: 10.1172/JCI111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Sims J. L., Berger S. J., Berger N. A. Poly(ADP-ribose) Polymerase inhibitors preserve nicotinamide adenine dinucleotide and adenosine 5'-triphosphate pools in DNA-damaged cells: mechanism of stimulation of unscheduled DNA synthesis. Biochemistry. 1983 Oct 25;22(22):5188–5194. doi: 10.1021/bi00291a019. [DOI] [PubMed] [Google Scholar]
- Skidmore C. J., Davies M. I., Goodwin P. M., Halldorsson H., Lewis P. J., Shall S., Zia'ee A. A. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979 Nov 1;101(1):135–142. doi: 10.1111/j.1432-1033.1979.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Smulson M. E., Schein P., Mullins D. W., Jr, Sudhakar S. A putative role for nicotinamide adenine dinucleotide-promoted nuclear protein modification in the antitumor activity of N-methyl-N-nitrosourea. Cancer Res. 1977 Sep;37(9):3006–3012. [PubMed] [Google Scholar]
- Smulson M. E., Sugimura T. Overview of poly(ADP-ribosyl)ation. Methods Enzymol. 1984;106:438–440. doi: 10.1016/0076-6879(84)06047-x. [DOI] [PubMed] [Google Scholar]
- Spragg R. G., Hinshaw D. B., Hyslop P. A., Schraufstätter I. U., Cochrane C. G. Alterations in adenosine triphosphate and energy charge in cultured endothelial and P388D1 cells after oxidant injury. J Clin Invest. 1985 Oct;76(4):1471–1476. doi: 10.1172/JCI112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi Man N., Shall S. The alkylating agent, dimethyl sulphate, stimulates ADP-ribosylation of histone H1 and other proteins in permeabilised mouse lymphoma (L1210) cells. Eur J Biochem. 1982 Aug;126(1):83–88. doi: 10.1111/j.1432-1033.1982.tb06749.x. [DOI] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman S. A., Stossel T. P. Effects of oxygen radical scavengers and antioxidants on phagocyte-induced mutagenesis. J Immunol. 1982 Jun;128(6):2770–2772. [PubMed] [Google Scholar]
- Weitzman S. A., Weitberg A. B., Clark E. P., Stossel T. P. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985 Mar 8;227(4691):1231–1233. doi: 10.1126/science.3975611. [DOI] [PubMed] [Google Scholar]
- Whish W. J., Davies M. I., Shall S. Stimulation of poly(ADP-ribose) polymerase activity by the anti-tumour antibiotic, streptozotocin. Biochem Biophys Res Commun. 1975 Jul 22;65(2):722–730. doi: 10.1016/s0006-291x(75)80205-1. [DOI] [PubMed] [Google Scholar]
- Wielckens K., George E., Pless T., Hilz H. Stimulation of poly(ADP-ribosyl)ation during Ehrlich ascites tumor cell "starvation" and suppression of concomitant DNA fragmentation by benzamide. J Biol Chem. 1983 Apr 10;258(7):4098–4104. [PubMed] [Google Scholar]
- Wielckens K., Schmidt A., George E., Bredehorst R., Hilz H. DNA fragmentation and NAD depletion. Their relation to the turnover of endogenous mono(ADP-ribosyl) and poly(ADP-ribosyl) proteins. J Biol Chem. 1982 Nov 10;257(21):12872–12877. [PubMed] [Google Scholar]
- Yamamoto H., Uchigata Y., Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. 1981 Nov 19;294(5838):284–286. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]


