Abstract
We report the first account of metabolically labelling N-acetylglucosamine, in conjunction with either N-acetylgalactosamine or N-acetylmannosamine using a combination of isonitrile- and azide-based chemistries. With the appropriately labelled fluorescent probe molecules, that react with either the azido or isonitrile groups, the method enabled co-visualisation of cancer cell glycoproteins.
Introduction
Glycans are complex biological macromolecules that decorate the cell surface in the form of glycoproteins or glycolipids. They play key roles in cell adhesion, proliferation and intracellular signalling. It has long been known that glycans also influence tumour progression, invasion and metastasis.1 In this context, changes in cell surface glycosylation influence the way cancer cells interact with the extracellular matrix, neighbouring cells and the epithelium of the target organ, during metastatic invasion.1-3 One of the key players in cancer progression is thought to be the monosaccharide sialic acid, which caps the end of O- and N-linked glycoproteins.1 Certain sialyltransferases are overexpressed in colorectal and breast cancers, increasing the total content of sialic acid on the cell surface.4 High levels of sialic acid have been correlated with metastatic disease and poor prognosis.5,6 Imaging glycosylation may therefore be of clinical value in this context. We have recently shown the use of small molecular probes for imaging glycosylation in vivo, with contrast achieved in tumours and other highly glycosylated tissues.7
Studying glycosylation remains challenging due to the complexity of the glycome.2 Metabolic glycan labelling has been a powerful tool for labelling cell surface glycans in vitro and in vivo.7-11 In this method, cells are fed with a non-natural monosaccharide carrying a small, inert chemical reporter group. This monosaccharide is then internalised by the cells and accepted by the biosynthetic machinery of glycoprotein synthesis in the Golgi and Endoplasmic Reticulum (ER). These modified glycoproteins are then presented on the cell surface. The azide group has been the most widely used reporter group due to its chemical stability, small size and bioorthogonal reactivity with alkynes and Staudinger phosphines.12-14 Other chemical reporters used for metabolic labelling include: alkynes,15,16 ketones,17 thiols,18 cyclopropenes19,20 and alkenes.21 We have recently shown that the isonitrile group can also be used as a novel chemical reporter group for glycan metabolic labelling.22
Azides incorporated in cell surface glycoproteins can be detected using either the Staudinger ligation,23 the copper catalysed [3 + 2] cycloaddition with alkynes (with biologically compatible catalysts)24 or the copper-free [3 + 2] cycloaddition with strained cyclooctynes.25 Cyclopropenes and alkenes, on the other hand, can react with tetrazines.20 We recently reported the incorporation of primary isonitrile sugars into cell surface glycoproteins and the subsequent detection of the latter by tetrazines, via [4 + 1] cycloaddition.22,26
Recently it has been shown that the cyclopropene–tetrazine and alkene–tetrazine reactions are bioorthogonal to the widely used azide–alkyne click chemistry. This enabled simultaneous visualisation of two metabolically labelled sugars on cell surfaces.19,21 In these two accounts of dual sugar labelling N-acetyl mannosamine was the monosaccharide bearing the cyclopropene19 or alkene21 tag. As with per-acetylated N-azidoacetyl mannosamine (Ac4ManNAz),13 these non-natural mannosamine derivatives are incorporated into cell surface glycoproteins as modified terminal sialic acids. The modified sialic acids were then detected using a fluorescent tetrazine. Dual sugar metabolic labelling was achieved by using these mannosamine derivatives in conjunction with per-acetylated N-azidoacetyl galactosamine (Ac4GalNAz).13,19,21
We wanted to explore whether we could use our newly developed isonitrile–tetrazine metabolic labelling chemistry to perform dual-glycan labelling on cancer cells. We have demonstrated that primary isonitrile derivatives of both mannosamine (Ac4ManN-n-Iso) and glucosamine (Ac4GlcN-n-Iso) are incorporated into cell surface glycoproteins, making them valuable tools for dual metabolic labelling studies. Importantly, the isonitrile derivative of glucosamine that we have recently introduced is the first example of a glucosamine analogue that shows good metabolic incorporation into cell surface glycoproteins.22 It has been reported that per-acetylated N-azidoacetylglucosamine (Ac4GlcNAz) shows poor metabolic labelling, which was thought to be due to low tolerance of glycosyltransferases for GlcNAz in the N-acetyl glucosamine salvage pathway.27 Labelling cell-surface N-acetyl glucosamine residues in the core of O-linked and N-linked glycans in parallel to sialic acid or galactosamine (via the azido–alkyne bioorthogonal chemistry) could give new insights into the dynamics of cell surface glycosylation. Unlike N-linked glycosylation where proteomic and enrichment methods have been available, O-linked glycosylation remains challenging to study.28,29
Results and discussion
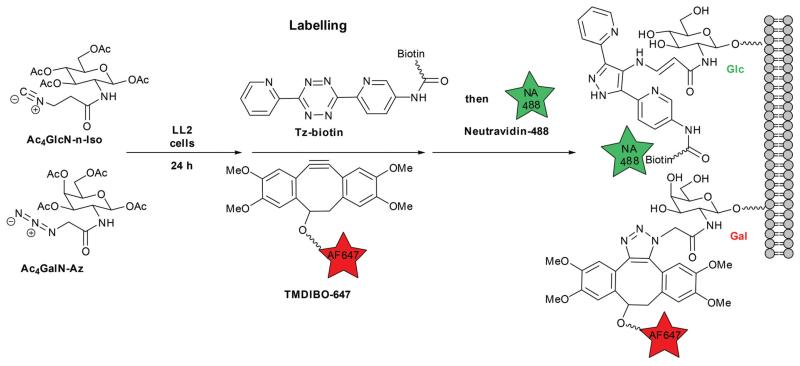
We investigated various isonitrile and azido sugar combinations for metabolic glycan labelling of Lewis Lung Carcinoma (LL2) cells. Detection of isonitrile-modified surface glycans was achieved using a two-step approach, whereby a biotinylated tetrazine (Tz-biotin, see ESI) was reacted with tumour cells, followed by detection with a green fluorescent neutravidin (NA488).22 This two-step approach enabled higher signal-to-background ratios (SBRs). The azido sugar, on the other hand, was detected using a far-red fluorescently-labelled strained cyclooctyne, tetramethoxydibenzocyclooctyne (TMDIBO-647).30
Our previous work showed the bioorthogonal nature of the isonitrile group as well as the orthogonality of the isonitrile/tetrazine reaction to the commonly used alkyne–azide chemistry under physiological conditions.22 Fig. 1 illustrates the dual-sugar labelling strategy for Ac4GalN-Az and Ac4GlcN-n-Iso (the same strategy was used for the other sugar combinations).
Fig. 1. Dual-sugar metabolic labelling with Ac4GlcN-n-Iso and Ac4GalN-Az on LL2 cancer cells.
Various monosaccharide combinations were tested and labelling was assessed by flow cytometry. LL2 cells were seeded at 2 × 104 cells cm−2 and incubated for 24 hours prior to sugar addition. Cells were then treated with either solvent vehicle (DMSO, <0.25% v/v in buffer) or an isonitrile sugar (200 μM) in combination with an azido sugar (50 μM). Single sugar controls were also used to compare dual sugar labelling SBRs with single sugar labelling. Cells were then incubated for a further 24 hours. Upon harvesting, cells were treated with Tz-biotin (200 μM) and TMDIBO-647 (10 μM) for 30 min at 37 °C followed by washes (3×) with cold FACS buffer (see ESI for details). The cells were then incubated with NA488 for 15 min, and washed again (2×).
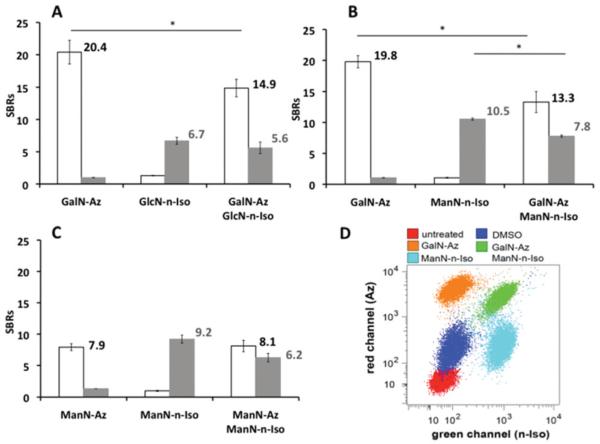
A cell-death marker (DAPI) was added to the final cell suspension (3.60 μM), prior to flow cytometry. Median fluorescent intensities (MFIs) were recorded for both the far-red and blue channels. Signal-to-background ratios (SBRs) were calculated from the ratios of the MFIs for single sugar and double sugar treated cells to the MFIs of cells treated with solvent vehicle alone (Fig. 2A and B). The SBR values show efficient dual sugar labelling for the Ac4GalN-Az/Ac4GlcN-n-Iso (Fig. 2A) and Ac4GalN-Az/Ac4ManN-n-Iso (Fig. 2B and D) combinations. Ac4GlcN-n-Iso generated lower cell labelling than Ac4ManN-n-Iso (SBRs for single-sugar controls 6.7 ± 0.5 and 10.5 ± 0.2 respectively), in accordance with our previous results.22 In the case of dual labelling, there was a clear decrease in SBR values for the azido sugar Ac4GalN-Az compared to the single sugar control (14.9 ± 1.3 vs. 20.4 ± 1.8, P = 0.0002, 2-tailed t-test). Furthermore, the isonitrile labelling ratios decreased slightly in the presence of N-azidoacetylgalactosamine (Fig. 2A and B), potentially due to the growth inhibiting properties of isonitrile sugars22 and/or possible competition between the sugars.
Fig. 2. Flow cytometry results for various isonitrile and azido sugars combinations.
SBRs: signal-to-background ratios (vs. control: cells treated with DMSO vehicle, <0.25% v/v). White bars = red channel (azide); grey bars = green channel (isonitrile). Error bars represent standard deviation of three repeats, 20 000 events per repeat. (A) Ac4GalN-Az and Ac4GlcN-n-Iso single-sugar controls and combined. (B) Ac4GalN-Az and Ac4ManN-n-Iso single sugar controls and combined. (C) Ac4ManN-n-Iso and Ac4ManN-Az (both 200 μM): single sugar controls and combined. (D) Red and green channel scatter plot comparing controls and dually labelled cells for Ac4GalN-Az and Ac4ManN-n-Iso. *P < 0.05, differences between SBRs were significant, two-tailed t-test.
To investigate whether ManN-n-Iso competes with ManN-Az in the sialic acid biosynthetic pathway, LL2 cells were treated with the same concentration of both sugars (200 μM) prior to labelling and flow cytometry analysis, Fig. 2C. In the dual sugar labelling scenario, the azido sialic acid labelling did not change whereas the isonitrile sialic acid labelling decreased a little. ManN-Az seemed to be slightly favoured over ManN-n-Iso as a substrate for glycan synthesis. It was also observed that the azido sialic acid labelling was not affected by the presence of Ac4GlcN-n-Iso (see Fig. S2). This observation suggests that decreases observed in dual-sugar SBRs are not solely due to cell growth inhibition by isonitriles. However the high concentration of Ac4ManNAz used in Fig. 2C (200 μM instead of the 50 μM used in the other experiments) had little effect on the extent of ManN-n-Iso labelling, so it seems there is not much direct competition between the precursors.
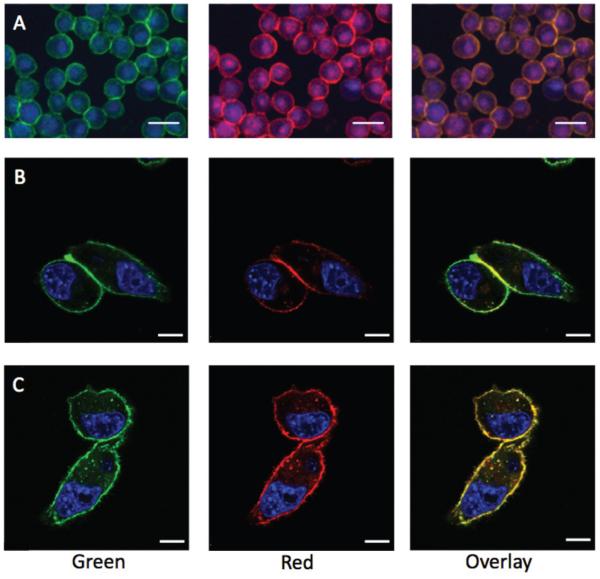
In order to visualise cell labelling and to investigate the influence of the probes on MFI values, quantitative epifluorescence microscopy was carried out with the Ac4GalN-Az/Ac4ManN-n-Iso combination as these two sugars showed the highest labelling of LL2 cells. The nuclear stain DAPI was used to distinguish individual cells. A lower concentration of TMDIBO-647 probe (5 μM) was used so that SBRs for both sugars would be in the same range, facilitating comparison of fluorescence intensities. Green and far-red fluorescence were visualised on cell membranes of cells incubated with both sugars. The labelling of both sugars seemed to colocalize, as can be seen on the overlay of both colour channels (see Fig. 3A). In addition, cells incubated with both sugars were labelled with various probe combinations to investigate whether the presence of both the tetrazine and the strained cyclooctyne during the 30 min incubation affected the extent of labelling. Individual green and red MFI values per cell are slightly higher for controls with single probes in comparison to cells treated with both Tz-biotin and TMDIBO-647, but were within the standard errors (see Fig. S5).
Fig. 3. Epifluorescence (A) and confocal microscopy (B–C) of dually labelled LL2 cells.
(A) Epifluorescence microscopy of Ac4ManN-n-Iso/Ac4GalN-Az treated cells. (B) Confocal microscopy of Ac4GlcN-n-Iso/Ac4ManN-Az treated cells. (C) Confocal microscopy of Ac4GlcN-n-Iso and Ac4GalN-Az treated cells. Far-red channel = azido sugars. Green channel = isonitrile sugars. Scale bars: 30 μm (A) and 10 μm (B–C). See Fig. S6 for control cells treated with DMSO vehicle, <0.25% v/v.
Finally, confocal microscopy was used to visualise dual labelled LL2 cells with Ac4GlcN-n-Iso as the isonitrile sugar. There has been no previous account of visualising metabolically incorporated N-acetylglucosamine in conjunction with N-acetylgalactosamine or N-acetyl mannosamine. Cells were first incubated with Ac4GlcN-n-Iso and Ac4ManN-Az for 24 hours, prior to labelling and fixing for microscopic visualisation. In the case of Ac4GlcN-n-Iso and Ac4ManN-Az (Fig. 3B), Ac4GlcN-n-Iso showed homogeneous labelling of the cell surface whereas Ac4ManN-Az, which is presented as azido-sialic acid on the cell surface, appeared to show maximal labelling at the cell-cell junctions. The Ac4GlcN-n-Iso and Ac4GalN-Az combination (Fig. 3C) showed similar distribution of both labelled sugars.
Conclusions
In conclusion, we have demonstrated that our recently developed isonitrile labelled sugars can be used in conjunction with conventional azido sugars for dual metabolic labelling of cancer cells. For the first time, cell surface azido galactosamine and azido sialic acid could be visualised in parallel to isonitrile-labelled glucosamine. We propose this simultaneous two-colour cell-labelling platform as a valuable tool for dynamic live-cell glycan imaging applications and for cell surface glycomics and analytical studies.
Supplementary Material
Acknowledgements
Many thanks to Joe C-H Kuo and Alexander Schreiner at the CRI for help and advice. This work was supported by Cancer Research UK (CRUK).
Notes and references
- 1.Fuster MM, Esko JD. Nat. Rev. Cancer. 2005;5:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 2.Dube DH, Bertozzi CR. Nat. Rev. Drug Discovery. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 3.Chang PV, Bertozzi CR. Chem Commun. 2012;48:8864. doi: 10.1039/c2cc31845h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider F, Kemmner W, Haensch W, Franke G, Gretschel S, Karsten U, Schlag PM. Cancer Res. 2001;61:4605–4611. [PubMed] [Google Scholar]
- 5.Bos PD, Zhang XHF, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massagué J. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varki NM, Varki A. Lab. Invest. 2007;87:851–857. doi: 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neves AA, Stöckmann H, Wainman YA, Kuo JC-H, Fawcett S, Leeper FJ, Brindle KM. Bioconjugate Chem. 2013;24:934–941. doi: 10.1021/bc300621n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neves AA, Stöckmann H, Harmston RR, Pryor HJ, Alam IS, Ireland-Zecchini H, Lewis DY, Lyons SK, Leeper FJ, Brindle KM. FASEB J. 2011;25:2528–2537. doi: 10.1096/fj.10-178590. [DOI] [PubMed] [Google Scholar]
- 9.Boyce M, Bertozzi CR. Nat. Methods. 2011;8:638–642. doi: 10.1038/nmeth.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehnert KW, Baskin JM, Laughlin ST, Beahm BJ, Naidu NN, Amacher SL, Bertozzi CR. ChemBio-Chem. 2012;13:353–357. doi: 10.1002/cbic.201100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang PV, Chen X, Smyrniotis C, Xenakis A, Hu T, Bertozzi CR, Wu P. Angew. Chem., Int. Ed. 2009;48:4030–4033. doi: 10.1002/anie.200806319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. Proc. Natl. Acad. Sci. U. S. A. 2002;99:19–24. doi: 10.1073/pnas.012583299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dube DH, Prescher JA, Quang CN, Bertozzi CR. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4819–4824. doi: 10.1073/pnas.0506855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du J, Meledeo MA, Wang Z, Khanna HS, Paruchuri VDP, Yarema KJ. Glycobiology. 2009;19:1382–1401. doi: 10.1093/glycob/cwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu T-L, Hanson SR, Kishikawa K, Wang S-K, Sawa M, Wong C-H. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2614–2619. doi: 10.1073/pnas.0611307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng L, Hong S, Rong J, You Q, Dai P, Huang R, Tan Y, Hong W, Xie C, Zhao J, Chen X. J. Am. Chem. Soc. 2013;135:9244–9247. doi: 10.1021/ja402326z. [DOI] [PubMed] [Google Scholar]
- 17.Mahal LK. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 18.Sampathkumar S-G, Li AV, Jones MB, Sun Z, Yarema KJ. Nat. Chem. Biol. 2006;2:149–152. doi: 10.1038/nchembio770. [DOI] [PubMed] [Google Scholar]
- 19.Cole CM, Yang J, Šečkutė J, Devaraj NK. ChemBio-Chem. 2013;14:205–208. doi: 10.1002/cbic.201200719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Šečkutė J, Cole CM, Devaraj NK. Angew. Chem., Int. Ed. 2012;51:7476–7479. doi: 10.1002/anie.201202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niederwieser A, Späte A-K, Nguyen LD, Jüngst C, Reutter W, Wittmann V. Angew. Chem., Int. Ed. 2013;52:4265–4268. doi: 10.1002/anie.201208991. [DOI] [PubMed] [Google Scholar]
- 22.Stairs S, Neves AA, Stöckmann H, Wainman YA, Ireland-Zecchini H, Brindle KM, Leeper FJ. Chem-BioChem. 2013;14:1063–1067. doi: 10.1002/cbic.201300130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Berkel SS, van Eldijk MB, van Hest JCM. Angew. Chem., Int. Ed. 2011;50:8806–8827. doi: 10.1002/anie.201008102. [DOI] [PubMed] [Google Scholar]
- 24.Besanceney-Webler C, Jiang H, Zheng T, Feng L, Soriano del Amo D, Wang W, Klivansky LM, Marlow FL, Liu Y, Wu P. Angew. Chem., Int. Ed. 2011;50:8051–8056. doi: 10.1002/anie.201101817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agard NJ, Prescher JA, Bertozzi CR. J. Am. Chem. Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 26.Stöckmann H, Neves AA, Stairs S, Brindle KM, Leeper FJ. Org. Biomol. Chem. 2011;9:7303. doi: 10.1039/c1ob06424j. [DOI] [PubMed] [Google Scholar]
- 27.Vocadlo DJ, Hang HC, Kim E-J, Hanover JA, Bertozzi CR. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9116–9121. doi: 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Li X-J, Martin DB, Aebersold R. Nat. Biotechnol. 2003;21:660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 29.Kaji H, Yamauchi Y, Takahashi N, Isobe T. Nat. Protoc. 2007;1:3019–3027. doi: 10.1038/nprot.2006.444. [DOI] [PubMed] [Google Scholar]
- 30.Stöckmann H, Neves AA, Stairs S, Ireland-Zecchini H, Brindle KM, Leeper FJ. Chem. Sci. 2011;2:932. doi: 10.1039/C0SC00631A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.