Abstract
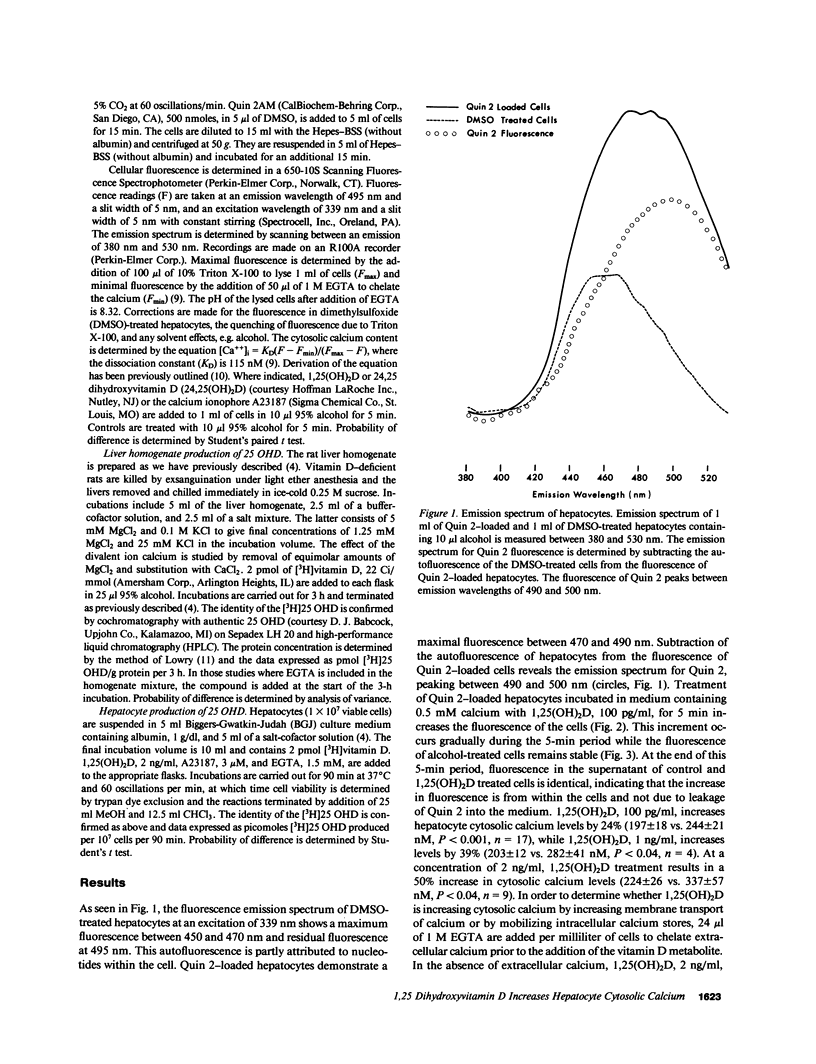
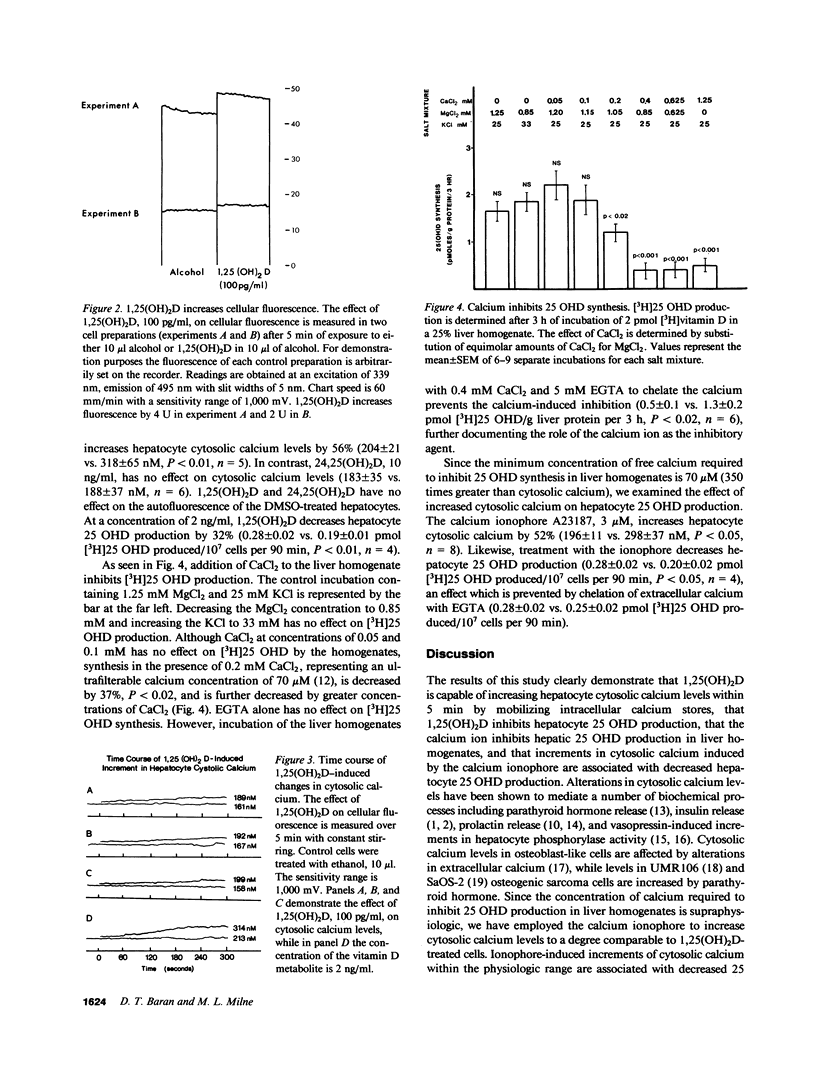
1,25 dihydroxyvitamin D (1,25(OH)2D) has been demonstrated to inhibit hepatic 25 hydroxyvitamin D (25 OHD) production. Changes in cytosolic calcium have been shown to regulate cellular processes. Using the fluorescent dye Quin 2, we have investigated the effects of 1,25(OH)2D and 24,25(OH)2D on cytosolic calcium levels in hepatocytes. 1,25(OH)2D exposure for 5 min increases cytosolic calcium levels by 24% at a concentration of 100 pg/ml, 39% at a concentration of 1 ng/ml, and 50% at a concentration of 2 ng/ml. The latter increment occurs in both the presence and absence of extracellular calcium, indicating that 1,25(OH)2D is mobilizing intracellular calcium pools. 24,25(OH)2D, 10 ng/ml, does not increase cytosolic calcium levels while the calcium ionophore A23187, 3 microM, increases levels by 52%. Calcium inhibits hepatic 25 OHD synthesis in liver homogenates in a dose-dependent fashion, which can be prevented by chelation of calcium with EGTA. 1,25(OH)2D and A23187 decrease hepatocyte 25 OHD synthesis. The inhibitory effect of A23187 can be prevented by chelation of extracellular calcium. The data demonstrate that 1,25(OH)2D increases hepatocyte cytosolic calcium, and that these increments in cytosolic calcium may regulate some of the hepatic actions of the vitamin D metabolite.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Thyrotropin-releasing hormone-induced spike and plateau in cytosolic free Ca2+ concentrations in pituitary cells. Relation to prolactin release. J Biol Chem. 1984 May 10;259(9):5827–5832. [PubMed] [Google Scholar]
- Baran D. T. Effect of phenobarbital treatment on metabolism of vitamin D by rat liver. Am J Physiol. 1983 Jul;245(1):E55–E59. doi: 10.1152/ajpendo.1983.245.1.E55. [DOI] [PubMed] [Google Scholar]
- Baran D. T., Fausto A. C., Roberts M. L., Karl I., Avioli L. V. Phenobarbital-induced alterations in the metabolism of [3H]vitamin D3 by the perfused rachitic rat liver in vitro. J Clin Invest. 1979 Oct;64(4):1112–1117. doi: 10.1172/JCI109550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran D. T., Milne M. L. 1,25 dihydroxyvitamin D-induced inhibition of 3H-25 hydroxyvitamin D production by the rachitic rat liver in vitro. Calcif Tissue Int. 1983 Jul;35(4-5):461–464. doi: 10.1007/BF02405077. [DOI] [PubMed] [Google Scholar]
- Bengoa J. M., Bolt M. J., Rosenberg I. H. Hepatic vitamin D 25-hydroxylase inhibition by cimetidine and isoniazid. J Lab Clin Med. 1984 Oct;104(4):546–552. [PubMed] [Google Scholar]
- Dueland S., Holmberg I., Berg T., Pedersen J. I. Uptake and 25-hydroxylation of vitamin D3 by isolated rat liver cells. J Biol Chem. 1981 Oct 25;256(20):10430–10434. [PubMed] [Google Scholar]
- Kadowaki S., Norman A. W. Demonstration that the vitamin D metabolite 1,25(OH)2-vitamin D3 and not 24R,25(OH)2-vitamin D3 is essential for normal insulin secretion in the perfused rat pancreas. Diabetes. 1985 Apr;34(4):315–320. doi: 10.2337/diab.34.4.315. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loré F., Di Cairano G., Periti P., Caniggia A. Effect of the administration of 1,25-dihydroxyvitamin D3 on serum levels of 25-hydroxyvitamin D in postmenopausal osteoporosis. Calcif Tissue Int. 1982;34(6):539–541. doi: 10.1007/BF02411300. [DOI] [PubMed] [Google Scholar]
- MacLaughlin J. A., Weiser M. M., Freedman R. A. Biphasic recovery of vitamin D-dependent Ca2+ uptake by rat intestinal Golgi membranes. Gastroenterology. 1980 Feb;78(2):325–332. [PubMed] [Google Scholar]
- Manolagas S. C., Deftos L. J. The vitamin D endocrine system and the hematolymphopoietic tissue. Ann Intern Med. 1984 Jan;100(1):144–146. doi: 10.7326/0003-4819-100-1-144. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Kowalchyk J. A. Evidence for the role of calcium and diacylglycerol as dual second messengers in thyrotropin-releasing hormone action: involvement of diacylglycerol. Endocrinology. 1984 Oct;115(4):1517–1526. doi: 10.1210/endo-115-4-1517. [DOI] [PubMed] [Google Scholar]
- Milne M. L., Baran D. T. Acute metabolic acidosis stimulates 3H-25 hydroxyvitamin D production by the rachitic rat liver. Calcif Tissue Int. 1985 Jan;37(1):77–81. doi: 10.1007/BF02557683. [DOI] [PubMed] [Google Scholar]
- Milne M. L., Baran D. T. End product inhibition of hepatic 25-hydroxyvitamin D production in the rat: specificity and kinetics. Arch Biochem Biophys. 1985 Nov 1;242(2):488–492. doi: 10.1016/0003-9861(85)90234-6. [DOI] [PubMed] [Google Scholar]
- Milne M., Baran D. T. Inhibitory effect of maternal alcohol ingestion on rat pup hepatic 25-hydroxyvitamin D production. Pediatr Res. 1985 Jan;19(1):102–104. doi: 10.1203/00006450-198501000-00027. [DOI] [PubMed] [Google Scholar]
- Nemere I., Szego C. M. Early actions of parathyroid hormone and 1,25-dihydroxycholecalciferol on isolated epithelial cells from rat intestine: I. Limited lysosomal enzyme release and calcium uptake. Endocrinology. 1981 Apr;108(4):1450–1462. doi: 10.1210/endo-108-4-1450. [DOI] [PubMed] [Google Scholar]
- Nemere I., Yoshimoto Y., Norman A. W. Calcium transport in perfused duodena from normal chicks: enhancement within fourteen minutes of exposure to 1,25-dihydroxyvitamin D3. Endocrinology. 1984 Oct;115(4):1476–1483. doi: 10.1210/endo-115-4-1476. [DOI] [PubMed] [Google Scholar]
- Shoback D., Thatcher J., Leombruno R., Brown E. Effects of extracellular Ca++ and Mg++ on cytosolic Ca++ and PTH release in dispersed bovine parathyroid cells. Endocrinology. 1983 Jul;113(1):424–426. doi: 10.1210/endo-113-1-424. [DOI] [PubMed] [Google Scholar]
- Siegel E. G., Wollheim C. B., Janjic D., Ribes G., Sharp G. W. Involvement of Ca2+ in the impaired glucose-induced insulin release from islets cultured at low glucose. Diabetes. 1983 Nov;32(11):993–1000. doi: 10.2337/diab.32.11.993. [DOI] [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Voltage-dependent calcium channels in pituitary cells in culture. II. Participation in thyrotropin-releasing hormone action on prolactin release. J Biol Chem. 1984 Jan 10;259(1):427–434. [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Thomas A. P., Marks J. S., Coll K. E., Williamson J. R. Quantitation and early kinetics of inositol lipid changes induced by vasopressin in isolated and cultured hepatocytes. J Biol Chem. 1983 May 10;258(9):5716–5725. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Pozzan T. Correlation between cytosolic free Ca2+ and insulin release in an insulin-secreting cell line. J Biol Chem. 1984 Feb 25;259(4):2262–2267. [PubMed] [Google Scholar]
- Wortsman J., Killam L. I., Traycoff R. B. A rapid method for the determination of ultrafilterable calcium in serum. J Lab Clin Med. 1981 Nov;98(5):691–696. [PubMed] [Google Scholar]


