Abstract
Visual systems have a rich history as model systems for the discovery and understanding of basic principles underlying neuronal connectivity. The compound eyes of insects consist of up to thousands of small unit eyes that are connected by photoreceptor axons to set up a visual map in the brain. The photoreceptor axon terminals thereby represent neighboring points seen in the environment in neighboring synaptic units in the brain. Neural superposition is a special case of such a wiring principle, where photoreceptors from different unit eyes that receive the same input converge upon the same synaptic units in the brain. This wiring principle is remarkable, because each photoreceptor in a single unit eye receives different input and each individual axon, among thousands others in the brain, must be sorted together with those few axons that have the same input. Key aspects of neural superposition have been described as early as 1907. Since then neuroscientists, evolutionary and developmental biologists have been fascinated by how such a complicated wiring principle could evolve, how it is genetically encoded, and how it is developmentally realized. In this review article, we will discuss current ideas about the evolutionary origin and developmental program of neural superposition. Our goal is to identify in what way the special case of neural superposition can help us answer more general questions about the evolution and development of genetically “hard-wired” synaptic connectivity in the brain.
Keywords: Drosophila, Visual System, synapse, brain wiring
INTRODUCTION
Connecting the Eye to the Brain: Neural Superposition and the Visual Map
Both the camera eye of vertebrates and the compound eye of insects capture a picture of the outside world. The spatial organization of this picture is mapped through axonal projections from the eye into optic ganglia in the brain, a concept called retinotopy. Retinotopic axonal projections thereby map neighboring points in the picture of the world to neighboring synaptic units in the brain. Both vertebrates and insects form such synaptic visual maps of the world in the brain (Figure 1A–D). Neural superposition is a particular case of visual mapping (Braitenberg, 1967; Kirschfeld, 1967; Vigier, 1907a, b, c) found in the visual systems of some insects of the order Diptera (Hennig, 1973).
Figure 1.
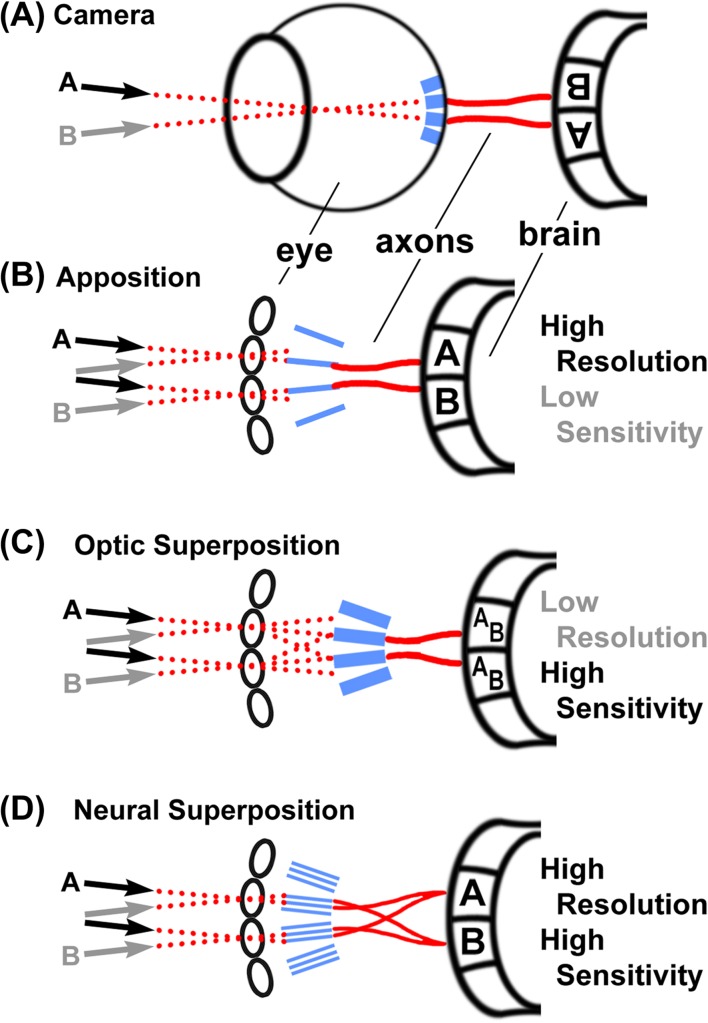
Comparison of visual systems. All light-sensitive elements (retina or rhabdoms) are shown in blue, light paths are shown as dotted red lines, and axonal connections between the eye and the brain are shown as solid red lines. (A) The camera eye of vertebrates produces an inverted image on the light-sensitive elements that is transmitted to the brain via optic nerves. (B) The apposition eye is the most common diurnal insect eye and produces an upright image on the light-sensitive rhabdoms as well as in the first optic neuropil, the lamina. Note that each individual ommatidium of the compound eye technically produces an inverted image on the rhabdom underneath that lens; however, the rhabdom is a single “fused” light guide for several retinula cells and only contributes a single pixel to the final image that is not further resolved. Apposition eyes are typically optimized for high resolution by “apposing” little overlapping visual fields of neighboring ommatidia based on small apertures and rhabdoms. (C) Optic superposition eyes comprise the reflectory and refractory superposition types. Input from several ommatidia is optically superimposed on individual rhabdoms, which increases sensitivity at the expense of resolution, typically in nocturnal insects, e.g., moths. (D) Neural superposition retains the high resolution of the apposition eye while increasing sensitivity by combining a number of input channels. This is achieved by separating the light sensitive elements (rhabdomeres) in the rhabdom and precise axonal wiring, as described in detail in Figures 2–4.
The architectures of insect visual systems can be categorized into three types: apposition, optic superposition, and neural superposition (Braitenberg, 1967; Greiner, 2006; Kirschfeld, 1967; Land, 2005; Land & Nilsson, 2002; Shaw, 1969) (Figure 1B–D). In all three architectures, the light-sensing elements are the rhabdomeres (marked blue in Figure 1B–D), morphological specializations of the photoreceptor neurons (retinula cells) under the lens (facet) of a single unit eye (ommatidium) of the compound eye. Most insects have apposition or optic superposition eyes and we need a basic understanding of these types to appreciate neural superposition. Both apposition and optic superposition eyes have a so-called fused rhabdom, i.e., the rhabdomeres of the contributing retinula cells are in direct contact with each other and function as a single, central light guide under the lens. Hence, all retinula cells in an ommatidium with a fused rhabdom receive input from the same field of view (Figure 1B, C).
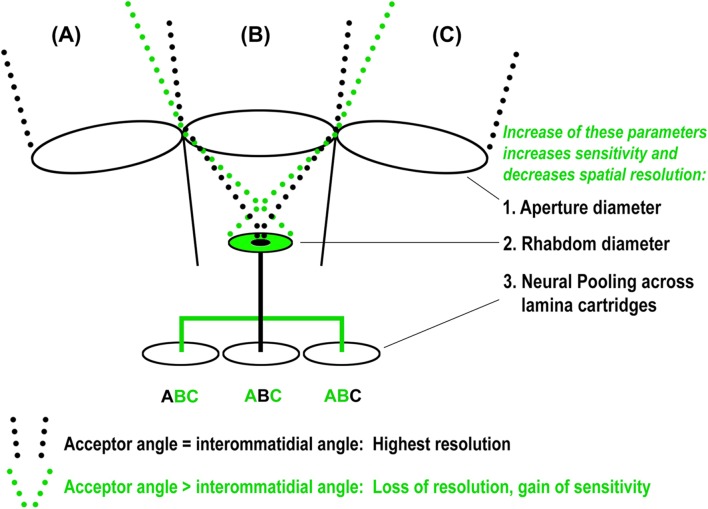
The size of the rhabdom and the lens aperture together define the retinula cell’s acceptor angle and field of view (dotted lines in Figure 2). The apposition eye maximizes its spatial resolution when the acceptor angle matches the angle between the individual ommatidia, because then the field of view of each ommatidium directly abuts (apposes) the field of view of the neighboring ommatidium (black dotted lines in Figure 2). The ommatidia in most diurnal (active during daylight) insects have a small fused rhabdom and a small lens aperture because they do not need high sensitivity and profit from a high spatial resolution. In contrast, optical superposition eyes exhibit an increase of the lens aperture and/or the rhabdom size that leads to overlapping (superimposed) fields of view and a loss in spatial resolution (green dotted lines in Figure 2). Optic superposition increases sensitivity at the cost of spatial resolution and is an adaptation to nocturnal life and dim light conditions (Land, 2005; Land & Nilsson, 2002; Warrant, 1999). In both apposition and optic superposition eyes, retinula cells form fused rhabdoms and their axons project as a bundle into the brain where they make connections in the same synaptic unit (Figure 1B, C) (Meinertzhagen, 1976). These synaptic units are called cartridges (Cajal & Sanchez, 1915). The apposition and optical superposition eyes are classic examples of retinotopy and simple axonal wiring diagrams.
Figure 2.
Limits of the ancestral apposition optics reveal the improvement potential for neural superposition. In an idealized, ancestral apposition eye each ommatidium sees a field of view (A, B, C) that directly abuts/apposes the field of view of its neighboring ommatidia. The aperture that defines this field of view only allows for a small maximum size of the light-sensitive rhabdom (small black disc). An increase of the rhabdom diameter (green disc) causes overlapping, instead of apposing, fields of view (dotted green light paths), which increases sensitivity at the expense of resolution. In the idealized (and indeed typical) apposition eye, little or no overlap between neighboring fields of view is preserved by connecting each rhabdom separately to an individual cartridge in the lamina (black line) with neighboring rhabdoms connected to neighboring cartridges. Sensitivity can be increased at the expense of resolution by distributing the input channel from a single rhabdom to surrounding cartridges (green line), in which case each cartridge receives input from several fields of view (green A,B,C). Both neural pooling and increased rhabdom size occur in nocturnal insects with typical diurnal apposition eyes. Note that for a given increase in neural pooling, a corresponding increase in rhabdom size will further increase sensitivity without loss of resolution.
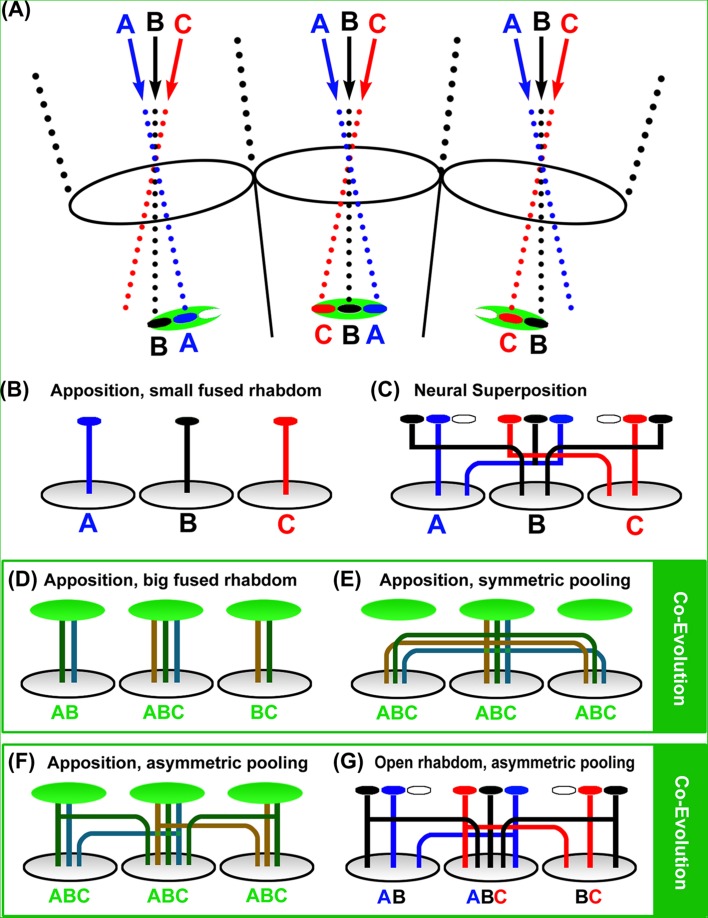
In neural superposition eyes, simple retinotopy of ommatidial axon bundles is replaced by a more complicated wiring diagram that combines individual axon terminals in the brain from retinula cells with the same visual axis in the eye (Figure 1D). Remarkably, neural superposition preserves the high resolution of an apposition eye with the same optics, but significantly increases sensitivity (Braitenberg, 1967; Kirschfeld, 1967; Vigier, 1908). How is that possible? A key to an understanding of neural superposition lies in the optics of the apposition eye (Figure 2). As described above, the size of a fused rhabdom in an apposition eye with optimal spatial resolution is limited to a small area that ensures an ommatidial field of view that has little or no overlap with the fields of view of neighboring ommatidia (central black disc/rhabdom in Figure 2). The small rhabdom size limits photon catch and thus sensitivity. For a given eye size an increase in the rhabdom diameter increases sensitivity at the cost of spatial resolution (green disc/rhabdom in Figure 2). Neural superposition eyes with the same small aperture utilize this additional space marked by the green disc in Figure 2 to increase their sensitivity. To avoid a loss of spatial resolution, the neural superposition eye separates different areas within the area marked by the green disc with separate rhabdomeres (Figure 3A). Separated rhabdomeres are also referred to as an open rhabdom and partially or fully open rhabdoms are found in all true flies (Diptera) (Dietrich, 1909; Osorio, 2007; Tuurala, 1963) (Figure 1D; Figure 3A, C). Since each of the separated rhabdomeres receive input via a different visual axis (a different point in the environment), neural superposition requires a dramatic rewiring of all axons carrying input from the same visual axis from different ommatidia into the same lamina cartridge (Figure 3C).
Figure 3.
Intermediate solutions suggest an evolutionary path from apposition to neural superposition. (A) Principle optics for differently sized open and fused rhabdoms. Note that these optics are based on an ideal apposition eye (as shown in Figure 2) in which the central disc represents the maximal sized rhabdom (blue for A, black for B, and red for C). The green disc denotes an enlarged (fused) rhabdom area that leads to increased sensitivity and a loss of resolution, as shown in Figure 2. The green disc also marks the area in which separate rhabdomeres in an open rhabdom are positioned (small red, black and blue discs). (B–G) Different wiring diagrams underneath the optics shown in (A). (B) In an idealized apposition eye, small fused rhabdoms receive input from fields of view with little or no overlap (small blue, black, and red discs) and this information is mapped via single retinula cell axon bundles to neighboring cartridges in the lamina. This type of apposition eye is considered ancestral (Nilsson, 1989) and most commonly found in diurnal insects. (C) Neural superposition is based on an open rhabdom in which separate rhabdomeres utilizes additional space around the small rhabdom of an equivalent apposition eye (red, black, and blue discs) and are wired according to their input without loss of resolution. (D) An increase in rhabdom size (green discs) leads to increased fields of view and a corresponding loss of resolution. Note that each of the axons of different retinula cells in the case of an enlarged fused rhabdom represents input from all overlapping fields of view seen by the big rhabdom. All axons under the big fused rhabdoms (green) are therefore colored in a green hue. (E) Insects with increased rhabdom size typically exhibit neural pooling in the lamina to further increase sensitivity. This arrangement is, for example, observed in the scorpionfly Panorpa, which is considered to represent an ancestral type of the true flies (Diptera) (Kristensen, 1981; Melzer et al., 1997). Note that symmetric neural pooling across all direct neighbors will actually lead to a further loss of resolution compared with the same-sized rhabdom apposition eye shown in (D). (F) similar to (E), but with asymmetric pooling such that cartridges receive preferential input from ommatidia on one side. In this hypothetical arrangement, cartridges still receive input from the same ommatidia with fused rhabdoms, but the input may be weighted differently compared with symmetric pooling such that less input is pooled from ommatidia peripheral to the central field of view. A representative of this arrangement may be the nocturnal bee Megalopta genalis, which exhibits increased rhabdom size and in at least one documented case asymmetric pooling of a retinula cell axon (Greiner et al., 2004, 2004). (G) Asymmetric pooling can be perfectly matched to different input channels, which requires the separation of the light-sensitive rhabdomeres (red, black, and blue discs). Compared with the large fused rhabdom in (E), this arrangement represents a substantial gain of resolution with only minor loss of sensitivity (due to the inter-rhabdomere space). A large variety of types of asymmetric pooling in conjunction with partially or completely open rhabdoms characterize numerous species of the polyphyletic suborder Nematocera (Land & Horwood, 2005; Melzer et al., 1997), as further discussed in the text and shown in Figure 5. Neural superposition can be interpreted as an extreme case of asymmetric pooling, where only a single axon input remains that projects to the particular cartridge collecting input from the same field of view (Melzer et al., 1997; Nilsson & Ro, 1994).
The most common neural superposition eye is found in all advanced flies (suborder Brachycera; includes Drosophila melanogaster). Here, retinula cells R1–R6 contribute larger, outer rhabdomeres, while R7 and R8 reside in the center and are stacked on top of each other (i.e., they see the same point in space). Since the outer and inner retinula cell rhabdomeres are arranged underneath a single lens, they receive light from seven different points in space (Figure 4). In Drosophila, R1–R6 are the primary motion detectors, whereas R7 and R8 may contribute to motion detection, but primarily transmit color information into the brain (Gao et al., 2008; Morante & Desplan, 2004; Wardill et al., 2012; Yamaguchi et al., 2008). Hence, R1–R6 axons form a primary visual map.
Figure 4.
Mapping of light-sensing rhabdomeres in the eye onto lamina cartridges in the first optic neuropil, the lamina, in neural superposition of advanced flies (Brachycera). (A) A single R1 in one ommatidium “sees” the same point in the environment as a single R2 in a neighboring ommatidium, a single R3 in a different neighboring ommatidium, etc. (marked in red in the eye). The six R1–R6 rhabdomeres that have the same visual field converge upon the same cartridge in the lamina (red dot). (B) R1–R6 in a single ommatidium see six different points in space through the separate rhabdomeres (red) and a seventh point through the central, stacked R7/R8 rhabdomeres (blue). The six R1–R6 input lines from a single ommatidium are separated into six separate cartridges in the lamina (red dots).
The first fascinating aspect of the neural superposition eye is the crystalline precision with which facets and rhabdomeres are arranged to ensure proper function. In the Drosophila type of neural superposition, the angle between the visual axes of neighboring R1–R6 rhabdomeres is closely matched by the angle between the facets (Braitenberg, 1967; Kirschfeld, 1967; Kirschfeld & Franceschini, 1968). Consequently, if an R1 photoreceptor sees a point “A” in the environment, there must be an R2 in exactly one neighboring ommatidium that sees the same point “A” in the environment (Figures 1D and 4A). In total, there is exactly one R1, R2, R3, R4, R5, and R6 (in six different ommatidia) that sees the same point in space. In all advanced flies R1–R6 are arranged in a trapezoidal pattern (Figure 4A). The summation of independent, parallel input channels with the same visual axis significantly increases sensitivity for the signal from that field of view as described above. Because the number of visual axes as well as the number of cartridges (i.e., pixels of the visual map) are the same in a neural superposition eye and apposition eye of the same size, we think the interpretation that neural superposition increases resolution (Moses, 2006) is less likely to be the case. Instead, the increased sensitivity afforded by neural superposition is considered to be advantageous under low light conditions (Land & Nilsson, 2002) and provides additional parallel input for efficient visual processing of the day-active, fast-flying flies.
How does the precision of the angular arrangement of light-sensitive elements and single eyes in the neural superposition eye translate to the wiring in the brain? Notably, already Vigier and Cajal (Cajal & Sanchez, 1915; Vigier, 1907c) observed that the axons from one ommatidium participate in the formation of different optical cartridges. As in the case of the apposition eye, the photoreceptor neurons residing in the same ommatidium form a bundle of their axons that together projects to the brain. In contrast to the apposition eye, these bundles consist of eight input lines that receive input via seven different visual axes (Figure 4B). In order for neural superposition to work, the bundle needs to untangle in such a way that precisely those R1–R6 axons from six different ommatidia that receive input through the same visual axis converge upon the same synaptic cartridge (while R7 and R8 project straight through the lamina into a deeper brain area) (Figure 4A). In the case of the well-studied genetic model organism Drosophila melanogaster, this means that R1–R6 axons from approximately 800 ommatidia (Ready et al., 1976) must unscramble the eye’s input by engaging in an enigmatic sorting process that forms a functional visual map. How this sorting process occurs developmentally is the second, and maybe most fascinating aspect of neural superposition. This wiring principle is not strictly retinotopic, since axons from different ommatidia intermingle to innervate synaptic cartridges that represent neighboring points in the visual environment. However, at the level of the visual map, it is beautifully simple: from the perspective of the postsynaptic neurons that receive input from R1–R6 that see the same point “A”, it does not matter how the complicated optics and wiring are developmentally resolved. This then is the challenge and the source of the fascination with neural superposition: how could such a complicated wiring pattern evolve, and how is it developmentally realized?
Of Codes and Cues
In 1940, Roger Sperry formulated the chemoaffinity theory (Meyer, 1998; Sperry, 1963). The idea that molecules can function as attractive or repulsive cues and direct axon pathfinding by determining axon targets has been hugely influential. Over many years, the idea has developed to include the concept of a molecular code: any given set of distinct attractive or repulsive molecular cues may define where exactly a specific axon will target and form synaptic connection. Neural superposition highlights a key conceptual limitation of this idea. At face value, every single one of the six times 800 axons that form the visual map in, for example, Drosophila could theoretically have a target that is determined by a unique molecular code. This seems unlikely: the amount of information needed to encode the six times 800 targets (codes) by distinct molecular cues would be the same as the information required to encode the molecular cues in the first place. In other words, we reason that thousands of distinct molecular codes are a theoretical solution, but not a simplification of the problem how to define thousands of targets; a large number of distinct codes only explains one complicated phenomenon (targeting specificity) with another, equally complicated phenomenon (molecular code specificity). So where is the information coming from? We speculate that the solution may lie in the iterative, self-similar organization of the visual map. We further hope that an understanding of the design principles that underlie such an organization may contribute to our understanding of the general problem of brain wiring by pinpointing rules that are sufficient to robustly establish a precise wiring pattern and thereby specify large numbers of synaptic contacts.
Like most products of evolution, the assembly pathway and final architecture are unlikely to resemble what an engineer would have designed. If the apposition eye can be assumed to be ancestral to the evolution of neural superposition (Land & Nilsson, 2002; Nilsson, 1989), then it may place constraints on the development of neural superposition. In the following two sections, we will therefore first review current knowledge about the evolutionary origin and existing intermediates between apposition and superposition eyes. In a second part, we will review current knowledge about visual map development for the specific case of neural superposition in Drosophila. Finally, we will discuss how the evolutionary constraints may impinge on the mechanisms underlying the developmental program.
THE EVOLUTION OF NEURAL SUPERPOSITION
From Apposition to Neural Superposition
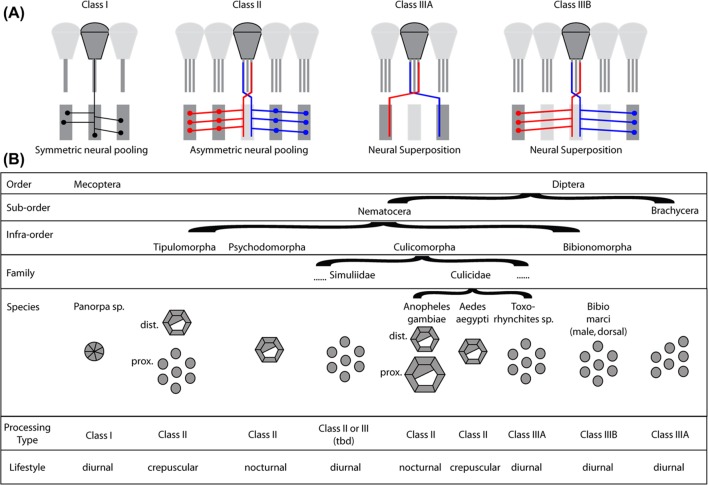
As described above, the neural superposition arrangement found in advanced flies (suborder Brachycera) requires a fundamental rewiring of the neural connections of the compound eye photoreceptors to the underlying visual neuropils when compared with the arrangement found in most other insects. This poses an intriguing evolutionary problem. For flying insects, a functioning optical system is a survival prerequisite for both the ancestral precursors and for the species with the new trait. A long succession of nonfunctional intermediates is highly unlikely. The trapezoidal rhabdom pattern and its corresponding wiring are shared by all advanced flies, making it likely that the transition to this specific type of neural superposition happened only once early in the evolution of advanced flies (Shaw, 1990) and constitutes an apomorphic trait. It certainly was a very successful adaptation, as the advanced flies subsequently radiated to become the most prominent suborder of Diptera with more than twice as many extant (currently living) families and species as the (paraphyletic) suborder Nematocera, which includes midges and mosquitoes (Wiegmann et al., 2011). There are strong indications that other variants of neural superposition have evolved independently within the Nematocera in some mosquitoes (Culicidae) (Land & Horwood, 2005) and march flies (Bibionidae) (Zeil, 1979, 1983). However, the neuronal morphology and synaptic connectivity has not yet been studied in detail in these cases. In this section, we provide an overview of the current model for the evolution of neural superposition from apposition eyes based on the analyses of extant species.
The fly’s visual system is most likely descended from a visual system of the apposition type with a fused rhabdom and retinula cell inputs from each ommatidium into its own individual lamina cartridge (Figures 1B and 3B) (Horridge & Meinertzhagen, 1970b; Nilsson, 1989). As outlined above, this eye type has limited sensitivity and is typically found in diurnal insects. An increase in sensitivity would offer a selective advantage for insects that explore dim light conditions and ultimately adapt to a nocturnal lifestyle. Optical superposition eyes are a widespread solution that mostly increases sensitivity at the expense of spatial resolution, for example, in nocturnal moths (Figure 1C) (Land & Nilsson, 2002). Alternative solutions to increase sensitivity at the expense of spatial resolution include an increase of rhabdom size and/or the pooling of inputs from several ommatidia at the level of the lamina (Figure 2). Remarkably, some grasshoppers can change the diameter of their fused rhabdom in a circadian rhythm more than three-fold (Horridge et al., 1981; Williams, 1982; neural pooling was postulated as beneficial for low light activity of insects with apposition-type eyes (Warrant, 1999) and has been used to explain the behavior of night-active bees (Greiner et al., 2004). Changes in rhabdom size or neural pooling in the lamina favor the co-evolutionary change of the other (Figure 3D, E). An increase in rhabdom size leads to overlapping fields of view; hence, a precise wiring to single cartridges provides little or no advantage (Figures 2 and 3D). Conversely, neural pooling of inputs from several ommatidia also leads to overlapping visual fields and removes the selective advantage of a small rhabdom (Figure 3E). An architecture suitable for symmetric neural pooling through retinula cell axons (Figure 3E) can be found in the scorpionfly Panorpa (Melzer, 1994; Melzer et al., 1997). Panorpa could represent the ancestral situation for the Diptera, since the Mecoptera are considered the sister group for Diptera (Kristensen, 1981). The compound eyes of Mecoptera have fused rhabdoms (Figure 5) (Chen et al., 2012).
Figure 5.
Types of neural pooling and rhabdom organization in scorpionflies and true flies. (A) Classes of neural pooling and neural superposition in extant (currently living) scorpionflies (Mecoptera), and true flies (Diptera). Class I: Symmetric neural pooling by collaterals of retinula cell axons with no preferential orientation. Class II: Asymmetric neural pooling by collaterals of retinula axons with preferential orientations to neighboring cartridges, as described in Nematocera for Tipulidae (Nilsson & Ro, 1994) and Chaoboridae (Melzer et al., 1997). Every retinula axon innervates more than one target cartridge. Class IIIA: Neural superposition as found in advanced flies (Brachycera), including Drosophila. Retinula cell axons directly connect to one target cartridge without entering the cartridge of origin. Class IIIB: Neural superposition as found in the dorsal eyes of Bibio marci males (Zeil, 1979, 1983). Retinula cell axons innervate their specific target cartridge by collaterals while remaining associated with the cartridge of origin. (B) Overview of the rhabdom architectures and their known or predicted neural processing and general lifestyle in Mecoptera and Diptera. Transitions to partially and fully open rhabdom architectures have developed in several branches of the Nematocera and the full range of optical and neural solutions can be found even within a single family (Culicidae). Dipteran rhabdom drawings for Nematocera are based on (Land et al., 1999; Osorio, 2007; Seifert & Smola, 1990) and for Mecoptera on (Chen et al., 2012). The information for the known or predicted neural processing type is taken from (Melzer, 1994) (Mecoptera), (Melzer et al., 1997) (Tipulidae, Culicomorpha), (Land & Horwood, 2005) (Anopheles gambiae and Toxorhynchites brevipalpis) and (Zeil, 1979, 1983) (Bibio marci).
The theory of the evolution of neural superposition through neural pooling for night vision was initiated by Shaw and Meinertzhagen (Meinertzhagen, 1991; Shaw, 1989), has been elaborated in detail by Nilsson and Ro (1994), and corroborated, among others, by Land and Horwood (2005) and is summarized by Osorio (2007). In low light situations it is advantageous to sacrifice spatial resolution for sensitivity. For the many species of Nematocera that are nocturnal or active during twilight (crepuscular) this clearly constitutes a favorable adaptation. Optical indications for eyes that would allow neural pooling can be found in many nematoceran groups (Land et al., 1999; Nilsson & Ro, 1994). In many groups, neuronal structures suitable for neural pooling have been found in the form of photoreceptor axon collaterals that extend into neighboring cartridges (Figure 5A, Class II) (Land & Horwood, 2005; Melzer et al., 1997; Zeil, 1983; Melzer & Paulus, 1993). In the phantom midge Chaoborus crystallinus, the synaptic connections in the lamina neuropil analyzed from EM serial sections reveal that each of the retinula cells R1–R6 provides inputs to four neighboring cartridges in a clearly defined pattern through collateral fibers (Melzer et al., 1997). The capacity for neural pooling may constitute the first step in the transition from an apposition eye to the neural superposition eye.
Neural pooling in the lamina is linked to the evolutionary separation of rhabdomeres. All neural superposition eyes have an open rhabdom. Open rhabdoms have evolved at least five times independently (Osorio, 2007). The gene eyes shut/spacemaker (eys) encodes an extracellular protein that plays a key role in the formation of the open rhabdom. eys mutants exhibit a fused rhabdom, providing a genetic basis and potential evolutionary handle for the transformation of fused to open rhabdoms (Zelhof et al., 2006). Among the mosquitoes, Anopheles gambiae lacks eys expression, has a fused rhabdom, and is nocturnal; in contrast, the large diurnal Toxorhynchites brevipalpis expresses eys, and has both an open rhabdom and neural superposition wiring (Land & Horwood, 2005). However, without neuronal rewiring in the lamina, the actions of eys would degrade the high spatial resolution of an apposition eye, as the separation of visual inputs of the single retinula cells results in a bigger visual field per ommatidium, and all this information would be fed into just one input channel (the corresponding lamina cartridge). It is therefore likely that neural pooling preceded the opening of the rhabdom in all cases of its convergent evolution.
Numerous intermediates of partially to fully separated rhabdomeres can be found in beetles, heteropteran bugs, earwigs, and of course flies; interestingly, they all have in common a separation of a central pair of retinula cells from six peripheral ones. This arrangement can be used to combine vision with high spatial resolution through the central rhabdomeres and vision with increased sensitivity by adding the peripheral photoreceptors through the use of an adjustable aperture (Nilsson & Ro, 1994). An adjustable pigment aperture is found in all four insects studied by Nilsson and Ro (1994). It is typically regulated in a circadian rhythm that opens the aperture at night, thereby exposing all rhabdomeres to increase sensitivity at the expense of resolution, and closes the aperture during the day, thereby increasing spatial resolution at the expense of sensitivity. Typically, the rhabdom has the ability to move down when the aperture is narrowed, consistent with a reduced field of view for increased spatial resolution (Figure 2). These mechanisms make sense of the commonly found separation of central from peripheral rhabdomeres in non-neural superposition eyes with open rhabdoms. Further consistent with this mechanism, the tenebrionid beetle and the earwig exhibit a circle of fused outer rhabdomeres, while the central rhabdomeres are separated from the outer ones (Nilsson & Ro, 1994).
The idea of separate input channels for daylight vision (photopic) and night vision (scotopic) was originally suggested by Hanström (1927), subsequently described for the aquatic bug (Ioannides & Horridge, 1975) and related to the “long visual fibers” (R7/8) and “short visual fibers” (R1–R6) (Meinertzhagen, 1976; Strausfeld, 1976). The idea implies that the central rhabdomeres are responsible for high-resolution daytime vision, whereas the peripheral R1–R6 are recruited as additional detectors for low-resolution night vision. This functional separation may be different from advanced flies like Drosophila, in which R7/8 have a key role in color vision while R1–R6 are prominently implicated in motion detection. If the photopic/scotopic detection configuration represents an ancestral stage to neural superposition in advanced flies, then the new functional roles or R7/8 in advanced flies may be a secondary development (Shaw & Meinertzhagen, 1986).
The transition from fully fused rhabdoms to the open rhabdoms with two central retinula cells frequently found in Nematocera also marks a change from a cylindrical rhabdom shape to a filled cone shape that can collect light over a significantly wider angle (Land et al., 1997, 1999). The open rhabdom of Brachycera is again cylindrical, but with the rhabdomeres of the retinula cells fully separated over their entire length by the extracellular space created by the actions of eys (Zelhof et al., 2006). This extracellular space may have first arisen in the proximal (lower) parts of conical rhabdoms as found in many nematoceran groups (Seifert & Smola, 1990) to generate a wider lower cone diameter before fully separating the rhabdomeres throughout the whole rhabdom.
The open rhabdom architecture profits from asymmetric projections of retinula cell axons to the lamina (Figure 1D and 3G). If symmetric pooling of retinula cell axons, as seems to be the case in the scorpionfly Panorpa (Figures 3E and 5A, B), is ancestral, then the emergence of open rhabdoms may coincide with asymmetric pooling (Figure 3F, G and 5A, B). Indeed, aquatic Hemiptera have open rhabdoms and branched retinula cell axons that project to cartridges on one side of the cartridge directly underneath the ommatidium from which the axon derived (Meinertzhagen, 1976; Strausfeld, 1976; Wolburg-Buchholz, 1979). The symmetric pooling found in Panorpa and the asymmetric pooling in Nematocera indicates a transition from an initial symmetrical neural pooling situation (Figure 5A, Class I) to asymmetrical neural pooling (Figure 5A, Class II). Such a transition may have initially come about to facilitate axon targeting in larger pooling fields, in keeping with the longer collaterals found in Nematocera.
The separation of rhabdomeres and asymmetric pooling in the lamina to match the spatially restricted input hence appear to be co-evolutionary traits (Figure 3F, G). However, it is not clear whether the separation of the rhabdomeres or the asymmetric pooling occurred first. Separation of central from outer rhabdomeres together in the presence of symmetric pooling can provide a selection advantage when combined with the adjustable pigment aperture (Nilsson & Ro, 1994). On the other hand, asymmetric pooling even without separation of rhabdomeres changes the weights of input channels, such that cartridges receive more input from the associated ommatidium and its direct neighbors and less from further removed neighbors than in the case of symmetric pooling (PRH, unpublished) (Figure 3F). An example of this arrangement may be the nocturnal bee Megalopta genalis, which exhibits increased rhabdom size and, in at least one documented case, asymmetric pooling of a retinula cell axon (Greiner et al., 2004, 2005). Within the Nematocera many species exhibit fused, partially or fully open rhabdoms that are correlated with their nocturnal or diurnal lifestyle (Figure 5) (Land et al., 1999; Seifert & Smola, 1990). Whereas an open rhabdom would be disadvantageous in a conventionally wired eye of the apposition type, it is advantageous in an asymmetric neural pooling visual system. Here, spatial resolution can be increased by the presence of partially or fully open rhabdoms, because the visual fields become more defined if the pooled retinula cells share similar optical orientations (Osorio, 2007). A subsequent further reduction of retinula cell inputs to only those with identical optical axes leads to neural superposition (Figures 3G and 5). Such a design is advantageous for the diurnal lifestyle that is found in most brachyceran species. In addition to the neural superposition eyes of brachycerans, similar transitions have taken place at least twice more in different nematoceran groups, the Bibionidae (Zeil, 1979) and the Culicidae (Land & Horwood, 2005) in combination with a diurnal lifestyle (Figure 5). The asymmetric neural pooling systems of ommatidia with partially open rhabdoms found in many extant Nematocera can therefore be assumed to reflect the ancestral conditions for the development of neural superposition in the Brachycera.
The main lamina neuron cell types found in the visual systems of Nematocera and of brachyceran flies are very similar (Fischbach & Dittrich, 1989; Melzer & Paulus, 1993; Zeil, 1983). Changes in function have arisen at the level of synaptic connectivity, not through the creation of new cell types (Shaw & Meinertzhagen, 1986; Shaw & Moore, 1989). This conservation of cell types and of the overall architecture of the ommatidia as well as of the lamina cartridges made it possible that the ancestral and the derived states (i.e., the apposition eye and the neural superposition eye) look anatomically very similar even though they are fundamentally different. This apparent similarity masks the extent of changes that were necessary in between, but these evolutionary intermediates can be appreciated in the Nematocera. It is interesting to note that in the evolution of all known neural superposition solutions found in the Diptera the intermediate between two visual systems of high spatial resolution (apposition and neural superposition) is very likely an evolutionarily favored system of low spatial resolution.
In summary, the apposition eye likely evolved into the radically different wiring of the neural superposition eye through three distinct steps: (1) increase of sensitivity through neural pooling (advantageous in dim light and for nocturnal life style; Figure 3D–E), (2) the emergence of asymmetric pooling and the opening of the rhabdom (Figure 3F–G), and (3) the restriction of neural pooling to increase spatial resolution for those insects that returned to a diurnal life style (Figure 3G, C). Neural superposition resulted from the co-evolution of the separation of rhabdomeres and the restriction of neural pooling in nocturnal insects that came back to the light.
The Trapezoid Question
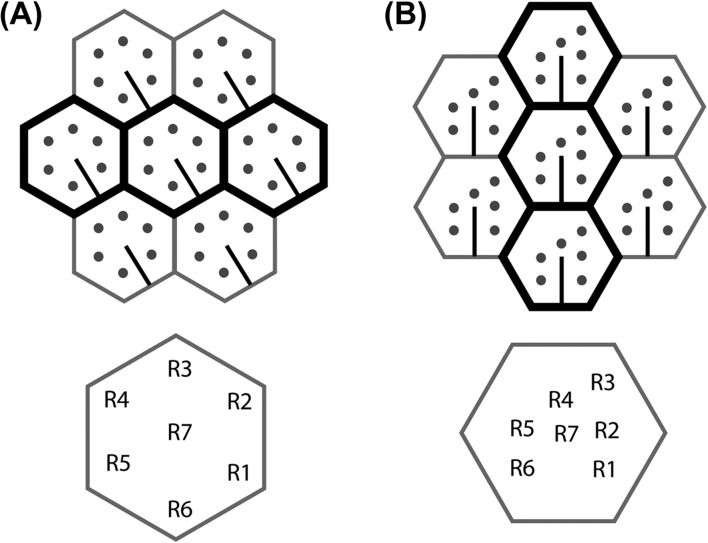
The rhabdomeres of all brachyceran flies, but no other extant Diptera, are arranged in a distinctive asymmetric trapezoidal pattern (Figure 4). The trapezoidal pattern is matched by the retinula axon redistribution to the lamina cartridges (Meinertzhagen & O’Neil, 1991; Shaw & Meinertzhagen, 1986; Shaw & Moore, 1989). The pattern is not only shared by all extant members of the Brachycera, but can already be found in a 45-million-years old sample (Tanaka et al., 2009). This observation supports existing ideas about the conservation of the cellular organization of the fly eye and the underlying neural superposition from early on in the evolution of the Brachycera (Shaw, 1990). This distinctive rhabdomere distribution pattern has become a popular model for planar cell polarity (Eaton, 1997; Schweisguth, 2005; Wolff & Ready, 1993) as it can be easily recognized by the R3/R4 cell asymmetry in the ommatidia (Figures 4 and 6B).
Figure 6.
Comparison of the orientations of ommatidia and rhabdoms in Brachycera and the phantom midge Chaoborus crystallinus (Culicomorpha). (A) Chaoborus crystallinus (Melzer, R.R and Zimmerman, T. unpublished result) (B) Brachycera. Positions of peripheral retinula cells R1–R6 are shown as round dots and the orientation of the cell body of R7 relative to its contribution to the central rhabdomere is shown as a line. The orientation of R7 shows that the ommatidia of Brachycera and Chaoborus differ in their orientations by 30°. Hence, the arrangement in (B) can be obtained by rotating (A) 30° and rearranging the rhabdomeres in a trapezoidal position. Note that adjacent ommatidia align horizontally in Chaoborus and many other Nematocera (A), but vertically in all Brachycera (B). These horizontal and vertical ommatidial alignments are highlighted by bold black hexagons. Retinula cell identities are shown below.
An asymmetric pattern does not constitute a prerequisite for neural superposition, as evidenced by the existing symmetrical rhabdomere distributions of Bibionidae and Toxorhynchites (Figure 5). The distinctive brachyceran rhabdomere pattern could be linked to the following finding: In the brachyceran compound eye the ommatidia are generally positioned along different axes than in Nematocera. This is due to the fact that, relative to the orientation in many insect orders and also still in their nematoceran sister group, the facet eyes in brachyceran flies are arranged with a 30° difference of the axes (Figure 6). Ommatidial rows in Nematocera are arranged horizontally (as is also the case in many other insect groups like bees and locusts) and in Brachycera vertically (Horridge, 2005; Land, 1997), at least in small eyes like those of Drosophila and in the anterior regions of bigger eyes like Musca (Braitenberg, 1970; Stavenga, 1975). This 30°-axis difference is not a repositioning of the whole eye relative to the head, but it happens inside the organ at the level of the single ommatidia. Taking the eye equator (the line of mirror symmetry between the dorsal and ventral halves of the eye) as reference for the ommatidial orientation, this is evident in the positions of the single retinula cells which are different at least between the nematoceran Chaoborus (Melzer, R.R and Zimmerman, T., unpublished result) and Brachycera. A partial rotation of the whole ommatidial unit relative to its immediate surroundings and neighboring ommatidia could therefore have been the initial cause for the distortion of the rhabdomere positions into the distinctive trapezoidal pattern of brachyceran eyes (Figure 6).
THE DEVELOPMENT OF NEURAL SUPERPOSITION
The fly eye has served for decades as a powerful model for the study of cell specification, organ development, and pattern formation, which are extensively discussed elsewhere (Baker, 2007; Carthew, 2007; Chan et al., 2011; Roignant & Treisman, 2009; Tsachaki & Sprecher, 2012; Wolff & Ready, 1991, 1993). Here, we will focus on the basic principles that set the stage for neural superposition wiring as it is found in Brachycera and best studied in the genetic model organism Drosophila melanogaster.
Axon Pathfinding from the Eye to the Lamina
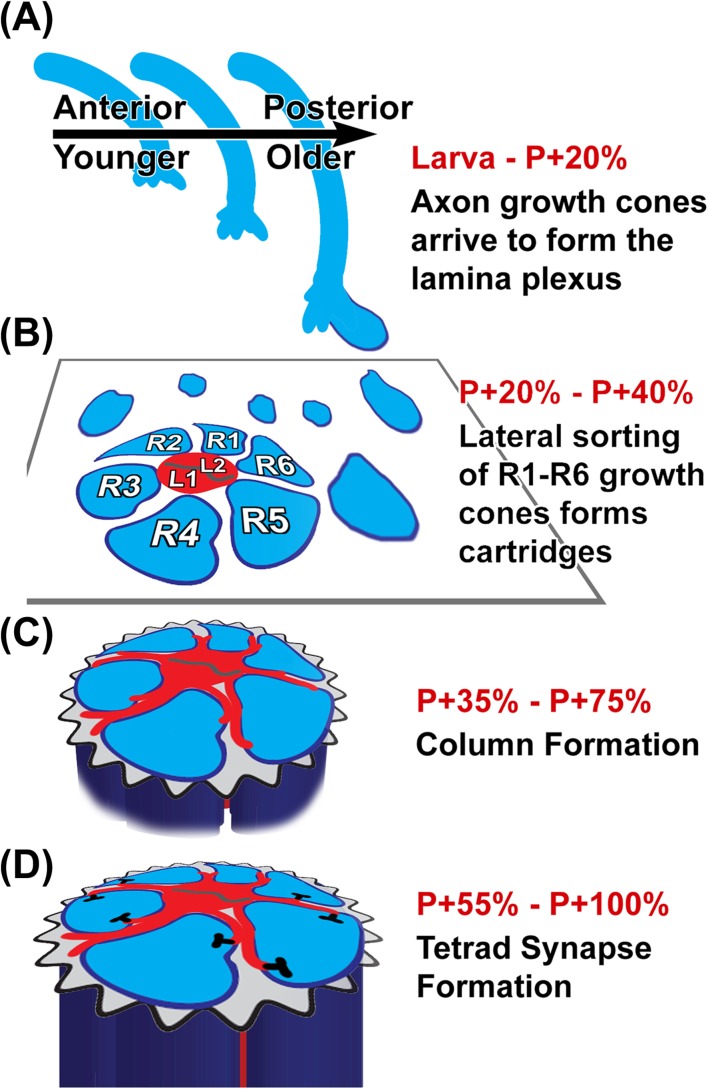
The morphogenesis of the developing eye disc orchestrates the initial timing of axonal connections between the eye and lamina. Retinula cells differentiate and pattern the fly eye in a temporal wave. A morphogenetic furrow sweeps across the developing eye disc during larval stages, patterning the hexagonal array of the compound eye in its wake. The photoreceptor neuron R8 differentiates first, followed sequentially by the pairs R2/R5, R3/R4, R1/R6, and eventually R7. The differentiation process of all cells from the posterior to the anterior margin takes around 2 days (Roignant & Treisman, 2009; Tomlinson & Ready, 1987; Wolff & Ready, 1991).
Retinula cells send out axonal processes shortly after differentiation beginning with R8 and followed by all other subtypes (Tomlinson & Ready, 1987). The axons are subsequently ensheathed by glia cells that divide the axons in ommatidial bundles (Meinertzhagen & Hanson, 1993). Each retinula cell axon bundle twists 180° between the retina and lamina plexus such that the axon terminals are 180° degrees rotated relative to their arrangement when leaving the ommatidium. This bundle rotation is very precise and clockwise for the right eye from the dorsal retina, counterclockwise from the ventral retina, and vice versa for the left eye (Braitenberg, 1967; Meinertzhagen & Hanson, 1993; Trujillo-Cenoz & Melamed, 1966). The axons terminate between two layers of glia cells where their growth cones form the lamina plexus (Clandinin & Zipursky, 2002; Fischbach & Hiesinger, 2008; Poeck et al., 2001). The lamina plexus will give rise to the lamina neuropil, where R1–R6 establish the visual map through synaptic connections with lamina neurons (Cajal, 1909; Vigier, 1908). R7 and R8 axons project through the lamina into the deeper medulla neuropil, where they terminate in the layers that in the adult will become M6 and M3, respectively (Fischbach & Dittrich, 1989).
Several molecular cues have been identified that are required for the correct targeting of R1–R6 in the lamina and R7/R8 in the medulla. For example, the ubiquitin-specific protease Nonstop is required for the development of the glial cells that provide the initial R1–R6 target (Martin et al., 1995; Poeck et al., 2001). On R1–R6 growth cones, the receptor tyrosine phosphatase PTP69D is required for correct targeting (Garrity et al., 1999). The nuclear protein Brakeless functions in all retinula cells and represses the function of the transcription factor Runt in R2 and R5. Interestingly, loss of this repression in only these two retinula cells is sufficient to mistarget all R1–R6 to the medulla (Edwards & Meinertzhagen, 2009; Kaminker et al., 2002; Rao et al., 2000; Senti et al., 2000; Tayler & Garrity, 2003). Together, these studies reveal a hierarchical dependence of retinula cell–glia and retinula cell–retinula cell interactions that determine the initial axon targeting to the lamina.
Rows of retinula cell axons arrive sequentially in the optic lobe, in the wake of the developmental wave of retinula cell differentiation in the eye disc. The axon bundles thereby establish a grid that represents a prerequisite for subsequent visual map formation. It is not entirely clear how each row of retinula cell bundles defasciculates from the optic stalk in an evenly spaced manner to form a precisely patterned rhomboidal grid (Figure 4). This grid is established by the photoreceptors themselves and not the lamina neurons, since the arriving bundles induce the initiation of differentiation of lamina neurons through the secretion of Hedgehog (Huang & Kunes, 1996). However, glia play a critical role in the targeting of the bundles and probably in the establishment of the grid (Hadjieconomou et al., 2011). In the lamina plexus, R1–R6 axons defasciculate and extend into lamina cartridges where they find their synaptic partners, the lamina neurons (Figure 7). The rhomboidal grid is a prerequisite for this sorting process of R1–R6 growth cones to establish neural superposition.
Figure 7.
Development of neural superposition axon projection pattern as observed in Drosophila. (A) Retinula cell axons arrive in the optic lobe during a temporal wave in the wake of photoreceptor differentiation in the developing eye disc (Tomlinson & Ready, 1987; Wolff & Ready, 1991, 1993). The intermediate target for retinula axons are two layers of glial cells in the optic lobe. (B) The arrival of retinula axon bundles is followed by a lateral growth cone sorting process. The growth cones form a new layer perpendicular to the axons, between the layers of glia cells, called the lamina plexus. Sorting the correct R1–R6 growth cones with the same field of view from six different ommatidia predetermines synaptic partners (Clandinin & Zipursky, 2002; Hiesinger et al., 2006). (C) After growth cone sorting into cartridges that receive input from the same field of view, each retinula terminal elongates proximally for up to 30 μm (Meinertzhagen & Hanson, 1993). (D) Lastly, synapses form between the postsynaptic lamina monopolar cells (red) and the presynaptic retinula cell columns (blue), obeying a minimal spacing rule (Meinertzhagen & Hu, 1996).
R1–R6 Growth Cone Sorting in the Lamina Plexus
The arrival of ommatidial axon bundles in the lamina plexus marks the end of photoreceptor target finding in the case of the apposition eye. In contrast, in the neural superposition eye, the most important and least understood developmental process is about to begin: How do thousands of R1–R6 growth cones leave their origination bundle to identify new targets in other cartridges, thereby exchanging growth cones with neighboring cartridges? This enigmatic shuffling process has fascinated scientists for decades (Clandinin & Zipursky, 2000; Fischbach & Hiesinger, 2008; Hiesinger et al., 2006; Horridge & Meinertzhagen, 1970a; Meinertzhagen & Hanson, 1993; Trujillo-Cenoz & Melamed, 1973).
As described above, rows of axons arrive in a temporal wave in the lamina plexus throughout larval development and into the first day of pupal development. However, it is unclear how long the developmental wave persists in the lamina plexus. Cross-sectional images of growth cones in the lamina plexus after 20% of pupal development (P + 20%) reveal no morphological differences between growth cones from axons that arrived earlier or later during development (Hiesinger et al., 2006; Schwabe et al., 2013). Based on this observation, it has been argued that the growth cone sorting process that establishes neural superposition in the lamina plexus occurs synchronously for all retinula cells (Schwabe et al., 2013). This important notion implies a break of the asymmetry of the temporal wave after axon arrival and a transition to synchronous morphogenesis of the growth cone sorting process. The precise time point or mechanism of this asymmetry break has not been determined and it is unclear whether it applies equally to all photoreceptors and lamina neurons involved in the establishment of neural superposition.
R1–R6 growth cone shapes have first been described for different developmental stages using transmission electron microscopy and reviewed extensively by Meinertzhagen and Hanson (1993). According to ultrastructural analyses, only few morphological changes are apparent between P + 12.5% and P + 24.5%. Between P + 25% and P + 32% filopodial extensions become more pronounced and especially R3 elongates rather suddenly in the direction of its distinct trajectory. At P + 50% distinct cartridges are apparent in the ultrastructure (Meinertzhagen & Hanson, 1993). A more recent analysis of R1–R6 growth cone shapes at distinct time points in fixed preparations by Schwabe et al. (2013) revealed a clear, subtype-specific polarization of the growth cones starting at P + 20%. According to this study, growth cones first polarize and subsequently begin to extend to their targets around P + 32%.
Mutant analyses in Drosophila have provided key insights into the establishment of neural superposition. Clandinin and Zipursky (2000) showed that the bundle rotation predetermines the growth cone trajectories, as evidenced by the following analyses of mutant phenotypes. In the frizzled mutant, individual ommatidia are rotated by 180°. Remarkably, this ommatidial rotation results in a perfect 180° rotation of the trapezoidal projection trajectories of R1–R6. This important experiment revealed that the projection direction is autonomously encoded by the orientation of the bundle, i.e., not determined by other growth cones in the lamina plexus. In contrast, in nemo mutants, in which ommatidia are rotated up to 45°, no corresponding angular change of R1–R6 growth cone trajectories was observed (Clandinin and Zipursky, 2000). This experiment suggests that the target grid in the lamina plexus places constraints on the orientation of the trapezoidal R1–R6 growth cone trajectories; it may allow a full 180° flip, but no partial rotations (compare Figure 4). Further mutational analyses in the same study revealed a dependency of R1, R2, R5, and R6 targeting on R3 and R4, whereas R3 and R4 themselves target independently of R1 and R6. In contrast, R2 and R5 targeting seemed to be affected by loss of R1 and R6 (Clandinin & Zipursky, 2000). These findings suggest a hierarchical pattern formation process, the rules of which remain unresolved.
Since these seminal studies, numerous mutants, predominantly affecting cell adhesion molecules, have been identified that are required for R1–R6 sorting in the lamina plexus, including N-Cadherin (Lee et al., 2001), D-Lar (Clandinin et al., 2001; Maurel-Zaffran et al., 2001), the protocadherin Flamingo (Lee et al., 2003; Senti et al., 2003), among numerous others (reviewed in Hadjieconomou et al., 2011; Mast et al., 2006; Ting & Lee, 2007). Of these, arguably the most informative studies for our understanding of the development of neural superposition come from studies by Clandinin and colleagues on the protocadherin Flamingo. Individual R1–R6 cells that lack Flamingo exhibit surprisingly normal targeting behavior if they are surrounded by wild type cells. In contrast, wild type growth cones neighboring the flamingo mutant growth cones exhibit specific mistargeting defects (Chen & Clandinin, 2008). These findings indicate that the precise trajectory of growth cone polarization and targeting is nonautonomously determined by the neighboring growth cones in the origination bundle. This idea was recently developed further to include the concept of a network of differential adhesion through differential levels of Flamingo both within the same growth cone as well as between ommatidial bundles (Schwabe et al., 2013).
Mutant analyses of several other cell adhesion molecules reveal partially penetrant phenotypes for several steps of the developmental program (Figure 7). These include a cell-autonomous role for Dlar, Liprin-alpha, and N-Cadherin in R1–R6 targeting (Clandinin et al., 2001; Maurel-Zaffran et al., 2001; Prakash et al., 2005; Prakash et al., 2009). None of these studies suggested a molecular code for target matching, while they are consistent with an iterative pattern formation process (Chan et al., 2011). The precise role of interactions between R1–R6 growth cones and postsynaptic lamina neurons is unknown. However, at least one secreted protein, the anaplastic lymphome kinase (alk) ligand Jelly Belly (Jeb), has been characterized that anterogradely signals to lamina neurons and is required for R1–R6 targeting (Bazigou et al., 2007). How these different molecular signaling events interplay to set up the dynamic interplay of R1–R6 growth cone sorting remains unclear.
R1–R6 Growth Cone Sorting Predetermines Synaptic Columns and Partners
At P + 40% R1–R6 growth cones have established contact with their target cartridges (Meinertzhagen & Hanson, 1993; Schwabe et al., 2013). While the entire preceding sorting process occurred in a two-dimensional array, R1–R6 growth cones now commence a “dive in” process that creates tubular columns perpendicular to the lamina plexus. During this column formation process, each R1–R6 growth cone in the correct target cartridge elongates through the proximal glia cell layer toward the center of the brain. The final R1–R6 terminals exhibit a column length of ∼25 μm. The fate of the original lamina plexus, which remains distal to the expanding columns in the lamina, has received comparably little attention and its adult function, if any, is unknown.
In the final arrangement, R1–R6 form a more (e.g., Calliphora) or less (e.g., Drosophila) circular arrangement of R1–R6 in the periphery of the cartridge while the main postsynaptic lamina neurons L1 and L2 reside in the cartridge center (Figure 7). R1–R6 are organized in stereotypic rotational sequence R1-2-3-4-5-6 as determined in 1970 through the precise reconstruction of hundreds of retinula cell axons in Calliphora (Horridge & Meinertzhagen, 1970a). Similarly, perfect stereotypy was revealed through dye labeling of all retinula cells in an ommatidium of the house fly Musca domestica (Picaud et al., 1990). In addition, the apparent stereotypic arrangement of L3 between R5 and R6 in the lamina column further supports a stereotypic arrangement of all cartridge elements (Meinertzhagen & O’Neil, 1991; Rivera-Alba et al., 2011). Since the R1–R6 in a cartridge represent input from the same field of view, it is not entirely clear whether such stereotypy has a functional significance.
The stereotypic arrangement of six terminals per cartridge is altered at the equator that separates the axonal projections from the dorsal and ventral half of the eye. On each side of the equator reside two rows of cartridges with eight terminals and one with seven terminals per cartridge (Braitenberg, 1967; Horridge & Meinertzhagen, 1970a). It has therefore been argued that the equator is a region of increased sensitivity (Hardie, 1985). R1–R6 terminals in equatorial cartridges with 7 or 8 terminals per cartridge are also arranged in a stereotypic rotational organization with a characteristic complement of terminals (Horridge & Meinertzhagen, 1970a). Interestingly, Horridge and Meinertzhagen (1970a) also found that within a population of 650 R1–R6 axon terminals, not a single one projected to the wrong cartridge. In contrast, 10 of these 650 exhibited misplacements in the rotational organization within the correct cartridge, most of which occurred around the equator. Further analyses of more than 500 R1–R6 axon terminals around the equator in another single Calliphora specimen revealed an increased error rate specifically at places where the equator is not a straight line (Meinertzhagen, 1972). Based on these seminal studies, Meinertzhagen (1972) concluded that these errors reveal simple rules for the sorting of R1–R6 in cartridges that are not consistent with a rigid specification of terminal location.
The columnar cartridge organization has recently been described in terms of a quantitative model for “wiring economy” (Rivera-Alba et al., 2011). The placement of neurons in a lamina cartridge can be explained by minimizing lengths of connections between neurons and preventing them from taking the same physical space (volume exclusion) through optimization of a cost function that takes into consideration wiring economy and volume exclusion. Rivera-Alba et al. (2011) argue that R1–R6 should occupy the periphery of the cartridge to minimize obstruction of other neuronal connections due to their larger diameter. Since R1–R6 terminals form more synapses with L1 and L2 than any other cells, the wiring economy model offers a plausible explanation why L1 and L2 are in the center of the cartridge.
L1 and L2 extend numerous filopodia that intercalate between the R1–R6 columns and initiate synapse formation (Meinertzhagen & Hanson, 1993; Meinertzhagen et al., 2000) (Figure 7). Synapse formation is characterized by the appearance of presynaptic densities, so-called “T-bars,” which become apparent in the ultrastructure only after P + 50% (Meinertzhagen & Hanson, 1993). In the adult visual map, each R1–R6 columnar terminal forms approximately 50 synapses (Meinertzhagen & Hu, 1996; Meinertzhagen & Sorra, 2001). R1–R6 form so-called tetrad synapses in which each presynaptic site is opposed by four postsynaptic spines. The assembly sequence of these tetrad synapses follows a distinct sequence and always includes at least one L1 and one L2 (Meinertzhagen & Hu, 1996; Meinertzhagen & O′Neil, 1991; Meinertzhagen et al., 2000). The synapses obey a spacing rule that ensures that in wild type each synaptic contact site is on average at least 1 μm apart from any other synapse (Meinertzhagen & Hu, 1996). Other postsynaptic cell types include amacrine cells and lamina widefield neurons, but their role in the development of neural superposition is unclear.
The assembly sequence of R1–R6 tetrad synapses is invariable and precise with respect to the cell types involved (Meinertzhagen et al., 2000). However, mutant analyses have revealed that the synapse formation program is blind with respect to whether the preceding growth cone sorting is correct or faulty (Hiesinger et al., 2006). Specifically, individual R1–R6 terminals in adult cartridges that formed after incorrect growth cone sorting still form on average the correct number of synapses. Since L1 and L2 initiate synapse formation through the extension of filopodial contact with R1–R6 it is conceivable that either the lamina neurons or the photoreceptors determine this precise number of synapses. This question has been addressed through the analysis of mis-wired cartridges. Such cartridges in different mutants may contain as few as one or more than ten retinula cell terminals. The precise number of synapses per retinula cell terminal is independent of such flawed cartridge compositions (Hiesinger et al., 2006). Synapse number per R1–R6 terminal has also been analyzed in cartridges of systematically different composition in the wild type (Frohlich & Meinertzhagen, 1987). Together, these findings support the idea that synapse formation is a genetically separable developmental program that is based on cell types present in cartridges, but blind toward the earlier growth cone sorting process and with respect to the retinula cell number or subtypes in a cartridge. The same study also showed that this synapse formation program is unaffected by the loss of electrical or synaptic activity. Hence, the primary Drosophila visual map is an example for a genetically “hard-wired” brain region and neural circuit (Hiesinger et al., 2006), even though individual synaptic structures undergo plastic changes, e.g., in response to light (Rybak & Meinertzhagen, 1997).
Evolutionary lessons for the development of neural superposition
Extensive anatomical and genetic studies on the development of neural superposition have revealed a series of genetically separable steps (Figure 7):
axon pathfinding from the eye to the lamina occurs in a temporal wave until P + 20%,
growth cone sorting occurs laterally, perpendicular to the original axon bundles, in the lamina plexus layer between P + 20% and P + 40%,
retinula cell growth cones elongate proximally to form columns, and lamina neurons initiate synaptic partner selection after P + 35%,
synapses form between P + 55% and eclosion.
Of these four steps, numbers (1) and (2) are genetically separated in mutants of guidance receptors/cell adhesion molecules (e.g., flamingo). Similarly, steps (2) and (4) are genetically separated by numerous mutants; to our knowledge, all neural superposition mutants analyzed to date ultrastructurally still have tetrad synapses. These findings give rise to the idea that the seemingly complicated developmental program underlying the synapse-specific wiring of neural superposition is encoded by the concatenation of much simpler genetically encoded developmental subprograms (Chan et al., 2011; Hiesinger et al., 2006). Of these subprograms, step (2), the lateral sorting of growth cones in the lamina, predetermines synaptic partners. This makes sense in light of the evolutionary origin: growth cone sorting is a process that has been intercalated in the series of developmental steps in the neural superposition eye during its evolution from the apposition eye, which develops through steps (1), (3), and (4) only.
As described above, neural superposition most likely evolved through transition from apposition eyes through neural pooling in the lamina. In Diptera, neural pooling manifests itself as lateral filopodial connections between R1–R6 terminals. Neural pooling variants that may resemble ancestral retinula cell wiring can be found in Nematocera (Land & Horwood, 2005; Melzer et al., 1997) (Figure 5). Is neural pooling the evolutionary ancestor of lateral growth cone sorting in the lamina plexus? The development of the lateral filopodial connections in non-fly species has, to our knowledge, not been studied. However, individual long filopodia of R1–R6 growth cones between P + 20% and P + 35% that span up to two cartridge diameters have been described based on ultrastructural analyses in Drosophila (Meinertzhagen & Hanson, 1993). Land & Horwood (2005) point out the difference between collateral extension between retinula cell terminals in Nematocera as opposed to the redistribution of axons out of the bundle into neighboring cartridges seen in Drosophila. However, it remains tempting to speculate that developmental filopodial dynamics seen in Drosophila resemble the existing neural pooling structures in Nematocera.
A second important evolutionary lesson regards the trapezoidal arrangement of the R1–R6 growth cones. A hexagonal arrangement of neural superposition wiring in the lamina may be ancestral to the trapezoidal projections found in Brachycera. The trapezoidal arrangement may be a secondary consequence of a 30° difference in ommatidial alignment that exists between Nematocera (which have the original orientation) and advanced flies (which have a changed orientation). In this hypothetical model, neural superposition in advanced flies evolved first through neural pooling in the lamina, second through opening of the rhabdom and asymmetric restriction of filopodial connections in the lamina, and third through ommatidial rotation, which would require a trapezoidal correction of filopodial contacts in the lamina. We note that this model is currently only a speculation as the correct order of steps around the ommatidial rotation remains unresolved and the trapezoidal pattern may also have arisen prior to the restriction of filopodial connections. However, the proposed series of evolutionary steps makes testable predictions for developmental constraints. For example, based on this model, we propose that any set of rules that governs the R1–R6 growth cone sorting process in neural superposition should be consistent with and adaptable to a sorting process in either hexagonal or trapezoidal arrangements. An understanding of the development of neural superposition in light of its evolution thereby may offer a unique opportunity to understand the rules that determine synapse- specific connectivity in this system.
Lastly, a more general lesson concerns the rules underlying brain wiring across species. The evolutionary and developmental mechanisms described in this review provide examples for how seemingly complex architectures can arise through simple, genetically encoded rules. Such rules must be consistent in both the evolutionary and developmental context. For example, a transition from symmetric to asymmetric pooling in the visual map must be evolutionarily selectable and realizable in the framework of the developmental process that established neural pooling in the first place. Maybe not surprisingly, neural pruning is a major pattern formation process found in the refinement of sensory maps and other neural circuitry throughout the animal kingdom. If the beautiful pattern formation processes in the visual systems of insects are any indication, then sculpting of neural circuitry through hierarchical, interdependent, and iterative pattern formation processes may not be an exception, but a basic principle underlying brain development.
ACKNOWLEDGEMENTS
We would like to thank all members of the Hiesinger lab, Tom Clandinin, Ian Meinertzhagen, Claude Desplan, and Roland Melzer for discussion.
Footnotes
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The work was supported by grants from the National Institute of Health to PRH (RO1EY018884, RO1EY02 3333) and the Welch Foundation to PRH (I-1657). This work was further supported by grant from the National Institute of Health to SJA (R01CA133253) and LFW (R01GM071794) and the Welch Foundation to SJA (I-1619) and LFW (I-1644). ML was supported by a Green Center for Systems Biology Postdoctoral Fellowship.
References
- 1.Baker N. E. Patterning signals and proliferation in Drosophila imaginal discs. Curr Opin Genet Dev. (2007);17:287–293. doi: 10.1016/j.gde.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Bazigou E., Apitz H., Johansson J., Loren C. E., Hirst E. M., Chen P. L., et al. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. (2007);128:961–975. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Braitenberg V. Patterns of projection in the visual system of the fly. I. Retina-lamina projections. Exp Brain Res. (1967);3:271–298. doi: 10.1007/BF00235589. [DOI] [PubMed] [Google Scholar]
- 4.Braitenberg V. Ordnung und Orientierung im Sehseystem der Fliege. Kybernetik. (1970);7:235–242. [PubMed] [Google Scholar]
- 5.Cajal S. R. Nota sobre la structural de la retina de la Mosca. Trab Lab Invest Biol Univ Madr. (1909);7:217–227. [Google Scholar]
- 6.Cajal S. R., Sanchez D. Contribucion al conocimiento de los centros nerviosos de los insectos. Parte 1. retina y centros opticos. Trab Lab Invest Biol Univ Madr. (1915);13:1–168. [Google Scholar]
- 7.Carthew R. W. Pattern formation in the Drosophila eye. Curr Opin Genet Dev. (2007);17:309–313. doi: 10.1016/j.gde.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan C. C., Epstein D., Hiesinger P. R. Intracellular trafficking in Drosophila visual system development: a basis for pattern formation through simple mechanisms. Dev Neurobiol. (2011);71:1227–1245. doi: 10.1002/dneu.20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen P. L., Clandinin T. R. The cadherin Flamingo mediates level-dependent interactions that guide photoreceptor target choice in Drosophila. Neuron. (2008);58:26–33. doi: 10.1016/j.neuron.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q., Wei Y., Hua B. Ultrastructural comparison of the compound eyes of Sinopanorpa and Panorpa (Mecoptera: Panorpidae) Micron. (2012);43:893–901. doi: 10.1016/j.micron.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Clandinin T. R., Lee C. H., Herman T., Lee R. C., Yang A. Y., Ovasapyan S., Zipursky S. L. Drosophila LAR regulates R1–R6 and R7 target specificity in the visual system. Neuron. (2001);32:237–248. doi: 10.1016/s0896-6273(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 12.Clandinin T. R., Zipursky S. L. Afferent growth cone interactions control synaptic specificity in the Drosophila visual system. Neuron. (2000);28:427–436. doi: 10.1016/s0896-6273(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 13.Clandinin T. R., Zipursky S. L. Making connections in the fly visual system. Neuron. (2002);35:827–841. doi: 10.1016/s0896-6273(02)00876-0. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich W. Die Facettenaugen der Dipteren. Z Wiss Zool. (1909);92:465–539. [Google Scholar]
- 15.Eaton S. Planar polarization of Drosophila and vertebrate epithelia. Curr Opin Cell Biol. (1997);9:860–866. doi: 10.1016/s0955-0674(97)80089-0. [DOI] [PubMed] [Google Scholar]
- 16.Edwards T. N., Meinertzhagen I. A. Photoreceptor neurons find new synaptic targets when misdirected by overexpressing runt in Drosophila. J Neurosci. (2009);29:828–841. doi: 10.1523/JNEUROSCI.1022-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischbach K. F., Dittrich A. P. M. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell tissue res. (1989);258:441–475. [Google Scholar]
- 18.Fischbach K. F., Hiesinger P. R. Optic lobe development. Adv Exp Med Biol. (2008);628:115–136. doi: 10.1007/978-0-387-78261-4_8. [DOI] [PubMed] [Google Scholar]
- 19.Frohlich A., Meinertzhagen I. A. Regulation of synaptic frequency: comparison of the effects of hypoinnervation with those of hyperinnervation in the fly’s compound eye. J neurobiol. (1987);18:343–357. doi: 10.1002/neu.480180403. [DOI] [PubMed] [Google Scholar]
- 20.Gao S., Takemura S. Y., Ting C. Y., Huang S., Lu Z., Luan H., et al. The neural substrate of spectral preference in Drosophila. Neuron. (2008);60:328–342. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrity P. A., Lee C. H., Salecker I., Robertson H. C., Desai C. J., Zinn K., Zipursky S. L. Retinal axon target selection in Drosophila is regulated by a receptor protein tyrosine phosphatase. Neuron. (1999);22:707–717. doi: 10.1016/s0896-6273(00)80730-8. [DOI] [PubMed] [Google Scholar]
- 22.Greiner B. Adaptations for nocturnal vision in insect apposition eyes. Int Rev Cytol. (2006);250:1–46. doi: 10.1016/S0074-7696(06)50001-4. [DOI] [PubMed] [Google Scholar]
- 23.Greiner B., Ribi W. A., Warrant E. J. A neural network to improve dim-light vision? Dendritic fields of first-order interneurons in the nocturnal bee Megalopta genalis. Cell Tissue Res. (2005);322:313–320. doi: 10.1007/s00441-005-0034-y. [DOI] [PubMed] [Google Scholar]
- 24.Greiner B., Ribi W. A., Wcislo W. T., Warrant E. J. Neural organisation in the first optic ganglion of the nocturnal bee Megalopta genalis. Cell Tissue Res. (2004);318:429–437. doi: 10.1007/s00441-004-0945-z. [DOI] [PubMed] [Google Scholar]
- 25.Hadjieconomou D., Timofeev K., Salecker I. A step-by-step guide to visual circuit assembly in Drosophila. Curr Opin Neurobiol. (2011);21:76–84. doi: 10.1016/j.conb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Hardie R. C. Functional Organization of the Fly Retina. In: Autrum H., Ottoson D., Perl E., Schmidt R., Shimazu H., Willis W., editors. Progress in Sensory Physiology, New York: Springer Berlin Heidelberg; (1985). pp. 1–79. [Google Scholar]
- 27.Hennig W. Diptera (Zweifluegler). Handbuch der Zoologie. (2) Vol. 4. Berlin: Walter de Gruyter; (1973). [Google Scholar]
- 28.Hiesinger P. R., Zhai R. G., Zhou Y., Koh T. W., Mehta S. Q., Schulze K. L., et al. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol. (2006);16:1835–1843. doi: 10.1016/j.cub.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horridge A. The spatial resolutions of the apposition compound eye and its neuro-sensory feature detectors: observation versus theory. J Insect Physiol. (2005);51:243–266. doi: 10.1016/j.jinsphys.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Horridge G. A., Duniec J., Marcelja L. A 24-hour cycle in single locust and manti photoreceptors. J Exp Biol. (1981);91:307–322. [Google Scholar]
- 31.Horridge G. A., Meinertzhagen I. A. The accuracy of the patterns of connexions of the first- and second-order neurons of the visual system of Calliphora. Proc R Soc Lond B Biol Sci. (1970a);175:69–82. doi: 10.1098/rspb.1970.0012. [DOI] [PubMed] [Google Scholar]
- 32.Horridge G. A., Meinertzhagen I. A. The exact projection of the visual fields upon the first and second ganglia of the insect eye. Z Vergl Physiol. (1970b);66:369–378. [Google Scholar]
- 33.Huang Z., Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. (1996);86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- 34.Ioannides A. C., Horridge G. A. The organization of visual fields in the hemipteran acone eye. Proc R Soc Lond B Biol Sci. (1975);190:373–391. doi: 10.1098/rspb.1975.0101. [DOI] [PubMed] [Google Scholar]
- 35.Kaminker J. S., Canon J., Salecker I., Banerjee U. Control of photoreceptor axon target choice by transcriptional repression of Runt. Nat Neurosci. (2002);5:746–750. doi: 10.1038/nn889. [DOI] [PubMed] [Google Scholar]
- 36.Kirschfeld K. [The projection of the optical environment on the screen of the rhabdomere in the compound eye of the Musca] Exp Brain Res. (1967);3:248–270. doi: 10.1007/BF00235588. [DOI] [PubMed] [Google Scholar]
- 37.Kirschfeld K., Franceschini N. [Optical characteristics of ommatidia in the complex eye of Musca] Kybernetik. (1968);5:47–52. doi: 10.1007/BF00272694. [DOI] [PubMed] [Google Scholar]
- 38.Kristensen N. P. Phylogeny of insect orders. Ann Rev Entomol. (1981);26:135–157. [Google Scholar]
- 39.Land M. F. Visual acuity in insects. Annu Rev Entomol. (1997);42:147–177. doi: 10.1146/annurev.ento.42.1.147. [DOI] [PubMed] [Google Scholar]
- 40.Land M. F. The optical structures of animal eyes. Curr Biol. (2005);15:R319–R323. doi: 10.1016/j.cub.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 41.Land M. F., Gibson G., Horwood J. Mosquito eye design: conical rhabdoms are matched to wide aperture lenses. Proc Biol Sci. (1997);264:1183–1187. [Google Scholar]
- 42.Land M. F., Gibson G., Horwood J., Zeil J. Fundamental differences in the optical structure of the eyes of nocturnal and diurnal mosquitoes. J Comp Physiol A. (1999);185:91–103. [Google Scholar]
- 43.Land M. F., Horwood J. Different retina-lamina projections in mosquitoes with fused and open rhabdoms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. (2005);191:639–647. doi: 10.1007/s00359-005-0616-x. [DOI] [PubMed] [Google Scholar]
- 44.Land M. F., Nilsson D. E. Animal Eyes. Oxford, UK: Oxford University Press; (2002). [Google Scholar]
- 45.Lee C. H., Herman T., Clandinin T. R., Lee R., Zipursky S. L. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. (2001);30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 46.Lee R. C., Clandinin T. R., Lee C. H., Chen P. L., Meinertzhagen I. A., Zipursky S. L. The protocadherin Flamingo is required for axon target selection in the Drosophila visual system. Nat Neurosci. (2003);6:557–563. doi: 10.1038/nn1063. [DOI] [PubMed] [Google Scholar]
- 47.Martin K. A., Poeck B., Roth H., Ebens A. J., Ballard L. C., Zipursky S. L. Mutations disrupting neuronal connectivity in the Drosophila visual system. Neuron. (1995);14:229–240. doi: 10.1016/0896-6273(95)90281-3. [DOI] [PubMed] [Google Scholar]
- 48.Mast J. D., Prakash S., Chen P. L., Clandinin T. R. The mechanisms and molecules that connect photoreceptor axons to their targets in Drosophila. Semin Cell Dev Biol. (2006);17:42–49. doi: 10.1016/j.semcdb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Maurel-Zaffran C., Suzuki T., Gahmon G., Treisman J. E., Dickson B. J. Cell-autonomous and -nonautonomous functions of LAR in R7 photoreceptor axon targeting. Neuron. (2001);32:225–235. doi: 10.1016/s0896-6273(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 50.Meinertzhagen I. A. Erroneous projection of retinula axons beneath a dislocation in the retinal equator of Calliphora. Brain Res. (1972);41:39–49. doi: 10.1016/0006-8993(72)90615-4. [DOI] [PubMed] [Google Scholar]
- 51.Meinertzhagen I. A. The organization of perpendicular fibre pathways in the insect optic lobe. Philos Trans R Soc Lond B Biol Sci. (1976);274:555–594. doi: 10.1098/rstb.1976.0064. [DOI] [PubMed] [Google Scholar]
- 52.Meinertzhagen I. A. Evolution of the cellular organization of the compound eye and optic lobe. In: Cronly-Dillon J.R., Gregory R.L., editors. Vision and visual dysfunction. London: Macmillan Press; (1991). pp. 341–363. [Google Scholar]
- 53.Meinertzhagen I. A., Hanson T. >The development of the optic lobe (1993) [Google Scholar]
- 54.Meinertzhagen I. A., Hu X. Evidence for site selection during synaptogenesis: the surface distribution of synaptic sites in photoreceptor terminals of the files Musca and Drosophila. Cell Mol Neurobiol. (1996);16:677–698. doi: 10.1007/BF02151904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meinertzhagen I. A., O’Neil S. D. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol. (1991);305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- 56.Meinertzhagen I. A., Piper S. T., Sun X. J., Frohlich A. Neurite morphogenesis of identified visual interneurons and its relationship to photoreceptor synaptogenesis in the flies, Musca domestica and Drosophila melanogaster. Eur J Neurosci. (2000);12:1342–1356. doi: 10.1046/j.1460-9568.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- 57.Meinertzhagen I. A., Sorra K. E. Synaptic organization in the fly’s optic lamina: few cells, many synapses and divergent microcircuits. Prog Brain Res. (2001);131:53–69. doi: 10.1016/s0079-6123(01)31007-5. [DOI] [PubMed] [Google Scholar]
- 58.Melzer R. R. Optic lobes of the larval and imaginal scorpionfly Panorpa vulgaris (Mecoptera, Panorpidae): A neuroanatomical study of neuropil organization, retinula axons, and lamina monopolar cells. Cell Tissue Res. (1994);275:283–290. [Google Scholar]
- 59.Melzer R. R., Paulus H. F. Neuroanatomische Studien an Mucken (Diptera, Nematocera): ein Beitrag zur vergleichenden Analyse neuronaler Strukturen. Verh Dtsch Zool Ges. (1993);86:221. [Google Scholar]
- 60.Melzer R. R., Zimmermann T., Smola U. Modification of dispersal patterns of branched photoreceptor axons and the evolution of neural superposition. Cell Mol Life Sci. (1997);53:242–247. [Google Scholar]
- 61.Meyer R. L. Roger Sperry and his chemoaffinity hypothesis. Neuropsychologia. (1998);36:957–980. doi: 10.1016/s0028-3932(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 62.Morante J., Desplan C. Building a projection map for photoreceptor neurons in the Drosophila optic lobes. Seminars in cell & developmental biology. (2004);15:137–143. doi: 10.1016/j.semcdb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Moses K. Evolutionary biology: fly eyes get the whole picture. Nature. (2006);443:638–639. doi: 10.1038/nature05209. [DOI] [PubMed] [Google Scholar]
- 64.Nilsson D. E. Optics and evolution of the compound eye. In: Stavenga D.G., Hardie R.C., editors. Facets of vision. Berlin: Springer; (1989). pp. 30–73. [Google Scholar]
- 65.Nilsson D. E., Ro A.-I. Did neuronal pooling for night vision lead to the evolution of neural superposition eyes? J Comp Physiol A. (1994);175:289–302. [Google Scholar]
- 66.Osorio D. Spam and the evolution of the fly’s eye. BioEssays. (2007);29:111–115. doi: 10.1002/bies.20533. [DOI] [PubMed] [Google Scholar]
- 67.Picaud S., Wunderer H., Franceschini N. Dye-induced photopermeabilization and photodegeneration: a lesion technique useful for neuronal tracing. J Neurosci Methods. (1990);33:101–112. doi: 10.1016/0165-0270(90)90014-7. [DOI] [PubMed] [Google Scholar]
- 68.Poeck B., Fischer S., Gunning D., Zipursky S. L., Salecker I. Glial cells mediate target layer selection of retinal axons in the developing visual system of Drosophila. Neuron. (2001);29:99–113. doi: 10.1016/s0896-6273(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 69.Prakash S., Caldwell J. C., Eberl D. F., Clandinin T. R. Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat Neurosci. (2005);8:443–450. doi: 10.1038/nn1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prakash S., McLendon H. M., Dubreuil C. I., Ghose A., Hwa J., Dennehy K. A., et al. Complex interactions amongst N-cadherin, DLAR, and Liprin-alpha regulate Drosophila photoreceptor axon targeting. Dev Biol. (2009);336:10–19. doi: 10.1016/j.ydbio.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rao Y., Pang P., Ruan W., Gunning D., Zipursky S. L. Brakeless is required for photoreceptor growth-cone targeting in Drosophila. Proc Natl Acad Sci U S A. (2000);97:5966–5971. doi: 10.1073/pnas.110135297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ready D. F., Hanson T. E., Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. (1976);53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- 73.Rivera-Alba M., Vitaladevuni S. N., Mishchenko Y., Lu Z., Takemura S. Y., Scheffer L., et al. Wiring economy and volume exclusion determine neuronal placement in the Drosophila brain. Curr Biol. (2011);21:2000–2005. doi: 10.1016/j.cub.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roignant J. Y., Treisman J. E. Pattern formation in the Drosophila eye disc. Int J Dev Biol. (2009);53:795–804. doi: 10.1387/ijdb.072483jr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rybak J., Meinertzhagen I. A. The effects of light reversals on photoreceptor synaptogenesis in the fly Musca domestica. Eur J Neurosci. (1997);9:319–333. doi: 10.1111/j.1460-9568.1997.tb01402.x. [DOI] [PubMed] [Google Scholar]
- 76.Schwabe T., Neuert H., Clandinin T. R. A network of cadherin-mediated interactions polarizes growth cones to determine targeting specificity. Cell. (2013);154:351–364. doi: 10.1016/j.cell.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schweisguth F. Temporal regulation of planar cell polarity: insights from the Drosophila eye. Cell. (2005);121:497–499. doi: 10.1016/j.cell.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Seifert P., Smola U. Adaptive structural changes indicate an evolutionary progression towards the open rhabdom in diptera. J Evol Biol. (1990);3:225–242. [Google Scholar]
- 79.Senti K., Keleman K., Eisenhaber F., Dickson B. J. Brakeless is required for lamina targeting of R1–R6 axons in the Drosophila visual system. Development. (2000);127:2291–2301. doi: 10.1242/dev.127.11.2291. [DOI] [PubMed] [Google Scholar]
- 80.Senti K. A., Usui T., Boucke K., Greber U., Uemura T., Dickson B. J. Flamingo regulates R8 axon-axon and axon-target interactions in the Drosophila visual system. Curr Biol. (2003);13:828–832. doi: 10.1016/s0960-9822(03)00291-4. [DOI] [PubMed] [Google Scholar]
- 81.Shaw S. R. Optics of arthropod compound eye. Science. (1969);165:88–90. doi: 10.1126/science.165.3888.88. [DOI] [PubMed] [Google Scholar]
- 82.Shaw S. R. The retina-lamina pathway in insects, particularly Diptera, viewed from an evolutionary perspective. In: Stavenga D.G., Hardie R.C., editors. Facets of vision. Berlin: Springer; (1989). pp. 186–212. [Google Scholar]
- 83.Shaw S. R. The photoreceptor axon projection and its evolution in the neural superposition eyes of some primitive brachyceran diptera. Brain Behav Evol. (1990);35:107–125. doi: 10.1159/000115860. [DOI] [PubMed] [Google Scholar]
- 84.Shaw S. R., Meinertzhagen I. A. Evolutionary progression at synaptic connections made by identified homologous neurones. Proc Natl Acad Sci U S A. (1986);83:7961–7965. doi: 10.1073/pnas.83.20.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shaw S. R., Moore D. Evolutionary remodeling in a visual system through extensive changes in the synaptic connectivity of homologous neurons. Vis Neurosci. (1989);3:405–410. doi: 10.1017/s0952523800005903. [DOI] [PubMed] [Google Scholar]
- 86.Sperry R. W. Chemoaffinity in the Orderly Growth of Nerve Fiber Patterns and Connections. Proc Natl Acad Sci U S A. (1963);50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stavenga D. G. The Neural Superposition Eye and Its Optical Demands. J Comp Physiol. (1975);102:297–304. [Google Scholar]
- 88.Strausfeld N. J. Berlin: Springer Verlag; (1976). Atlas of an insect brain. [Google Scholar]
- 89.Tanaka G., Parker A. R., Siveter D. J., Maeda H., Furutani M. An exceptionally well-preserved Eocene dolichopodid fly eye: function and evolutionary significance. Proc Biol Sci. (2009);276:1015–1019. doi: 10.1098/rspb.2008.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tayler T. D., Garrity P. A. Axon targeting in the Drosophila visual system. Curr Opin Neurobiol. (2003);13:90–95. doi: 10.1016/s0959-4388(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 91.Ting C. Y., Lee C. H. Visual circuit development in Drosophila. Curr Opin Neurobiol. (2007);17:65–72. doi: 10.1016/j.conb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 92.Tomlinson A., Ready D. F. Neuronal differentiation in Drosophila ommatidium. Dev Biol. (1987);120:366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 93.Trujillo-Cenoz O., Melamed J. Compound eye of dipterans: anatomical basis for integration–an electron microscope study. J Ultrastruct Res. (1966);16:395–398. doi: 10.1016/s0022-5320(66)80071-0. [DOI] [PubMed] [Google Scholar]
- 94.Trujillo-Cenoz O., Melamed J. The development of the retina-lamina complex in muscoid flies. J Ultrastruct Res. (1973);42:554–581. doi: 10.1016/s0022-5320(73)80027-9. [DOI] [PubMed] [Google Scholar]
- 95.Tsachaki M., Sprecher S. G. Genetic and developmental mechanisms underlying the formation of the Drosophila compound eye. Dev Dyn. (2012);241:40–56. doi: 10.1002/dvdy.22738. [DOI] [PubMed] [Google Scholar]
- 96.Tuurala O. Bau und photomechanische Erscheinungen im Auge einiger Chironomiden (Dipt.) Ann Entomol Fenn. (1963);29:209–217. [Google Scholar]
- 97.Vigier P. Mecanisme de la synthese des impressions rescueillies par les yeux composes des Dipteres. C R Acad Sci (Paris) (1907a);63:122–124. [Google Scholar]
- 98.Vigier P. Sur la reception de l’exitant lumineux dans le yeux composes des Insectes, en prticulier chez les Muscides. C R Acad Sci (Paris) (1907b);63:633–636. [Google Scholar]
- 99.Vigier P. Sur les terminations photoreceptrices dans les yeux composes des Muscides. C R Acad Sci (Paris) (1907c);63:532–536. [Google Scholar]
- 100.Vigier P. Sur l’existence reele et le role des appendices piriformes des neurons. Le neurons perioptique des Dipteres. CR Soc Biol (Paris) (1908);64:959–961. [Google Scholar]
- 101.Wardill T. J., List O., Li X., Dongre S., McCulloch M., Ting C. Y., et al. Multiple spectral inputs improve motion discrimination in the Drosophila visual system. Science. (2012);336:925–931. doi: 10.1126/science.1215317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Warrant E. J. Seeing better at night: life style, eye design and the optimum strategy of spatial and temporal summation. Vision Res. (1999);39:1611–1630. doi: 10.1016/s0042-6989(98)00262-4. [DOI] [PubMed] [Google Scholar]
- 103.Wiegmann B. M., Trautwein M. D., Winkler I. S., Barr N. B., Kim J. W., Lambkin C., et al. Episodic radiations in the fly tree of life. Proc Natl Acad Sci U S A. (2011);108:5690–5695. doi: 10.1073/pnas.1012675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williams D. S. Ommatidial structure in relation to turnover of photoreceptor membrane in the locust. Cell Tissue Res. (1982);225:595–617. doi: 10.1007/BF00214807. [DOI] [PubMed] [Google Scholar]
- 105.Wolburg-Buchholz K. The organization of the lamina ganglionaris of the hemipteran insects, Notonecta glauca, Corixa punctata and Gerris lacustris. Cell Tissue Res. (1979);197:39–59. doi: 10.1007/BF00233552. [DOI] [PubMed] [Google Scholar]
- 106.Wolff T., Ready D. F. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. (1991);113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- 107.Wolff T., Ready D. F. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory: Cold Spring Harbor Laboratory Press; (1993). Pattern formation in the Drosophila retina; pp. 1277–1325. [Google Scholar]
- 108.Yamaguchi S., Wolf R., Desplan C., Heisenberg M. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci U S A. (2008);105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeil J. A new kind of superposition eyee: the compound eye of male Bibionidae. Nature. (1979);278:249–250. [Google Scholar]
- 110.Zeil J. Sexual dimorphism in the visual system of flies: the divided brain of male Bibionidae (Diptera) Cell Tissue Res. (1983);229:591–610. doi: 10.1007/BF00207700. [DOI] [PubMed] [Google Scholar]
- 111.Zelhof A. C., Hardy R. W., Becker A., Zuker C. S. Transforming the architecture of compound eyes. Nature. (2006);443:696–699. doi: 10.1038/nature05128. [DOI] [PubMed] [Google Scholar]