Abstract
Oxidative stress is induced by a wide range of environmental factors including UV stress, pathogen invasion (hypersensitive reaction), herbicide action and oxygen shortage. Oxygen deprivation stress in plant cells is distinguished by three physiologically different states: transient hypoxia, anoxia and reoxygenation. Generation of reactive oxygen species (ROS) is characteristic for hypoxia and especially for reoxygenation. Of the ROS, hydrogen peroxide (H2O2) and superoxide (O2·–) are both produced in a number of cellular reactions, including the iron‐catalysed Fenton reaction, and by various enzymes such as lipoxygenases, peroxidases, NADPH oxidase and xanthine oxidase. The main cellular components susceptible to damage by free radicals are lipids (peroxidation of unsaturated fatty acids in membranes), proteins (denaturation), carbohydrates and nucleic acids. Consequences of hypoxia‐induced oxidative stress depend on tissue and/or species (i.e. their tolerance to anoxia), on membrane properties, on endogenous antioxidant content and on the ability to induce the response in the antioxidant system. Effective utilization of energy resources (starch, sugars) and the switch to anaerobic metabolism and the preservation of the redox status of the cell are vital for survival. The formation of ROS is prevented by an antioxidant system: low molecular mass antioxidants (ascorbic acid, glutathione, tocopherols), enzymes regenerating the reduced forms of antioxidants, and ROS‐interacting enzymes such as SOD, peroxidases and catalases. In plant tissues many phenolic compounds (in addition to tocopherols) are potential antioxidants: flavonoids, tannins and lignin precursors may work as ROS‐scavenging compounds. Antioxidants act as a cooperative network, employing a series of redox reactions. Interactions between ascorbic acid and glutathione, and ascorbic acid and phenolic compounds are well known. Under oxygen deprivation stress some contradictory results on the antioxidant status have been obtained. Experiments on overexpression of antioxidant production do not always result in the enhancement of the antioxidative defence, and hence increased antioxidative capacity does not always correlate positively with the degree of protection. Here we present a consideration of factors which possibly affect the effectiveness of antioxidant protection under oxygen deprivation as well as under other environmental stresses. Such aspects as compartmentalization of ROS formation and antioxidant localization, synthesis and transport of antioxidants, the ability to induce the antioxidant defense and cooperation (and/or compensation) between different antioxidant systems are the determinants of the competence of the antioxidant system.
Keywords: Key words: Review, anoxia, hypoxia, reactive oxygen species, antioxidant, lipid peroxidation, adaptation, oxygen deprivation stress.
INTRODUCTION
Lack of oxygen or anoxia is a common environmental challenge which plants have to face throughout their life. Winter ice encasement, seed imbibition, spring floods and excess of rainfall are examples of natural conditions leading to root hypoxia or anoxia. Low oxygen concentration can also be a normal attribute of a plants’ natural environment. Wetland species and aquatic plants have developed adaptative structural and metabolic features to combat oxygen deficiency. A decrease in adenylate energy charge, cytoplasmic acidification, anaerobic fermentation, elevation in cytosolic Ca2+ concentration, changes in the redox state and a decrease in the membrane barrier function, are the main features caused by lack of oxygen (reviewed by Richard et al., 1994; Ratcliffe, 1995; Crawford and Braendle, 1996; Drew, 1997; Vartapetian and Jackson, 1997; Tadege et al., 1999). Regulation of anoxic metabolism is complex and not all the features are well established. In the recent paper by Gout et al. (2001) a cytoplasmic acidification process has been temporally resolved in sycamore (Acer pseudoplatanus) cell culture by NMR (nuclear magnetic resonance). The immediate response of cytoplasmic pH was solely dependent on proton‐releasing metabolization of the nucleoside triphosphate pool; the long‐term regulation (after 20 min of anoxia) involves lactate synthesis, succinate, malate, amino acid metabolism and ethanolic fermentation (Gout et al., 2001).
Under natural conditions anoxic stress includes several transition states (hypoxia, anoxia and reoxygenation) characterized by different O2 concentrations. Excessive generation of reactive oxygen species (ROS), i.e. under oxidative stress, is an integral part of many stress situations, including hypoxia. Hydrogen peroxide accumulation under hypoxic conditions has been shown in the roots and leaves of Hordeum vulgare (Kalashnikov et al., 1994) and in wheat roots (Biemelt et al., 2000). The presence of H2O2 in the apoplast and in association with the plasma membrane has been visualized by transmission electron microscopy under hypoxic conditions in four plant species (Blokhina et al., 2001). In these experiments H2O2 was probably of enzymatic origin considering the low oxygen concentration in the system and the positive effects of the various inhibitors of H2O2‐producing enzymes. Indirect evidence of ROS formation (i.e. lipid peroxidation products) under low oxygen has been detected (Hunter et al., 1983; Crawford et al., 1994; Yan et al., 1996; Chirkova et al., 1998; Blokhina et al., 1999).
The phenomenon of cross‐tolerance to various environmental stresses suggests the existence of a common factor, which provides crosstalk between different signalling pathways. ROS have recently been considered as possible signalling molecules in the detection of the surrounding oxygen concentration (Semenza, 1999). It has been suggested also that ROS and oxygen concentration (including hypoxia) can be sensed via the same mechanism. Several models employ direct sensing of oxygen (via haemoglobin or protein SH oxidation) or ROS sensing. There are two models which suggest either a decrease in ROS under oxygen deprivation (low NADPH oxidase activity) or an increase in ROS due to the inhibition of the mitochondrial electron transport chain.
SOURCES OF ROS IN PLANT CELLS
Molecular oxygen is relatively unreactive (Elstner, 1987) due to its electron configuration. Activation of oxygen (i.e. the first univalent reduction step) is energy dependent and requires an electron donation. The subsequent one‐electron reduction steps are not energy dependent and can occur spontaneously or require appropriate e–/H+ donors. In biological systems transition metal ions (Fe2+, Cu+) and semiquinones can act as e– donors. Four‐electron reduction of oxygen in the respiratory electron transport chain (ETC) is always accompanied with a partial one‐ to three‐electron reduction, yielding the formation of ROS. This term includes not only free radicals (superoxide radical, O2·–, and hydroxyl radical, OH·), but also molecules such as hydrogen peroxide (H2O2), singlet oxygen (1O2) and ozone (O3). Both O2·– and the hydroperoxyl radical HO2· undergo spontaneous dismutation to produce H2O2. Although H2O2 is less reactive than O2·–, in the presence of reduced transition metals such as Fe2+ in a chelated form (which is the case in biological systems), the formation of OH· can occur in the Fenton reaction.
Mechanisms for the generation of ROS in biological systems are represented by both non‐enzymatic and enzymatic reactions. The partition between these two pathways under oxygen deprivation stress can be regulated by the oxygen concentration in the system. Non‐enzymatic one electron O2 reduction can occur at about 10–4 m and higher oxygen concentrations (Skulachev, 1997), while in very low O2 concentrations plant terminal oxidases (Km 10–6 m for oxygen) and the formation of ROS via mitochondrial ETC still remain functional.
Among enzymatic sources of ROS, xanthine oxidase (XO), an enzyme responsible for the initial activation of dioxygen should be mentioned. As electron donors XO can use xanthine, hypoxanthine or acetaldehyde (Bolwell and Wojtaszek, 1997). The latter has been shown to accumulate under oxygen deprivation (Pfister‐Sieber and Braendle, 1994) and can represent a possible source for hypoxia‐stimulated ROS production. The next enzymatic step is the dismutation of the superoxide anion by superoxide dismutase (SOD, EC.1.15.1.1) to yield H2O2. Due to its relative stability the level of H2O2 is regulated enzymatically by an array of catalases (CAT) and peroxidases localized in almost all compartments of the plant cell. Peroxidases, besides their main function in H2O2 elimination, can also catalyse O2·– and H2O2 formation by a complex reaction in which NADH is oxidized using trace amounts of H2O2 first produced by the non‐enzymatic breakdown of NADH. Next, the NAD· radical formed reduces O2 to O2·–, some of which dismutates to H2O2 and O2 (Lamb and Dixon, 1997). Thus, peroxidases and catalases play an important role in the fine regulation of ROS concentration in the cell through activation and deactivation of H2O2 (Elstner, 1987). Lipoxygenase (LOX, linoleate:oxygen oxidoreductase, EC.1.13.11.12) reaction is another possible source of ROS and other radicals. It catalyses the hydroperoxidation of polyunsaturated fatty acids (PUFA) (Rosahl, 1996). The hydroperoxyderivatives of PUFA can undergo autocatalytic degradation, producing radicals and thus initiating the chain reaction of lipid peroxidation (LP). In addition LOX‐mediated formation of singlet oxygen (Kanofsky and Axelrod, 1986) or superoxide (Lynch and Thompson, 1984) has been shown. A specific LOX activity increase and its positive correlation with the duration of anoxia have been detected in potato cells (Pavelic et al., 2000).
Several apoplastic enzymes may also lead to ROS production under normal and stress conditions. Other oxidases, responsible for the two‐electron transfer to dioxygen (amino acid oxidases and glucose oxidase) can contribute to H2O2 accumulation. Also an extracellular germin‐like oxalate oxidase catalyses the formation of H2O2 and CO2 from oxalate in the presence of oxygen (Bolwell and Wojtaszek, 1997). Amine oxidases catalyse the oxidation of biogenic amines to the corresponding aldehyde with a release of NH3 and H2O2. Data on polyamine (putrescine) accumulation under anoxia in rice and wheat shoots (Reggiani and Bertani, 1989) and predominant localization of amine oxidase in the apoplast, suggest amine oxidase participation in H2O2 production under oxygen deprivation.
ROS can be also formed as by‐products in the electron transport chains of chloroplasts (Asada, 1999), mitochondria and the plasma membrane (cytochrome b‐mediated electron transfer) (Elstner, 1987). Plant mitochondrial ETC, with its redox‐active electron carriers, is considered as the most probable candidate for intracellular ROS formation. Mitochondria have been shown to produce ROS (superoxide anion O2·– and the succeeding H2O2) due to the electron leakage at the ubiquinone site—the ubiquinone:cytochrome b region (Gille and Nohl, 2001)—and at the matrix side of complex I (NADH dehydrogenase) (Chakraborti et al., 1999; Möller, 2001). Hydrogen peroxide generation by higher plant mitochondria and its regulation by uncoupling of ETC and oxidative phosphorylation have been demonstrated by Braidot et al. (1999).
Lipid peroxidation is a natural metabolic process under normal aerobic conditions and it is one of the most investigated consequences of ROS action on membrane structure and function. PUFA, the main components of membrane lipids, are susceptible to peroxidation. The initiation phase of LP includes activation of O2 (see above) which is rate limiting. Hydroxyl radicals and singlet oxygen can react with the methylene groups of PUFA forming conjugated dienes, lipid peroxy radicals and hydroperoxides (Smirnoff, 1995):
PUFA–H + X· → PUFA· + X–H
PUFA· + O2 → PUFA–OO·
The peroxyl radical formed is highly reactive and is able to propagate the chain reaction:
PUFA–OO· + PUFA–H → PUFA–OOH + PUFA·
The formation of conjugated dienes occurs when free radicals attack the hydrogens of methylene groups separating double bonds and leading to a rearrangement of the bonds (Recknagel and Glende, 1984). The lipid hydroperoxides produced (PUFA–OOH) can undergo reductive cleavage by reduced metals, such as Fe2+, according to the following equation:
Fe2+ complex + PUFA–OOH → Fe3+ complex + OH– + PUFA–O·
The lipid alkoxyl radical produced, PUFA–O., can initiate additional chain reactions (Buettner, 1993):
PUFA–O· + PUFA–H → PUFA–OH + PUFA·
The multi‐stage character of the process, i.e. branching of chain reactions, allows several ways of regulation (Shewfelt and Purvis, 1995). Among the regulated properties are the structure of the membranes: composition and organization of lipids inside the bilayer in a way which prevents LP (Merzlyak, 1989), the degree of PUFA unsaturation, mobility of lipids within the bilayer, localization of the peroxidative process in a particular membrane and the preventive antioxidant system (ROS scavenging and LP product detoxification). The idea of LP as a solely destructive process has changed during the last decade. It has been shown that lipid hydroperoxides and oxygenated products of lipid degradation as well as LP initiators (i.e. ROS) can participate in the signal transduction cascade (Tarchevskii, 1992).
EFFECT OF ANOXIA ON MEMBRANE STRUCTURE AND FUNCTION
Lipid and membrane integrity during oxygen deprivation are among the key factors in the survival of plants. Under anoxia a decrease in membrane integrity is a symptom of injury, and it can be measured as changes in the lipid content and composition (Hetherington et al., 1982; Chirkova et al., 1989), as activation of lipid peroxidation (Crawford et al., 1994; Crawford and Braendle, 1996; Chirkova et al., 1998; Blokhina et al., 1999), as enhanced electrolyte leakage (Chirkova et al., 1991a, b) and as a decrease in adenylate energy charge (Chirkova et al., 1984; Hanhijärvi and Fagerstedt, 1994, 1995). Since de novo lipid synthesis is energy dependent, and could hardly occur under anoxia, the preservation of membrane lipids is the most efficient way to maintain functional membranes. In previous studies it has been shown that anoxia‐tolerant plant species such as Acorus calamus and Schoenoplectus lacustris are able to preserve their polar lipids during anoxia and in post‐anoxia, while in anoxia‐sensitive plants (e.g. Iris germanica) a significant decrease in polar lipids and a simultaneous increase in free fatty acids (FFA) occur during anoxic stress with markedly enhanced lipid peroxidation during reoxygenation (Henzi and Braendle, 1993).
A decrease in unsaturated to saturated fatty acid ratio under anoxia may represent a result of LP and, at the same time sets limits for substrates of LP, the PUFA. This is the case in the anoxia‐tolerant Acorus calamus, where a decrease in linolenic acid (18:3) is compensated by linoleic (18:2) and oleic (18:0) acids under oxygen deprivation. The original lipid composition is recovered during 2 d of re‐aeration (Pfister‐Sieber and Braendle, 1994). Similar results have been obtained for the anoxia‐tolerant and ‐intolerant cereals rice and wheat, respectively (Chirkova et al., 1989). On the other hand, no significant qualitative and quantitative changes have been detected in the composition of fatty acids in anaerobically treated rice seedlings (Generosova et al., 1998). In that study it was postulated that the reduction of unsaturated fatty acids esterified in lipids was of no significance as a mechanism of plant adaptation to anaerobic conditions. The key role in survival was assigned to energy metabolism (Generosova et al., 1998). Indeed, a correlation exists between the leakage of electrolytes (i.e. membrane damage) under low ATP and a release of FFA from anoxic tissue (Crawford and Braendle, 1996). The role of ATP in the maintenance of membrane lipid integrity under anoxia has been confirmed by Rawyler et al. (1999) in potato cell culture. It has been shown that, when the rate of ATP synthesis falls below 10 µmol g–1 fresh weight h–1, the integrity of membranes cannot be preserved and FFA are liberated via lipolytic acyl hydrolase (Rawyler et al., 1999). In general, lipids of anoxia‐tolerant plants are more preserved during oxygen deprivation in respect to the composition and the degree of unsaturation. During recent years evidence has accumulated on the importance of lipid metabolism, and especially on unsaturated fatty acids, in the induction of defence reactions under biotic and abiotic stresses. Linolenic acid (18:3) has been shown to be a precursor of jasmonic acid, a signal transducer in defence reactions in plant–pathogen interactions (Rickauer et al., 1997). FFA, liberated during membrane breakdown under stress conditions, are not only the substrates for LP, but can act also as uncouplers in mitochondrial ETC (Skulachev, 1998).
Lipid hydroperoxides, formed as a result of LP, can affect membrane properties, i.e. increase hydrophilicity of the internal side of the bilayer (Frenkel, 1991). This phenomenon is very important for the termination of LP, since increased hydrophilicity of the membrane favours the regeneration of tocopherol by ascorbate.
Reoxygenation injury is a well‐documented fact for both animal and plant tissues. Indeed, under anoxia‐saturated electron transport components, the highly reduced intracellular environment (including transition metal ions), and low energy supply are factors favourable for ROS generation. Formation of free radicals within minutes after restoration of the oxygen supply has been shown by electron paramagnetic resonance (EPR) spectroscopy in the rhizodermis of the anoxia‐intolerant I. germanica, while in the tolerant I. pseudacorus no signal was detected (Crawford et al., 1994). An investigation on the dynamics of LP [changes in conjugated dienes, trienes and thiobarbituric acid reactive susbstances (TBARS)] in the same plant species confirmed this observation: neither dienes nor TBARS production was detected in the anoxia‐tolerant I. pseudacorus, with the exception of a 45‐d anoxic treatment (Blokhina et al., 1999). Accumulation of various LP products as a result of reoxygenation has been observed in the roots of the anoxia‐intolerant wheat and ‐tolerant rice, the latter showing higher membrane stability and lower level of LP after several days of anoxia (Chirkova et al., 1998; Blokhina et al., 1999). The length of the anoxic/hypoxic treatment has been shown to affect the intensity of LP in post‐anoxia. Cultured potato cells are known to exhibit a two‐phase response to anoxia in respect to lipid hydrolysis: no FFA release has been detected up to 12 h under oxygen deprivation, while after 12 h intensive liberation of FFA sustained by lipolytic acid hydrolase has been observed. This behaviour was mirrored by post‐anoxic LP: negligible after short‐term anoxia and elevated after lipid hydrolysis had occurred (Pavelic et al., 2000).
The existence of anoxia‐inducible changes in plant metabolism implies that plant cells sense anoxic conditions and respond to them quickly by glycolytic production of ATP and the regeneration of NAD(P)+ (Richard et al., 1994). Impairment of membrane structure and function under anoxia contribute to ROS‐induced post‐anoxic injury. This causes peroxidation of lipid membranes, depletion of reduced glutathione, an increase in cytosolic Ca2+ concentration, oxidation of protein thiol groups and membrane depolarization.
THE ROLE OF HYPOXIC PRETREATMENT IN PLANT ADAPTATION TO ANOXIA
Hypoxic pretreatment of plants prior to anoxia leads to increased survival (Waters et al., 1991; Xia and Roberts, 1994; reviewed by Drew, 1997; Vartapetian and Jackson, 1997). The minimal duration of hypoxia required for the acclimation has been estimated at 2–4 h for the root tips of maize seedlings (Chang et al., 2000). The biochemical and physiological features induced by this pretreatment suggest the involvement of several systems for increased stress tolerance. Of these, one is aimed at the maintenance of energy resources through the support of sugar utilization and ATP formation via the glycolytic pathway, while avoiding lactate accumulation and cytoplasmic acidosis. The majority of the genes induced codes for enzymes involved in starch and glucose mobilization, glycolysis and ethanol fermentation (Russel and Sachs, 1991; Chirkova and Voitzekovskaya, 1999). For example, anaerobic induction of enolase (2‐phospho‐d‐glycerate hydratase, EC 4.2.1.11), an integral enzyme in glycolysis, which catalyses the interconversion of 2‐phosphoglycerate to phosphoenolpyruvic acid (PEP), has been reported in maize (Lal et al., 1998). Some other glycolytic and fermentation pathway enzymes, such as alcohol dehydrogenase (ADH, EC 1.1.1.1), glucose phosphate isomerase, pyruvate decarboxylase (PDC, EC 4.1.1.1), and sucrose synthase have been characterized as hypoxically induced in maize. ADH and PDC, enzymes of ethanolic fermentation, were induced by hypoxic pretreatment in rice cultivars with different tolerance to anoxia (Ellis and Setter, 1999). Interestingly, both abscisic acid (ABA) and hypoxic pretreatment of Lactuca sativa L. seedlings have resulted in increased survival of roots and elevated ADH activity (Kato‐Noguchi, 2000). However, endogenous ABA level did not respond to hypoxic pretreatment, suggesting that ABA was not involved in hypoxia‐induced anoxia tolerance. The crucial role of protein synthesis under hypoxic conditions, but not under anoxia, has been shown in root tips of maize seedlings (Chang et al., 2000). Among 46 individual proteins analysed, four anaerobic proteins have been identified: ADH1, enolase, glyceraldehyde‐3‐phosphate dehydrogenase and PDC. The rate of their synthesis under hypoxia was enhanced (or comparable) under normoxic conditions. As expected, cycloheximide treatment during hypoxic acclimation (but not under anoxia) resulted in decreased anoxia tolerance (Chang et al., 2000). Interestingly, low oxygen (5 %) treatment of arabidopsis plants resulted in higher tolerance to hypoxia (0·1 % O2) but not anoxia (Ellis et al., 1999). In these experiments differential response of shoots and roots was observed. In conclusion, early (hypoxic) induction of the ethanolic fermentation pathway and sugar utilization allows the maintenance of the energy status through regeneration of NADH and, hence, improves anoxia tolerance. Under natural conditions oxygen concentration would decrease gradually, and hence anoxia is always preceeded by hypoxia.
Another metabolic feature that has been shown to be up‐regulated (though not always) under lack of oxygen is the antioxidant system. In an investigation on SOD activity and expression under hypoxia, anoxia and subsequent re‐aeration, the appearance of additional isozymes has been shown under anoxia. Judged by a cycloheximide treatment, this activity could not be attributed to de novo synthesis (Biemelt et al., 2000). It has been shown also that anoxic pretreatment protected soybean cells from H2O2‐induced cell death. Such resistance was associated with up‐regulation of peroxidases and alternative oxidase (Amor et al., 2000). The beneficial effect of alternative oxidase protein accumulation under anoxia is due to electron flow bifurcation and reduced probability of ROS formation under subsequent reoxygenation. It has been known for a long time that the main damage caused by anoxic stress occurs during re‐admission of oxygen. Some ROS formation can take place in hypoxic tissues as a result of over reduction of redox chains. Hence, anoxic stress is always accompanied to some extent by oxidative stress (generation of ROS) and its consequences. Induction of some components of the antioxidant system by hypoxic pretreatment can be due to such ROS accumulation and signalling (Lander, 1997; Semenza, 1999).
ANTIOXIDANT SYSTEMS
To control the level of ROS and to protect cells under stress conditions, plant tissues contain several enzymes scavenging ROS (SOD, CAT, peroxidases and glutathione peroxidase), detoxifying LP products (glutathione S‐transferases, phospholipid‐hydroperoxide glutathione peroxidase and ascorbate peroxidase), and a network of low molecular mass antioxidants (ascorbate, glutathione, phenolic compounds and tocopherols). In addition, a whole array of enzymes is needed for the regeneration of the active forms of the antioxidants (monodehydroascorbate reductase, dehydroascorbate reductase and glutathione reductase).
Superoxide dismutase activity under various stress conditions
Enhanced formation of ROS under stress conditions induces both protective responses and cellular damage. The scavenging of O2·– is achieved through an upstream enzyme, SOD, which catalyses the dismutation of superoxide to H2O2. This reaction has a 10 000‐fold faster rate than spontaneous dismutation (Bowler et al., 1992). The enzyme is present in all aerobic organisms and in all subcellular compartments susceptible of oxidative stress (Bowler et al., 1992). Recently, a new type of SOD with Ni in the active centre has been described in Streptomyces (Kim et al., 1996). The other three types of this enzyme, classified by their metal cofactor, can be found in living organisms, and they are the structurally similar FeSOD (prokaryotic organisms, chloroplast stroma) and MnSOD (prokaryotic organisms and the mitochondrion of eukaryotes); and the structurally unrelated Cu/ZnSOD (cytosolic and chloroplast enzyme, gram‐negative bacteria). These isoenzymes differ in their sensitivity to H2O2 and KCN (Bannister et al., 1987). All three enzymes are nuclear encoded, and SOD genes have been shown to be sensitive to environmental stresses, presumably as a consequence of increased ROS formation. This has been shown in an experiment with corn (Zea mays), where a 7‐d flooding treatment resulted in a significant increase in TBARS content, membrane permeability and the production of superoxide anion‐radical and hydrogen peroxide in the leaves (Yan et al., 1996). An excessive accumulation of superoxide due to the reduced activity of SOD under flooding stress was shown also (Yan et al., 1996). In anoxically treated wheat and rice roots the activity of SOD has been determined without a prolonged re‐oxygenation period, immediately after termination of the anoxic treatment. In the course of this experiment the activity decreased in wheat under both aeration and anoxia, but in the anoxic samples this decline was slower. As a result, after 3 d of anoxia the activity was 65 % higher than in the control roots. In the more anoxia‐tolerant rice, anoxia did not affect SOD activity (Chirkova et al., 1998). Similar results have been reported by Pavelic et al. (2000) for potato cell culture during the post‐anoxic period: only 60 % of initial specific SOD activity remained after 3 h reoxygenation. In cereals the activity of SOD has been found to decline depending on the duration of the anoxic treatment, while in Iris pseudacorus a 14‐fold increase was observed during a reoxygenation period (Monk et al., 1989). An increase in total SOD activity has been also detected in wheat roots under anoxia but not under hypoxia. The degree of increase positively correlated with duration of anoxia (Biemelt et al., 2000). Induction of SOD activity under hypoxia by 40–60 % in the roots and leaves of Hordeum vulgare has been shown by Kalashnikov et al. (1994).
Hence, investigations of SOD activity in different plant species under hypoxia (submergence) and/or anoxia have resulted in contradictory observations (Table 2). The explanation can be found in different tolerance to anoxia between species and experimental set‐up (e.g. a prolonged reoxygenation period in the case of Iris spp., while in cereal roots activity of the enzyme was determined immediately after anoxia). The formation of ROS already under hypoxic conditions and during the oxidative burst after re‐admission of oxygen could cause rapid substrate overload of constitutive SOD, while induction was hindered probably by other factors [e.g. time, activity of downstream enzymes in the ROS‐detoxification cascade, inhibition by the end product (H2O2) and consequences of anoxic metabolism]. Observations on SOD activity in different plant species under several stress conditions (drought, salinity and high/low temperature) suggest that different mechanisms may be involved in oxidative stress injury (Yu and Rengel, 1999a, b). Activation of oxygen may proceed through different mechanisms, not necessarily producing a substrate for SOD. Changes in O2 electronic configuration can lead to the formation of highly reactive singlet oxygen (1O2). Comparison of drought and water stress effects on tolerant and intolerant wheat genotypes suggests that different mechanisms can participate in ROS detoxification. For example, water stress did not affect SOD activity, while under drought conditions a significant increase was detected (Sairam et al., 1998). In another experiment, oxidative stress conditions combined with cold acclimation of cold‐resistant and unresistant wheat cultivars, SOD activity in the leaves and in the roots was unaffected by the low temperature treatment but plants exhibited higher guaiacol peroxidase activity (Scebba et al., 1998). Inefficiency of ROS‐detoxifying enzymes (SOD, CAT, ascorbate peroxidase and non‐specific peroxidase) has been shown under water deficit‐induced oxidative stress in rice (Boo and Jung, 1999). In this paper a decrease in enzymatic activity was accompanied by LP, chlorophyll bleaching, loss of ascorbic acid (AA), reduced glutathione (GSH), α‐tocopherol and carotenoids in stressed plants. The authors suggested the formation of a certain strong pro‐oxidant, which is neither superoxide nor H2O2 under the conditions of water deficit (Boo and Jung, 1999). The ability of plants to overcome oxidative stress only partly relies on the induction of SOD activity and other factors can regulate the availability of the substrate for SOD. Diversification of the pathways of ROS formation, compartmentalization of oxidative processes (charged ROS cannot penetrate the membrane) and compartmentalization of SOD isozymes. It is also possible that in different plant species and tissues different mechanisms are involved in the protection against oxidative stress.
Table 2.
Differential response of SOD to oxygen deprivation stress
| Plant* | Site | Stress | SOD activity | Reference |
| Iris pseudacorus L. (t) | Rhizomes | Anoxia + reaeration | Increase | Monk et al. (1987) |
| Lotus (Nelumbo nucifera Gaertn.) (t) | Seedlings | Hypoxia + reoxygenation | Increase | Ushimaru et al. (2001) |
| Rice (Oryza sativa L.) (t) | Roots | Anoxia | Decline | Chirkova et al. (1998) |
| Rice (Oryza sativa L.) (t) | Seedlings | Hypoxia (submerged plants) | Plastidic SOD decline; mitochondrial SOD decline | Ushimaru et al. (1999) |
| Iris germanica L. (i) | Rhizomes | Anoxia + reaeration | Decline | Monk et al. (1987) |
| Soybean (Glycine max (L.) Merr.) (i) | Seedlings | Anoxia | Increase | Van Toai and Bolles (1991) |
| Barley (Hordeum vulgare L.) (i) | Roots | Hypoxia | Increase | Kalashnikov et al. (1994) |
| Narrow‐leaved lupin (Lupinus angustifolius L.) | Waterlogging + reoxygenation | FeSOD and Cu/Zn SOD, increase; MnSOD, decline | Yu and Rengel (1999a) | |
| Wheat (Triticum aestivum L.) (i) | Roots | Hypoxia | Unaffected | Biemelt et al. (2000) |
| Anoxia | Increase | |||
| Wheat (Triticum aestivum L.) (i) | Roots | Anoxia | Decline | Chirkova et al. (1998) |
| Maize (Zea mays L.) (i) | Hypoxia (submerged plants) | Decline | Yan et al. (1996) | |
| Potato (Solanum tuberosum L.) (i) | Cell culture | Anoxia + reoxygenation | Decline | Pavelic et al. (2000) |
* (t), Plants tolerant to oxygen deprivation stress; (i), plants intolerant to oxygen deprivation stress.
Catalase and peroxidases
The intracellular level of H2O2 is regulated by a wide range of enzymes, the most important being catalase (reviewed by Willekens et al., 1995) and peroxidases. Catalase functions through an intermediate catalase–H2O2 complex (Compound I) and produces water and dioxygen (catalase action) or can decay to the inactive Compound II. In the presence of an appropriate substrate Compound I drives the peroxidatic reaction. Compound I is a much more effective oxidant than H2O2 itself, thus the reaction of Compound I with another H2O2 molecule (catalase action) represents a one‐electron transfer, which splits peroxide and produces another strong oxidant, the hydroxyl radical (OH·)(Elstner, 1987). OH· is a very strong oxidant and can initiate radical chain reactions with organic molecules, particularly with PUFA in membrane lipids.
Under anoxia a differential response of the peroxidase system has been observed in coleoptiles and roots of rice seedlings. There was a decrease in activity of cell wall‐bound guaiacol and syringaldazine peroxidase activities, while soluble peroxidase activity was not affected in coleoptiles. In contrast anoxia‐grown roots showed an increase in the cell wall‐bound peroxidases (Lee and Lin, 1995). Acclimation to anoxia has been shown to be dependent, at least partly, on peroxidases, which have been up‐regulated by anoxic stress (Amor et al., 2000). In rice seedlings ADH and SOD activities responded non‐significantly to submergence, while catalase activity increased upon re‐admission of oxygen (Ushimaru et al., 1999). However, under strict anoxia in bakers yeast (Saccharomyces cerevisiae) the expression of peroxisomal catalase A was down‐regulated by anoxia (Skoneczny and Rytka, 2000).
Phospholipid hydroperoxide glutathione peroxidase
Phospholipid hydroperoxide glutathione peroxidase (PHGPX) is a key enzyme in the protection of the membranes exposed to oxidative stress and it is inducible under various stress conditions. The enzyme catalyses the regeneration of phospholipid hydroperoxides at the expense of GSH and is localized in the cytosol and the inner membrane of mitochondria of animal cells. PHGPX can also react with H2O2 but this is a very slow process. Until now, most of the investigations have been performed on animal tissues. Recently, a cDNA clone homologous to PHGPX has been isolated from tobacco, maize, soybean and arabidopsis (Sugimoto et al., 1997). The PHGPX protein and its encoding gene csa have been isolated and characterized in citrus. It has been shown that csa is directly induced by the substrate of PHGPX under heat, cold and salt stresses, and that this induction occurs mainly via the production of ROS (Avsian‐Kretchmer et al., 1999).
ANTIOXIDANT AND NON‐ANTIOXIDANT FUNCTIONS OF GLUTATHIONE, ASCORBATE AND TOCOPHEROL UNDER PHYSIOLOGICAL AND STRESS CONDITIONS
Glutathione
A tripeptide glutathione (γ‐glutamylcysteinylglycine) is an abundant compound in plant tissues. It has been found virtually in all cell compartments: cytosol, endoplasmic reticulum, vacuole and mitochondria (Jimenez et al., 1998), where GSH executes multiple functions. GSH is the main storage form of sulfur, and it acts as a potent detoxifier of xenobiotics through GSH‐conjugation, and can serve as a precursor of phytochelatins (reviewed by Noctor et al., 1998b; May et al., 1998). Together with its oxidized form (GSSG) glutathione maintains a redox balance in the cellular compartments. The latter property is of great biological importance, since it allows fine‐tuning of the cellular redox environment under normal conditions and upon the onset of stress, and provides the basis for GSH stress signalling. Indeed, the role for GSH in redox regulation of gene expression has been described in many papers (e.g. Wingate et al., 1988; Alscher, 1989). Due to redox properties of the GSH/GSSG pair and reduced SH‐group of GSH, it can participate in the regulation of the cell cycle (Sanchez‐Fernandez et al., 1997).
Functioning of GSH as antioxidant under oxidative stress has received much attention during the last decade. A central nucleophilic cysteine residue is responsible for high reductive potential of GSH. It scavenges cytotoxic H2O2, and reacts non‐enzymatically with other ROS: singlet oxygen, superoxide radical and hydroxyl radical (Larson, 1988). The central role of GSH in the antioxidative defence is due to its ability to regenerate another powerful water‐soluble antioxidant, ascorbic acid, via the ascorbate–glutathione cycle (Foyer and Halliwell, 1976; Noctor and Foyer, 1998).
Ascorbic acid
AA is one of the most studied and powerful antioxidants (reviewed by Smirnoff, 1996; Noctor and Foyer, 1998; Arrigoni and de Tullio, 2000; Horemans et al., 2000b; Smirnoff, 2000). It has been detected in the majority of plant cell types, organelles and in the apoplast. Under physiological conditions AA exists mostly in the reduced form (90 % of the ascorbate pool) in leaves and chloroplasts (Smirnoff, 2000); and its intracellular concentration can build up to millimolar range (e.g. 20 mm in the cytosol and 20–300 mm in the chloroplast stroma (Foyer and Lelandais, 1996). The ability to donate electrons in a wide range of enzymatic and non‐enzymatic reactions makes AA the main ROS‐detoxifying compound in the aqueous phase. AA can directly scavenge superoxide, hydroxyl radicals and singlet oxygen and reduce H2O2 to water via ascorbate peroxidase reaction (Noctor and Foyer, 1998). In chloro plasts, AA acts as a cofactor of violaxantin de‐epoxidase thus sustaining dissipation of excess exitation energy (Smirnoff, 2000). AA regenerates tocopherol from tocopheroxyl radical providing membrane protection (Thomas et al., 1992). In addition, AA carries out a number of non‐antioxidant functions in the cell. It has been implicated in the regulation of the cell division, cell cycle progression from G1 to S phase (Liso et al., 1988; Smirnoff, 1996) and cell elongation (De Tullio et al., 1999).
Tocopherol
Tocopherols and tocotrienols are essential components of biological membranes where they have both antioxidant and non‐antioxidant functions (Kagan, 1989). There are four tocopherol and tocotrienol isomers (α‐, β‐, γ‐, δ‐) which structurally consist of a chroman head group and a phytyl side chain giving vitamin E compounds amphipathic character (Kamal‐Eldin and Appelqvist, 1996). Relative antioxidant activity of the tocopherol isomers in vivo is α > β > γ > δ which is due to the methylation pattern and the amount of methyl groups attached to the phenolic ring of the polar head structure. Hence, α‐tocopherol with its three methyl substituents has the highest antioxidant activity of tocopherols (Kamal‐Eldin and Appelqvist, 1996). Though antioxidant activity of tocotrienols vs. tocopherols is far less studied, α‐tocotrienol is proven to be a better antioxidant than α‐tocopherol in a membrane environment (Packer et al., 2001). Tocopherols, synthesized only by plants and algae, are found in all parts of plants (Janiszowska and Pennock, 1976). Chloroplast membranes of higher plants contain α‐tocopherol as the predominant tocopherol isomer, and are hence well protected against photooxidative damage (Fryer, 1992). There is also evidence that α‐tocopherol quinone, existing solely in chloroplast membranes, shows antioxidant properties similar to those of α‐tocopherol (Kruk et al., 1997).
Vitamin E is a chain‐breaking antioxidant, i.e. it is able to repair oxidizing radicals directly, preventing the chain propagation step during lipid autoxidation (Serbinova and Packer, 1994). It reacts with alkoxy radicals (LO·), lipid peroxyl radicals (LOO·) and with alkyl radicals (L·), derived from PUFA oxidation (Kamal‐Eldin and Appelqvist, 1996; Buettner, 1993). The reaction between vitamin E and lipid radical occurs in the membrane‐water interphase where vitamin E donates a hydrogen ion to lipid radical with consequent tocopheroxyl radical (TOH·) formation (Buettner, 1993). Regeneration of the TOH· back to its reduced form can be achieved by vitamin C (ascorbate), reduced glutathione (Fryer, 1992) or coenzyme Q (Kagan et al., 2000). In addition, tocopherols act as chemical scavengers of oxygen radicals, especially singlet oxygen (via irreversible oxidation of tocopherol), and as physical deactivators of singlet oxygen by charge transfer mechanism (Fryer, 1992).
TOH· formation sustains prooxidant action of tocopherol. At high concentration tocopherols act as prooxidant synergists with transition metal ions, lipid peroxides or other oxidizing agents (Kamal‐Eldin and Appelqvist, 1996). It has been clearly shown, that prooxidant function of tocopherol on low density lipoprotein was clearly inhibited in vitro by antioxidants (ascorbate or ubiquinol) (Upston et al., 1999).
In addition to antioxidant functions vitamin E has several non‐antioxidant functions in membranes. Tocopherols have been suggested to stabilize membrane structures. Earlier studies have shown that α‐tocopherol modulates membrane fluidity in a similar manner to cholesterol, and also membrane permeability to small ions and molecules (Fryer, 1992). In recent studies α‐tocopherol has been shown to decrease the permeability of digalactosyldiacylglycerol vesicles for glucose and protons (Berglund et al., 1999). There is also recent evidence of interaction between PS II with α‐tocopherol and α‐tocopherol quinone (Kruk et al., 2000). Complexation of tocopherol with free fatty acids and lysophospholipids protects membrane structures against their deleterious effects. The process is of great physiological relevance, since phospholipid hydrolysis products are characteristics of pathological events such as hypoxia, ischaemia or stress damage (Kagan, 1989). In addition, several other non‐antioxidant functions of α‐tocopherol have been described such as protein kinase C inhibition, inhibition of cell proliferation, etc. as reviewed by Azzi and Stocker (2000).
Phenolic compounds as antioxidants
Phenolics are diverse secondary metabolites (flavonoids, tannins, hydroxycinnamate esters and lignin) abundant in plant tissues (reviewed by Grace and Logan, 2000). Polyphenols possess ideal structural chemistry for free radical scavenging activity, and they have been shown to be more effective antioxidants in vitro than tocopherols and ascorbate. Antioxidative properties of polyphenols arise from their high reactivity as hydrogen or electron donors, and from the ability of the polyphenol‐derived radical to stabilize and delocalize the unpaired electron (chain‐breaking function), and from their ability to chelate transition metal ions (termination of the Fenton reaction) (Rice‐Evans et al., 1997). Another mechanism underlying the antioxidative properties of phenolics is the ability of flavonoids to alter peroxidation kinetics by modification of the lipid packing order and to decrease fluidity of the membranes (Arora et al., 2000). These changes could sterically hinder diffusion of free radicals and restrict peroxidative reactions. Moreover, it has been shown recently that phenolic compounds can be involved in the hydrogen peroxide scavenging cascade in plant cells (Takahama and Oniki, 1997). According to our unpublished results the content of condensed tannins (flavonols), as measured by high performance liquid chromatography, was 100 times higher in I. pseudacorus rhizomes than in those of I. germanica. The effect of anoxia on the flavonol content (a decrease after 35 d of treatment) suggests their participation in the antioxidative defence in I. pseudacorus rhizomes.
ANTIOXIDANT STATUS UNDER OXYGEN DEPRIVATION
Data on antioxidant levels and the activity of antioxidant‐regenerating enzymes are somewhat contradictory, both decreases and increases in antioxidative capacity of the tissues have been reported. Such diversification partly arises from the response specificity of a particular plant species and from different experimental conditions (stress treatment, duration of stress, assay procedure and parameters measured). A large‐scale investigation on monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR) activities, and AA and GSH contents in 11 species with contrasting tolerance to anoxia has revealed an increase in MDHAR and/or DHAR in the anoxia‐tolerant plants after several days of anoxic treatment. In the intolerant plants activities were very low or without any changes. GSH decreased significantly during the post‐anoxic period, while AA showed increased values in the tolerant species (Wollenweber‐Ratzer and Crawford, 1994). An investigation on the antioxidative defence system in the roots of wheat seedlings under root hypoxia or whole plant anoxia (Biemelt et al., 1998) has revealed a significant increase in the reduced forms of ascorbate and glutathione. Nevertheless, a rapid decrease in the redox state of both antioxidants was observed during reaeration. The activities of MDHAR, DHAR and glutathione reductase (GR) decreased slightly or remained unaltered under hypoxia, while anoxia caused a significant inhibition of enzyme activities (Biemelt et al., 1998). Inhibition of GR, ascorbate peroxidase (APX), CAT and SOD activities has been shown also by Yan et al. (1996) in corn leaves under prolonged flooding, while a short‐term treatment led to an increase in the activities. Induction of enzymes involved in the ascorbate‐dependent antioxidative system (APX, MDHAR, DHAR) has been shown for anaerobically germinated rice seedlings after transfer to air. In submerged seedlings (i.e. under hypoxic conditions) the activities of antioxidative enzymes were lower compared with air‐germinated controls (measured as changes in the protein levels of enzymes) (Ushimaru et al., 1997). The imposition of anoxia and subsequent reoxygenation caused a decrease both in the content of ascorbate and in its reduction state in the roots of cereals and the rhizomes of Iris spp. (Blokhina et al., 2000). Prolongation of the anoxic treatment led to a decline in the antioxidant level, both reduced and oxidized forms, in all plants tested. A decrease in the AA/DHA ratio indicated a shift in the reduction state of the ascorbate pool under oxygen deprivation.
Less information is available on tocopherol status under oxygen deprivation. Since oxidative stress is non‐specific and many diverse environmental stress factors, e.g. light, drought, chilling temperature and flooding, affect plant tissues enhancing production of ROS in chloroplasts and inducing photo‐oxidation of thylakoid membranes (Elstner and Osswald, 1994), the response of tocopherols to other abiotic stresses will be discussed. In an experiment where isolated spinach thylakoids and thylakoids with an exogenously added high concentration of α‐tocopherol were exposed either to photosynthetically active radiation (PAR) or to UV‐B light, lipid peroxidation occurred only in normal thylakoids while no peroxidation was detected in membranes with high amounts of α‐tocopherol. According to the results, there was no decrease in endogenous α‐tocopherol in normal thylakoids, while in artificially treated thylakoids α‐tocopherol contents decreased though no significant lipid peroxidation could be detected (DeLong and Steffen, 1998). The latter results contradict previous studies on lipid peroxidation since increased peroxidation of membranes has been described to occur only after significant amounts of membrane α‐tocopherol have been depleted (Thomas et al., 1989; Shewfelt and Purvis, 1995).
During drought, plants show a general response to stress by increasing tocopherol and carotenoid contents in photo synthetic tissues (Munné‐Bosch and Alegre, 2000a) which is accompanied by a similar sized rise in total glutathione pool and a depletion of ascorbate at least in many grass species (Price and Hendry, 1989). Substantial increases in α‐tocopherol during water‐stress have been detected in leaves of Rosmarinus officinalis L. (Munné‐Bosch and Alegre, 2000a), Melissa officinalis L. (Munné‐Bosch and Alegre, 2000b) and Fagus sylvatica L. (García‐Plazaola and Becerril, 2000). Enhanced activity of the xanthophyll cycle measured as increases in de‐epoxidized xanthophylls (antheraxanthin and zeaxanthin) during drought is also a feature shared in water‐stressed plant species. Rosemary plants also have species‐specific antioxidants, abietane diterpenes, known for their function in inhibiting lipid peroxidation and superoxide generation in chloroplasts and microsomes, which are consumed during drought‐stress in scavenging oxygen radicals (Munné‐Bosch et al., 1999).
There is evidence that the chilling‐tolerance of plants is correlated with increasing amounts of antioxidants and increasing activity of radical scavenging enzymes. A chilling‐tolerant maize genotype has been shown to contain higher amounts of both α‐tocopherol and glutathione and higher GR activity than a chilling‐sensitive maize genotype (Leipner et al., 1999). It is known that ascorbate regenerates tocopherols from their radical forms (Buettner, 1993). However, artificially increased ascorbate content in maize leaves did not improve the preservation of endogenous tocopherol during high light and chilling stress, but the high ascorbate content increased the usage of glutathione (Leipner et al., 2000).
Studies on vitamin E in the underground parts of plants during stress are scarce, which might in part be a consequence of the fact that generally the predominating tocopherol isomer of plant tissues, α‐tocopherol, is mainly localized in chloroplasts (Kamal‐Eldin and Appelqvist, 1996), and tocopherol synthesis is described to take place only in chloroplasts and chromoplasts (Schulz et al., 1991). During long‐term anoxic stress vitamin E contents in the rhizomes of two iris species, highly anoxia‐tolerant Iris pseudacorus and anoxia‐sensitive I. germanica, have been determined. Tocopherols (α‐ and β‐) were identified in both iris species, β‐tocopherol being the predominant tocopherol isomer especially in rhizomes of I. germanica which also possessed markedly higher total tocopherol content than I. pseudacorus. Anoxia caused a decrease in tocopherol isomers in both iris species (Blokhina et al., 2000).
The vitamin E composition in rhizomes of the iris species is unique since there are no previous reports of plant species having β‐tocopherol as the main tocopherol isomer in vegetative tissues. In addition, according to mass spectrometry the identified isomer is β‐dehydrotocopherol with one double bond in its phytyl side chain (Blokhina et al., 2000), while tocopherols have saturated phytyl chains. Dehydrotocopherols have been found previously in etiolated shoots of maize and barley (Threlfall and Whistance, 1977). There is evidence that tocopherol isomers differ from each other in their functional properties. When the effectiveness of tocopherol isomers in quenching of singlet oxygen was studied, α‐and β‐tocopherols were equally effective in quenching singlet oxygen physically, but β‐tocopherol showed almost no chemical reactivity with singlet oxygen, while α‐tocopherol had the highest chemical reactivity of tocopherol isomers (Kaiser et al., 1990). Inhibition of protein kinase C activity and cell proliferation is a specific non‐antioxidant function of α‐tocopherol in animal cells. β‐Tocopherol lacks this ability but when the two isomers are present together β‐tocopherol prevents the inhibitory effect of α‐tocopherol (Azzi and Stocker, 2000).
Though the total tocopherol content was higher in I. germanica than in the more anoxia‐tolerant I. pseudacorus (Blokhina et al., 2000), this could not prevent the massive lipid degradation in I. germanica during anoxia found by Henzi and Braendle (1993). There are also earlier reports suggesting that anoxia causes more pronounced lipid peroxidation in the rhizomes of I. germanica than in I. pseudacorus during reaeration (Hunter et al., 1983; Blokhina et al., 1999). In anaerobically germinated rice seedlings a three‐fold increase in tocopherol and low TBARS formation have been observed (Ushimaru et al., 1994). However, an anoxia‐induced elevation in the tocopherol level observed in the anoxia‐intolerant wheat and oat seedlings could not be detected in rice seedlings subjected to anoxia (Chirkova et al., 1998). There are in vitro studies suggesting that under anaerobic conditions a radical‐initiated reaction between linoleic acid hydroperoxide or methyl linoleate hydroperoxide and α‐tocopherol occurs, forming an addition compound of the two reactants, the reaction being terminated in the presence of air (Gardner et al., 1972). Carbon‐centred radicals are also formed during anaerobic conditions, tending to add to the oxygen of tocopheroxyl radicals forming 6‐O‐lipid alkyl‐chromanol adducts (Kamal‐Eldin and Appelqvist, 1996).
FACTORS AFFECTING THE ANTIOXIDANT DEFENCE SYSTEM
Considering the experimental data discussed above, it is difficult to delineate a universal mechanism for the whole antioxidant system response to anoxia. It is necessary to discuss other factors involved in the protective machinery of plants under oxygen deprivation with a particular emphasis on the antioxidant system.
Oxygen deprivation stress‐specific factors
Metabolic changes specifically induced by anoxia (a drop in cytosolic pH, a decrease in adenylate energy charge, membrane lipid peroxidation, excess of NADH) may alter the antioxidant status of the tissue. One of the most important consequences of energy limitation under anoxia is the altered redox state of the cell. When oxygen—the terminal electron acceptor of ETC—is unavailable, intermediate electron carriers become reduced. This process in turn affects redox‐active metabolic reactions. Indeed, the ability to maintain redox characteristics of the cell (i.e. NADH/NAD+ ratio) unaltered for a prolonged period has been shown for the anoxia‐tolerant rice (Chirkova et al., 1992) and is considered important for plant survival under anoxia. A decrease in NADH/NAD+ has been observed in the anoxia‐intolerant wheat and bean (Chirkova et al., 1992). The redox changes can affect other redox‐dependent reactions, i.e. the oxidation state of ferrous ions—the promoters of ROS generation (through the Fenton reaction) and peroxidation of lipids. If oxygen deprivation persists, the need for oxidized NAD+ and ATP leads to the fermentation pathway, where both LDH and ADH can regenerate NAD+. Among the possible targets of oxygen deprivation stress in respect of the antioxidant system are de novo antioxidant synthesis, intra‐ and intercellular transport, recycling of antioxidants and impaired cooperation of the antioxidant network.
Compartmentalization of lipophilic antioxidants
ROS (with the exception of H2O2) are charged species and cannot penetrate biological membranes, hence local antioxidant protection is more important than an overall increase in antioxidants. In a model of lipid peroxidation in tissue disorders, Shewfelt and Purvis (1995) emphasize the importance of compartmentalization within the cell. The fate of the tissue may rely on the antioxidant capacity of a specific membrane structure (Shewfelt and Purvis, 1995). In previous studies on the compartmentalization of tocopherols in photosynthetic tissues tocopherol has been localized in chloroplasts and plastids, while other tocopherol isomers have been found in chloroplasts, mitochondria and microsomes (Janiszowska and Korczak, 1980). Some evidence exists on the importance of compartmentalization of other lipophilic antioxidants. In a recent study on the chloro plast localized antioxidant carnosic acid of rosemary (Rosmarinus officinalis) leaves, it was shown that, after ROS scavenging, the carnosic acid metabolites are transferred to the plasma membrane (Munné‐Bosch and Alegre, 2001). Identification of the cell structures affected primarily in oxidative stress, as well as the localization pattern of different antioxidants especially in non‐photosynthesizing plant organs, are still poorly studied areas. Accumulation of antioxidants and ROS in different cell compartments could lead to lowered antioxidant defence, and hence would require fine tuning of cellular metabolism to achieve protection. Under severe stress conditions such a regulatory mechanism can be impaired.
Is there a possibility for de novo synthesis of antioxidants under particular stress conditions?
Limitations for GSH biosynthesis under oxygen deprivation mainly arise from the restriction of the energy supply. Two ATP‐dependent steps represent the GSH biosynthetic pathway: synthesis of γ‐glutamylcysteine catalysed by γ‐ECS (γ‐glutamylcysteine synthase, EC 6.3.2.2), and glycine addition to γ‐glutamylcysteine catalysed by glutathione synthetase (May et al., 1998). GSH is synthesized in both the chloroplasts and the cytosol (Noctor et al., 1998b). Besides ATP availability, several other factors affect GSH biosynthesis: cysteine supply, GSH turnover (since GSH is a feedback inhibitor of γ‐ECS), GSH conjugation (see below) and environmental factors. A decline in ATP content observed under anoxia (Chirkova, 1988; Hanhijärvi and Fagerstedt, 1995; Rawyler et al., 1999) increases the probability of ROS formation in the ETC of mitochondria and, at the same time, inhibits an energy‐dependent step in GSH biosynthesis. The key enzyme in GSH biosynthesis, γ‐ECS, requires ATP as a cofactor and has an alkaline pH optimum of 8–8·4 (Noctor et al., 1998a).
The AA biosynthetic pathway in plants has been elucidated recently (Wheeler et al., 1998; Conklin, 2001; reviewed by Smirnoff et al., 2001). The pathway proceeds through d‐glucose ⇔ GDP‐d‐mannose ⇔ GDP‐l‐galactose ⇒ l‐galactose ⇒ l‐galactono‐1,4‐lactone, the latter being an immediate precursor of l‐ascorbate (non‐inversion pathway, i.e. no inversion of glucose carbon skeleton occurs); while in animals AA biosynthesis involves the conversion of derivatives of d‐glucose (Loewus, 1980). Another possible non‐inversion route involves the following reactions: d‐glucose ⇒ d‐glucosone ⇒ l‐sorbosone ⇒ AA (Loewus et al., 1990). Nevertheless, evidence exists on the possibility of AA biosynthesis through other pathways (Davey et al., 1999). The final step in AA biosynthesis occurs in the inner mitochondrial membrane and is catalysed by l‐galactono‐γ‐lactone dehydrogenase (GAL, EC 1.3.2.3), an enzyme with specificity to cytochrome c as an electron acceptor (Bartoli et al., 2000). Association of AA biosynthesis with the functional activity of mitochondrial ETC sets a limit to AA synthesis under lack of oxygen due to saturation of ETC and reduction of cytochrome c. Another factor, unfavourable for AA synthesis, is anoxia‐induced cytoplasmic acidosis, which can affect the activity of GAL (Ostergaard et al., 1997).
Efficient transport of antioxidants from the biosynthetic sites and/or undamaged tissue to the ROS‐producing compartment
Under physiological pH the reduced form of AA is negatively charged, and therefore cannot freely diffuse through the biological membranes. In contrast, dehydro ascorbic acid (DHA) is more likely to penetrate the membrane. In plants, the AA biosynthetic site is localized on the inner mitochondrial membrane (Wheeler et al., 1998; Conklin et al., 1999) and, hence, AA should be transported out from the mitochondria to the cytosol, chloroplast, and across the plasma membrane to the apoplast to provide antioxidative defence. Until now, a mitochondrial transporter has not been characterized. Evidence has been accumulating on the existence of both AA and DHA specific transporters on the plant plasma membrane. DHA appears to be the preferred form of transport from the apoplast to the cytosol in Phaseolus vulgaris (Horemans et al., 1998a) and in Nicotiana tabacum (Horemans et al., 1998b). The Km values for intercellular transport of AA (90 μm) and DHA (20 μm) through high‐affinity carriers also suggest that DHA is more readily taken up by the cell (Smirnoff, 1996). Recently, the existence of an AA/DHA exchanger on the plant plasma membrane has been suggested (Horemans et al., 2000a). The mechanism of exchange employs the proton‐electrochemical gradient across the plasma membrane, as shown with uncoupler [carbonylcyanide‐3‐chlorophenylhydrazone (CCCP)] experi ments, while DHA uptake occurs via facilitated diffusion and shows no dependence on proton and ion gradients. The pathways of AA (DHA) transport are of crucial importance under anoxia, since the inner mitochondrial membrane potential dissipates after a short lag phase, sustained by ATP hydrolysis via F1F0‐ATPase, and hence, only proton gradient‐independent transport is possible. In our experiments (Blokhina et al., 2000) DHA was the main form in the ascorbate pool of cereal roots (AA/DHA ratios between 0·2 and 0·8), a fact that can be partly explained by the preferred transport of DHA from shoots to roots. It is not clear whether AA biosynthesis occurs in the plant root mitochondria to the same extent as it does in green tissues, where more precursors are available. However, expression of l‐galactono‐γ‐lactone dehydrogenase mRNA has been found in tobacco leaves, shoots and roots in almost equal quantities (Yabuta et al., 2000). Besides, high‐level irradiance has been shown to have a stimulating effect on ascorbate accumulation in leaves and the chloroplasts (Foyer et al., 1991), and dark‐induced ascorbate deficiency has been described in leaf cell walls of Phaseolus vulgaris (Moldau et al., 1998). It is also possible that intercellular transport of DHA can act as a signal of redox imbalance under stress, a condition that is known to induce defence responses.
Less is known about the glutathione transport mechanism in plant tissues. Most of the studies are focused on the function of glutathione‐S‐transferases (GST, EC 2.5.1.18) in herbicide detoxification, conjugation of GSH to cytotoxic compounds arising from oxidative stress, pathogen attack and heavy metals (reviewed by Marrs, 1996). The onset of hypoxia and subsequent reoxygenation is manifested by enhanced ROS formation and LP. Peroxidation products such as membrane lipid hydroperoxides (e.g. 4‐hydroxy alkenals), epoxides, organic hydroperoxides (Alin et al., 1985) and oxidative products of DNA degradation (base propanols) are the substrates of GST; they can be conjugated to GSH and detoxified (Marrs, 1996; Dixon et al., 1998). In plant tissues GSH‐conjugates are transported from the cytosol into the vacuole for storage via ATP‐binding cassette transporters (Rea, 1999), including the GS‐X pump. Conjugation to GSH serves as a specific ‘tag’ for recognition, transport and sequestration of endogenous and stress‐specific metabolites. ATP‐dependent transport of GSSG, but not GSH into barley vacuoles via glutathione S‐conjugate ATPase has been described (Tommasini et al., 1993). However, under energy limitation during hypoxia/anoxia the translocation to vacuole can be inhibited, while GSH conjugation can still occur. In addition, ascorbate peroxidase‐mediated conjugation of GSH to unsaturated phenylpropanoids (trans‐cinnamic and para‐coumaric acids) has been shown in plants. GSH‐conjugate is presumably formed via peroxidase‐dependent formation of thiyl free radicals that react with the alkyl double bond (Dean and Devarenne, 1997). Conjugation of GSH to LP products can lead to the depletion of the total glutathione pool, since glutathione turnover will be repressed. Indeed, exhaustion of the glutathione pool under anoxia and reoxygenation was not accompanied with concurrent increase in GSSG (Blokhina et al., 2000).
Other pathways for glutathione transport have been described in animal tissues and yeast. In rabbit kidney mitochondria (Cheng et al., 2000) and in yeast (Cummings et al., 2000) uptake of GSH by dicarboxylate and 2‐oxoglutarate carriers in the inner mitochondrial membrane has been demonstrated. The first high affinity plasma membrane GSH transporter (Km 54 µm) different from glutathione‐conjugate pumps and dicarboxylate transporters has been identified in the yeast Saccharomyces cerevisiae. The transporter protein shares homology with S. pombe and with five proteins from Arabidopsis thaliana (Bourbouloux et al., 2000).
Cooperation between different antioxidant systems
It is very important for plant survival under stress conditions that antioxidants can work in co‐operation, thus providing better defence and regeneration of the active reduced forms. The most studied example of the antioxidant network is the ascorbate–glutathione (Halliwell–Asada) pathway in the chloroplasts, where it provides photoprotection (Noctor and Foyer, 1998) by removing H2O2. Recently, components of this cycle have been detected in other cell compartments (Jimenez et al., 1998).
Ascorbate works in co‐operation not only with gluta thione, but also takes part in the regeneration of α‐tocopherol, providing synergetic protection of the membranes (Thomas et al., 1992). Tocopherol has been reported to be in direct interaction also with reduced glutathione (Fryer, 1992) and reduced coenzyme Q (Buettner, 1993). In a recent paper by Kagan et al. (2000) it was suggested that tocopherol and reduced coenzyme Q, when present together in a membrane, show combined antioxidant activity which is markedly synergetic.
Recently, redox coupling of plant phenolics with ascorbate in the H2O2–peroxidase system has been shown (Takahama and Oniki, 1997; Yamasaki and Grace, 1998). It takes place in the vacuole, where H2O2 diffuses and can be reduced by peroxidases using phenolics as primary electron donors. Both AA and the monodehydroascorbic acid radical can reduce phenoxyl radicals generated by this oxidation. If regeneration of AA is performed in the cytosol and AA is supplied back to the vacuole, a peroxidase/phenolics/AA system could function in vacuoles and scavenge H2O2 (Yamasaki and Grace, 1998). This mechanism is specific for plant tissues and can improve stress tolerance under oxidative stress.
Species and tissue specificity adds to the already complex antioxidant response. It is also important to carry out experiments under strictly controlled conditions with respect to oxygen concentration and to distinguish between hypoxia and anoxia. Accumulating data suggest that low oxygen concentration plays a crucial role in the induction of anoxic metabolism, i.e. triggers the expression of genes responsible for anaerobic fermentation, sugar utilization (Chang et al., 2000) and antioxidant defence. Another important point for the experimental set‐up is the unavoidable reoxygenation period, when most of anoxia‐induced damage has been shown to occur (Crawford et al., 1994). To clarify the situation ROS‐levels should be detected (e.g. by electron spin resonance spectrometry) in similar conditions.
Hypoxic tissues exhibit possibilities for enhanced ROS production, accumulation of LP substrates (FFA) and LP itself. These possibilities rise from mitochondria‐dependent ROS generation, acetaldehyde dependent O2‐formation via XO, lipoxygenase action on membrane lipids and finally from lipolytic acyl hydrolase‐catalysed liberation of FFA, which underpins a burst in LP on return to normoxia. Short‐term oxygen deprivation stress possibly causes limited accumulation of ROS and peroxidized lipids. At this stage the rate of ROS formation and the degree of lipid peroxidation can be regulated by constitutive endogenous antioxidants. This in part can explain the lack of antioxidant system induction under oxygen deprivation in some experiments. It is noteworthy, that accumulation of ROS and LP products already under hypoxic conditions can bear a signal for low oxygen concentration in the tissue.
Prolonged anoxic treatment will emphasize anoxia‐ specific metabolic changes which, in turn, will abolish antioxidant synthesis, transport and turnover. As a consequence, depleted antioxidants and decreased activity of the antioxidant‐associated enzymes will be unable to cope with the overflow of ROS and on‐going peroxidative chain reactions during reoxygenation. On the restoration of normoxia, enzymatic ROS formation and LP will be overwhelmed by chemical oxidations in an uncontrolled manner. The scope of membrane damage and cell fate are probably determined by the degree of hypoxia (anoxia)‐induced changes in metabolism (i.e. drop in adenylate energy charge, cytoplasmic acidosis, amount of ethanol and acetaldehyde produced) and in membrane structures (i.e. depending on the duration of oxygen deprivation) and on plants’ tolerance to anoxia.
ACKNOWLEDGEMENTS
This work was funded by the Academy of Finland and the Finnish Ministry of Education as a part of the Center of Excellence on Plant Biology (project no. 164346). The Finnish Cultural Foundation provided a grant for E.V. which is gratefully acknowledged.
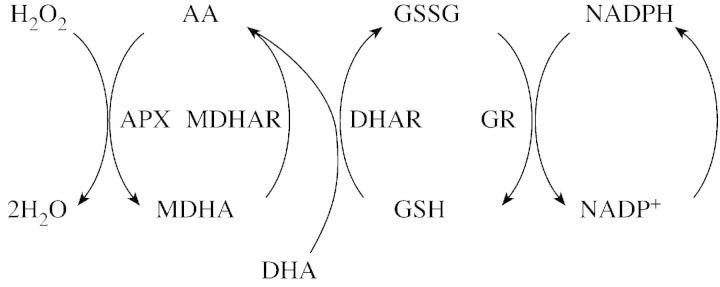
Fig. 1. Ascorbate‐glutathione cycle (Halliwell–Asada pathway). APX, ascorbate‐peroxidase; MDHAR, monodehydroascorbate reductase; DHAR, dehydroascorbate reductase; GR, glutathione reductase. According to May et al. (1998).
Table 1.
ROS scavenging and detoxifying enzymes
| Enzyme | EC number | Reaction catalysed |
| Superoxide dismutase | 1.15.1.1 | O2·– + O2·– + 2H+ ⇔ 2H2O2 + O2 |
| Catalase | 1.11.1.6 | 2H2O2 ⇔ O2 + 2H2O |
| Glutathione peroxidase | 1.11.1.12 | 2GSH + PUFA–OOH ⇔ GSSG + PUFA + 2H2O |
| Glutathione S‐transferases | 2.5.1.18 | RX + GSH ⇔ HX + R‐S‐GSH* |
| Phospholipid‐hydroperoxide glutathione peroxidase | 1.11.1.9 | 2GSH + PUFA‐OOH (H2O2) ⇔ GSSG + 2H2O† |
| Ascorbate peroxidase | 1.11.1.11 | AA + H2O2 ⇔ DHA + 2H2O |
| Guaiacol type peroxidase | 1.11.1.7 | Donor + H2O2 ⇔ oxidized donor + 2H2O‡ |
| Monodehydroascorbate reductase | 1.6.5.4 | NADH + 2MDHA ⇔ NAD+ + 2AA |
| Dehydroascorbate reductase | 1.8.5.1 | 2GSH + DHA ⇔ GSSG + AA |
| Glutathione reductase | 1.6.4.2 | NADPH + GSSG ⇔ NADP+ + 2GSH |
* R may be an aliphatic, aromatic or heterocyclic group; X may be a sulfate, nitrite or halide group.
† Reaction with H2O2 is slow.
‡ AA acts as an electron donor (Mehlhorn et al., 1996).
Supplementary Material
Received: 6 August 2001; Returned for revision: 20 November 2001; Accepted: 16 January 2002
References
- AlinP, Jensson H, Guthenberg C, Danielson UH, Tahir MK, Mannervik B.1985. Purification of major basic glutathione transferase isoenzymes from rat liver by use of affinity chromatography and fast protein liquid chromatofocusing. Analytical Biochemistry 146: 313–320. [DOI] [PubMed] [Google Scholar]
- AlscherRG.1989. Biosynthesis and antioxidant function of glutathione in plants. Physiologia Plantarum 77: 457–464. [Google Scholar]
- AmorY, Chevion M, Levine A.2000. Anoxia pretreatment protects soybean cells against H2O2‐induced cell death: possible involvement of peroxidases and of alternative oxidase. FEBS Letters 477: 175–180. [DOI] [PubMed] [Google Scholar]
- AroraA, Byrem TM, Nair MG, Strasburg GM.2000. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Archives of Biochemistry and Biophysics 373: 102–109. [DOI] [PubMed] [Google Scholar]
- ArrigoniO, De Tullio MC.2000. The role of ascorbic acid in cell metabolism: between gene‐directed functions and unpredictable chemical reactions. Journal of Plant Physiology 157: 481–488. [Google Scholar]
- AsadaK.1999. The water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50: 601–639. [DOI] [PubMed] [Google Scholar]
- Avsian‐KretchmerO, Eshdat Y, Gueta‐Dahan Y, Ben‐Hayyim G.1999. Regulation of stress‐induced phospholipid hydroperoxide glutathione peroxidase expression in citrus. Planta 209: 469–477. [DOI] [PubMed] [Google Scholar]
- AzziA, Stocker A.2000. Vitamin E: non‐antioxidant roles. Progress in Lipid Research 39: 231–255. [DOI] [PubMed] [Google Scholar]
- BannisterJV, Bannister WH, Rotilio G.1987. Aspects of the structure, function and applications of superoxide dismutase. Critical Reviews in Biochemistry 22: 111–180 [DOI] [PubMed] [Google Scholar]
- BartoliCG, Pastori GM, Foyer CH.2000. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiology 123: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BerglundAH, Nilsson R, Liljenberg C.1999. Permeability of large unilamellar digalactosyldiacylglycerol vesicles for protons and glucose—influence of α‐tocopherol, β‐carotene, zeaxanthin and cholesterol. Plant Physiology and Biochemistry 37: 179–186. [Google Scholar]
- BiemeltS, Keetman U Albrecht G.1998. Re‐aeration following hypoxia or anoxia leads to activation of the antioxidative defense system in roots of wheat seedlings. Plant Physiology 116: 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BiemeltS, Keetman U, Mock H‐P, Grimm B.2000. Expression and activity of isoenzymes of superoxide dismutase in wheat roots in response to hypoxia and anoxia. Plant Cell and Environment 23: 135–144. [Google Scholar]
- BlokhinaOB, Fagerstedt KV, Chirkova TV.1999. Relationships between lipid peroxidation and anoxia tolerance in a range of species during post‐anoxic reaeration. Physiologia Plantarum 105: 625–632. [Google Scholar]
- BlokhinaOB, Virolainen E, Fagerstedt KV, Hoikkala A, Wähälä K, Chirkova TV.2000. Antioxidant status of anoxia‐tolerant and ‐intolerant plant species under anoxia and reaeration. Physiologia Plantarum 109: 396–403. [Google Scholar]
- BlokhinaOB, Chirkova TV, Fagerstedt KV.2001. Anoxic stress leads to hydrogen peroxide formation in plant cells. Journal of Experimental Botany 52: 1–12. [PubMed] [Google Scholar]
- BolwellGP, Wojtaszek P.1997. Mechanisms for generation of reactive oxygen species in plant defence—a broad perspective. Physiological and Molecular Plant Pathology 51: 347–366. [Google Scholar]
- BooYC, Jung J.1999. Water deficit‐induced oxidative stress and antioxidative defenses in rice plants. Journal of Plant Physiology 155: 255–261. [Google Scholar]
- BourboulouxA, Shahi P, Chakladar A, Delrot S, Bachhawat AH.2000. Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae Journal of Biological Chemistry 275: 13259–13265. [DOI] [PubMed] [Google Scholar]
- BowlerC, Van Montagu M, Inze D.1992. Superoxide dismutase and stress tolerance. Annual Review of Plant Physiology and Plant Molecular Biology 43: 83–116. [Google Scholar]
- BraidotE, Petrussa E, Vianello A, Macri F.1999. Hydrogen peroxide generation by higher plant mitochondria oxidizing complex I of complex II substrates. FEBS Letters 451: 347–350. [DOI] [PubMed] [Google Scholar]
- BuettnerGR.1993. The pecking order of free radicals and antioxidants: lipid peroxidation, α‐tocopherol, and ascorbate. Archives of Biochemistry and Biophysics 300: 535–543. [DOI] [PubMed] [Google Scholar]
- ChakrabortiT, Das S, Mondal M, Roychoudhury S, Chakraborti S.1999. Oxidant, mitochondria and calcium: an overview. Cellular Signalling 11: 77–85. [DOI] [PubMed] [Google Scholar]
- ChangWWP, Huang L, Shen M, Webster C, Burlingame AL, Roberts JKM.2000. Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low‐oxygen environment, and identification of proteins by mass spectrometry. Plant Physiology 122: 295–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChengZ, Putt DA, Lash LH.2000. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: further evidence for the role of dicarboxylate and 2‐oxoglutarate carriers in mitochondrial glutathione transport. Archives of Biochemistry and Biophysics 373: 193–202. [DOI] [PubMed] [Google Scholar]
- ChirkovaTV.1988. Plant adaptation to hypoxia and anoxia. Leningrad: Leningrad State University Press.
- ChirkovaTV, Voitzekovskaya SA.1999. Plant protein synthesis under hypoxia and anoxia. Uspekhi Sovremennoi. Biologii 119: 178–189. [Google Scholar]
- ChirkovaTV, Zhukova TM. Tretiakov AL.1984. Adenine nucleotides in wheat and rice seedlings under aeration and anoxia. Vestnik LGU 15: 74–81 (in Russian). [Google Scholar]
- ChirkovaTV, Sinyutina NF, Blyudzin YA, Barsky IE, Smetannikova SV.1989. Phospholipid fatty acids of root mitochondria and microsomes from rice and wheat seedlings exposed to aeration or anaerobiosis. Russian Journal of Plant Physiology 36: 126–134. [Google Scholar]
- ChirkovaTV, Zhukova TM, Goncharova NN.1991a Method of determination of plant resistance to oxygen shortage. Russian Journal of Plant Physiology 38: 359–364. [Google Scholar]
- ChirkovaTV, Zhukova TM, Goncharova NN.1991b Specificity of membrane permeability in wheat and rice seedlings under anoxia. Fiziologia i Biokhimia Kulturnux Rastenii 23: 541–546 (in Russian). [Google Scholar]
- ChirkovaTV, Zhukova TM, Bugrova MP.1992. Redox reactions of plant cells in response to short‐term anaerobiosis. Vestnik SPBGU 3: 82–86 (in Russian). [Google Scholar]
- ChirkovaTV, Novitskaya LO, Blokhina OB.1998. Lipid peroxidation and antioxidant systems under anoxia in plants differing in their tolerance to oxygen deficiency.Russian Journal of Plant Physiology 45: 55–62. [Google Scholar]
- ConklinPL.2001. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell and Environment 24: 383–394. [Google Scholar]
- ConklinPL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL.1999. Genetic evidence for the role of GDP‐mannose in plant ascorbic acid (vitamin C) biosynthesis. Proceedings of the National Academy of Sciences of the USA 96: 4198–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CrawfordRMM, Braendle R.1996. Oxygen deprivation stress in a changing environment. Journal of Experimental Botany 47: 145–159. [Google Scholar]
- CrawfordRMM, Walton JC, Wollenweber‐Ratzer B.1994. Similarities between post‐ischaemic injury to animal tissues and post anoxic injury in plants. Proceedings of the Royal Society of Edinburgh 102B, 325–332. [Google Scholar]
- CummingsBS, Angeles R, McCauley RB, Lash LH.2000. Role of voltage‐dependent channels in glutathione transport into yeast mitochondria. Biochemical and Biophysical Research Communications 276: 990–944. [DOI] [PubMed] [Google Scholar]
- DaveyMW, Gilot C, Persiau G, Østergaard J, Han Y, Bauw GC, Van Montagu M.1999. Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiology 121: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeanJV, Devarenne TP.1997. Peroxidase‐mediated conjugation of glutathione to unsaturated phenylpropanoids—evidence against glutathione S‐transferase involvement. Physiologia Plantarum 99: 271–278. [Google Scholar]
- DeLongJM, Steffen KL.1998. Lipid peroxidation and α‐tocopherol content in α‐tocopherol‐supplemented thylakoid membranes during UV‐B exposure. Environmental and Experimental Botany 39: 177–185. [Google Scholar]
- DeTullioMC, Paciolla C, Dalla Vecchia F, Rascio N, D’Emerico S, De Gara L, Liso R, Arrigoni O.1999. Changes in onion root development induced by the inhibition of peptidyl‐prolyl hydroxylase and influence of the ascorbate system on cell division and elongation. Planta 209: 424–434. [DOI] [PubMed] [Google Scholar]
- DixonDP, Cummins I, Cole DJ, Edwards R.1998. Glutathione‐mediated detoxification systems in plants. Current Opinion in Plant Biology 1: 258–266. [DOI] [PubMed] [Google Scholar]
- DrewMC.1997. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Plant Molecular Biology 48: 223–250. [DOI] [PubMed] [Google Scholar]
- EllisMH, Setter TL.1999. Hypoxia induces anoxia tolerance in completely submerged rice seedlings. Journal of Plant Physiology 154: 219–230. [Google Scholar]
- EllisMH, Dennis ES, Peacock WL.1999. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiology 119: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElstnerEF.1987. Metabolism of activated oxygen species. In: Davies DD, ed. Biochemistry of plants, Vol. 11. London: Academic Press, 253–315.
- ElstnerEF, Osswald W.1994. Mechanisms of oxygen activation during plant stress. Proceedings of the Royal Society of Edinburgh 102B: 131–154. [Google Scholar]
- FoyerCH, Halliwell B.1976. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133: 21–25. [DOI] [PubMed] [Google Scholar]
- FoyerCH, Lelandais MA.1996. A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaves mesophyll cells. Journal of Plant Physiology 148: 391–398. [Google Scholar]
- FoyerCH, Lelandais M, Edwards EA, Mullineaux PM.1991. The role of ascorbate in plants, interactions with photosynthesis, and regulatory significance. In: Pell E, Steffen K, eds. Active Oxygen/Oxidative Stress and Plant Metabolism: Proceedings of the 6th Annual Penn State Symposium inPlant Physiology, 23–25 May 1991 Rockville, MD: American Society of Plant Physiologists, 131–144.
- FrenkelC.1991. Disruption of macromolecular hydration—a possible origin of chilling destabilisation of biopolymers. Trends in Food Science and Technology 2: 39–41. [Google Scholar]
- FryerMJ.1992. The antioxidant effects of thylakoid vitamin E (α‐tocopherol). Plant Cell and Environment 15: 381–392. [Google Scholar]
- García‐PlazaolaJI, Becerril JM.2000. Effects of drought on photoprotective mechanisms in European beech (Fagus sylvatica L.) seedlings from different provenances. Trees 14: 485–490. [Google Scholar]
- GardnerHW, Eskins K, Grams GW, Inglett GE.1972. Radical addition of linoleic hydroperoxides to α‐tocopherol or the analogous hydroxychroman. Lipids 7: 324–334. [Google Scholar]
- GenerosovaIP, Krasavina MS, Polyakova LI., Burmistrova NA, Lyubomilova MV, Vartapetian BB.1998. On some molecular aspects of adaptation of Oryza sativa seedlings to anoxia. Russian Journal of Plant Physiology 45: 227–233. [Google Scholar]
- GilleL, Nohl H.2001. The ubiquinol/bc1 redox couple regulates mitochondrial oxygen radical formation. Archives of Biochemistry and Biophysics 388: 34–38. [DOI] [PubMed] [Google Scholar]
- GoutE, Boisson AM, Aubert S, Douce R, Bligny R.2001. Origin of the cytoplasmic pH changes during anaerobic stress in higher plant cells. Carbon‐13 and phosphorus‐31 nuclear magnetic resonance studies. Plant Physiology 125: 912–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GraceS, Logan BA.2000. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philosophical Transactions of the Royal Society of London B 355: 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HanhijärviAM, Fagerstedt KV.1994. Comparison of the effect of natural and experimental anoxia on carbohydrate and energy metabolism in Iris pseudacorus rhizomes. Physiologia Plantarum 90: 437–444. [Google Scholar]
- HanhijärviAM, Fagerstedt KV.1995. Comparison of carbohydrate utilization and energy charge in the yellow flag iris (Iris pseudacorus) and garden iris (Iris germanica) under anoxia. Physiologia Plantarum 93: 493–497. [Google Scholar]
- HenziT, Braendle R.1993. Long term survival of rhizomatous species under oxygen deprivation. In: Jackson MB, Black CR, eds. Interacting stresses on plants in a changing climate, NATO ASI Series, Vol. I 16. Berlin, Heidelberg: Springer‐Verlag, 305–314.
- HetheringtonAM, Hunter MI, Crawford RMM.1982. Contrasting effects of anoxia on rhizome lipids of Iris species. Phytochemistry 21: 1275–1278. [Google Scholar]
- HoremansN, Asard H, Van Gestelen P, Caubergs RJ.1998a Facilitated diffusion drives transport of oxidised ascorbate molecules into purified plasma membrane vesicles of Phaseolus vulgaris Physiologia Plantarum 104: 783–789. [Google Scholar]
- HoremansN, Potters G, Caubergs RJ, Asard H.1998b Transport of ascorbate into protoplasts of Nicotiana tabacum Bright Yellow‐2 cell line. Protoplasma 205: 114–121. [Google Scholar]
- HoremansN, Foyer CH, Asard H.2000a Transport and action of ascorbate at the plant plasma membrane. Trends in Plant Sciences 5: 263–267. [DOI] [PubMed] [Google Scholar]
- HoremansN, Foyer CH, Potters G, Asard H.2000b Ascorbate function and associated transport systems in plants. Plant Physiology and Biochemistry 38: 531–540. [Google Scholar]
- HunterMIS, Hetherington AM, Crawford RMM.1983. Lipid peroxidation—a factor in anoxia intolerance in Iris species? Phytochemistry 22: 1145–1147. [Google Scholar]
- JaniszowskaW, Korczak G.1980. The intracellular distribution of tocopherols in Calendula officinalis leaves. Phytochemistry 19: 1391–1392. [Google Scholar]
- JaniszowskaW, Pennock JF.1976. The biochemistry of vitamin E in plants. Vitamins and Hormones 34: 77–105. [DOI] [PubMed] [Google Scholar]
- JimenezA, Hernandez JA, Pastori G, del Rio LA, Sevilla F.1998. Role of the ascorbate‐glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiology 118: 1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KaganVE.1989. Tocopherol stabilizes membrane against phospholipase A, free fatty acids, and lysophospholipids. In: Diplock AT, Machlin J, Packer L, Pryor WA, eds. Vitamin E: biochemistry and health implications, Vol. 570. New York, NY: Annals of the New York Academy of Sciences, 121–135. [DOI] [PubMed]
- KaganVE, Fabisiak JP, Quinn PJ.2000. Coenzyme Q and vitamin E need each other as antioxidants. Protoplasma 214: 11–18. [Google Scholar]
- KaiserS, Di Mascio P, Murphy ME, Sies H.1990. Physical and chemical scavenging of singlet molecular oxygen by tocopherols. Archives of Biochemistry and Biophysics 277: 101–108. [DOI] [PubMed] [Google Scholar]
- KalashnikovJuE, Balakhnina TI, Zakrzhevsky DA.1994. Effect of soil hypoxia on activation of oxygen and the system of protection from oxidative destruction in roots and leaves of Hordeum vulgare Russian Journal of Plant Physiology 41: 583–588. [Google Scholar]
- Kamal‐EldinA, Appelqvist L‐Å.1996. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31: 671–701. [DOI] [PubMed] [Google Scholar]
- KanofskyJR, Axelrod B.1986. Singlet oxygen production by soybean lipoxygenase isozymes. Journal of Biological Chemistry 261: 1099–1104. [PubMed] [Google Scholar]
- Kato‐NoguchiH.2000. Abscisic acid and hypoxic induction of anoxia tolerance in roots of lettuce seedlings. Journal of Experimental Botany 51: 1939–1944. [DOI] [PubMed] [Google Scholar]
- KimFJ, Kim HP, Hah YC, Roe JH.1996. Differential expression of superoxide dismutases containing Ni and Fe/Zn in Streptomyces coelicolor European Journal of Biochemistry 241: 178–185. [DOI] [PubMed] [Google Scholar]
- KrukJ, Jemiola‐Rzeminska M, Strzalka K.1997. Plastoquinol and α‐tocopherol quinol are more active than ubiquinol and α‐tocopherol in inhibition of lipid peroxidation. Chemistry and Physics of Lipids 87: 73–80. [Google Scholar]
- KrukJ, Schmid GH, Strzalka K.2000. Interaction of α‐tocopherol quinone, α‐tocopherol and other prenyllipids with photosystem. II. Plant Physiology and Biochemistry 38: 271–277. [Google Scholar]
- LalSK, Lee C, Sachs MM.1998. Differential regulation of enolase during anaerobiosis in maize. Plant Physiology 118: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LambC, Dixon RA.1997. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology 48: 251–275. [DOI] [PubMed] [Google Scholar]
- LanderHM.1997. An essential role for free radicals and derived species in signal transduction. FASEB Journal 11: 118–124. [PubMed] [Google Scholar]
- LarsonRA.1988. The antioxidants of higher plants. Phytochemistry 27: 969–978. [Google Scholar]
- LeeTM, Lin YN.1995. Changes in soluble and cell wall‐bound peroxidase activities with growth in anoxia‐treated rice (Oryza sativa L.) coleoptiles and roots. Plant Science 106: 1–7. [Google Scholar]
- LeipnerJ, Fracheboud Y, Stamp P.1999. Effect of growing season on the photosynthetic apparatus and leaf antioxidative defenses in two maize genotypes of different chilling tolerance. Environmental and Experimental Botany 42: 129–139. [Google Scholar]
- LeipnerJ, Stamp P, Fracheboud Y.2000. Artificially increased ascorbate content affects zeaxanthin formation but not thermal energy dissipation or degradation of antioxidants during cold‐induced photooxidative stress in maize leaves. Planta 210: 964–969. [DOI] [PubMed] [Google Scholar]
- LisoR, Innocenti AM, Bitonti MB, Arrigoni O.1988. Ascorbic acid‐induced progression of quiescent centre cells from G1 to S phase. New Phytologist 110: 469–471. [Google Scholar]
- LoewusFA.1980. L‐ascorbic acid: metabolism, biosynthesis, function. In: Preiss J, ed. Carbohydrates, structure and function. The biochemistry of plants, Vol. 3. New York: Academic Press, 77–79.
- LoewusMW, Bedgar DR, Saito K, Loewus FA.1990. Conversion of L‐sorbosone to L‐ascorbic acid by a NADP‐dependent dehydrogenase in bean and spinach leaf. Plant Physiology 94: 1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LynchDV, Thompson JE.1984. Lipoxygenase‐mediated production of superoxide anion in senescing plant tissue. FEBS Letters 173: 251–254. [Google Scholar]
- MarrsKA.1996. The functions and regulation of glutathione S‐transferases in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 127–158. [DOI] [PubMed] [Google Scholar]
- MayMJ, Vernoux T, Leaver C, Van Montagu M, Inze D.1998. Glutathione homeostasis in plants: implications for environmental sensing and plant development. Journal of Experimental Botany 49: 649–667. [Google Scholar]
- MehlhornH, Lelandais M, Korth HG, Foyer CH.1996. Ascorbate is the natural substrate for plant peroxidases? FEBS Letters 378: 203–206. [DOI] [PubMed] [Google Scholar]
- MerzlyakMN.1989. Activated oxygen and oxidative processes in plant cell membranes. Itogi Nauki Tekhhiki, Ser. Plant Physiology 6: 1–167.
- MoldauH, Bichele I, Huve K.1998. Dark‐induced ascorbate deficiency in leaf cell walls increases plasmalemma injury under ozone. Planta 207: 60–66. [Google Scholar]
- MöllerIM.2001. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Physiology and Plant Molecular Biology 52: 561–91. [DOI] [PubMed] [Google Scholar]
- MonkLS, Fagerstedt KV, Crawford RMM.1987. Superoxide dismutase as an anaerobic polypeptide—a key factor in recovery from oxygen deprivation in Iris pseudacorus? Plant Physiology 85:1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MonkLS, Fagerstedt KV, Crawford RMM.1989. Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiologia Plantarum 76: 456–459. [Google Scholar]
- Munné‐BoschS, Alegre L.2000a Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 210: 925–931. [DOI] [PubMed] [Google Scholar]
- Munné‐BoschS, Alegre L.2000b The significance of β‐carotene, α‐tocopherol and the xanthophyll cycle in droughted Melissa officinalis plants. Australian Journal of Plant Physiology 27: 139–146. [Google Scholar]
- Munné‐BoschS, Alegre L.2001. Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. Plant Physiology 125: 1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné‐BoschS, Schwarz K, Alegre L.1999. Enhanced formation of α‐tocopherol and highly oxidized abietane diterpenes in water‐stressed rosemary plants. Plant Physiology 121: 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NoctorG, Foyer CH.1998. Ascorbate and glutathione: keeping active oxygen under control.Annual Review of Plant Physiology and Plant Molecular Biology 49: 249–279. [DOI] [PubMed] [Google Scholar]
- NoctorG, Arisi ACM, Jouanin L, Foyer CH.1998a Manipulation of glutathione and amino acid biosynthesis in the chloroplast. Plant Physiology 118: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NoctorG, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH.1998b Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. Journal of Experimental Botany 49: 623–647. [Google Scholar]
- OstergaardJ, Persiau G, Davey MW, Bauw G, Van Montagu M.1997. Isolation of a cDNA coding for L‐galactono‐gamma‐lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants. Purification, characterization, cDNA cloning, and expression in yeast. Journal of Biological Chemistry 272: 30009–30016. [DOI] [PubMed] [Google Scholar]
- PackerL, Weber SU, Rimbach G.2001. Molecular aspects of α‐tocotrienol antioxidant action and cell signalling. Journal of Nutrition 131: 369S–373S. [DOI] [PubMed] [Google Scholar]
- PavelicD, Arpagaus S, Rawyler A, Braendle R.2000. Impact of post‐anoxia stress on membrane lipids of anoxia pretreated potato cells. A re‐appraisal. Plant Physiology 124: 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister‐SieberM, Braendle R.1994. Aspects of plant behavior under anoxia and post‐anoxia. Proceedings of the Royal Society of Edinburgh 102B: 313–324. [Google Scholar]
- PriceAH, Hendry GAF.1989. Stress and the role of activated oxygen scavengers and protective enzymes in plants subjected to drought. Biochemical Society Transactions 17: 493–494. [Google Scholar]
- RatcliffeRG.1995. Metabolic aspects of the anoxic response in plant tissue. In: Smirnoff N, ed. Environment and plant metabolism: flexibility and acclimation. Oxford: BIOS Scientific Publishers, 111–127.
- RawylerA, Pavelic D, Gianiazzi C, Oberson J, Braendle R.1999. Membrane lipid integrity relies on a threshold of ATP production rate in potato cell cultures submitted to anoxia. Plant Physiology 120: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ReaPA.1999. MRP subfamily ABC transporters from plants and yeast. Journal of Experimental Botany 50: 895–913. [Google Scholar]
- RecknagelRO, Glende EA, Jr.1984. Spectrophotometric detection of lipid conjugated dienes. Methods in Enzymology 105: 331–337. [DOI] [PubMed] [Google Scholar]
- ReggianiR, Bertani A.1989. Effect of decreasing oxygen concentration on polyamine metabolism in rice and wheat shoots. Journal of Plant Physiology 135: 375–377. [Google Scholar]
- Rice‐EvansCA, Miller NJ, Paganga G.1997. Antioxidant properties of phenolic compounds. Trends in Plant Sciences 2: 152–159. [Google Scholar]
- RichardB, Couce I, Raymond P, Saglio PH, Saint‐Ges V, Pradet A.1994. Plant metabolism under hypoxia and anoxia. Plant Physiology and Biochemistry 32: 1–10. [Google Scholar]
- RickauerM, Brodschelm W, Bottin A, Veronesi C, Grimal H, Esquerretugaye MT.1997. The jasmonate pathway is involved differentially in the regulation of different defense responses in tobacco cells. Planta 202: 155–162. [Google Scholar]
- RosahlS.1996. Lipoxygenases in plants—their role in development and stress response. Zeitschrift fur Naturforschung 51c: 123–138. [DOI] [PubMed] [Google Scholar]
- RusselDA, Sachs MM.1991. The maize cytosolic glyceraldehyde‐3‐phosphate dehydrogenase gene family: organ specific expression and genetic analysis. Molecular and General Genetics 229: 219–228. [DOI] [PubMed] [Google Scholar]
- SairamRK, Deshmukh PS, Saxena DC.1998. Role of antioxidant systems in wheat genotype tolerance to water stress. Biologia Plantarum 41: 387–394. [Google Scholar]
- Sanchez‐FernandezR, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inze D, May MJ.1997. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proceedings of the National Academy of Sciences of the USA 94: 2745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ScebbaF, Sebastiani L, Vitagliano C.1998. Changes in activity of antioxidative enzymes in wheat (Triticum aestivum) seedlings under cold acclimation. Physiologia Plantarum 104: 747–752. [Google Scholar]
- SchultzG, Heintze A, Hoppe P, Hagelstein P, Görlach J, Meereis K, Schwanke U, Preiss M.1991. Tocopherol and carotenoid synthesis in chloroplasts: tight linkage to plastidic carbon metabolism in developing chloroplasts. In: Pell E, Steffen K, eds. Active Oxygen/Oxidative Stress and Plant Metabolism: Proceedings of the 6th Annual Penn State Symposium in Plant Physiology 23–25 May 1991 Rockville, MD: American Society of Plant Physiologists, 156–170.
- SemenzaGL.1999. Perspectives on oxygen sensing. Cell 98: 281–284. [DOI] [PubMed] [Google Scholar]
- SerbinovaEA, Packer L.1994. Antioxidant properties of α‐tocopherol and α‐tocotrienol. Methods in Enzymology 234: 354–366. [DOI] [PubMed] [Google Scholar]
- ShewfeltRL, Purvis AC.1995. Toward a comprehensive model for lipid peroxidation in plant tissue disorders. HortScience 30: 213–218. [Google Scholar]
- SkonecznyM, Rytka J.2000. Oxygen and haem regulate the synthesis of peroxisomal proteins: catalase A, acyl‐CoA oxidase and Pex 1p in the yeast Saccharomyces cerevisiae; the regulation of these proteins by oxygen is not mediated by haem. Biochemical Journal 350: 313–319. [PMC free article] [PubMed] [Google Scholar]
- SkulachevVP.1997. Membrane‐linked systems preventing superoxide formation. Bioscience Reports 17: 347–366. [DOI] [PubMed] [Google Scholar]
- SkulachevVP.1998. Cytochrome c in the apoptotic and antioxidant cascades. FEBS Letters 423: 275–280. [DOI] [PubMed] [Google Scholar]
- SmirnoffN.1995. Antioxidant systems and plant response to the environment. In: Smirnoff N, ed. Environment and plant metabolism: flexibility and acclimation. Oxford: BIOS Scientific Publishers, 217–243.
- SmirnoffN.1996. The function and metabolism of ascorbic acid in plants. Annals of Botany 78: 661–669. [Google Scholar]
- SmirnoffN.2000. Ascorbic acid: metabolism and functions of a multi‐facetted molecule. Current Opinion in Plant Biology 3: 229–235. [PubMed] [Google Scholar]
- SmirnoffN, Conklin PL, Loewus FA.2001. Biosynthesis of ascorbic acid in plants: a renaissance. Annual Review of Plant Physiology and Plant Molecular Biology 52: 437–467. [DOI] [PubMed] [Google Scholar]
- SugimotoM, Furui S, Suzuki Y.1997. Molecular cloning and characterisation of a cDNA encoding putative phospholipid hydroperoxide glutathione peroxidase from spinach. Bioscience, Biotechnology and Biochemistry 61: 1379–1381. [DOI] [PubMed] [Google Scholar]
- TadegeM, Dupuis I, Kuhlemeier C.1999. Ethanolic fermentation: new functions for an old pathway. Trends in Plant Sciences 4: 320–325. [DOI] [PubMed] [Google Scholar]
- TakahamaU, Oniki T.1997. A peroxide/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells.Physiologia Plantarum 101: 845–852. [Google Scholar]
- TarchevskiiIA.1992. Regulatory role of degradation of biopolymers and lipids. Fiziologiya Rastenii 39: 1215–1223. [Google Scholar]
- ThomasCE, McLean LR, Parker RA, Ohlweiler DF.1992. Ascorbate and phenolic antioxidant interactions in prevention of liposomal oxidation. Lipids 27: 543–550. [DOI] [PubMed] [Google Scholar]
- ThomasSM, Gebicki JM, Dean RT.1989. Radical initiated α‐tocopherol depletion and lipid peroxidation in mitochondrial membranes. Biochimica et Biophysica Acta 1002: 189–197. [DOI] [PubMed] [Google Scholar]
- ThrelfallDR, Whistance GR.1977. Dehydrophylloquinone, α‐hydrotocopherolquinone and dehydrotocopherols from etiolated maize and barley shoots. Phytochemistry 16: 1903–1907. [Google Scholar]
- TommasiniR, Martinoia E, Grill E, Dietz KJ, Amrhein N.1993. Transport of oxidized glutathione into barley vacuoles: evidence for the involvement of the glutathione‐S‐conjugate ATPase. Zeitschrift für Naturforschung. C. 48c: 867–871. [Google Scholar]
- UpstonJM, Terentis AC, Stocker R.1999. Tocopherol‐mediated peroxidation of lipoproteins: implications for vitamin E as a potential antiatherogenic supplement. FASEB Journal 13: 977–994. [DOI] [PubMed] [Google Scholar]
- UshimaruT, Shibazaka M, Tsuji H.1994. Resistance to oxidative injury in submerged rice seedlings after exposure to air. Plant Cell Physiology 35: 211–218. [Google Scholar]
- UshimaruT, Maki Y, Sano S, Koshiba K, Asada K, Tsuji H.1997. Induction of enzymes involved in the ascorbate‐dependent antioxidative system, namely ascorbate peroxidase, mono dehydroascorbate reductase and dehydroascorbate reductase, after exposure to air of rice (Oryza sativa) seedlings germinated under water. Plant and Cell Physiology 38: 541–549. [Google Scholar]
- UshimaruT, Kanematsu S, Shibasaka M, Tsuji H.1999. Effect of hypoxia on the antioxidative enzymes in aerobically grown rice (Oryza sativa) seedlings. Physiologia Plantarum 107: 181–187. [Google Scholar]
- UshimaruT, Kanematsu S, Katayama M, Tsuji H.2001. Antioxidative enzymes in seedlings of Nelumbo nucifera germinated under water. Physiologia Plantarum 112: 39–46. [DOI] [PubMed] [Google Scholar]
- Van ToaiTT, Bolles CS.1991. Postanoxic injury in soybean (Glycine max) seedlings. Plant Physiology 97: 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VartapetianBB, Jackson MB.1997. Plant adaptations to anaerobic stress. Annals of Botany 79 (Suppl. A): 3–20. [Google Scholar]
- WatersI, Morrell S, Greenway H, Colmer TD.1991. Effects of anoxia on wheat seedlings. II. Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation, and sugar levels. Journal of Experimental Botany 42: 1437–1447. [Google Scholar]
- WheelerGL, Jones MA, Smirnoff N.1998. The biosynthetic pathway of vitamin C in higher plants. Nature 393: 365–369. [DOI] [PubMed] [Google Scholar]
- WillekensH, Inze D, Van Montagu M, Van Camp W.1995. Catalase in plants. Molecular Breeding 1: 207–228. [Google Scholar]
- WingateVPM, Lawton MA, Lamb CJ.1988. Glutathione causes a massive and selective induction of plant defence genes. Plant Physiology 87: 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenweber‐RatzerB, Crawford RMM.1994. Enzymatic defence against post‐anoxic injury in higher plants. Proceedings of the Royal Society of Edinburgh 102B: 381–390. [Google Scholar]
- XiaJH, Roberts JKM.1994. Improved cytoplasmic pH regulation, increased lactate efflux, and reduced cytoplasmic lactate levels are biochemical traits expressed in root tips of whole maize seedlings acclimated to low oxygen environment. Plant Physiology 105: 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- YabutaY, Yoshimura K, Takeda T, Shigeoka S.2000. Molecular characterization of tobacco mitochondrial L‐galactono‐gamma‐lactone dehydrogenase and its expression in Escherichia coli Plant and Cell Physiology 41: 666–675. [DOI] [PubMed] [Google Scholar]
- YamasakiH, Grace SC.1998. EPR detection of phytophenoxyl radicals stabilized by zinc ions: evidence for the redox coupling of plant phenolics with ascorbate in the H2O2‐peroxidase system. FEBS Letters 422: 377–380. [DOI] [PubMed] [Google Scholar]
- YanB. Dai Q, Liu X, Huang S, Wang Z.1996. Flooding‐induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant and Soil 179: 261–268. [Google Scholar]
- YuQ, Rengel Z.1999a Drought and salinity differentially influence activities of superoxide dismutase in narrow‐leafed lupins. Plant Science 142: 1–11. [Google Scholar]
- YuQ, Rengel Z.1999b Waterlogging influences plant growth and activities of superoxide dismutases in narrow‐leafed lupin and transgenic tobacco plants. Journal of Plant Physiology 155: 431–438. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


