Abstract
Rumex palustris has the capacity to respond to complete submergence with hyponastic (upward) growth and stimulated elongation of petioles. These adaptive responses allow survival of this plant in habitats with sustained high water levels by re‐establishing contact with the aerial environment. Accumulated ethylene in submerged petioles interacts with ethylene receptor proteins and operates as a reliable sensor for the under‐water environment. Further downstream in the transduction pathway, a fast and substantial decrease of the endogenous abscisic acid concentration and a certain threshold level of endogenous auxin and gibberellin are required for hyponastic growth and petiole elongation. Interactions of these plant hormones results in a significant increase of the in vitro cell wall extensibility in submerged petioles. Furthermore, the pattern of transcript accumulation of a R. palustris α‐expansin gene correlated with the pattern of petiole elongation upon submergence.
Keywords: Key words: Review, Rumex palustris Sm., ethylene, auxin, gibberellins, abscisic acid, submergence, cell walls, expansins
Introduction
Transient flooding with fresh water is a world‐wide phenomenon in river floodplains and wetlands as well as other terrestrial ecosystems. It frequently results in complete submergence of plants. In general this excess of water has a negative impact on growth and survival of most terrestrial plants, especially when flooding occurs during the growing season of a plant (Blom and Voesenek, 1996; Colmer et al., 1998). In an ecological perspective, flooding has a severe impact on the distribution patterns of plant species and vegetation in flood‐prone environments (Blom, 1999). Flooding can be seen as an environmental filter, sorting species according to the traits they possess to cope with this stress.
Submergence is stressful for higher plants because it inhibits the entry of atmospheric oxygen to plant leaves and roots due to the very slow rate of diffusion of gases in water. The greatly reduced flux of oxygen is much too slow to support respiration, resulting in energy deficits and, eventually, death of cells and tissues in non‐adapted plants (Jackson and Armstrong, 1999). The slow diffusion rate of gases through water also causes accumulation of endogenously produced gases, such as ethylene, in submerged plants. The enhanced concentrations of this phytohormone can result in a severe inhibition of root growth as shown for plant species belonging to the genus Rumex (Visser et al., 1997).
A way out of the problem of oxygen shortage is a highly coordinated enhancement in upward growth of shoot organs such as stems and petioles. This fast growth, demonstrated by a restricted number of plant species (Ridge, 1987), returns the shoot to the atmosphere above the water surface, allowing exchange of gases between the plant and atmosphere. In this way, the plant can resume aerobic metabolic activity required for long‐term survival. Thus, the shoot functions as a ‘snorkel’ that facilitates the entrance of oxygen and the outward ventilation of gases (Voesenek and Blom, 1999).
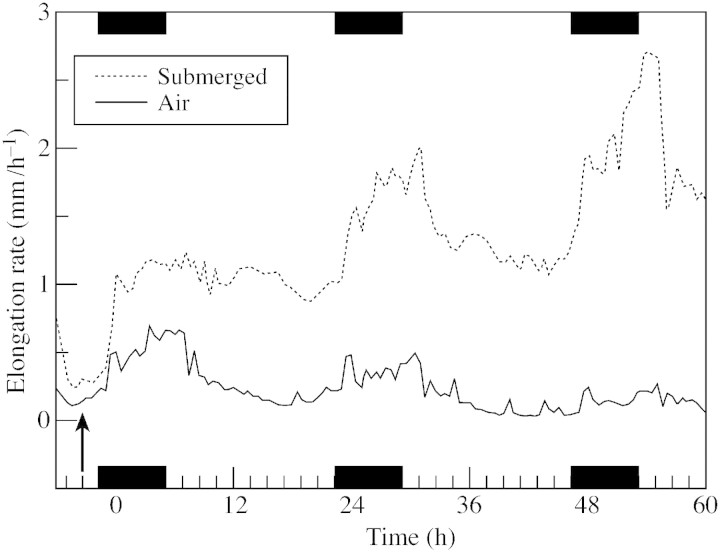
The dicotyledonous terrestrial plant Rumex palustris is used intensively to study acclimatic responses to complete submergence. Within a few hours of submergence, the orientation of rosette leaves changes from prostrate to almost vertical (hyponastic growth). Furthermore, the youngest petioles demonstrate a strong enhancement in growth rate that lasts several days (Voesenek and Blom, 1989) (Figs 1 and 2). In existing petioles this growth response is almost completely attributable to increased cell expansion (Voesenek et al., 1990).
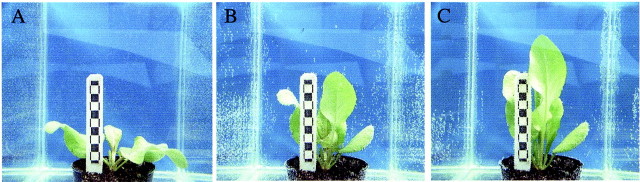
Fig. 1. Submergence‐induced hyponastic growth and petiole elongation in Rumex palustris. At the start of the submergence treatment plants had an age of 28 days. The length of the black and white squares on the bar is 5 mm. A, Plant submerged for 7 min; B, plant submerged for 8 h; C, plant submerged for 50 h.
Fig. 2. Mean elongation rate of Rumex palustris leaves (petiole + lamina, n = 3) under submerged and air grown (control) conditions. A linear displacement transducer was connected to the tip of the lamina of the fifth leaf (youngest) of intact plants placed in a growth room with a day and night temperature of 20 °C and a photosynthetic photon flux density of 200 µmol m–2 s–1. Dark periods (8 h) are indicated by black bars. Arrow indicates onset of submergence.
The signal‐transduction pathway in R. palustris that results in submergence‐induced petiole elongation is composed of three major events. First, submergence has to be sensed in a quick and reliable manner. Hereafter, the submergence‐induced signal is transduced through the integrated action of plant hormones. Finally, cells in the petiole perceive the signal and start a cascade of reactions resulting in hyponastic growth and elongation of the entire petiole (Voesenek and Blom, 1999).
It is the aim of our research team to elucidate the signal‐transduction pathway that leads to submergence‐induced petiole elongation in R. palustris. This knowledge will improve insight into basic processes that regulate growth in higher plants. We also aim to elucidate the rate‐limiting steps in plant species and/or organs that do not demonstrate enhanced rates of shoot elongation upon submergence.
This paper provides an update on the regulation of submergence‐induced petiole elongation in R. palustris and the concerted action in this process of ethylene, auxin, abscisic acid and gibberellins. A model will be presented that shows our current view and hypotheses on the network of events that lead to enhanced petiole elongation when the dicot R. palustris is submerged.
Sensing of submergence
Gases diffuse approx. 10 000 times slower in water than in air (Jackson, 1985). In non‐photosynthesizing organs of submerged plants, such as roots, this results in a significant increase in ethylene and carbon dioxide concentrations, whereas the levels of oxygen strongly decrease (Jackson and Armstrong, 1999). However, the endogenous gas concentration is less predictable in submerged photosynthesizing tissue, such as stems and petioles. In internodal air spaces of deepwater rice (Oryza sativa L. cv. Habiganj Aman II), the oxygen and carbon dioxide concentrations followed a diurnal pattern with high oxygen and low carbon dioxide levels during the light period. Ethylene increases in this species gradually with no clear diurnal pattern (Stünzi and Kende, 1989). With respect to oxygen, a similar light‐dependent pattern was observed in petioles of R. palustris (Rijnders et al., 2000). Also in Rumex, ethylene increases independently of the light level. An increase from 0·05 µl l–1 to more than 1 µl l–1 was measured within 1 h of complete submergence (Banga et al., 1996).
Both carbon dioxide and oxygen are unreliable indicators of submergence in tissues that have the capacity to photosynthesize. Ethylene, however, is produced by nearly all plant organs if some oxygen is present and it is hardly catabolized. In conclusion, in shoot tissue, ethylene is the most reliable indicator of the submerged status.
In plants, ethylene is synthesized from methionine via S‐adenosyl‐l‐methionine (AdoMet) and 1‐aminocyclopropane‐1‐carboxylic acid (ACC). The conversion of AdoMet to ACC is catalysed by ACC synthase, whereas ACC is converted to ethylene, carbon dioxide and hydrogen cyanide by ACC oxidase in an oxygen‐dependent process (Kende, 1993). When submerged, shoots of R. palustris produce ethylene at a rate which is similar to that of non‐submerged controls (Voesenek et al., 1993; Banga et al., 1996) despite a reduction of the oxygen concentration in submerged petioles (Rijnders et al., 2000). This can only be realized if the rate of ethylene biosynthesis is regulated by submergence‐induced signals. On a molecular level, cDNAs corresponding to an ACC synthase gene (RP‐ACS1) and an ACC oxidase gene (RP‐ACO1) are up‐regulated during complete submergence (Vriezen et al., 1999a, b). On a protein level, submergence induces also a significant increase in the in vitro activity of ACC synthase (Huibers and Voesenek, unpublished results) and ACC oxidase (Vriezen et al., 1999b) in R. palustris. We conclude, that transcription, translation and possibly also post‐translational events of the last two enzymes in the ethylene biosynthesis pathway maintain continued ethylene production and, thus, signal strength in environments with sub‐ambient oxygen concentrations.
During the last few years, enormous progress has been achieved in unravelling the mechanism by which Arabidopsis thaliana perceives ethylene. Ethylene is perceived by a family of five membrane‐bound receptors that show a high similarity to two‐component receptors known from prokaryotic organisms (Chang et al., 1993; Stepanova and Ecker, 2000). These proteins are active in the absence of ethylene and negatively regulate ethylene responses. In the presence of ethylene, receptors are switched to a signalling inactive state, allowing ethylene responses to proceed (Hua and Meyerowitz, 1998). The consequence of this model of action is that an increase in mRNA coding for ethylene receptors and a subsequent increase in receptor protein will de‐sensitize the tissue to ethylene. In this respect, it is surprising to notice that some receptor genes are up‐regulated by ethylene. In arabidopsis, this was interpreted as a mechanism to shut down ethylene signalling quickly when ethylene levels decrease (Hall et al., 2000), as happens during de‐submergence of R. palustris. A cDNA homologous to the arabidopsis ERS genes was isolated recently from a cDNA library of 24‐h submerged shoot tissue of R. palustris. The transcript level was strongly up‐regulated within 4 h of submergence; fast down‐regulation was observed upon de‐submergence (Vriezen et al., 1997). This submergence response could be mimicked by reduced oxygen concentrations (3 %) and enhanced ethylene levels (5 µl l–1) (Voesenek et al., 1997; Vriezen et al., 1997). If the ethylene receptor in Rumex operates in a similar way as described for arabidopsis, an increase in ERS transcript probably leads to a de‐sensitization of the tissue to ethylene. Probably this has no effect on the shoot elongation response since over‐saturating ethylene concentrations (4–8 µl l–1) (Voesenek et al., 1993; Banga et al., 1996) are reached in submerged shoots of these plants.
Submergence‐induced petiole elongation in R. palustris can partially be mimicked by enhanced ethylene concentrations or reduced oxygen levels applied to intact plants in a controlled atmosphere (Voesenek et al., 1997). When applied together, the highest growth rate was observed. The response to low oxygen was ethylene dependent since the ethylene biosynthesis inhibitor AVG and the ethylene action inhibitor norbornadiene could inhibit low oxygen‐induced petiole elongation. This inhibition could be rescued by addition of ACC or ethylene, respectively. This interaction between low oxygen and ethylene was not caused by a low oxygen‐induced increase in ethylene production but was related to an increase in ethylene sensitivity (Voesenek et al., 1997).
Transduction of submergence signal
One of the earliest detectable effects of submergence in R. palustris is a very fast decline of the endogenous abscisic acid (ABA) concentration. Within 1 h of submergence the ABA level in petioles is reduced by 80%. This response could be mimicked by exposure of plants to exogenous ethylene, demonstrating the interaction between ethylene and ABA. Since ABA, added to submerged Rumex plants, severely inhibited elongation of petioles we hypothesize that the fast endogenous decline of ABA in submerged plants is a prerequisite for enhanced petiole elongation (Benschop, Jackson and Voesenek, unpublished results). A similar role for ABA is decribed for the elongation of internodes of deepwater rice (Hoffmann‐Benning and Kende, 1992).
It is tempting to compare these results with an interaction between ethylene and ABA found in arabidopsis during sugar‐induced feedback inhibition of photosynthesis (Zhou et al., 1998; Huijser et al., 2000; Smeekens, 2000). The recessive gin1 mutant in arabidopsis is insensitive to glucose repression of cotyledon greening and expansion, shoot development, floral transition and gene expression. GIN1 acts downstream of the sensor hexokinase in the glucose signalling pathway (Zhou et al., 1998). Furthermore, ABI4, a gene that encodes a transcription factor in ABA signal transduction (Finkelstein et al., 1998), acts downstream of GIN1 in this pathway (Huijser et al., 2000). The gin1 phenotype could be mimicked by addition of ACC to wild‐type plants. As expected, glucose insensitivity was also demonstrated in the constitutive ethylene signalling mutant ctr1 and in the ethylene overproducer eto1 (Zhou et al., 1998). These data suggest that ethylene can regulate ABA‐mediated inhibition of reserve mobilization during germination. Via a similar cascade it is possible that during submergence‐induced shoot elongation in R. palustris ethylene stimulates the breakdown of starch via a down‐regulation of ABA action. We assume that sugars are necessary to fuel submergence‐induced petiole elongation and to provide building units for cell wall synthesis accompanying elongation. Starch breakdown in response to submergence is also described for elongating internodes of deepwater rice (Raskin and Kende, 1984a).
Submergence‐induced petiole elongation in R. palustris also depends on the concentration of at least one other group of plant hormones: gibberellins (GAs). Application of the GA biosynthesis inhibitor paclobutrazol partially decreases the elongation response upon submergence. This reduced elongation could be rescued by addition of GA3 (Rijnders et al., 1997). Submergence induces an increase in the concentration of the bioactive GA1 and not in GA4, indicating that GA1 is probably the most important gibberellin in submergence‐induced petiole elongation in R. palustris. However, the first significant increase in GA1 was observed only 6 h after submergence indicating that the early hours of submergence‐induced petiole elongation do not require an increase in the endogenous level of GA1 (Benschop and Voesenek, unpublished results). The up‐regulation of GA1 after 24 h of submergence could be mimicked by 5 µl l–1 ethylene (Rijnders et al., 1997). This observation indicates an interaction between ethylene and GA, although an intermediate role for ABA cannot be excluded in this interaction. This interaction between ethylene and GA is further strengthened by the observation that petiole responsiveness to exogenous GA3 increases in the presence of 5 µl l–1 ethylene (Rijnders et al., 1997).
The antagonism between GA and ABA is earlier described for submergence‐induced elongation in internodes of deepwater rice. The decline in endogenous ABA upon submergence explained, at least in part, the increase in tissue sensitivity to GA in this plant. Together with an increase in the endogenous GA1 concentration this lead to an increased GA signal strength (Raskin and Kende, 1984b; Hoffmann‐Benning and Kende, 1992).
We hypothesize that an increase in hydrolytic enzyme activity is required for fast underwater elongation of R. palustris. This will produce sugars, via the breakdown of starch in shoot and tap root, needed for cell wall synthesis that must accompany cell elongation. A decline in the starch concentration in Rumex shoots was observed within 24 h of submergence (Cox and Voesenek, unpublished results). Since the concentration of GA1 is not up‐regulated in R. palustris during the first hours of stimulated petiole elongation, we assume that a fast decrease of ABA is essential for an increase in the GA1/ABA ratio and thus an increase in GA signal strength. This in turn may regulate the required hydrolytic enzyme activity in shoots of Rumex.
Some aquatic and semi‐aquatic plant species (e.g. Ranunculus sceleratus, Nymphoides peltata) require auxin for maximal submergence‐induced elongation (Voesenek and Blom, 1999). Neither removal of the lamina (putative auxin source) nor addition of N‐1‐naphthylphthalamic acid (NPA), an inhibitor of polar auxin transport, had any effect on the submergence‐induced elongation of R. palustris petioles as measured over a response time of 48 h (Rijnders et al., 1996). However, with these relatively long‐lasting experiments, we cannot exclude the possibility that auxin does play a significant role during the first hour of submergence‐induced petiole elongation. This becomes extra relevant if we take into account that internode elongation in Pisum sativum requires an interaction between auxin and GA (Ross et al., 2000). Decapitation (removal of auxin source) results in a reduction of the concentration of mRNA that codes for an enzyme involved in the synthesis of active GA1 (GA 3β‐hydroxylase) and in a reduction of the concentration of GA1 in pea internodes. This down‐regulation was completely rescued by application of indole‐3‐acetic acid (IAA) to the remaining ‘stump’ of the shoot internode of decapitated plants. These results show that at least in pea internode elongation, biosynthesis of GA1 requires a certain threshold of endogenous IAA.
Differential and non‐differential growth
The first visible effect of submergence on the phenotype of R. palustris is hyponastic growth of petioles and leaf blades (Fig. 1). This differential growth response starts within 2 h of submergence and is finished after 6–8 h (Cox and Voesenek, unpublished results). By this time the youngest leaves have an almost vertical orientation. The putative functional significance of this hyponastic growth lies in the reduction of the distance of the photosynthesizing tissue to the water surface. Preliminary experiments indicate that hyponastic growth during submergence is induced by ethylene and that it requires auxin. It is not yet known how these two hormones interact in hyponastic growth.
An interaction between auxin and ethylene is described for waterlogging‐induced formation of adventitious roots in R. palustris (Visser et al., 1996). Waterlogged roots of R. palustris accumulate ethylene to a level that induces formation of adventitious roots in non‐waterlogged plants. Inhibition of ethylene production decreased the number of adventitious roots induced by waterlogging, indicating that high ethylene is required for the intiation of these roots. Ethylene promotes also the development of adventitious roots at the nodes of deepwater rice (Bleecker et al., 1987; Lorbiecke and Sauter, 1999). The endogenous auxin concentration did not change in Rumex during waterlogging‐induced adventitious rooting. However, a continuous basipetal transport of auxin from the shoot to the rooting zone (upper part taproot) is required for adventious root formation. Furthermore, it was shown for R. palustris that exogenous auxin could induce root formation in the presence of an ethylene biosynthesis inhibitor. However, applied ethylene was unable to induce adventitious roots in the presence of an auxin transport inhibitor. Waterlogging‐induced formation of adventitious roots in R. palustris can be explained by a model in which high ethylene concentrations sensitize the root‐forming tissue in the upper part of the taproot to endogenous IAA (Visser et al., 1996).
To reach the water surface as quickly as possible, it is of utmost importance that differential growth switches to elongation of the entire petiole. Submergence‐induced petiole elongation is equally distributed over the entire organ, as demonstrated by an experiment in which the most responsive petiole (youngest) was marked from top to base to distinguish six segments of 2 mm (Rijnders et al., 1996). During submergence‐induced petiole elongation, no new cells were formed, indicating that the increase in petiole length upon submergence is completely attributable to increased cell expansion (Voesenek et al., 1990).
For sustained cell elongation and water uptake, cell wall loosening and synthesis of cell wall components is required. There is general consensus that elongation of plant cells is ultimately controlled by the extensibility of cell walls (McQueen‐Mason and Rochange, 1999). Cell walls extend much faster at acidic pH than at neutral pH (Rayle and Cleland, 1992). This ‘acid growth’ requires expansins, a class of cell wall proteins, as mediators of extension. Expansins appear to break hydrogen bonds between cellulose and other cell wall polysaccharides, thereby permitting turgor‐driven cell enlargement (Cosgrove, 2000). Treatment of R. palustris with fusicoccin, a powerful enhancer of the plasmalemma H+‐ATPase activity, stimulated petiole elongation (Vriezen and Voesenek, unpublished results).
An α‐expansin cDNA (Rp‐EXP1) was isolated from a R. palustris cDNA library constructed from the two youngest leaves of plants submerged for 24 h. The transcript level in petioles increased within 2 h of submergence; de‐submergence induced a fast decline of the concentration of mRNA (Vriezen et al., 2000). When R. palustris was treated with exogenous ethylene (5 µl l–1) an up‐regulation of Rp‐EXP1 gene expression was already observed after 1 h. In situ mRNA hybridization showed that Rp‐EXP1 mRNA was detectable in vascular bundles, parenchyma cells, epidermis and in the angular collenchyma indicating that all cell types in the petiole express this gene (Vriezen et al., 2000), as presumably required to provide a coordinated elongation response of all tissues in the petioles.
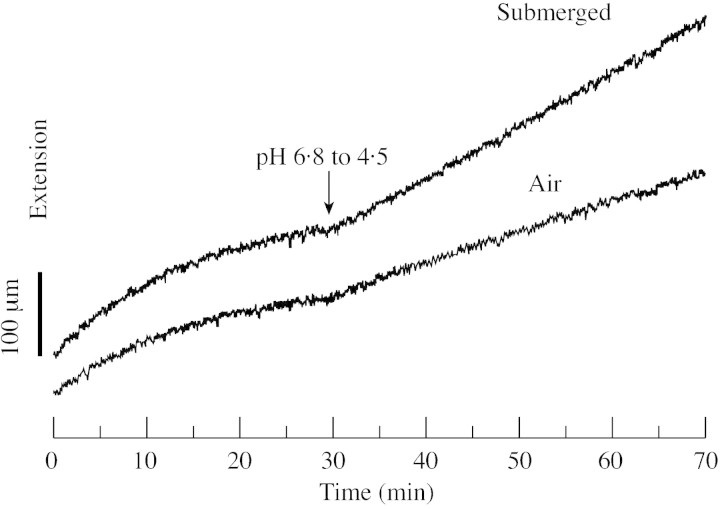
The increase in Rp‐EXP1 transcript upon submergence correlates with the extensibility of isolated cell walls of R. palustris as measured with the extensometer assay (Vreeburg and Voesenek, unpublished results). An example of a typical extensometer trace from petioles of previously submerged or non‐submerged plants after 8 h of treatment, is shown in Fig. 3. Heat‐killed petioles demonstrated no acid‐induced extension (data not shown).
Fig. 3. Representative traces of acid‐induced extension of cell walls from submerged (8 h) and control petioles of Rumex palustris. To measure cell wall extentensibility, intact petioles (length approx. 10 mm) were frozen, thawed, abraded and pressed prior to clamping in the extensometer. Petioles were clamped under an applied force of 30 g and extension was recorded with a linear displacement transducer. Heat‐killed petioles (15 s in boiling water) showed no detectable acid‐induced extension.
The expression of α‐expansins is also studied in deepwater rice and correlated in this species with acid‐inducible cell wall extensibility (Cho and Kende, 1997). Recently, it was shown for deepwater rice that the expression of β‐expansins also correlated with rapid internode elongation under water (Lee and Kende, 2001).
Synthesis
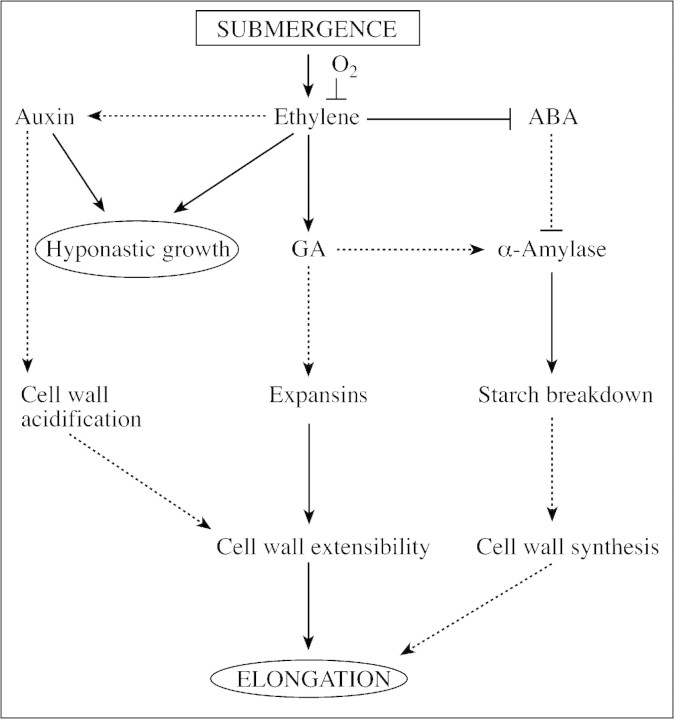
The signal transduction and response network in Fig. 4 presents our current view and hypotheses on how submergence‐induced petiole elongation in Rumex palustris is regulated. Ethylene accumulates in submerged Rumex plants and signals the plant that its outside environment is changed from air to water. Hereafter, interactions between ethylene and ABA, ethylene and GA, and possibly also between ethylene and auxin and between ABA and GA regulates petiole elongation in a highly coordinated way. We hypothesize that cell wall acidification might be an important process to induce elongation within a few hours of submergence. This acidification response could be mediated by auxin and perhaps also ABA. It is reported that ABA can inhibit H+ pumps and 14‐3‐3 proteins in guard cells (Schroeder et al., 2001). We therefore cannot exclude the possibility that a reduced ABA content, as observed in submerged R. palustris, might stimulate acidification of the apoplast. More indirectly a reduction in ABA might result in an increased GA signal strength. Via this route GA‐enhanced starch breakdown in the shoot can produce sugars to fuel proton pump activity and deliver building blocks for cell wall synthesis.
Fig. 4. Signal transduction and response network involved in submergence‐induced petiole elongation of the flooding‐tolerant dicot Rumex palustris. Arrows represent positive regulation and bars represent negative regulations. Dashed lines are hypothetical interactions.
However, to continue the fast petiole elongation rate for several days more than only acidification is required. As petioles of R. palustris mature, they loose the capacity to elongate in response to submergence or exogenous ethylene (Voesenek and Blom, 1989). This might be related to an increase in cross‐linking of the cell wall matrix (Cosgrove, 1998). A decrease of in vitro acid‐induced extensibility was observed for non‐submerged control petioles (Vreeburg and Voesenek, unpublished data). We here speculate that the increased expression of a member of the expansin gene family in submerged petioles (Vriezen et al., 2000) counteracts this developmental decline in extensibility and thus enhances long‐term elongation.
So far, an overview is given of components and their sequence involved in submergence‐induced petiole elongation in R. palustris. On top of these data, we have indications that the rate of petiole elongation is also under tight environmental control. In this fine‐tuning mechanism oxygen plays a major role. Ambient oxygen concentrations inhibit ethylene‐mediated petiole elongation; maximal elongation was observed at concentrations between 3 and 10 % oxygen (Voesenek et al., 1997). This mechanism results in a quick restoration of shoot–atmosphere contact when plants are opposed to submergence conditions that result in low endogenous oxygen concentrations in the shoot. Such conditions can occur when underwater photosynthesis is inhibited by large amounts of suspended fine sediment in the flood‐water.
Some plant species like R. palustris and deepwater rice (for reviews, see Kende et al., 1998; Sauter, 2000) are stimulated in growth when exposed to submergence or ethylene. Others, sometimes closely related species, however, are significantly reduced in growth rate under similar conditions. It is still an enigma how congenial species such as those belonging to the genus Rumex can have such an opposite response to ethylene. A better understanding of this variation among species is important to comprehend distribution patterns and species abundance in natural environments. Rumex acetosa is flooding intolerant, and its distribution is restricted to rarely flooded habitats. Furthermore, it lacks traits such as aerenchyma formation, development of adventitious roots and ethylene‐driven petiole elongation that increase survival in flooding prone environments (Blom and Voesenek, 1996). R. acetosa differs in some aspects of the signalling network resulting in shoot elongation from R. palustris. The non‐elongating R. acetosa lacks the fast decline in ABA (Benschop, Jackson and Voesenek, unpublished results), misses an up‐regulation of GA1 (Rijnders et al., 1997) and expansin transcript concentration is not enhanced upon submergence or ethylene application (Vriezen et al., 2000). We have evidence that GA1 is not the rate‐limiting step causing lack of submergence‐induced petiole elongation in R. acetosa. Addition of GA to submerged or ethylene‐exposed plants could not rescue its non‐elongating phenotype (Rijnders et al., 1997). It is not yet clear whether ABA and/or expansins are important to explain the inability of petioles of R. acetosa to elongate under water.
Acknowledgements
This work was supported by the NWO‐PIONIER grant (800·84·470) as given to L. A. C. J. Voesenek and an EU Research Training Network grant (RTN1‐1999–00086) given to J. Bou. We furthermore thank Rob Welschen, Wim Huibers and Niels Wagemaker for technical assistance and Tim Colmer and Hendrik Poorter for critically reading this manuscript.
Supplementary Material
Received: 6 August 2001; Returned for revision: 29 October 2001; Accepted: 21 December 2001
References
- BangaM, Slaa EJ, Blom CWPM, Voesenek LACJ.1996. Ethylene biosynthesis and accumulation under drained and submerged conditions: a comparative study of two Rumex species. Plant Physiology 112: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BleeckerAB, Rose‐John S, Kende H.1987. An evaluation of 2,5‐norbornadiene as a reversible inhibitor of ethylene action in deepwater rice. Plant Physiology 84: 395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BlomCWPM.1999. Adaptations to flooding stress: from plant community to molecule. Plant Biology 1: 261–273. [Google Scholar]
- BlomCWPM, Voesenek LACJ.1996. Flooding: the survival strategies of plants. Trends in Ecology and Evolution 11: 290–295. [DOI] [PubMed] [Google Scholar]
- ChangC, Kwok SF, Bleecker AB, Meyerowitz EM.1993. Arabidopsis ethylene‐response gene ETR1: similarity of product to two‐component regulators. Science 262: 539–544. [DOI] [PubMed] [Google Scholar]
- ChoH‐T, Kende H.1997. Expression of expansin genes is correlated with growth in deepwater rice. Plant Cell 9: 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ColmerTD, Gibberd MR, Wiengweera A, Tinh TK.1998. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany 49: 1431–1436. [Google Scholar]
- CosgroveDJ.1998. Cell wall loosening by expansins. Plant Physiology 118: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CosgroveDJ.2000. Loosening of plant cell walls by expansins. Nature 407: 321–326. [DOI] [PubMed] [Google Scholar]
- FinkelsteinRR, Wang ML, Lynch TJ, Rao S, Goodman HM.1998. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein.Plant Cell 10: 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HallAE, Findell JL, Schaller GE, Sisler EC, Bleecker AB.2000. Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiology 123: 1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann‐BenningS, Kende H.1992. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiology 99: 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HuaJ, Meyerowitz EM.1998. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana Cell 94: 261–271. [DOI] [PubMed] [Google Scholar]
- HuijserC, Kortstee A, Pego J, Weisbeek P, Wisman E, Smeekens S.2000. The Arabidopsis SUCROSE UNCOUPLED‐6 gene is identical to ABSCISIC ACID INSENSITIVE‐4: involvement of abscisic acid in sugar responses. Plant Journal 23: 577–585. [DOI] [PubMed] [Google Scholar]
- JacksonMB.1985. Ethylene and responses of plants to soil waterlogging and submergence. Annual Review of Plant Physiology 36: 145–174. [Google Scholar]
- JacksonMB, Armstrong W.1999. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology 1: 274–287. [Google Scholar]
- KendeH.1993. Ethylene biosynthesis. Annual Review of Plant Physiology and Molecular Biology 44: 283–307. [Google Scholar]
- KendeH, Van de Knaap E, Cho, H‐T 1998. Deepwater rice: a model plant to study stem elongation. Plant Physiology 118: 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeeY, Kende H.2001. Expression of β‐expansins is correlated with internodal elongation in deepwater rice. Plant Physiology 127: 645–654. [PMC free article] [PubMed] [Google Scholar]
- LorbieckeR, Sauter M.1999. Adventitious root growth and cell‐cycle induction in deepwater rice. Plant Physiology 119: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen‐MasonSJ, Rochange F.1999. Expansins in plant growth and development: an update on an emerging topic. Plant Biology 1: 19–25. [Google Scholar]
- RaskinI, Kende H.1984a Effect of submergence on translocation, starch content and amylolytic activity in deepwater rice. Planta 162: 556–559. [DOI] [PubMed] [Google Scholar]
- RaskinI, Kende H.1984b The role of gibberellin in the growth response of submerged deepwater rice. Plant Physiology 76: 947–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RayleDL, Cleland RE 1992. The acid growth theory of auxin‐induced cell elongation is alive and well. Plant Physiology 99: 1271–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RidgeI 1987. Ethylene and growth control in amphibious plants. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific.
- RijndersJGHM, Barendse GWM, Blom CWPM, Voesenek LACJ.1996. The contrasting role of auxin in submergence‐induced petiole elongation in two species from frequently flooded wetlands. Physiologia Plantarum 96: 467–473. [Google Scholar]
- RijndersJGHM, Yang Y‐Y, Kamiya Y, Takahashi N, Barendse GWM, Blom CWPM, Voesenek LACJ 1997. Ethylene enhances glibberellin levels and petiole sensitivity in flooding‐tolerant Rumex palustris but not in flooding‐intolerant R. acetosa Planta 203: 20–25. [Google Scholar]
- RijndersJGHM, Armstrong W, Darwent MJ, Blom CWPM, Voesenek LACJ 2000. The role of oxygen in submergence‐induced petiole elongation in Rumex palustris: in situ measurements of oxygen in petioles of intact plants using micro‐electrodes. New Phytologist 147: 497–504. [DOI] [PubMed] [Google Scholar]
- RossJJ, O’Neill DP, Smith JJ, Kerckhoffs LHJ, Elliott RC.2000. Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant Journal 21: 547–552. [DOI] [PubMed] [Google Scholar]
- SauterM.2000. Rice in deep water: ‘How to take heed against a sea of troubles’. Naturwissenschaften 87: 289–303. [DOI] [PubMed] [Google Scholar]
- SchroederJI, Kwak JM, Allen GJ.2001. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410: 327–330. [DOI] [PubMed] [Google Scholar]
- SmeekensS.2000. Sugar‐induced signal transduction.Annual Review of Plant Physiology and Molecular Biology 51: 49–81. [DOI] [PubMed] [Google Scholar]
- StepanovaAN, Ecker JR.2000. Ethylene signaling: from mutants to molecules. Current Opinion in Plant Biology 3: 353–360. [DOI] [PubMed] [Google Scholar]
- StünziJT, Kende H.1989. Gas composition in the internal air spaces of deepwater rice in relation to growth induced by submergence. Plant Cell Physiology 30: 49–56. [Google Scholar]
- VisserEJW, Cohen JD, Barendse GWM, Blom CWPM, Voesenek LACJ.1996. An ethylene‐mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiology 112: 1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VisserEJW, Nabben RHM, Blom CWPM, Voesenek LACJ 1997. Elongation by primary lateral roots and adventitious roots during conditions of hypoxia and high ethylene concentration. Plant, Cell and Environment 20: 647–653. [Google Scholar]
- VoesenekLACJ, Blom CWPM.1989. Growth responses of Rumex species in relation to submergence and ethylene. Plant, Cell and Environment 12: 433–439. [Google Scholar]
- VoesenekLACJ, Blom CWPM 1999. Stimulated shoot elongation: a mechanism of semi‐aquatic plants to avoid submergence stress. In: Lerner HR, ed. Plant responses to environmental stresses: from phytohormones to genome reorganization. New York: Marcel Dekker.
- VoesenekLACJ, Perik PJM, Blom CWPM, Sassen MMA.1990. Petiole elongation in Rumex during submergence and ethylene exposure: the relative contributions of cell division and cell expansion. Journal of Plant Growth Regulation 9: 13–17. [Google Scholar]
- VoesenekLACJ, Banga M, Thier RH, Mudde CM, Harren FJM, Barendse GWM, Blom CWPM.1993. Submergence‐induced ethylene synthesis entrapment, and growth in two plant species with contrasting flooding resistances. Plant Physiology 103: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VoesenekLACJ, Vriezen WH, Smekens MJE, Huitink FHM, Bögemann GM, Blom CWPM 1997. Ethylene sensitivity and response sensor expression in petioles of Rumex species at low O2 and high CO2 concentrations. Plant Physiology 114: 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VriezenWH, Van Rijn CPE, Voesenek LACJ, Mariani C.1997. A homolog of the Arabidopsis thaliana ERS gene is actively regulated in Rumex palustris upon flooding. The Plant Journal 11: 1265–1271. [DOI] [PubMed] [Google Scholar]
- VriezenWH, Voesenek LACJ, Mariani C.1999a Ethylene biosynthesis in Rumex palustris upon flooding. In: Kanellis AK, Chang C, Klee H, Bleecker AB, Pech JC, Grierson D, eds. Biology and biotechnology of the plant hormone ethylene. II. Dordrecht: Kluwer Academic Publishers.
- VriezenWH, Hulzink R, Mariani C, Voesenek LACJ 1999b 1‐Amino cyclopropane‐1‐carboxylate oxidase activity limits ethylene biosynthesis in Rumex palustris during submergence. Plant Physiology 121: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VriezenWH, De Graaf B, Mariani C, Voesenek LACJ.2000. Submergence induces expansin gene expression in flooding‐tolerant Rumex palustris and not in flooding‐intolerant R. acetosa Planta 210: 956–963. [DOI] [PubMed] [Google Scholar]
- ZhouL, Jang JC, Jones TL, Sheen J.1998. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose‐insensitive mutant. Proceedings of the National Academy of Sciences of the USA 95: 10294–10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.