Abstract
With increasing prevalence of childhood obesity, non-alcoholic fatty liver disease (NAFLD) has emerged as the most common cause of liver disease among children and adolescents in industrialized countries. It is generally recognized that both genetic and environmental risk factors contribute to the pathogenesis of NAFLD. Recently, there has been a growing body of evidence to implicate altered gut microbiota in the development of NAFLD through the gut-liver axis. The first line of prevention and treatment of NAFLD in children should be intensive lifestyle interventions such as changes in diet and physical activity. Recent advances have been focused on limitation of dietary fructose and supplementation of antioxidants, omega-3 fatty acids, and prebiotics/probiotics. Convincing evidences from both animal models and human studies have shown that reduction of dietary fructose and supplement of vitamin E, omega-3 fatty acids, and prebiotics/probiotics improve NAFLD.
Keywords: non-alcoholic fatty liver disease (NAFLD), nutrition, children
1. Introduction
Obesity has been increasing significantly worldwide over the past three decades and has become a major public health concern. According to the latest National Health and Nutrition Examination Survey [1,2], the prevalence of obesity in United States is 35.5% among adult men, 35.8% among adult women, and 16.9% in children and adolescents 2–19 years old. Recently Ng et al. (2004) showed that the world-wide prevalence of overweight and obesity (body mass index ≥ 25 kg/m2 in adults >18 years old) between 1980 and 2013 increased from 28.8% to 36.9% in men, and from 29.8% to 38.0% in women. Based on the International Obesity Task Force definition, the prevalence for children in developed countries also increased remarkably from 16.9% to 23.8% for boys and from 16.2% to 22.6% for girls. It is also reported that the prevalence for children in developing countries increased from 8.1% to 12.9% for boys and from 8.4% to 13.4% for girls [3].
It is well known that obesity is associated with major complications involving all major organ systems. A recent systematic review and meta-analysis showed that relative to normal weight, both obesity (all grades) and Grades 2 and 3 obesity were associated with significantly higher all-cause mortality [4].
With increasing prevalence of childhood obesity, non-alcoholic fatty liver disease (NAFLD) has emerged as the most common cause of liver disease among children and adolescents in industrialized countries [5,6]. NAFLD is defined as hepatic fat infiltration >5% of hepatocytes on liver biopsy, with no excessive alcohol intake and no evidence of viral, autoimmune, or drug-induced liver disease. NAFLD refers to a spectrum of liver diseases ranging from simple fat infiltration (steatosis) to advanced non-alcoholic steatohepatitis (NASH, steatosis with liver inflammation), and fibrosis. In children, its biopsy-proven prevalence in the United States (as revealed at autopsy after accidents) ranges from 9.6% in normal weight individuals up to 38% in obese ones [7]. In specialized pediatric obesity centers in Germany, Austria, and Switzerland, NAFLD (defined as aspartate aminotransferase (AST) and/or alanine transaminase (ALT) > 50 UL−1) was present in 11% of the study population of 16,390 children, but predominantly in boys, in those with extreme obesity, and in those ≥12 years of age [8]. In a study of 748 schoolchildren in Taiwan, the rates of NAFLD assessed by ultrasonography were 3% in the normal-weight, 25% in the overweight, and 76% in the obese children [9]. Twenty-two percent of obese children had abnormal ALT levels. It appears that NASH occurs more in obese children in Taiwan (22%) than in Europe (11%), and this racial and ethnic difference requires further investigation. It is clear that NAFLD prevalence in children increases with age and is more common among boys [6,8,10]. In the US, Hispanic children have the highest NAFLD prevalence, whereas African American children are less affected [11].
NAFLD in children, as in adults, is associated with severe obesity and metabolic syndrome. It is believed that insulin resistance plays a key role in the development of metabolic syndrome, thus called “the syndrome of insulin resistance”, which traditionally includes abdominal obesity, type-2 diabetes, dyslipidemia, and hypertension. NAFLD/NASH has been proposed to be included in this syndrome. In this review, we will briefly summarize the current understanding of the pathogenesis of NAFLD, the current management guidelines in children and the new insights into nutritional interventions.
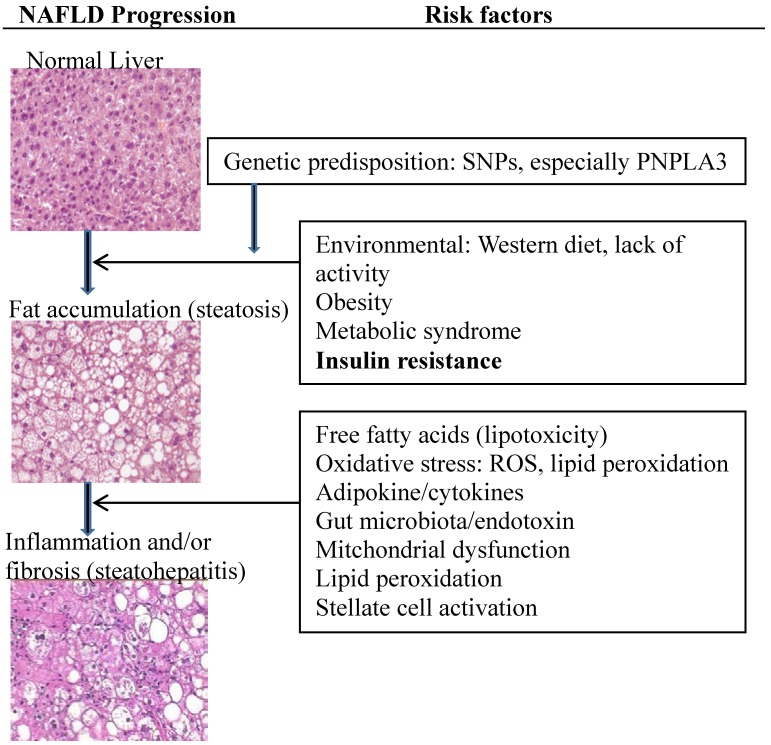
2. Current Understanding of NAFLD Pathogenesis (Figure 1)
Figure 1.
Current understanding of non-alcoholic fatty liver disease (NAFLD) pathogenesis. In individuals with genetic predisposition, insulin resistance plays a crucial role in NAFLD and other factors including nutritional factors, adipose tissue, and the immune system may be necessary for NAFLD manifestation and progression. SNPs: single nucleotide polymorphisms; ROS: reactive oxygen species.
The pathogenesis of NAFLD remains incompletely understood. Like other complex diseases, both genetic and environmental factors contribute to NAFLD development and progression. Recent genomic studies have identified many variants (single nucleotide polymorphisms, SNPs) in genes controlling lipid metabolism, pro-inflammatory cytokines, fibrotic mediators, and oxidative stress, in patients with NAFLD. The most important one is the patatin-like phospholipase domain-containing 3 gene (PNPLA3) [12]. PNPLA3 rs738409 variant contributes to ancestry-related and inter-individual differences in hepatic fat content and may confer susceptibility to NAFLD in obese children across multiple ethnic groups [13]. Other variants have been identified in genes including glucokinase regulatory protein (GCKR), apolipoprotein C3 (APOC3), neurocan (NCAN), lysophospholipase-like 1 (LYPLAL1), protein phosphatase 1 regulatory subunit 3b (PPP1R3B), group-specific component (GC), lymphocyte cytosolic protein-1 (LCP1), solute carrier family 38 member 8 (SLC38A8), lipid phosphate phosphatase-related protein type 4 (LPPR4), sorting and assembly machinery component (SAMM50), parvin beta (PARVB) and farnesyl-diphosphate farnesyltransferase 1 (FDFT1) [14].
The two-hit hypothesis was initially proposed to explain the pathogenesis of NASH. In individuals with genetic predisposition, the “first hit” results in liver fat accumulation due to insulin resistance, obesity, and adipokine/cytokine productions. Oxidative stress, endotoxins, and apoptosis represent a “second hit” to further amplify liver injury and NASH progression. More recently, Tilg & Moschen (2010) proposed a multiple parallel hits hypothesis, suggesting that gut-derived and adipose tissue–derived factors may play a central role [15]. Both hypotheses recognized the crucial role of insulin resistance in NAFLD and that other factors including genetic determinants, nutritional factors, adipose tissue and the immune system may be necessary for NAFLD manifestation and progression.
The development of NAFLD involves complex interactions between alterations in nutrient metabolism, hormonal dysregulation, and the onset of inflammation in multiple organ systems [16]. Insulin resistance increases free fatty acid influx and lipogenesis and induces oxidative stress and stellate cell activation. There are increased adipocytokines (TNF-α, IL-8 and visfatin) and decreased adiponectin in patients with NAFLD/NASH [17]. Impaired mitochondrial function is thought to contribute to NAFLD and insulin resistance [18]. Hepatocellular lipid accumulation, together with high reactive oxygen species (ROS) production, lipid peroxidation, and proinflammatory cytokines, lead to DNA damage and eventual cell death, known as “mitochondrial dysfunction”, which is now believed to be a major determinant in hepatocellular inflammation.
There has been emerging data to indicate the metabolites of free fatty acids cause hepatic lipotoxicity, which contributes to the pathogenesis of NASH [19,20]. Animal studies and a limited number of human studies strongly suggest that triglyceride accumulation does not cause insulin resistance or hepatocellular injury and may actually be a protective response to prevent lipotoxicity from free fatty acid-derived metabolites. This new lipotoxicity liver injury hypothesis proposes that insulin resistance facilitates an excessive flow of free fatty acids to the liver, resulting in increased production of lipotoxic intermediates and eventually NASH, through oxidative stress, mitochondrial dysfunction, adiponectin, and other complex pathways.
Recently, there has been a growing body of evidence to implicate the gut microbiota in NAFLD. Gut microbiota is thought to contribute to the development of NAFLD through the gut-liver axis [21]. Obesity and metabolic syndrome are definitive risk factors for NAFLD and are associated with alteration of gut microbiota. It has been shown that obese patients have altered gut microbiota with an increase in the relative proportion of Bacteroidales and Clostridiales. Most recently, Zhu et al. (2013) demonstrated an increased abundance of alcohol-producing microbiota in NASH patients. Bacterial overgrowth and increased intestinal permeability have been observed in patients with NAFLD [22]. Gut-derived bacterial products such as endotoxin (lipopolysaccharides, LPS) and bacterial DNA are delivered to the liver through the portal vein and activate toll-like receptors (TLRs), mainly TLR4 and TLR9, and their down-stream cytokines and chemokines, leading to the development and progression of NAFLD.
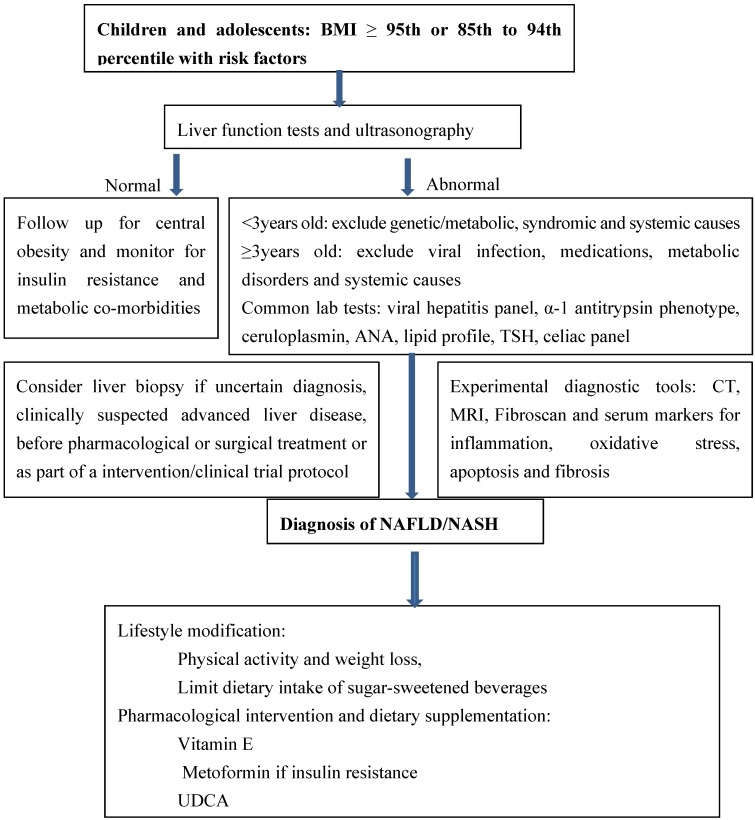
3. NAFLD in Children: Current Management Guidelines (Figure 2)
Figure 2.
Management algorithm for children with suspected non-alcoholic fatty liver disease (NAFLD). UDCA: ursodeoxycholic acid.
Diagnosis and management guidelines for NAFLD in children were recently published [6,23]. Infants and children <3 years old with fatty liver are less likely to have NAFLD and should be tested for genetic, metabolic, syndromic, and systemic causes, such as fatty acid oxidation defects, lysosomal storage diseases and peroxisomal disorders, in addition to those causes considered for adults. In older children and teenagers, metabolic, infectious, toxic, and systemic causes should also be considered for differential diagnosis.
Ultrasonography is the only imaging technique used for NAFLD screening in children because it is safe, non-invasive, widely available, relatively inexpensive, and candetect evidence of portal hypertension. However, low sensitivity of ultrasonography was reported when hepatic fat content was less than 30% [24]. In addition, ultrasonography is operator dependent and cannot distinguish liver steatosis from fibrosis. The use of computer tomography (CT) in children is not clinically recommended due to exposure to ionizing radiation. Magnetic resonance imaging (MRI) is not cost-effective. A new device, fibroscan, has not yet been validated in children.
Liver biopsy is also not recommended as a screening procedure due to its invasive nature and high cost. For diagnosis, liver biopsy should be considered as the last test after noninvasive biochemical and metabolic tests. However, liver biopsy is recommended to exclude other treatable diseases, in cases of clinically suspected advanced liver disease, before pharmacological/surgical treatment, and as part of a structured intervention protocol or clinical research trial [6].
Recommendations for pediatric treatment options are limited by a small number of randomized clinical trials and insufficient information on the natural history of the condition to assess risk-benefit ratios [23]. Since most pediatric NAFLD patients are obese, intensive lifestyle modification, including dietary changes and physical exercise, should be the first line of treatment. In children with poor adherence to lifestyle changes, pharmacological interventions and dietary supplementations, including antioxidants (vitamin E), insulin sensitizers (metoformin), ursodeoxycholic acid (UDCA), omega-3 docosahexaenoic acid (DHA), and probiotics, may be tried.
4. Nutritional Interventions
The diet of children with NAFLD is characterized by over consumption of fructose, soft drinks, meat, saturated fat, and cholesterol, and low consumption of fiber, fish, omega 3 fats, and vitamin E [25]. So far, weight loss, though hard to achieve, is still the biggest and best-known treatment [26]. Recent advances have been focused on dietary fructose, antioxidants, omega-3 fatty acids, and pre-/pro-biotics.
4.1. Role of Weight Loss
Randomized controlled trial in adults showed that 7%–10% weight loss by a combination of diet, exercise, and behavior modification for 48 weeks significantly improved NASH histological activity score and other clinical parameters [27]. An earlier randomized controlled study demonstrated that 12-month lifestyle intervention with diet and increased physical activity induced an average weight loss of 4.75 kg and was associated with a significant improvement in liver histology and laboratory abnormalities in pediatric NAFLD [28]. In a cohort study of 144 children with NAFLD, Koot et al. (2011) also demonstrated that a lifestyle intervention (physical exercise, dietary change, and behavioral modification) of 6 months significantly improved hepatic steatosis and serum aminotransferases [29]. A multidisciplinary lifestyle intervention long-term follow-up study showed that the greatest decrease of NAFLD prevalence was observed in children with the greatest overweight reduction [30]. Grønbæk et al. (2012) observed that a 10-week “weight loss camp” (moderate exercise for 1 hour/day and energy intake restriction) in 117 obese children resulted in an average weight loss of 7.1 kg and markedly improved ultrasonographic liver steatosis and reduced liver transaminases and insulin sensitivity [31]. Due to the difficulty in adhering to long-term physical exercise and behavioral changes, the role of weight loss as an effective treatment on NAFLD in children may be limited.
4.2. Role of Dietary Fructose
Fructose is generally not consumed alone but commonly found in large quantities in processed foods and beverages [32]. High-fructose corn syrup usually contains 53%–55% fructose and 42% glucose. After consumption, fructose is absorbed in the small intestine through GLUT5 transporter and then taken up by the liver, testes, and other organs. Consumption of a fructose-rich diet has been shown to induce de novo lipogenesis leading to elevated triglyceride and cholesterol levels and dyslipidemia [33].
In healthy humans, a 4-week moderate fructose supplementation (1.5 g/kg/day) increased plasma triacyllglycerol and glucose concentrations and a 7-day high-fructose diet (3.5 g/kg/day) caused dyslipidemia and ectopic lipid deposition in liver and muscle [34,35]. Faeh et al. (2005) showed that a high fructose diet (3 g/kg/day) for 6 days in healthy men induced dyslipedemia and hepatic and adipose tissue insulin resistance [36]. In an exploratory trial, Silbernagel et al. (2011) did not find any difference in glucose metabolism and body fat composition in healthy participants receiving 150 g/day of either high fructose or glucose for 4 weeks. They did show that plasma triacyllglycerol markedly increased in the fructose group, but not in the glucose group [37].
Rats fed the high-fat-high-fructose diet exhibited significantly higher plasma triglycerides, non-esterified fatty acids, insulin, and indexes of hepatic insulin resistance compared with rats fed a low-fat or a high-fat diet, suggesting the deleterious effect of fructose supplementation on liver steatosis and glucose homeostasis [38]. To explore the mechanism underlying fructose-induced lipid accumulation, Sapp et al. (2014) showed that fructose treatment of zebrafish induced hepatic lipid accumulation, inflammation, and oxidative stress through activation of the target of rapamycin complex 1 (Torc1) pathway, which was also confirmed in liver samples from patients with NAFLD and NASH [39]. Toll-like receptors (TLR)-4 is the receptor for gut microbiota-derived endotoxins. TLR4-mutant mice fed with a fructose-enriched diet had significantly less hepatic steatosis, MyD88, and TNFα level in comparison to fructose-fed wild type mice, supporting the role of endotoxins/TLR4 signaling in fructose-induced NAFLD [40].
High fructose consumption is associated with the development of NAFLD in human studies [41,42,43], likely through increasing intestinal permeability and translocation of endotoxin [40,44]. Consumption of fructose in patients with NAFLD was nearly two to three times higher than in controls [365 kcal vs. 170 kcal] [42]. In 31 NAFLD patients without classic risk factors, 80% consumed an excessive amount of soft drink beverages (more than 50 g/day of added sugar) for 36 months, compared with only 20% in healthy controls [45]. Most recently, Sullivan et al. (2014) showed that children with NAFLD absorbed and metabolized fructose more effectively than lean subjects. Fructose ingestion was associated with an exacerbated metabolic profile [46]. In a 4-week randomized, controlled, double-blinded beverage intervention study, Jin et al. (2014) demonstrated that reduction of dietary fructose in Hispanic-American adolescents with NAFLD improved several important factors related to cardiovascular disease risk, including adipose insulin sensitivity, high sensitivity C-reactive protein and low-density lipoprotein oxidation [47].
4.3. Role of Antioxidants and Omega-3 Fatty Acids
Antioxidants can reduce oxidative stress and protect cell membranes from lipid peroxidation. The largest adult trial showed significant improvements in the aminotransferase levels, hepatic steatosis, lobular inflammation, and the total NAFLD activity score in the vitamin E group compared to the placebo group [48]. In contrast, the pediatric randomized controlled trial (RCT) study, TONIC, showed α- vitamin E treatment did not attain a significant and sustained decrease in ALT levels compared to placebo [49]. However, it was better than placebo in inducing resolution of a histologically borderline or defined NASH and in improving hepatocellular ballooning and NAFLD activity histological score. A recent systemic review and meta-analysis concluded that adjuvant vitamin E does not have a significant effect on NAFLD in children in comparison to placebo [50]. Vitamin E has been used widely in children with NAFLD even though more confirmatory studies are needed.
Dietary supplement of omega-3 long-chain polyunsaturated fatty acids, containing both DHA and eicosapentaenoic acid (EPA), has produced mixed results in adults with NAFLD. Earlier studies reported significant improvement in the biochemical and ultrasonographic features of liver steatosis [51,52]. However, a recent randomized, double blind placebo-controlled trial in adults with NAFLD only showed a trend towards improvement in liver fat percentage and no improvement in the fibrosis scores with DHA + EPA treatment for 15–18 months [53]. This is in contrast to a pediatric double-blind randomized controlled clinical trial [54], which demonstrated that DHA supplementation improved liver steatosis and insulin sensitivity in children with NAFLD. Interestingly, a study conducted by the Nonalcoholic Steatohepatitis Clinical Research Network demonstrated that lack of fish and long-chain ω-3 fatty acid intake in children was associated with greater portal (p = 0.03 and p = 0.10, respectively) and lobular inflammation (p = 0.09 and p = 0.004, respectively) after controlling for potential confounding factors [55]. Those studies provide a strong rationale that children with NAFLD should be encouraged to consume the recommended amount of fish per week or be supplemented with DHA.
4.4. Role of Prebiotics and Probiotics
Given the accumulating evidence of the possible fundamental role of gut derived microbial factors in the development and/or progression of NAFLD, prebiotics and probiotics have been utilized to modify gut microbiota as preventive or therapeutic strategies for this pathological condition.
Prebiotics are non-digestible dietary fibers that stimulate the growth and activity of intestinal bacteria. In animal studies, Cani et al. (2009) showed that genetically obese mice fed with prebiotics (oligofructose, a mix of fermentable dietary fibers) exhibited a lower plasma lipopolysaccharide (LPS) and cytokines (TNFα, IL1b, IL1α, IL6, and INFγ) levels and reduced intestinal permeability through a glucagon-like peptide-2 (GLP-2)-dependent mechanism [56]. Fukuda et al. (2011) demonstrated that prebiotic treatment of chemo-induced carcinogenic rats modulated intestinal microbiota and down-regulated TLR4 [57]. Lactulose is considered a prebiotic and has the ability to promote the growth of certain intestinal bacteria such as Lactobacillus and Bifidobacterium [58]. In rats with steatohepatitis induced by high-fat diet, lactulose treatment decreased hepatic inflammation activity and serum endotoxin levels [59]. In a randomized, controlled, double-blind, prospective clinical trial, Holscher et al. (2012) demonstrated that infants fed with formula containing prebiotics, galacto-oligosaccharides, and fructo-oligosaccharides (9:1 ratio), increased abundance and proportion of bifidobacteria [60]. In an earlier clinical study in patients with biopsy-proven NASH, Daubioul et al. (2005) showed that dietary supplementation of oligofructose 16 g/day for 8 weeks significantly decreased serum aminotransferases and insulin levels [61].
Probiotics are live commensal microorganisms that beneficially modulate the host’s gut microbiota. Both animal and human studies have shown beneficial effects of probiotics on NAFLD. In a mouse model of NAFLD, treatment with VSL#3, a probiotic food supplement, improved liver histology, reduced hepatic total fatty acid content, and decreased serum alanine aminotransferase (ALT) levels, which were associated with decreased hepatic expression of TNF-α mRNA and reduced activity of Jun N-terminal kinase (JNK) [62]. In another study, VSL#3 treatment ameliorated methionine-choline deficient diet (MCDD)-induced liver fibrosis in mice [63]. Xu et al. (2012) demonstrated that oral supplementation with Bifidobacterium significantly attenuated hepatic fat accumulation without improvement in intestinal permeability in rat nonalcoholic fatty liver disease model [64]. Karahan et al. (2012) showed that MCDD-induced NASH in rats improved after treatment with probiotic mixture containing 6 or 13 bacterial strains that were isolated from the healthy human stool samples, likely in part through modulation of TNF-α activity [65]. Dextran sulphate sodium (DSS) treatment of Apolipoprotein E-deficiency mice caused liver histopathological features of steatohepatitis, which were prevented by VSL#3 treatment, through modulation of the expression of nuclear receptors, peroxisome proliferator-activated receptor-γ, Farnesoid-X-receptors, and vitamin D receptor [66]. In human studies, Aller et al. (2011) reported that patients with NAFLD had improvement of liver aminotransferases after 3 months’ treatment with Lactobacillus bulgaricus and Streptococcus thermophilus [67]. Most recently, Alisi et al. (2014) performed a double-blind RCT of VSL#3 vs. placebo in obese children with biopsy-proven NAFLD and found that a 4-month supplement of VSL#3 significantly improved fatty liver and significantly reduced body mass index from 27.1 to 24.9 kg/m2, representing an 8.1% weight loss [68].
5. Conclusions and Future Perspectives
The growing obesity epidemic is believed to be a main driver of the increase of pediatric NAFLD. Although the pathogenesis of fatty liver in children and adolescents is not fully understood, it is generally recognized that both genetic and environmental risk factors contribute to the pathogenesis of NAFLD. The first line of prevention and treatment of NAFLD should focus on lifestyle interventions such as changes in diet and physical activity. However, it may be necessary to engage in or combine with pharmacological interventions and dietary supplementation, given the difficulty of compliance with lifestyle changes. Many published studies of treatment interventions, including fructose reduction, vitamin E, omega-3 fatty acids, prebiotics, and probiotics, on NAFLD only showed success in a limited percentage of participants. Furthermore, many of those studies were preliminary with small sample sizes and often without comparison with standard care and without long-term follow-up.
One of the key future research directions in the NAFLD field may be to use state of the art next generation sequence technology such as RNA-seq for the identification of new and significant genes or transcripts in the key pathobiology network and pathway involved in the development of hepatic steatosis, initiation of hepatic inflammation and fibrosis, transition to NASH, and progression to liver cirrhosis and hepatocellular carcinoma. Newly uncovered significant genes or transcripts will be valuable to shed new mechanistic insights into the pathogenesis of NAFLD, and they can be used for novel diagnostic/prognostic biomarkers and drug target development for NAFLD. Now that whole genome sequencing of individual subjects to identify genome wide SNPs has become feasible, information on individual genome-wide SNPs may provide a better understanding of unique host susceptibility factors and the drug response variation in NAFLD and NASH to form a solid basis for pharmacogenomics-based studies on specific drugs used for treating those conditions. We are entering the “omic” era. Other prospective “omics” research areas include metabolomic, lipidomic, and proteomics studies of both human samples and samples from animal models in NAFLD and NASH, which will advance the understanding of NAFLD pathogenesis. The integration of all these “omic” information may contribute to an improved and personalized nutrition regimen for early prevention and dietary treatment for both NAFLD and NASH.
Author Contributions
All authors have contributed to the writing and editing of manuscript drafts and approved the final version of manuscript.
Conflicts of Interest
Authors do not have any conflict of interest.
References
- 1.Flegal K.M., Carroll M.D., Kit B.K., Ogden C.L. Prevalence of obesity and trends in the distribution of: Body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng M., Fleming T., Robinson M., Thomson B, Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C., Abera S.F., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal K.M., Kit B.K., Orpana H., Graubard B.I. Association of all-cause mortality with overweight andobesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day C.P. Non-alcoholic fatty liver disease: A massive problem. Clin. Med. 2011;11:176–178. doi: 10.7861/clinmedicine.11-2-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vajro P., Lenta S., Socha P., Dhawan A., McKiernan P., Baumann U., Durmaz O., Lacaille F., McLin V., Nobili V. Diagnosis of Nonalcoholic Fatty Liver Disease in Children and Adolescents: Position Paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 2012;54:700–713. doi: 10.1097/MPG.0b013e318252a13f. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer J.B., Deutsch R., Kahen T., Lavine J.E., Stanley C., Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 8.Wiegand S., Keller K.M., Röbl M., L’Allemand D., Reinehr T., Widhalm K., Holl R.W. Obese boys at increased risk for nonalcoholic liver disease: Evaluation of 16,390 overweight or obese children and adolescents. Int. J. Obes. (Lond.) 2010;34:1468–1474. doi: 10.1038/ijo.2010.106. [DOI] [PubMed] [Google Scholar]
- 9.Huang S.C., Yang Y.J. Serum retinol-binding protein 4 is independently associated with pediatric NAFLD and fasting triglyceride level. J. Pediatr. Gastroenterol. Nutr. 2013;56:145–150. doi: 10.1097/MPG.0b013e3182722aee. [DOI] [PubMed] [Google Scholar]
- 10.Graham R.C., Burke A., Stettler N. Ethnic and sex differences in the association between metabolic syndrome and suspected nonalcoholic fatty liver disease in a nationally representative sample of US adolescents. J. Pediatr. Gastroenterol. Nutr. 2009;49:442–449. doi: 10.1097/MPG.0b013e31819f73b4. [DOI] [PubMed] [Google Scholar]
- 11.Fraser A., Longnecker M.P., Lawlor D.A. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–1820. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A., Boerwinkle E., Cohen J.C., Hobbs H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y.C., Chang P.F., Chang M.H., Ni Y.H. Genetic variants in GCKR and PNPLA3 confer susceptibility to nonalcoholic fatty liver disease in obese individuals. Am. J. Clin. Nutr. 2014;99:869–874. doi: 10.3945/ajcn.113.079749. [DOI] [PubMed] [Google Scholar]
- 14.Marzuillo P., del Giudice E.M., Santoro N. Pediatric fatty liver disease: Role of ethnicity and genetics. World J. Gastroenterol. 2014;20:7347–7355. doi: 10.3748/wjg.v20.i23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 16.Lomonaco R., Sunny N.E., Bril F., Cusi K. Nonalcoholic fatty liver disease: Current issues and novel treatment approaches. Drugs. 2013;73:1–14. doi: 10.1007/s40265-012-0004-0. [DOI] [PubMed] [Google Scholar]
- 17.Jarrar M.H., Baranova A., Collantes R., Ranard B., Stepanova M., Bennett C., Fang Y., Elariny H., Goodman Z., Chandhoke V., et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2008;27:412–421. doi: 10.1111/j.1365-2036.2007.03586.x. [DOI] [PubMed] [Google Scholar]
- 18.García-Ruiz C., Baulies A., Mari M., García-Rovés P.M., Fernandez-Checa J.C. Mitochondrial dysfunction in non-alcoholic fatty liver disease and insulin resistance: Cause or consequence? Free Radic. Res. 2013;47:854–868. doi: 10.3109/10715762.2013.830717. [DOI] [PubMed] [Google Scholar]
- 19.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 20.Bechmann L.P., Kocabayoglu P., Sowa J.P., Sydor S., Best J., Schlattjan M., Beilfuss A., Schmitt J., Hannivoort R.A., Kilicarslan A., et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology. 2013;57:1394–1406. doi: 10.1002/hep.26225. [DOI] [PubMed] [Google Scholar]
- 21.Li D.Y., Yang M., Edwards S., Ye S.Q. Non-alcoholic fatty liver disease: For better or worse, blame gut microbiota? JPEN J. Parenter. Enteral. Nutr. 2013;37:787–793. doi: 10.1177/0148607113481623. [DOI] [PubMed] [Google Scholar]
- 22.Zhu L., Baker S.S., Gill C., Liu W., Alkhouri R., Baker R.D., Gill S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 23.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Charlton M., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 24.Saadeh S., Younossi Z.M., Remer E.M., Gramlich T., Ong J.P., Hurley M., Mullen K.D., Cooper J.N., Sheridan M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 25.Zelber-Sagi S., Ratziu V., Oren R. Nutrition and physical activity in NAFLD: An overview of the epidemiological evidence. World J. Gastroenterol. 2011;17:3377–3389. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilg H., Moschen A. Weight loss: Cornerstone in the treatment of non-alcoholic fatty liver disease. Minerva Gastroenterol. Dietol. 2010;56:159–167. [PubMed] [Google Scholar]
- 27.Promrat K., Kleiner D.E., Niemeier H.M., Jackvony E., Kearns M., Wands J.R., Fava J.L., Wing R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nobili V., Manco M., Devito R., di Ciommo V., Comparcola D., Sartorelli M.R., Piemonte F., Marcellini M., Angulo P. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: A randomized, controlled trial. Hepatology. 2008;48:119–128. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- 29.Koot B.G., van der Baan-Slootweg O.H., Tamminga-Smeulders C.L., Rijcken T.H., Korevaar J.C., van Aalderen W.M., Jansen P.L., Benninga M.A. Lifestyle intervention for non-alcoholic fatty liver disease: Prospective cohort study of its efficacy and factors related to improvement. Arch. Dis. Child. 2011;96:669–674. doi: 10.1136/adc.2010.199760. [DOI] [PubMed] [Google Scholar]
- 30.Reinehr T., Schmidt C., Toschke A.M., Andler W. Lifestyle intervention in obese children with non-alcoholic fatty liver disease: 2-year follow-up study. Arch. Dis. Child. 2009;94:437–442. doi: 10.1136/adc.2008.143594. [DOI] [PubMed] [Google Scholar]
- 31.Grønbæk H., Lange A., Birkebæk N.H., Holland-Fischer P., Solvig J., Hørlyck A., Kristensen K., Rittig S., Vilstrup H. Effect of a 10-week weight loss camp on fatty liver disease and insulin sensitivity in obese Danish children. J. Pediatr. Gastroenterol. Nutr. 2012;54:223–228. doi: 10.1097/MPG.0b013e31822cdedf. [DOI] [PubMed] [Google Scholar]
- 32.Bray G.A., Popkin B.M. Calorie-sweetened beverages and fructose: What have we learned 10 years later. Pediatr. Obes. 2013;8:242–248. doi: 10.1111/j.2047-6310.2013.00171.x. [DOI] [PubMed] [Google Scholar]
- 33.Caton P.W., Nayuni N.K., Khan N.Q., Wood E.G., Corde R. Fructose induces gluconeogenesis and lipogenesis through a SIRT1-dependent mechanism. J. Endocrinol. 2011;208:273–283. doi: 10.1530/JOE-10-0190. [DOI] [PubMed] [Google Scholar]
- 34.Lê K.A., Faeh D., Stettler R., Ith M., Kreis R., Vermathen P., Boesch C., Ravussin E., Tappy L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am. J. Clin. Nutr. 2006;84:1374–1379. doi: 10.1093/ajcn/84.6.1374. [DOI] [PubMed] [Google Scholar]
- 35.Lê K.A., Ith M., Kreis R., Faeh D., Bortolotti M., Tran C., Boesch C., Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009;89:1760–1765. doi: 10.3945/ajcn.2008.27336. [DOI] [PubMed] [Google Scholar]
- 36.Faeh D., Minehira K., Schwarz J.M., Periasamy R., Park S., Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54:1907–1913. doi: 10.2337/diabetes.54.7.1907. [DOI] [PubMed] [Google Scholar]
- 37.Silbernagel G., Machann J., Unmuth S., Schick F., Stefan N., Häring H.U., Fritsche A. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: An exploratory trial. Br. J. Nutr. 2011;106:79–86. doi: 10.1017/S000711451000574X. [DOI] [PubMed] [Google Scholar]
- 38.Crescenzo R., Bianco F., Coppola P., Mazzoli A., Tussellino M., Carotenuto R., Liverini G., Iossa S. Fructose supplementation worsens the deleterious effects of short-term high-fat feeding on hepatic steatosis and lipid metabolism in adult rats. Exp. Physiol. 2014 doi: 10.1113/expphysiol.2014.079632. in press. [DOI] [PubMed] [Google Scholar]
- 39.Sapp V., Gaffney L., EauClaire S.F., Matthews R.P. Fructose leads to hepatic steatosis in zebrafish that is reversed by mTOR inhibition. Hepatology. 2014 doi: 10.1002/hep.27284. [DOI] [PubMed] [Google Scholar]
- 40.Spruss A., Kanuri G., Wagnerberger S., Haub S., Bischoff S.C., Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 41.Vos M.B., Lavine J.E. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57:2525–2531. doi: 10.1002/hep.26299. [DOI] [PubMed] [Google Scholar]
- 42.Ouyang X., Cirillo P., Sautin Y., McCall S., Bruchette J.L., Diehl A.M., Johnson R.J., Abdelmalek M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thuy S., Ladurner R., Volynets V., Wagner S., Strahl S., Königsrainer A., Maier K.P., Bischoff S.C., Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J. Nutr. 2008;138:1452–1455. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 44.Bergheim I., Weber S., Vos M., Krämer S., Volynets V., Kaserouni S., McClain C.J., Bischoff S.C. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: Role of endotoxin. J. Hepatol. 2008;48:983–992. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 45.Assy N., Nasser G., Kamayse I., Nseir W., Beniashvili Z., Djibre A., Grosovski M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can. J. Gastroenterol. 2008;22:811–816. doi: 10.1155/2008/810961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan J.S., le M.T., Pan Z., Rivard C., Love-Osborne K., Robbins K., Johnson R.J., Sokol R.J., Sundaram S.S. Oral fructose absorption in obese children with non-alcoholic fatty liver disease. Pediatr. Obes. 2014 doi: 10.1111/ijpo.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin R., Welsh J.A., le N.A., Holzberg J., Sharma P., Martin D.R., Vos M.B. Dietary Fructose Reduction Improves Markers of Cardiovascular Disease Risk in Hispanic-American Adolescents with NAFLD. Nutrients. 2014;6:3187–3201. doi: 10.3390/nu6083187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., Neuschwander-Tetri B.A., Lavine J.E., Tonascia J., Unalp A., et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavine J.E., Schwimmer J.B., van Natta M.L., Molleston J.P., Murray K.F., Rosenthal P., Abrams S.H., Scheimann A.O., Sanyal A.J., Chalasani N., et al. Effect of vitamin E or metformin for Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents: The TONIC Randomized Controlled Trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarkhy A.A., Al-Hussaini A.A., Nobili V. Does vitamin E improve the outcomes of pediatric nonalcoholic fatty liver disease? A systematic review and meta-analysis. Saudi. J. Gastroenterol. 2014;20:143–153. doi: 10.4103/1319-3767.132983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capanni M., Calella F., Biagini M.R., Genise S., Raimondi L., Bedogni G., Svegliati-Baroni G., Sofi F., Milani S., Abbate R., et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: A pilot study. Aliment. Pharmacol. Ther. 2006;23:1143–1151. doi: 10.1111/j.1365-2036.2006.02885.x. [DOI] [PubMed] [Google Scholar]
- 52.Spadaro L., Magliocco O., Spampinato D., Piro S., Oliveri C., Alagona C., Papa G., Rabuazzo A.M., Purrello F. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig. Liver Dis. 2008;40:194–199. doi: 10.1016/j.dld.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Scorletti E., Bhatia L., McCormick K.G., Clough G.F., Nash K., Hodson L., Moyses H.E., Calder P.C., Byrne C.D. Effects of purified eicosapentaenoic and docosahexaenoic acids in non-alcoholic fatty liver disease: Results from the *WELCOME study. Hepatology. 2014 doi: 10.1002/hep.27289. [DOI] [PubMed] [Google Scholar]
- 54.Nobili V., Alisi A., Della Corte C., Risé P., Galli C., Agostoni C., Bedogni G. Docosahexaenoic acid for the treatment of fatty liver: Randomised controlled trial in children. Nutr. Metab. Cardiovasc. Dis. 2013;23:1066–1070. doi: 10.1016/j.numecd.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 55.St-Jules D.E., Watters C.A., Brunt E.M., Wilkens L.R., Novotny R., Belt P., Lavine J.E. Estimation of fish and ω-3 fatty acid intake in pediatric nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 2013;57:627–633. doi: 10.1097/MPG.0b013e3182a1df77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cani P.D., Possemiers S., van de Wiele T., Guiot Y., Everard A., Rottier O., Geurts L., Naslain D., Neyrinck A., Lambert D.M., et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukuda M., Komiyama Y., Mitsuyama K., Andoh A., Aoyama T., Matsumoto Y., Kanauchi O. Prebiotic treatment reduced preneoplastic lesions through the downregulation of toll like receptor 4 in a chemo-induced carcinogenic model. J. Clin. Biochem. Nutr. 2011;49:57–61. doi: 10.3164/jcbn.10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salminen S., Salminen E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scand. J. Gastroenterol. Suppl. 1997;222:45–48. doi: 10.1080/00365521.1997.11720717. [DOI] [PubMed] [Google Scholar]
- 59.Fan J.G., Xu Z.J., Wang G.L. Effect of lactulose on establishment of a rat non-alcoholic steatohepatitis model. World. J. Gastroenterol. 2005;11:5053–5056. doi: 10.3748/wjg.v11.i32.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holscher H.D., Faust K.L., Czerkies L.A., Litov R., Ziegler E.E., Lessin H., Hatch T., Sun S., Tappenden K.A. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN J. Parenter. Enteral. Nutr. 2012;36:95S–105S. doi: 10.1177/0148607111430087. [DOI] [PubMed] [Google Scholar]
- 61.Daubioul C.A., Horsmans Y., Lambert P., Danse E., Delzenne N.M. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: Results of a pilot study. Eur. J. Clin. Nutr. 2005;59:723–726. doi: 10.1038/sj.ejcn.1602127. [DOI] [PubMed] [Google Scholar]
- 62.Li Z., Yang S., Lin H., Huang J., Watkins P.A., Moser A.B., Desimone C., Song X.Y., Diehl A.M. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 63.Velayudham A., Dolganiuc A., Ellis M., Petrasek J., Kodys K., Mandrekar P., Szabo G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu R.Y., Wan Y.P., Fang Q.Y., Lu W., Cai W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J. Clin. Biochem. Nutr. 2012;50:72–77. doi: 10.3164/jcbn.11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karahan N., Işler M., Koyu A., Karahan A.G., Başyığıt Kiliç G., Cırış I.M., Sütçü R., Onaran I., Cam H., Keskın M. Effects of probiotics on methionine choline deficient diet-induced steatohepatitis in rats. Turk. J. Gastroenterol. 2012;23:110–121. doi: 10.4318/tjg.2012.0330. [DOI] [PubMed] [Google Scholar]
- 66.Mencarelli A., Cipriani S., Renga B., Bruno A., D’Amore C., Distrutti E., Fiorucci S. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aller R., de Luis D.A., Izaola O., Conde R., Gonzalez Sagrado M., Primo D., de La Fuente B., Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011;15:1090–1095. [PubMed] [Google Scholar]
- 68.Alisi A., Bedogni G., Baviera G., Giorgio V., Porro E., Paris C., Giammaria P., Reali L., Anania F., Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2014;39:1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]