Abstract
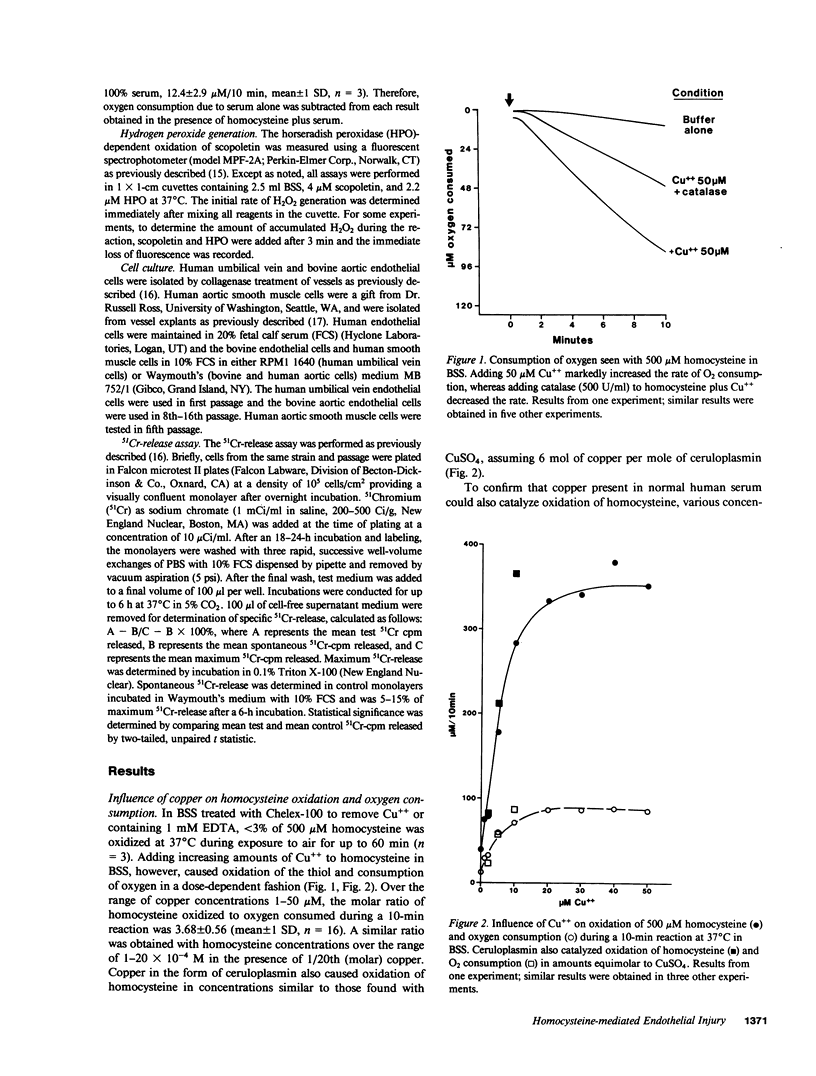
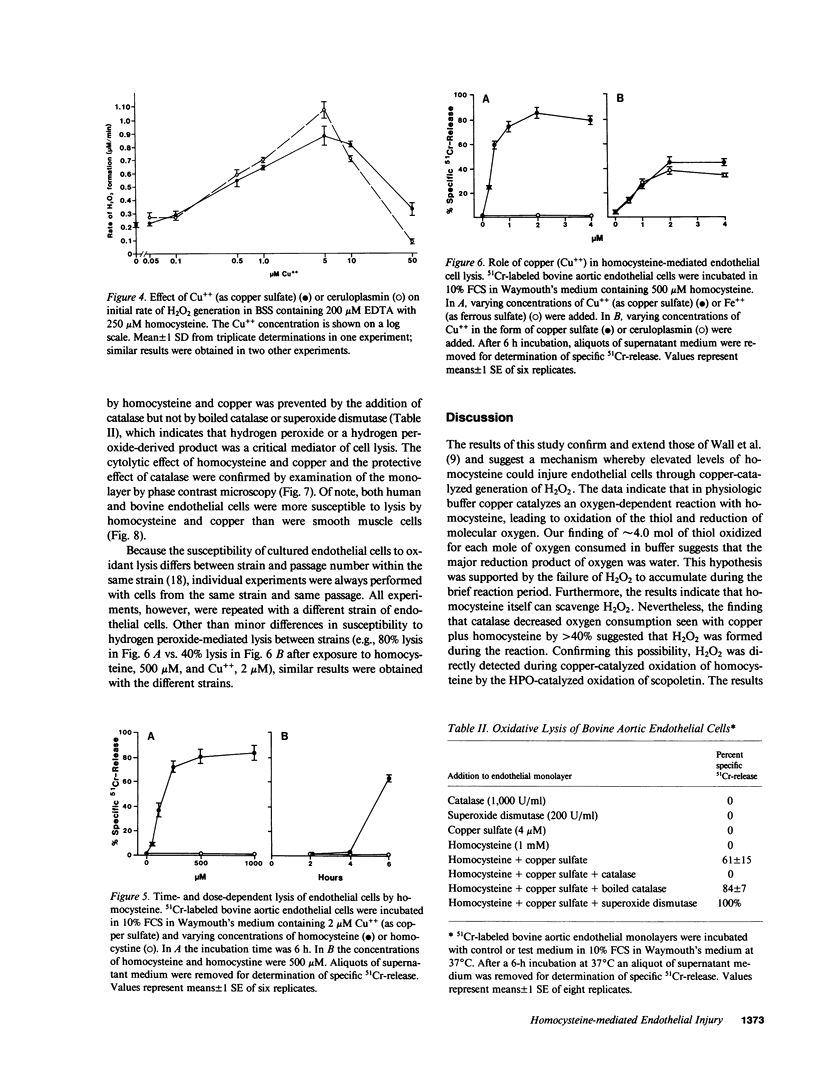
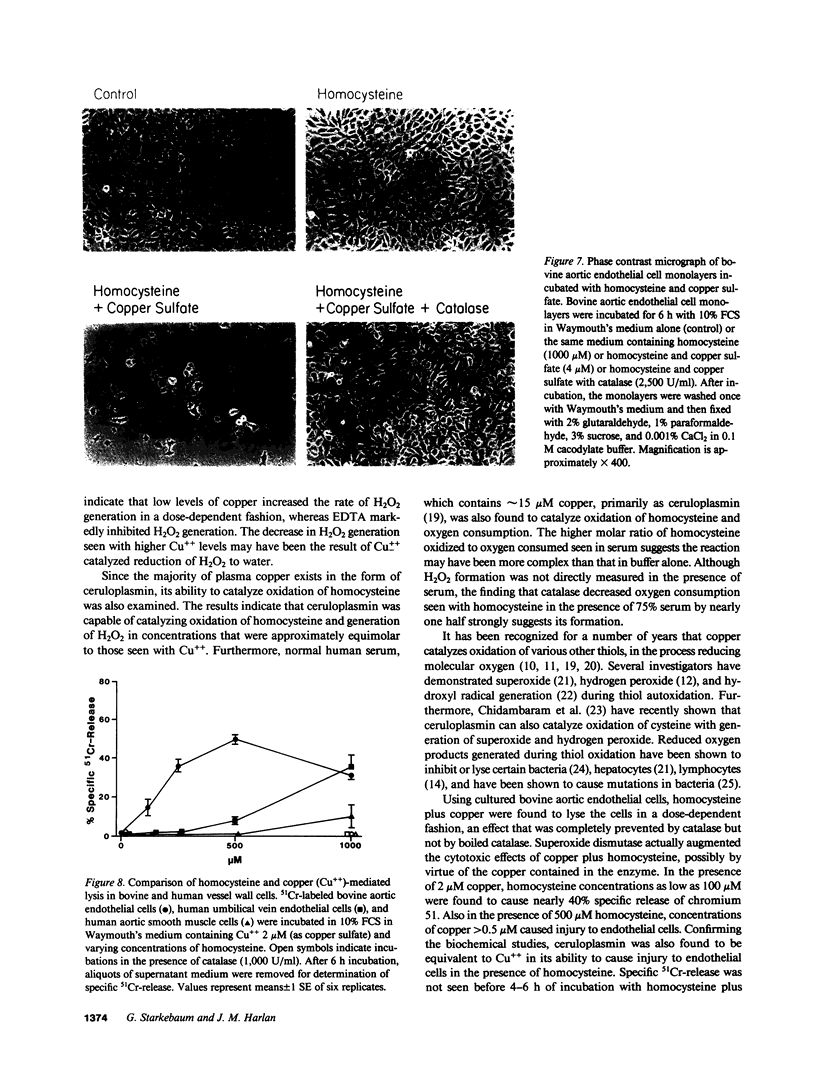
We have examined whether the toxic effects of homocysteine on cultured endothelial cells could result from the formation and action of hydrogen peroxide. In initial experiments with a cell-free system, micromolar amounts of copper were found to catalyze an oxygen-dependent oxidation of homocysteine. The molar ratio of homocysteine oxidized to oxygen consumed was approximately 4.0, which suggests that oxygen was reduced to water. The addition of catalase, however, decreased oxygen consumption by nearly one-half, which suggests that H2O2 was formed during the reaction. Confirming this hypothesis, H2O2 formation was detected using the horseradish peroxidase-dependent oxidation of fluorescent scopoletin. Ceruloplasmin was also found to catalyze oxidation of homocysteine and generation of H2O2 in molar amounts equivalent to copper sulfate. Finally, homocysteine oxidation was catalyzed by normal human serum in a concentration-dependent manner. Using cultured human and bovine endothelial cells, we found that homocysteine plus copper could lyse the cells in a dose-dependent manner, an effect that was completely prevented by catalase. Homocystine plus copper was not toxic to the cells. Specific injury to endothelial cells was seen only after 4 h of incubation with homocysteine plus copper. Confirming the biochemical studies, ceruloplasmin was also found to be equivalent to Cu++ in its ability to cause injury to endothelial cells in the presence of homocysteine. Since elevated levels of homocysteine have been implicated in premature development of atherosclerosis, these findings may be relevant to the mechanism of some types of chronic vascular injury.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boers G. H., Smals A. G., Trijbels F. J., Fowler B., Bakkeren J. A., Schoonderwaldt H. C., Kleijer W. J., Kloppenborg P. W. Heterozygosity for homocystinuria in premature peripheral and cerebral occlusive arterial disease. N Engl J Med. 1985 Sep 19;313(12):709–715. doi: 10.1056/NEJM198509193131201. [DOI] [PubMed] [Google Scholar]
- Boers G. H., Smals A. G., Trijbels F. J., Leermakers A. I., Kloppenborg P. W. Unique efficiency of methionine metabolism in premenopausal women may protect against vascular disease in the reproductive years. J Clin Invest. 1983 Dec;72(6):1971–1976. doi: 10.1172/JCI111161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattstrom L. E., Hardebo J. E., Hultberg B. L. Moderate homocysteinemia--a possible risk factor for arteriosclerotic cerebrovascular disease. Stroke. 1984 Nov-Dec;15(6):1012–1016. doi: 10.1161/01.str.15.6.1012. [DOI] [PubMed] [Google Scholar]
- Cavallini D., De Marco C., Duprè S., Rotilio G. The copper catalyzed oxidation of cysteine to cystine. Arch Biochem Biophys. 1969 Mar;130(1):354–361. doi: 10.1016/0003-9861(69)90044-7. [DOI] [PubMed] [Google Scholar]
- Chidambaram M. V., Zgirski A., Frieden E. The reaction of cysteine with ceruloplasmin copper. J Inorg Biochem. 1984 Jul;21(3):227–239. doi: 10.1016/0162-0134(84)83006-8. [DOI] [PubMed] [Google Scholar]
- Dudman N. P., Wilcken D. E. Increased plasma copper in patients with homocystinuria due to cystathionine beta-synthase deficiency. Clin Chim Acta. 1983 Jan 7;127(1):105–113. doi: 10.1016/0009-8981(83)90080-3. [DOI] [PubMed] [Google Scholar]
- Glatt H., Protić-SabljiC M., Oesch F. Mutagenicity of glutathione and cysteine in the Ames test. Science. 1983 May 27;220(4600):961–963. doi: 10.1126/science.6342137. [DOI] [PubMed] [Google Scholar]
- Gupta V. J., Wilcken D. E. The detection of cysteine-homocysteine mixed disulphide in plasma of normal fasting man. Eur J Clin Invest. 1978 Aug;8(4):205–207. doi: 10.1111/j.1365-2362.1978.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Harker L. A., Harlan J. M., Ross R. Effect of sulfinpyrazone on homocysteine-induced endothelial injury and arteriosclerosis in baboons. Circ Res. 1983 Dec;53(6):731–739. doi: 10.1161/01.res.53.6.731. [DOI] [PubMed] [Google Scholar]
- Harker L. A., Ross R., Slichter S. J., Scott C. R. Homocystine-induced arteriosclerosis. The role of endothelial cell injury and platelet response in its genesis. J Clin Invest. 1976 Sep;58(3):731–741. doi: 10.1172/JCI108520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J., Scott C. R., Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N Engl J Med. 1974 Sep 12;291(11):537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully K. S., Ragsdale B. D. Production of arteriosclerosis by homocysteinemia. Am J Pathol. 1970 Oct;61(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- McCully K. S. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969 Jul;56(1):111–128. [PMC free article] [PubMed] [Google Scholar]
- Misra H. P. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem. 1974 Apr 10;249(7):2151–2155. [PubMed] [Google Scholar]
- Nyberg G. K., Granberg G. P., Carlsson J. Bovine superoxide dismutase and copper ions potentiate the bactericidal effect of autoxidizing cysteine. Appl Environ Microbiol. 1979 Jul;38(1):29–34. doi: 10.1128/aem.38.1.29-34.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell-Tormey J., DeBoer C. J., Nathan C. F. Resistance of human tumor cells in vitro to oxidative cytolysis. J Clin Invest. 1985 Jul;76(1):80–86. doi: 10.1172/JCI111981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Saez G., Thornalley P. J., Hill H. A., Hems R., Bannister J. V. The production of free radicals during the autoxidation of cysteine and their effect on isolated rat hepatocytes. Biochim Biophys Acta. 1982 Oct 28;719(1):24–31. doi: 10.1016/0304-4165(82)90302-6. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968 Oct 24;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Starkebaum G., Root R. K. D-Penicillamine: analysis of the mechanism of copper-catalyzed hydrogen peroxide generation. J Immunol. 1985 May;134(5):3371–3378. [PubMed] [Google Scholar]
- Taylor L., Menconi M. J., Polgar P. The participation of hydroperoxides and oxygen radicals in the control of prostaglandin synthesis. J Biol Chem. 1983 Jun 10;258(11):6855–6857. [PubMed] [Google Scholar]
- Wall R. T., Harlan J. M., Harker L. A., Striker G. E. Homocysteine-induced endothelial cell injury in vitro: a model for the study of vascular injury. Thromb Res. 1980 Apr 1;18(1-2):113–121. doi: 10.1016/0049-3848(80)90175-9. [DOI] [PubMed] [Google Scholar]
- Warso M. A., Lands W. E. Presence of lipid hydroperoxide in human plasma. J Clin Invest. 1985 Feb;75(2):667–671. doi: 10.1172/JCI111745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton A. R., Montgomery M. E., Kent R. S. Effect of hydrogen peroxide on prostaglandin production and cellular integrity in cultured porcine aortic endothelial cells. J Clin Invest. 1985 Jul;76(1):295–302. doi: 10.1172/JCI111960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcken D. E., Gupta V. J., Betts A. K. Homocysteine in the plasma of renal transplant recipients: effects of cofactors for methionine metabolism. Clin Sci (Lond) 1981 Dec;61(6):743–749. doi: 10.1042/cs0610743. [DOI] [PubMed] [Google Scholar]
- Wilcken D. E., Gupta V. J. Cysteine-homocysteine mixed disulphide: differing plasma concentrations in normal men and women. Clin Sci (Lond) 1979 Aug;57(2):211–215. doi: 10.1042/cs0570211. [DOI] [PubMed] [Google Scholar]
- Wilcken D. E., Gupta V. J., Reddy S. G. Accumulation of sulphur-containing amino acids including cysteine-homocysteine in patients on maintenance haemodialysis. Clin Sci (Lond) 1980 May;58(5):427–430. doi: 10.1042/cs0580427. [DOI] [PubMed] [Google Scholar]
- Wilcken D. E., Gupta V. J. Sulphr containing amino acids in chronic renal failure with particular reference to homocystine and cysteine-homocysteine mixed disulphide. Eur J Clin Invest. 1979 Aug;9(4):301–307. doi: 10.1111/j.1365-2362.1979.tb00888.x. [DOI] [PubMed] [Google Scholar]
- de Groot P. G., Willems C., Boers G. H., Gonsalves M. D., van Aken W. G., van Mourik J. A. Endothelial cell dysfunction in homocystinuria. Eur J Clin Invest. 1983 Oct;13(5):405–410. doi: 10.1111/j.1365-2362.1983.tb00121.x. [DOI] [PubMed] [Google Scholar]






