Abstract
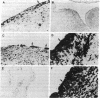
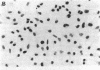
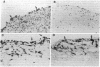
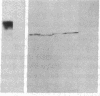
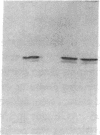
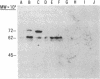
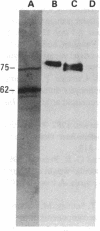
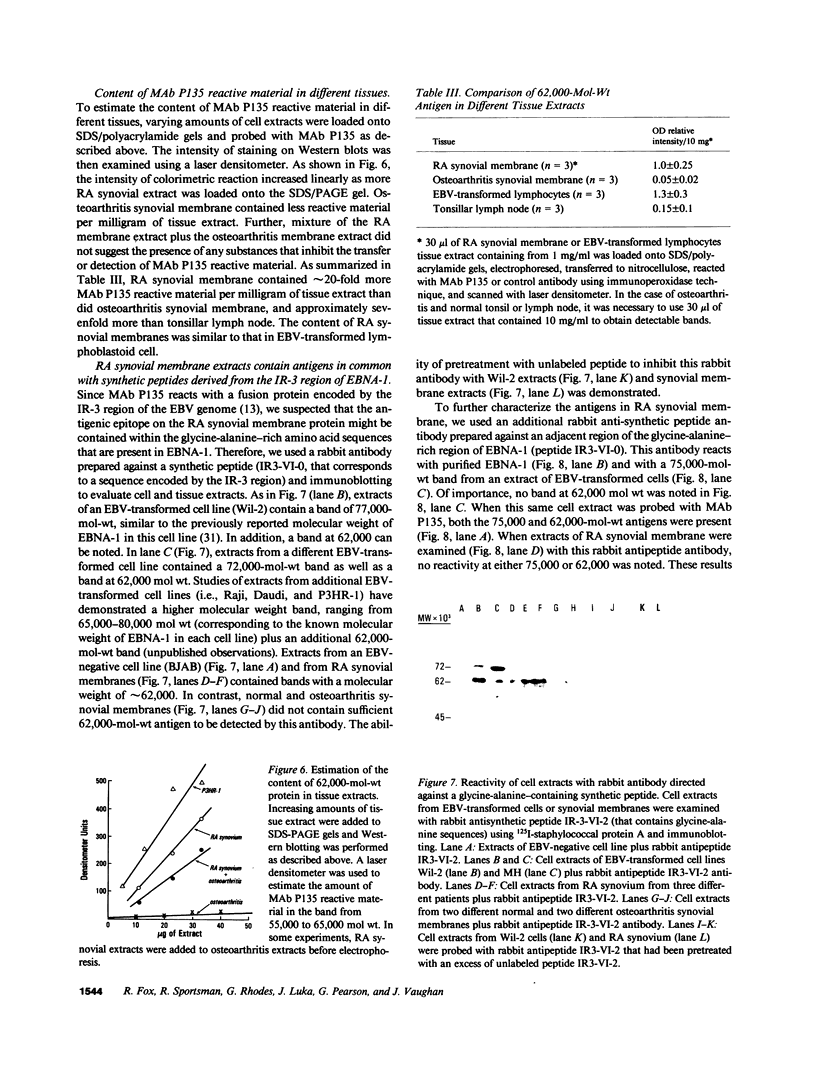
A monoclonal antibody, selected for reactivity with the Epstein-Barr virus (EBV)-encoded antigen EBNA-1, exhibited strong reactivity with the synovial lining cells in joint biopsies from 10 of 12 patients with rheumatoid arthritis (RA) and adherent cells eluted from these tissues. No staining of RA synovial membrane frozen tissue sections or eluted synovial-lining cells was obtained with monoclonal antibodies directed against other EBV-encoded antigens (anti-p160, anti-gp200/350) or with monoclonal antibodies directed against antigens encoded by cytomegalovirus, herpes simplex viruses, or human T cell leukemia virus type I. Among 12 osteoarthritis and normal synovial biopsies only rare reactive cells were noted. Characterization of the antigen(s) in RA synovium by the Western immunoblotting technique revealed a 62,000-molecular-weight (mol-wt) protein, in contrast to the 70,000-85,000-mol-wt EBNA-1 antigen found in EBV-transformed cells. The structural basis for the cross-reactivity of the RA synovial membrane 62,000-mol-wt protein and the EBNA-1 antigen appears to reside in the glycine-alanine rich region of these molecules. A rabbit antibody directed against a synthetic peptide (IR3-VI-2) derived from the glycine-alanine-rich region of EBNA-1 reacted with the 70,000-85,000-mol-wt EBNA-1 antigen in EBV-infected cells and with the 62,000-mol-wt molecule in RA synovial membrane extracts. Since strong antibody responses to EBNA-1 are known to exist in RA patients, these results suggest that immune responses to a cross-reactive antigen may play a role in the pathogenesis of RA.
Full text
PDF


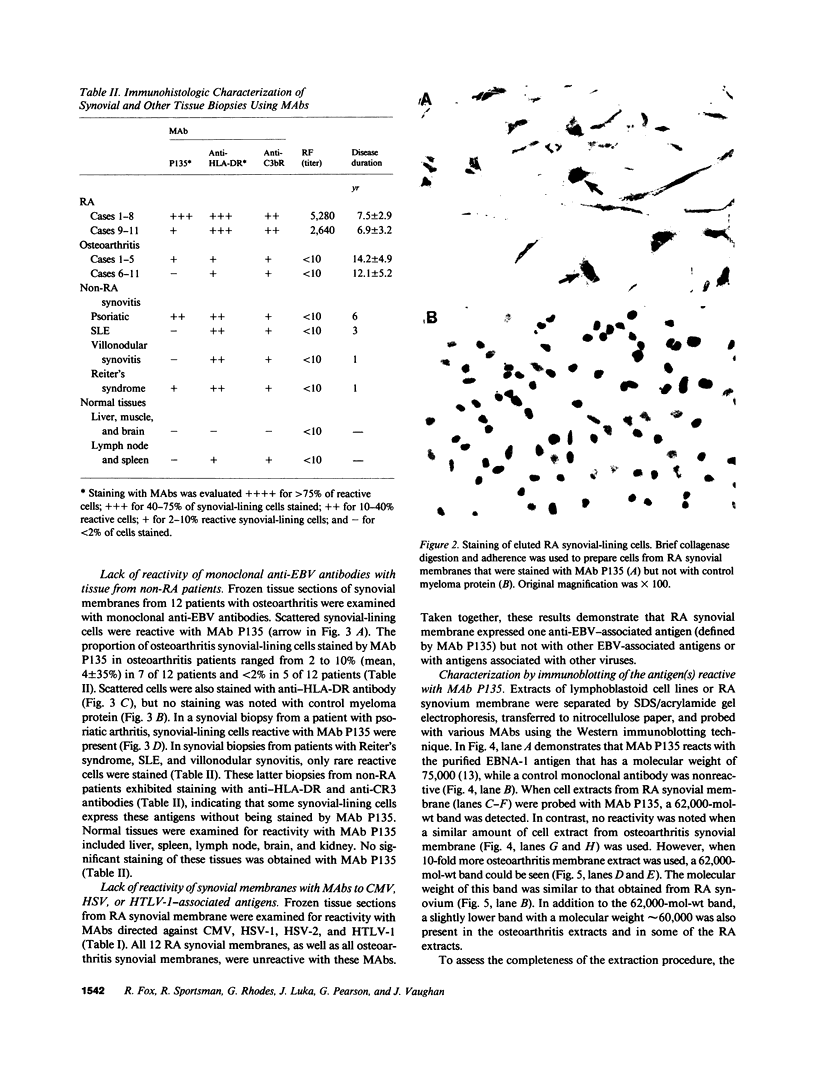




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alspaugh M. A., Shoji H., Nonoyama M. A search for rheumatoid arthritis-associated nuclear antigen and Epstein-Barr virus specific antigens or genomes in tissues and cells from patients with rheumatoid arthritis. Arthritis Rheum. 1983 Jun;26(6):712–720. doi: 10.1002/art.1780260603. [DOI] [PubMed] [Google Scholar]
- Aslpaugh M. A., Tan E. M. Serum antibody in rheumatoid arthritis reactive with a cell-associated antigen. Demonstration by precipitation and immunofluorescence. Arthritis Rheum. 1976 Jul-Aug;19(4):711–719. doi: 10.1002/1529-0131(197607/08)19:4<711::aid-art1780190409>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Billings P. B., Hoch S. O., White P. J., Carson D. A., Vaughan J. H. Antibodies to the Epstein-Barr virus nuclear antigen and to rheumatoid arthritis nuclear antigen identify the same polypeptide. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7104–7108. doi: 10.1073/pnas.80.23.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano M. A., Carson D. A., Niederman J. C., Feorino P., Vaughan J. H. Antibody to the rheumatoid arthritis nuclear antigen. Its relationship to in vivo Epstein-Barr virus infection. J Clin Invest. 1980 May;65(5):1238–1242. doi: 10.1172/JCI109779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Multiple, heart-cross-reactive epitopes of streptococcal M proteins. J Exp Med. 1985 Jan 1;161(1):113–122. doi: 10.1084/jem.161.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depper J. M., Bluestein H. G., Zvaifler N. J. Impaired regulation of Epstein-Barr virus-induced lymphocyte proliferation in rheumatoid arthritis is due to a T cell defect. J Immunol. 1981 Nov;127(5):1899–1902. [PubMed] [Google Scholar]
- Depper J. M., Zvaifler N. J. Epstein-Barr virus. Its relationship to the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 1981 Jun;24(6):755–761. doi: 10.1002/art.1780240601. [DOI] [PubMed] [Google Scholar]
- Ferrell P. B., Aitcheson C. T., Pearson G. R., Tan E. M. Seroepidemiological study of relationships between Epstein-Barr virus and rheumatoid arthritis. J Clin Invest. 1981 Mar;67(3):681–687. doi: 10.1172/JCI110083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Fox R. I., Thompson L. F., Huddlestone J. R. T gamma cells express T lymphocyte-associated antigens. J Immunol. 1981 May;126(5):2062–2063. [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B., Wroblewska Z., Frankel M. E., Koprowski H. Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2346–2350. doi: 10.1073/pnas.80.8.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. C., Corey L., McDougall J. K., Tolentino E., Nowinski R. C. Monoclonal antibodies to herpes simplex viruses: use in antigenic typing and rapid diagnosis. J Infect Dis. 1983 May;147(5):829–837. doi: 10.1093/infdis/147.5.829. [DOI] [PubMed] [Google Scholar]
- Goldstein L. C., McDougall J., Hackman R., Meyers J. D., Thomas E. D., Nowinski R. C. Monoclonal antibodies to cytomegalovirus: rapid identification of clinical isolates and preliminary use in diagnosis of cytomegalovirus pneumonia. Infect Immun. 1982 Oct;38(1):273–281. doi: 10.1128/iai.38.1.273-281.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Henderson A., Kieff E. Repeat array in Epstein-Barr virus DNA is related to cell DNA sequences interspersed on human chromosomes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5916–5920. doi: 10.1073/pnas.79.19.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Kieff E. One of two Epstein-Barr virus nuclear antigens contains a glycine-alanine copolymer domain. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5665–5669. doi: 10.1073/pnas.80.18.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F., Austin R. K., Hubert J. J., Bernardin J. E., Kasarda D. D. Possible role for a human adenovirus in the pathogenesis of celiac disease. J Exp Med. 1984 Nov 1;160(5):1544–1557. doi: 10.1084/jem.160.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay H. D., Horwitz D. A. Evidence by reactivity with hybridoma antibodies for a probable myeloid origin of peripheral blood cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Clin Invest. 1980 Oct;66(4):847–851. doi: 10.1172/JCI109923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Kabelitz D., Plöen L., Sundström C., Nilsson K., Wigren A., Wigzell H. Immune functions of human synovial cells. Phenotypic and T cell regulatory properties of macrophage-like cells that express HLA-DR. Arthritis Rheum. 1982 May;25(5):488–501. doi: 10.1002/art.1780250502. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Malmnäs Tjernlund U. K., Kabelitz D., Wigren A. Appearance of anti-HLA-DR-reactive cells in normal and rheumatoid synovial tissue. Scand J Immunol. 1981 Aug;14(2):183–192. doi: 10.1111/j.1365-3083.1981.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Reitamo S., Ranki A., Häyry P., Kankaanapä U., Wegelius O. Characterization of the immunocompetent cells of rheumatoid synovium from tissue sections and eluates. Arthritis Rheum. 1981 Jan;24(1):71–79. doi: 10.1002/art.1780240112. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Linder E., Kurki P., Andersson L. C. Autoantibody to "intermediate filament" in infectious mononucleosis. Clin Immunol Immunopathol. 1979 Dec;14(4):411–417. doi: 10.1016/0090-1229(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Luka J., Kreofsky T., Pearson G. R., Hennessy K., Kieff E. Identification and characterization of a cellular protein that cross-reacts with the Epstein-Barr virus nuclear antigen. J Virol. 1984 Dec;52(3):833–838. doi: 10.1128/jvi.52.3.833-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osung O. A., Chandra M., Holborow E. J. Antibody to intermediate filaments of the cytoskeleton. Ann Rheum Dis. 1982 Feb;41(1):69–73. doi: 10.1136/ard.41.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. R., Vroman B., Chase B., Sculley T., Hummel M., Kieff E. Identification of polypeptide components of the Epstein-Barr virus early antigen complex with monoclonal antibodies. J Virol. 1983 Jul;47(1):193–201. doi: 10.1128/jvi.47.1.193-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualtiere L. F., Chase R., Pearson G. R. Purification and biologic characterization of a major Epstein Barr virus-induced membrane glycoprotein. J Immunol. 1982 Aug;129(2):814–818. [PubMed] [Google Scholar]
- Reinherz E. L., Moretta L., Roper M., Breard J. M., Mingari M. C., Cooper M. D., Schlossman S. F. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. A comparison. J Exp Med. 1980 Apr 1;151(4):969–974. doi: 10.1084/jem.151.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G., Carson D. A., Valbracht J., Houghten R., Vaughan J. H. Human immune responses to synthetic peptides from the Epstein-Barr nuclear antigen. J Immunol. 1985 Jan;134(1):211–216. [PubMed] [Google Scholar]
- Rhodes G., Houghten R., Taulane J. P., Carson D., Vaughan J. The immune response to Epstein-Barr nuclear antigen: conformational and structural features of antibody binding to synthetic peptides. Mol Immunol. 1984 Nov;21(11):1047–1054. doi: 10.1016/0161-5890(84)90114-7. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Ruscetti F. W., Posner L. E., Poiesz B. J., Gallo R. C. Detection of the human T cell lymphoma virus p19 in cells of some patients with cutaneous T cell lymphoma and leukemia using a monoclonal antibody. J Exp Med. 1981 Dec 1;154(6):1957–1964. doi: 10.1084/jem.154.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter L., Carson D. A., Jensen F. C., Holbrook T. L., Vaughan J. H. In vitro effects of Epstein-Barr virus on peripheral blood mononuclear cells from patients with rheumatoid arthritis and normal subjects. J Exp Med. 1978 Nov 1;148(5):1429–1434. doi: 10.1084/jem.148.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportsman J. R., Elder J. H. A microanalytical protein assay using laser densitometry. Anal Biochem. 1984 Jun;139(2):298–300. doi: 10.1016/0003-2697(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Stefansson K., Dieperink M. E., Richman D. P., Gomez C. M., Marton L. S. Sharing of antigenic determinants between the nicotinic acetylcholine receptor and proteins in Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae. Possible role in the pathogenesis of myasthenia gravis. N Engl J Med. 1985 Jan 24;312(4):221–225. doi: 10.1056/NEJM198501243120407. [DOI] [PubMed] [Google Scholar]
- Toh B. H., Yildiz A., Sotelo J., Osung O., Holborow E. J., Kanakoudi F., Small J. V. Viral infections and IgM autoantibodies to cytoplasmic intermediate filaments. Clin Exp Immunol. 1979 Jul;37(1):76–82. [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Blaese R. M. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N Engl J Med. 1981 Nov 19;305(21):1238–1243. doi: 10.1056/NEJM198111193052102. [DOI] [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Yarchoan R., Heilman C. A., Pike S. E., De Seau V., Blaese R. M. Abnormally elevated frequency of Epstein-Barr virus-infected B cells in the blood of patients with rheumatoid arthritis. J Clin Invest. 1984 Jun;73(6):1789–1795. doi: 10.1172/JCI111388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman B., Luka J., Rodriguez M., Pearson G. R. Characterization of a major protein with a molecular weight of 160,000 associated with the viral capsid of Epstein-Barr virus. J Virol. 1985 Jan;53(1):107–113. doi: 10.1128/jvi.53.1.107-113.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Burmester G. R. Demonstration of Ia antigens on certain dendritic cells and on a novel elongate cell found in human synovial tissue. Scand J Immunol. 1981 Oct;14(4):439–444. doi: 10.1111/j.1365-3083.1981.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. L., Adamson T. C., 3rd, Vaughan J. H., Fox R. I. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1984 Jan;27(1):32–39. doi: 10.1002/art.1780270106. [DOI] [PubMed] [Google Scholar]
- van de Rijn I., Zabriskie J. B., McCarty M. Group A streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterization of the streptococcal antigen. J Exp Med. 1977 Aug 1;146(2):579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]