Abstract
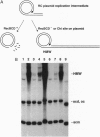
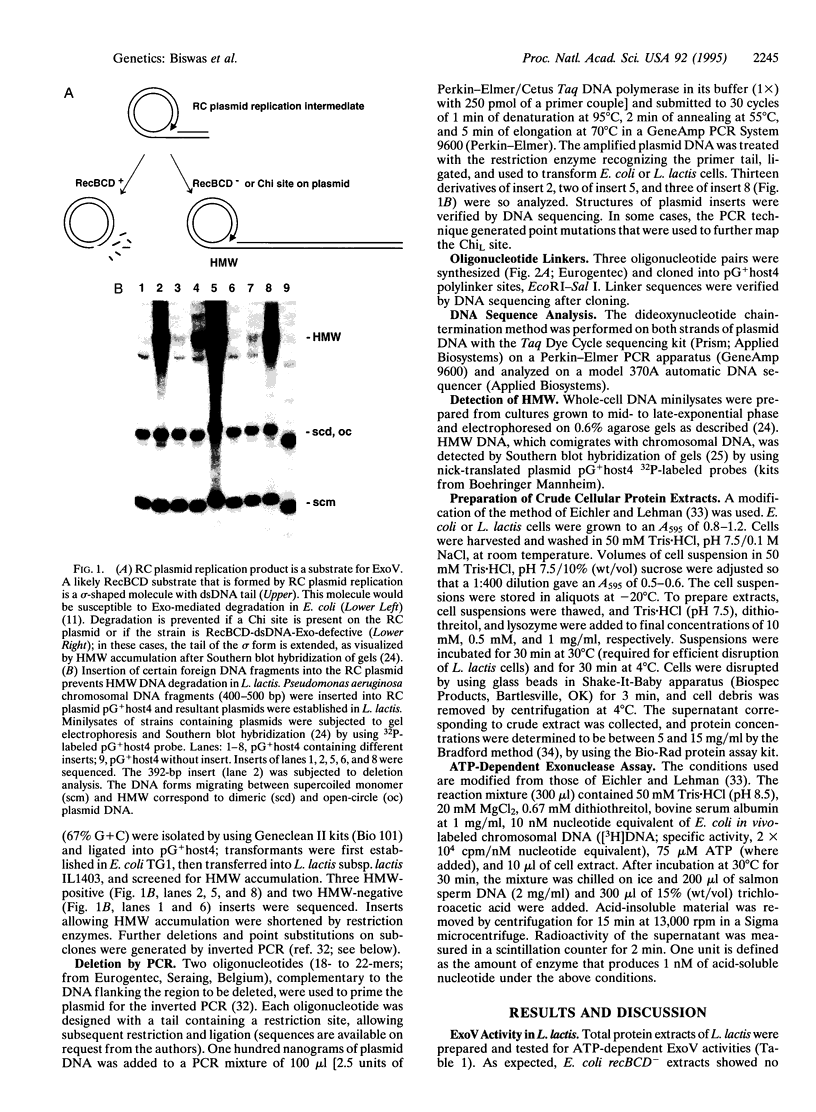
Linear DNA molecules are subject to degradation by various exonucleases in vivo unless their ends are protected. It has been demonstrated that a specific 8-bp sequence, 5'-GCTGGTGG-3', named Chi, can protect linear double-stranded DNA from the major Escherichia coli exonuclease RecBCD. Chi protects linear replication products of rolling-circle plasmids from RecBCD degradation in vivo, in agreement with observations in vitro. A unique 7-bp sequence, 5'-GCGCGTG-3', is shown to protect similar replication products from degradation in Lactococcus lactis strains but not in more distantly related Gram-positive bacteria. The properties of this sequence in L. lactis correspond to those of a Chi site. Linear plasmid replication products have been detected in numerous prokaryotes, suggesting the widespread existence of short species-specific sequences that preserve linear DNA from extensive degradation by host cell exonucleases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amundsen S. K., Neiman A. M., Thibodeaux S. M., Smith G. R. Genetic dissection of the biochemical activities of RecBCD enzyme. Genetics. 1990 Sep;126(1):25–40. doi: 10.1093/genetics/126.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell V. J., Treisman R. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 1994 Jul 15;8(14):1664–1677. doi: 10.1101/gad.8.14.1664. [DOI] [PubMed] [Google Scholar]
- Benedyk M. J., Mullen J. R., DiNardo S. odd-paired: a zinc finger pair-rule protein required for the timely activation of engrailed and wingless in Drosophila embryos. Genes Dev. 1994 Jan;8(1):105–117. doi: 10.1101/gad.8.1.105. [DOI] [PubMed] [Google Scholar]
- Burland V., Plunkett G., 3rd, Daniels D. L., Blattner F. R. DNA sequence and analysis of 136 kilobases of the Escherichia coli genome: organizational symmetry around the origin of replication. Genomics. 1993 Jun;16(3):551–561. doi: 10.1006/geno.1993.1230. [DOI] [PubMed] [Google Scholar]
- Chen Z., Brand N. J., Chen A., Chen S. J., Tong J. H., Wang Z. Y., Waxman S., Zelent A. Fusion between a novel Krüppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 1993 Mar;12(3):1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. C., Smith G. R. Cutting of chi-like sequences by the RecBCD enzyme of Escherichia coli. J Mol Biol. 1987 Apr 20;194(4):747–750. doi: 10.1016/0022-2836(87)90252-x. [DOI] [PubMed] [Google Scholar]
- Chopin A., Chopin M. C., Moillo-Batt A., Langella P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984 May;11(3):260–263. doi: 10.1016/0147-619x(84)90033-7. [DOI] [PubMed] [Google Scholar]
- Dabert P., Ehrlich S. D., Gruss A. Chi sequence protects against RecBCD degradation of DNA in vivo. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12073–12077. doi: 10.1073/pnas.89.24.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabert P., Ehrlich S. D., Gruss A. High-molecular-weight linear multimer formation by single-stranded DNA plasmids in Escherichia coli. J Bacteriol. 1992 Jan;174(1):173–178. doi: 10.1128/jb.174.1.173-178.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. B., Deich R. A., Sisco K. L., Smith H. O. An eleven-base-pair sequence determines the specificity of DNA uptake in Haemophilus transformation. Gene. 1980 Nov;11(3-4):311–318. doi: 10.1016/0378-1119(80)90071-2. [DOI] [PubMed] [Google Scholar]
- Dower N. A., Stahl F. W. Chi activity during transduction-associated recombination. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7033–7037. doi: 10.1073/pnas.78.11.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler D. C., Lehman I. R. On the role of ATP in phosphodiester bond hydrolysis catalyzed by the recBC deoxyribonuclease of Escherichia coli. J Biol Chem. 1977 Jan 25;252(2):499–503. [PubMed] [Google Scholar]
- Figdor M. C., Stern C. D. Segmental organization of embryonic diencephalon. Nature. 1993 Jun 17;363(6430):630–634. doi: 10.1038/363630a0. [DOI] [PubMed] [Google Scholar]
- Fraser M. J., Koa H., Chow T. Y. Neurospora endo-exonuclease is immunochemically related to the recC gene product of Escherichia coli. J Bacteriol. 1990 Jan;172(1):507–510. doi: 10.1128/jb.172.1.507-510.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser S., Keynes R., Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990 Mar 29;344(6265):431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S., DeFranco D. Lethal chromosomal deletions in the mouse, a model system for the study of development and regulation of postnatal gene expression. Bioessays. 1991 Nov;13(11):557–561. doi: 10.1002/bies.950131102. [DOI] [PubMed] [Google Scholar]
- Hemsley A., Arnheim N., Toney M. D., Cortopassi G., Galas D. J. A simple method for site-directed mutagenesis using the polymerase chain reaction. Nucleic Acids Res. 1989 Aug 25;17(16):6545–6551. doi: 10.1093/nar/17.16.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch J., Barbour A. G. Linear plasmids of Borrelia burgdorferi have a telomeric structure and sequence similar to those of a eukaryotic virus. J Bacteriol. 1991 Nov;173(22):7233–7239. doi: 10.1128/jb.173.22.7233-7239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holo H., Nes I. F. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Appl Environ Microbiol. 1989 Dec;55(12):3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., McBurney M. W., Rogers K. A., Kalnins V. I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982 Aug;94(2):253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey G., Ruppert S., Beermann F., Grund C., Tanguay R. M., Schütz G. Rescue of mice homozygous for lethal albino deletions: implications for an animal model for the human liver disease tyrosinemia type 1. Genes Dev. 1993 Dec;7(12A):2285–2297. doi: 10.1101/gad.7.12a.2285. [DOI] [PubMed] [Google Scholar]
- Kenter A. L., Birshtein B. K. Chi, a promoter of generalized recombination in lambda phage, is present in immunoglobulin genes. Nature. 1981 Oct 1;293(5831):402–404. doi: 10.1038/293402a0. [DOI] [PubMed] [Google Scholar]
- Kooistra J., Haijema B. J., Venema G. The Bacillus subtilis addAB genes are fully functional in Escherichia coli. Mol Microbiol. 1993 Mar;7(6):915–923. doi: 10.1111/j.1365-2958.1993.tb01182.x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krowczynska A. M., Rudders R. A., Krontiris T. G. The human minisatellite consensus at breakpoints of oncogene translocations. Nucleic Acids Res. 1990 Mar 11;18(5):1121–1127. doi: 10.1093/nar/18.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Kuzminov A., Schabtach E., Stahl F. W. Chi sites in combination with RecA protein increase the survival of linear DNA in Escherichia coli by inactivating exoV activity of RecBCD nuclease. EMBO J. 1994 Jun 15;13(12):2764–2776. doi: 10.1002/j.1460-2075.1994.tb06570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S. T., Stahl M. M., McMilin K. D., Stahl F. W. Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics. 1974 Jul;77(3):425–433. doi: 10.1093/genetics/77.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J. M., Louarn J., François V., Patte J. Analysis and possible role of hyperrecombination in the termination region of the Escherichia coli chromosome. J Bacteriol. 1991 Aug;173(16):5097–5104. doi: 10.1128/jb.173.16.5097-5104.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A., Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989 Feb 2;337(6206):424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Lumsden A. The cellular basis of segmentation in the developing hindbrain. Trends Neurosci. 1990 Aug;13(8):329–335. doi: 10.1016/0166-2236(90)90144-y. [DOI] [PubMed] [Google Scholar]
- Maguin E., Duwat P., Hege T., Ehrlich D., Gruss A. New thermosensitive plasmid for gram-positive bacteria. J Bacteriol. 1992 Sep;174(17):5633–5638. doi: 10.1128/jb.174.17.5633-5638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H., Nonchev S., Sham M. H., Muchamore I., Lumsden A., Krumlauf R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature. 1992 Dec 24;360(6406):737–741. doi: 10.1038/360737a0. [DOI] [PubMed] [Google Scholar]
- McKittrick N. H., Smith G. R. Activation of Chi recombinational hotspots by RecBCD-like enzymes from enteric bacteria. J Mol Biol. 1989 Dec 5;210(3):485–495. doi: 10.1016/0022-2836(89)90125-3. [DOI] [PubMed] [Google Scholar]
- Murphy K. C. Lambda Gam protein inhibits the helicase and chi-stimulated recombination activities of Escherichia coli RecBCD enzyme. J Bacteriol. 1991 Sep;173(18):5808–5821. doi: 10.1128/jb.173.18.5808-5821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. S., Stahl F. W. Chi and the RecBC D enzyme of Escherichia coli. Annu Rev Genet. 1994;28:49–70. doi: 10.1146/annurev.ge.28.120194.000405. [DOI] [PubMed] [Google Scholar]
- Ponticelli A. S., Schultz D. W., Taylor A. F., Smith G. R. Chi-dependent DNA strand cleavage by RecBC enzyme. Cell. 1985 May;41(1):145–151. doi: 10.1016/0092-8674(85)90069-8. [DOI] [PubMed] [Google Scholar]
- Puelles L., Rubenstein J. L. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 1993 Nov;16(11):472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Kelsey G., Schedl A., Schmid E., Thies E., Schütz G. Deficiency of an enzyme of tyrosine metabolism underlies altered gene expression in newborn liver of lethal albino mice. Genes Dev. 1992 Aug;6(8):1430–1443. doi: 10.1101/gad.6.8.1430. [DOI] [PubMed] [Google Scholar]
- Schneider-Maunoury S., Topilko P., Seitandou T., Levi G., Cohen-Tannoudji M., Pournin S., Babinet C., Charnay P. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993 Dec 17;75(6):1199–1214. doi: 10.1016/0092-8674(93)90329-o. [DOI] [PubMed] [Google Scholar]
- Sham M. H., Vesque C., Nonchev S., Marshall H., Frain M., Gupta R. D., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993 Jan 29;72(2):183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Sisco K. L., Smith H. O. Sequence-specific DNA uptake in Haemophilus transformation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):972–976. doi: 10.1073/pnas.76.2.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Kunes S. M., Schultz D. W., Taylor A., Triman K. L. Structure of chi hotspots of generalized recombination. Cell. 1981 May;24(2):429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Stahl F. W., Crasemann J. M., Stahl M. M. Rec-mediated recombinational hot spot activity in bacteriophage lambda. III. Chi mutations are site-mutations stimulating rec-mediated recombination. J Mol Biol. 1975 May 15;94(2):203–212. doi: 10.1016/0022-2836(75)90078-9. [DOI] [PubMed] [Google Scholar]
- Stahl F. W., Thomason L. C., Siddiqi I., Stahl M. M. Further tests of a recombination model in which chi removes the RecD subunit from the RecBCD enzyme of Escherichia coli. Genetics. 1990 Nov;126(3):519–533. doi: 10.1093/genetics/126.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatek P. J., Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 1993 Nov;7(11):2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler D. S., Sampson E., Siddiqi I., Rosenberg S. M., Thomason L. C., Stahl F. W., Stahl M. M. Recombination of bacteriophage lambda in recD mutants of Escherichia coli. Genome. 1989;31(1):53–67. doi: 10.1139/g89-013. [DOI] [PubMed] [Google Scholar]
- Topilko P., Schneider-Maunoury S., Levi G., Baron-Van Evercooren A., Chennoufi A. B., Seitanidou T., Babinet C., Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994 Oct 27;371(6500):796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Tönjes R. R., Xanthopoulos K. G., Darnell J. E., Jr, Paul D. Transcriptional control in hepatocytes of normal and c14CoS albino deletion mice. EMBO J. 1992 Jan;11(1):127–133. doi: 10.1002/j.1460-2075.1992.tb05035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret J. F., Alonso J. C. Generation of linear multigenome-length plasmid molecules in Bacillus subtilis. Nucleic Acids Res. 1987 Aug 25;15(16):6349–6367. doi: 10.1093/nar/15.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell R. P., Jr, de Thé H., Wang Z. Y., Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993 Jul 15;329(3):177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G. Molecular mechanisms of segmental patterning in the vertebrate hindbrain and neural crest. Bioessays. 1993 Aug;15(8):499–505. doi: 10.1002/bies.950150802. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Radding C. M. Partial purification and properties of an exonuclease inhibitor induced by bacteriophage Mu-1. J Virol. 1981 Aug;39(2):548–558. doi: 10.1128/jvi.39.2.548-558.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Saunders T. L., Bach F. H. Polymorphism of human Ia antigens generated by reciprocal intergenic exchange between two DR beta loci. Nature. 1986 Dec 18;324(6098):676–679. doi: 10.1038/324676a0. [DOI] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- Zelent A. Translocation of the RAR alpha locus to the PML or PLZF gene in acute promyelocytic leukaemia. Br J Haematol. 1994 Mar;86(3):451–460. doi: 10.1111/j.1365-2141.1994.tb04773.x. [DOI] [PubMed] [Google Scholar]
- el-Baradi T., Pieler T. Zinc finger proteins: what we know and what we would like to know. Mech Dev. 1991 Nov;35(3):155–169. doi: 10.1016/0925-4773(91)90015-x. [DOI] [PubMed] [Google Scholar]