Abstract
Epidemiological evidence supports a direct and causal association between lipoprotein(a) [Lp(a)] levels and coronary risk, but the nature of the association between Lp(a) levels and risk of type 2 diabetes (T2D) is unclear. In this study, we assessed the association of Lp(a) levels with risk of incident T2D, and tested whether Lp(a) levels are causally linked to T2D. We analysed data on 18,490 participants from the EPIC-Norfolk cohort that included adults aged 40-79 years at baseline 1993-1997. During average 10 years of follow-up, 593 participants developed incident T2D. Cox regression models were used to estimate the association between Lp(a) levels and T2D. In Mendelian randomisation analyses, based on EPIC-Norfolk combined with DIAGRAM data involving a total of 10,088 diabetes cases and 68,346 controls, we used a genetic variant (rs10455872) as an instrument to test whether the association between Lp(a) levels and T2D is causal. In adjusted analyses there was an inverse association between Lp(a) levels and T2D: hazard ratio (HR) was 0.63 (95% confidence interval 0.49-0.81; p-trend=0.003) comparing the top versus bottom quintile of Lp(a). In EPIC-Norfolk, a 1-SD increase in logLp(a) was associated with a lower risk of T2D (OR=0.88, 95%CI: 0.80-0.95). However, in Mendelian randomisation analyses, a 1-SD increase in logLp(a) due to rs10455872, which explained 26.8% of the variability in Lp(a) levels, was not associated with risk of T2D (OR=1.03, 95%CI: 0.96-1.10, p = 0.41). These prospective findings demonstrate a strong inverse association of Lp(a) levels with risk of T2D. However, a genetic variant that elevated Lp(a) levels was not associated with risk of T2D, suggesting that elevated Lp(a) levels are not causally associated with a lower risk of T2D.
Keywords: lipoprotein(a), type 2 diabetes, causal association, coronary heart disease, hazard ratio, Mendelian randomisation, prospective study
Introduction
Lipoprotein(a) [Lp(a)] is a low-density lipoprotein (LDL) like particle synthesized by the liver that contains an apolipoprotein B100 molecule covalently bound to a plasminogen-like glycoprotein, apolipoprotein(a) [apo(a)] (1, 2). Several meta-analyses of prospective epidemiological studies have demonstrated that elevated baseline concentrations of Lp(a) increase the risk of incident cardiovascular disease (CVD), particularly for coronary heart disease (CHD) (3-6). Recently, we reported a significant positive association of Lp(a) levels with coronary artery disease and peripheral arterial disease, but not with ischaemic stroke in the European Prospective Investigation of Cancer (EPIC)-Norfolk study (7). Based on the “common soil hypothesis” of the aetiology of diabetes and cardiovascular disease (8), it might be anticipated that Lp(a) levels would also be associated in a similar, direct manner with risk of T2D.
The only prospective study assessing the association of Lp(a) levels and T2D to date, the Women’s Health Study (WHS), suggested that Lp(a) levels were inversely associated with risk of T2D in women (9). In adjusted analyses, the hazard ratio was 0.78 (95%CI: 0.67-0.91) in a comparison of women in the top quintile versus those in the bottom quintile of the Lp(a) distribution. The findings were confirmed in a Danish population, where a similarly inverse association with prevalent diabetes was found in a cross-sectional analysis (9). In a meta-analysis of 34 prospective studies, it was reported that Lp(a) levels were 11% lower in people with a self-reported history of diabetes compared to those without (5). Other investigators have reported inconsistent results on the association of Lp(a) levels with T2D in small case-control or cross-sectional studies, with some but not all showing lower Lp(a) levels in patients with diabetes than among controls (10-13). There remains considerable uncertainty about the association between Lp(a) levels and risk of T2D.
Moreover, it is currently unknown whether any observed association between Lp(a) levels and risk of T2D is likely to be causal or due to residual confounding or reverse causality, as this has not yet been investigated. A likely causal nature of association between Lp(a) and CHD has been inferred by reports of associations of Lp(a)-related genetic variants with CHD risk (14-16), and a similar approach using a Mendelian randomisation experiment (17), could be applied to investigate a causal association between Lp(a) levels and T2D risk.
Our primary objective was to investigate the prospective association between baseline Lp(a) levels and incident T2D and to test for a causal association using the Mendelian randomisation approach. A secondary objective was to investigate the association of Lp(a) with incident CHD within the same longitudinal study, to enable a direct comparison of baseline Lp(a) concentration with both disease endpoints in a single study using the same methods, in order to gain a better understanding of the nature of the association.
Methods
Study Population
The EPIC-Norfolk study is a population-based cohort study, described in detail previously [18]. This study recruited 25,639 men and women at baseline (1993-1997), aged 40-79 years living in and around Norfolk, England. Written informed consent was obtained from all participants, and the study was approved by the Norwich district ethics committee. Since the baseline visit, there were three follow-up assessments: a postal questionnaire at 18 months, a repeat health-check visit (1998-2000), and a further postal questionnaire (2002-2004). After exclusion of individuals without information on the Lp(a) levels and covariates for multivariable analysis, data on 18,490 participants were available for current analyses.
Type 2 diabetes ascertainment
Incident cases of T2D were ascertained from the three follow-up phases of the study as well as through record linkage to external sources to the end of July 2006, which covers a mean of 9.8 ± 1.4 (SD) years of follow-up. Incident cases of diabetes were ascertained using multiple data sources including any self-report of diabetes diagnosed by a physician and of diabetes-specific medication at follow-up. In addition, record linkage with external sources was used to trace each participant for diabetes diagnosis including listing with general practice diabetes registers, hospital outpatient attendance or hospital admission data for diabetes-related admissions among study participants, and Office for National Statistics mortality data with coding for diabetes. Identification of cases through external data sources was independent of the study health check-up visit and questionnaire follow-up. Possible cases based solely on self-report, and not confirmed by another data source, did not qualify as a confirmed case of diabetes. A total of 593 incident diabetes cases were identified among the 17,908 eligible participants after we excluded prevalent diabetes cases (n=580).
Coronary heart disease ascertainment
Incident cases of CHD including fatal and non-fatal events were ascertained until 31st March 2008, which covers a mean of 11.4 ± 2.8 (SD) years of follow-up. All study participants were flagged for CHD related death with record linkage with the Office for National Statistics mortality data. CHD death was defined using International Classification of Disease (ICD) criteria 410-414 (ICD9) or I20-I25 (ICD10) for underlying cause of death. Additionally, data linkage with the East Norfolk Health Authority database identified all hospital contacts for study participants using their NHS number. Participants were identified as having a CHD event during follow-up if they had a hospital admission and/or died with CHD as cause of death through medical record inspection. A total of 1,978 CHD incident cases were identified among the 17,899 eligible participants after we excluded prevalent CHD cases (n=591).
Measurement of Lp(a) concentrations
Samples were stored frozen at −80°C since baseline collection. Levels of Lp(a) were measured in non-fasted serum samples by laboratory staff unaware of participants’ disease status. Baseline Lp(a) levels were measured by using a high-sensitivity immunoturbidimetric assay that is not affected by the number of kringle-IV type-2 repeats (KIV2) and is independent of the apolipoprotein(a) isoform. The reagents, calibrators, and controls for the measurement of Lp(a) were obtained from Randox Laboratories Ltd (Crumlin, County Antrim, UK).
Covariates
Participants completed a health and lifestyle questionnaire including data on medical history, smoking, alcohol intake, physical activity, use of medication, history of disease, and education and attended a clinic for a health examination at baseline. Body mass index (BMI) was calculated as weight (kilograms) divided by height (metres) squared. Current cigarette smoking status was categorized into 3 groups (never, former, current), physical activity status into 4 groups (active, moderately active, moderately inactive, inactive) as per a validated physical activity index (19), and educational level into 4 groups (none or primary, secondary O-level to age 16 years, secondary A-level to age 18years, and degree-level or equivalent). Blood pressure was measured using an Accutorr non-invasive blood pressure monitor. A non-fasting venous blood sample was examined for total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides using the RA1000 (Bayer Diagnostics, Basingstoke, UK), and low-density lipoprotein cholesterol (LDL-C) concentrations were calculated with the Friedewald formula (20).
Genetic association studies
We genotyped the variant rs10455872 in the EPIC-Norfolk study among 508 diabetes cases and 14,536 non-diabetic controls. To increase power to detect an association between rs10455872 and T2D, we also included publicly available DIAGRAM genetic data (http://www.diagram-consortium.org) with a total of 9,580 diabetes cases and 53,810 controls. In the EPIC-Norfolk study, rs10455872 explained 26.8% of the variation in Lp(a), allowing its use as an instrumental variable in Mendelian randomisation analysis. The SNP was genotyped using Custom TaqMan® SNP Genotyping Assays (Applied Biosystems, Warrington, UK). The genotyping assays were carried out using 10ng of genomic DNA in a 2.5μl reaction volume on 384-well plates using a G-Storm GS4 Thermal Cycler (GRI, Rayne, UK). The ABI PRISM® 7900HT Sequence Detection System (Applied Biosystems, Warrington, UK) was used for end-point detection and allele calling. The SNP passed the quality control criteria in the EPIC-Norfolk study (call rate > 95 %, blind duplicate concordance ≥ 95%). The frequency of rs10455872 in controls differed significantly from that expected under Hardy Weinberg Equilibrium (p-value < 0.0001).
Statistical Analyses
Baseline characteristics of participants were examined across quintiles of Lp(a) in the cohort. Differences were estimated using mean and SD for continuous variables, with P-values from an ANOVA test, or using number and percent for categorical variables, with χ2 test. Pearson correlation coefficients were used to assess the relationships of Lp(a) with other covariates. Cox proportional hazards regression model was used to estimate the associations between Lp(a) levels (quintiles) and time to incident diabetes as well as CHD. Model 1 was adjusted for age as the underlying timescale, and model 2 was further adjusted for sex and BMI. Model 3 additionally adjusted for alcohol use, smoking status, systolic and diastolic blood pressure, physical activity, education level, and family history of diabetes (for T2D endpoint) or family history of CHD (for CHD endpoint); Model 4 was additionally adjusted for total cholesterol, LDL-C, prevalent diabetes (only for CHD endpoint) or prevalent CHD and stroke (only for T2D endpoint), prevalent cancer, antihypertensive medication, lipid lowering drugs, and C-reactive protein. The P-value for trend was estimated by using quintile number as a predictor. Age, BMI, alcohol use, cholesterol levels, and blood pressure were analysed as continuous variables, whereas the rest of the covariates were analysed as categorical variables.
Further analyses including sensitivity and subgroup analyses were based on model 3. To assess the possibility of reverse association bias or association due to residual confounding, sensitivity analyses were performed by excluding all individuals with prevalent chronic diseases or/and with baseline HbA1c ≥ 6.5%, or incident T2D cases during the first 5 years of follow-up. We also assessed the possibility of survivor bias in exploratory analyses, assessing any deviation in proportionality of hazard over time. If survivor bias was present, we would expect any inverse association between Lp(a) and T2D to become stronger with analysis time. We tested for this by including standardised logLp(a) as a time varying covariate with analysis time as the underlying timescale, and log(analysis time) as the multiplier for the time varying covariate. We carried out a likelihood ratio test (LRT) for statistical significance of addition of this term into the model on a χ2 distribution (1 degree of freedom).
Previous studies have reported that LDL-C does not modify the association between Lp(a) and T2D [9] but that diabetes status and LDL-C concentrations may modify the association of Lp(a) with coronary disease (21-23). Thus, we examined for interaction between LDL-C concentration and Lp(a) on both endpoints of T2D and CHD, and for an interaction between presence of prevalent T2D and Lp(a) concentration and CHD. The interaction between sex and Lp(a) concentration on T2D and CHD was also assessed. Lastly, to investigate the nature of associations with both T2D and CHD, and any dose-response effect in greater detail, Lp(a) levels were divided into deciles with each decile group (decile two to ten) compared to the lowest decile group (decile one) as the reference category. Floating absolute risks were estimated, which were then plotted against the median Lp(a) levels in each decile. The LRT was used to assess whether the overall association was a linear or a quadratic shape.
Association of genetically elevated circulating Lp(a) with T2D – Mendelian randomisation
We used an instrumental variable method to obtain estimates of the association of Lp(a) levels and T2D implied from Mendelian randomisation. Briefly, an instrument is a variable (e.g. rs10455872) that is associated with an exposure of interest (e.g. Lp(a) levels), and is only related to the disease outcome (e.g. T2D) being studied through the exposure of interest. The instrument is assumed to be free of confounding and reverse causation, both of which may distort the association of interest. In our example, the instrumental variable rs10455872 is expected to act as a non-confounded and unbiased marker for Lp(a) levels (as alleles for the variant are allocated randomly at birth) and is used to estimate the causal effects of Lp(a) levels on T2D. Instrumental variable analysis was performed using a logistic control function estimator (24). The analysis was performed in two stages. In the first stage, we assessed the observational association between rs10455872 and Lp(a) levels in a linear regression model. In the second stage, the predicted values and residuals from the first stage were included as covariates in a logistic regression model with T2D as the outcome. Lp(a) concentration was log transformed in Mendelian randomisation analyses. The impact per 1-SD higher genetically elevated logLp(a) levels was estimated by using rs10455872 as an instrument, adjusted for age and sex. Analyses were performed in the EPIC-Norfolk study and DIAGRAM, separately, and then combined by fixed effects meta-analysis. The results from the EPIC-Norfolk study on the association between 1-SD change in logLp(a) levels and T2D risk were used as an estimate of the confounder-adjusted association for the observed estimate, performed using model 3, as described above.
All analyses were performed using stata/SE11.2 (Stata College Station, Texas). All statistical tests were 2-sided and used a significance level of P < 0.05, except where indicated.
Results
Among 18,490 participants (8,248 men and 10,242 women) the mean age at recruitment was 59.2 (SD 9.2) years. BMI was similar in men [26.4 kg/m2 (SD 3.2)] and women [26.1 kg/m2 (SD 4.2)]. Median Lp(a) level was 11.6 mg/dL (range between 2.0 mg/dL and 175.0 mg/dL). Pearson correlation coefficients suggested modest correlations between baseline Lp(a) levels and lifestyle characteristics and risk factors (physical activity, smoking, education levels, BMI, and family history of diabetes), and inflammatory markers (e.g., C-reactive protein) (all coefficients < 0.2). Table 1 shows baseline characteristics of the study participants by quintiles of serum Lp(a). Lp(a) levels were inversely associated with family history of diabetes and incident diabetes, and were generally positively associated with age, CHD risk factors, prevalent cancer and C-reactive protein.
Table 1. Baseline characteristics of study participants by quintiles of serum lipoprotein(a) in the EPIC-Norfolk study (n=18,490).
| Q1 (n=3,708) | Q2 (n=3,695) | Q3 (n=3,693) | Q4 (n=3,696) | Q5 (n=3,698) | P-value | |
|---|---|---|---|---|---|---|
| Lp(a) (mg/dL): median (range) | 3.9 (2.0-5.3) | 7.1 (5.3-9.0) | 11.6 (9.0-15.4) | 21.8 (15.4-35.4) | 53.5 (35.4-175.0) | |
| Age (years) | 58.4 (9.1) | 58.6 (9.2) | 59.4 (9.2) | 60.0 (9.0) | 59.5 (9.1) | <0.0001 |
| Women (%)^ | 2018 (54.4) | 2020 (54.7) | 2044 (55.4) | 2061 (55.8) | 2099 (56.8) | 0.3 |
| BMI (kg/m2) | 26.4 (4.0) | 26.1 (3.8) | 26.2 (3.7) | 26.3 (3.7) | 26.2 (3.8) | 0.6 |
| Alcohol (units/week) | 7.5 (9.5) | 7.2 (9.5) | 6.9 (9.3) | 6.6 (8.9) | 6.9 (9.3) | <0.0001 |
| Smoking status | ||||||
| Current smoker (%) | 419 (11.4) | 428 (11.7) | 435 (11.9) | 399 (10.9) | 396 (10.8) | |
| Former smoker (%) | 1536 (41.7) | 1526 (41.7) | 1559 (42.6) | 1542 (42.1) | 1520 (41.4) | |
| Never smoker (%) | 1725 (46.9) | 1708 (46.6) | 1669 (45.6) | 1718 (47.0) | 1756 (47.8) | 0.3 |
| Family history of diabetes (%) | 509 (13.7) | 504 (13.7) | 481 (13.0) | 473 (12.8) | 419 (11.3) | 0.02 |
| Family history of CHD (%) | 1233 (33.3) | 1306 (35.4) | 1320 (35.8) | 1376 (37.3) | 1506 (40.8) | <0.0001 |
| Incident diabetes (%) | 159 (4.3) | 101 (2.7) | 112 (3.0) | 123 (3.3) | 98 (2.7) | <0.0001 |
| Incident CHD (%) | 390 (10.5) | 419 (11.3) | 472 (12.8) | 507 (13.7) | 614 (16.6) | <0.0001 |
| Prevalent diabetes (%) | 123 (3.3) | 116 (3.1) | 118 (3.2) | 102 (2.8) | 121 (3.3) | 0.7 |
| Prevalent cancer (%) | 176 (4.8) | 186 (5.0) | 205 (5.6) | 199 (5.4) | 186 (5.0) | 0.5 |
| Prevalent CHD (%) | 97 (2.6) | 109 (3.0) | 110 (3.0) | 114 (3.1) | 161 (4.4) | <0.0001 |
| Prevalent stroke (%) | 44 (1.2) | 46 (1.3) | 56 (1.5) | 51 (1.4) | 60 (1.6) | 0.5 |
| Lipid parameters | ||||||
| Total cholesterol (mmol/L) | 6.0 (1.1) | 6.0 (1.1) | 6.2 (1.1) | 6.3 (1.2) | 6.5 (1.1) | <0.0001 |
| HDL-cholesterol (mmol/L) | 1.4 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 1.5 (0.5) | 0.07 |
| LDL-cholesterol (mmol/L) | 3.7 (1.0) | 3.8 (1.0) | 4.0 (1.0) | 4.1 (1.0) | 4.2 (1.0) | <0.0001 |
| Triglycerides (mmol/L)* | 1.9 (1.8-1.9) | 1.8 (1.7-1.8) | 1.8 (1.7-1.8) | 1.8 (1.7-1.8) | 1.8 (1.7-1.8) | 0.4 |
| antihypertensive medication | 630 (17.0) | 614 (16.6) | 631 (17.1) | 762 (20.6) | 719 (19.4) | <0.0001 |
| Lipid lowering drugs (%) | 40 (1.1) | 36 (1.0) | 54 (1.5) | 61 (1.7) | 70 (1.9) | 0.003 |
| Systolic BP (mm Hg) | 134.5 (17.8) | 134.5 (18.3) | 135.4 (18.6) | 136.4 (18.5) | 134.6 (18.2) | 0.03 |
| Diastolic BP (mm Hg) | 82.1 (11.0) | 82.1 (11.4) | 82.2 (11.3) | 82.8 (11.1) | 81.9 (11.1) | 0.2 |
| Highest education | ||||||
| None or primary school (%) | 1256 (33.9) | 1312 (35.5) | 1387 (37.6) | 1403 (38.0) | 1421 (38.5) | |
| O-level or equivalent (%) | 386 (10.4) | 384 (10.4) | 379 (10.3) | 389 (10.5) | 356 (9.6) | |
| A-level or equivalent (%) | 1559 (42.1) | 1507 (40.8) | 1462 (39.6) | 1439 (39.0) | 1459 (39.5) | |
| Degree or higher (%) | 504 (13.6) | 490 (13.3) | 464 (12.6) | 463 (12.5) | 459 (12.4) | 0.01 |
| Physical activity | ||||||
| Inactive (%) | 1047 (28.2) | 1065 (28.8) | 1146 (31.0) | 1097 (29.7) | 1164 (31.5) | |
| Moderately inactive (%) | 1106 (29.8) | 1113 (30.1) | 995 (26.9) | 1072 (29.0) | 1019 (27.6) | |
| Moderately active (%) | 858 (23.1) | 835 (22.6) | 855 (23.2) | 850 (23.0) | 812 (22.0) | |
| Active (%) | 697 (18.8) | 682 (18.5) | 697 (18.9) | 677 (18.3) | 703 (19.0) | 0.04 |
| CRP (mg/L)* | 2.9 (2.7-3.1) | 2.9 (2.7-3.1) | 3.1 (2.9-3.3) | 3.4 (3.1-3.6) | 3.2 (3.0-3.4) | <0.0001 |
| HbAlc (%) (mmol/mol)‡ | 5.2 (33) | 5.2 (33) | 5.3 (34) | 5.3 (34) | 5.3 (34) | <0.0001 |
Data are either mean (SD) or number (%); IQR: interquartile range
Data are geometric mean (95% confidence interval); P-value is the test for proportions for categorical variables or the test for linear trend for continuous variables; BP: blood pressure
number is different.
Only 7405 participants have HbAlc data.
Association with type 2 diabetes
At baseline, case participants were older, included fewer women and had greater BMI than non cases, with mean age 61.9 (SD 8.3) years in cases versus 58.9 (SD 9.2) years in non-cases. Women represented 45.4% of diabetes cases and 56.1% of non-cases, and mean BMI was 29.4 kg/m2 (SD 4.3) in cases versus 26.1 kg/m2 (SD 3.7) in non-cases. The median levels of Lp(a) were lower in diabetes cases (10.8 mg/dL, interquartile range 5.0-26.2 mg/dL) than in non-cases (11.6 mg/dL, interquartile range 6.3-27.7 mg/dL).
In a comparison of individuals with baseline Lp(a) values in the top quintile vs. the bottom quintile, the hazard ratio (HR) for T2D was 0.56 (95%CI: 0.44 - 0.72), p-trend < 0.0001 after adjustment for age as underlying timescale (Table 2). The inverse association was slightly attenuated but statistically significant after further adjustment for several lifestyle and diabetes risk factors (model 3, HR 0.63, 95% CI: 0.49 – 0.81, p-trend=0.003). Further adjustments (model 4) made no material difference (model 4, HR 0.64, 95%CI: 0.49 – 0.85). There was no evidence for interaction by sex (p = 0.50). A per quintile increase in Lp(a) was associated with a 9% reduction in incident diabetes, with a HR of 0.91 (95%CI: 0.85-0.97) per quintile (model 3). We found no threshold effect within the lowest quintile (2 to < 5.3 mg/dL) of the Lp(a) distribution. In the overall sample, we also found no threshold effect when we compared participants with Lp(a) levels above and below 3, 4 or 5 mg/dL.
Table 2. Association of Lp(a) levels with incident type 2 diabetes in the EPIC-Norfolk study.
| Q1 (n=3,583) | Q2 (n=3,579) | Q3 (n=3,575) | Q4 (n=3,594) | Q5 (n=3,577) | Per quintile effect | P for trend | |
|---|---|---|---|---|---|---|---|
| Lp(a) (mg/dL): median (range) | 3.9 (2.0-5.3) | 7.1 (5.3-9.0) | 11.6 (9.0-15.4) | 21.8 (15.4-35.4) | 53.5 (35.4-175.0) | ||
| No. of cases | 159 | 101 | 112 | 123 | 98 | ||
| No. of non-cases | 3424 | 3478 | 3463 | 3471 | 3479 | ||
| Model 1 (n=17,908) | 1 | 0.62 (0.48-0.79) | 0.65 (0.51-0.83) | 0.69 (0.55-0.87) | 0.56 (0.44-0.72) | 0.91 (0.85-0.97) | <0.0001 |
| Model 2 (n=17,908) | 1 | 0.65 (0.50-0.83) | 0.70 (0.54-0.88) | 0.72 (0.57-0.91) | 0.61 (0.47-0.79) | 0.92 (0.87-0.98) | 0.001 |
| Model 3 (n=17,579) | 1 | 0.63 (0.49-0.82) | 0.68 (0.53-0.87) | 0.71 (0.56-0.90) | 0.63 (0.49-0.81) | 0.93 (0.87-0.99) | 0.003 |
| Model 4 (n=16,430) | 1 | 0.66 (0.50-0.86) | 0.70 (0.54-0.92) | 0.75 (0.58-0.97) | 0.64 (0.49-0.85) | 0.95 (0.88-1.02) | 0.01 |
Data show hazard ratio (HR) and 95% confidence intervals (95% CIs). P for trend is from Cox regression models with quintiles
Model 1: adjusted for age as underlying timescale
Model 2: model 1 additionally adjusted for sex and BMI
Model 3: model 2 additionally adjusted for alcohol, smoking status, systolic and diastolic blood pressure, family history of diabetes, physical activity, and education level
Model 4: model 3 additionally adjusted for total cholesterol, LDL-C, prevalent cancer, prevalent CHD, prevalent stroke, antihypertensive medication, lipid lowering drugs, and C-reative protein (CRP)
Sensitivity analyses were performed using model 3:
1. If prevalent cancer, prevalent MI, and prevalent stroke cases were excluded (n=1713), the HRs were 0.68 (0.50-0.92), 0.70 (0.52-0.93), 0.79 (0.60-1.03), and 0.73 (0.55-0.99) from Q2 to Q5, respectively.
2. If only individuals with HbA1c levels ≥ 6.5% (48 mmol/mol) (n=180) were excluded, the HRs were 0.64 (0.48-0.84), 0.70 (0.54-0.91), 0.76 (0.59-0.98), and 0.66 (0.50-0.86) from Q2 to Q5, respectively.
3. If incident T2D diagnosed < 5 years of follow-up (n=157) and individuals with HbA1C levels ≥ 6.5% (48 mmol/mol) were excluded, the HRs were 0.65 (0.48-0.89), 0.68 (0.50-0.93), 0.75 (0.56-1.01), and 0.67 (0.49-0.91) from Q2 to Q5, respectively.
4. If incident CVD diagnosed <5 years of follow-up (n=977) were excluded, the HRs were 0.62 (0.48-0.81), 0.69 (0.54-0.89), 0.65 (0.51-0.84), 0.64 (0.49-0.83) from Q2 to Q5, respectively.
The results of sensitivity analyses were not materially different from the main analyses (Table 2). When we repeated our analyses after excluding all individuals (n=1,713) with prevalent CHD, prevalent stroke, and prevalent cancers, the findings remained relatively unchanged; the HRs for quintiles 2-5 versus the first quintile were 0.68 (95%CI: 0.50-0.92), 0.70 (95%CI: 0.52-0.93), 0.79 (95%CI: 0.60-1.03), and 0.73 (95%CI: 0.55-0.99), respectively. Exclusion of individuals with baseline HbA1C>6.5% (n=180) and those with incident diabetes during the first 5 years of follow-up (n=157) did not alter the observed association. We also excluded incident CHD and stroke events occurring during the first five years of follow-up (n=977), but the inverse associations still persisted (data not shown). Exploratory analyses assessing variation of HR with time suggested that addition of the time varying covariate to the model was not statistically significant (p=0.35), although the HR associated with a 1SD increase in logLp(a) was noted to be attenuated towards the null (HR=1.00, 95% CI 0.75, 1.34) following addition of this parameter to the model. Finally, we performed an analysis stratified by baseline LDL-C levels (above and below median LDL-C of 3.9 mmol/L). There was no evidence for interaction by LDL-C levels (p = 0.42).
Association with coronary heart disease
Median levels of Lp(a) were higher in CHD cases (14.1 mg/dL, interquartile range 7.2-36.0 mg/dL) than in non-cases (11.3 mg/dL, interquartile range 6.1-26.5 mg/dL). In a comparison of individuals with baseline Lp(a) values in the top quintile vs. the bottom quintile, the HR for CHD was 1.45 (95%CI: 1.26 – 1.67), p-trend < 0.001 after adjustment for age as underlying timescale (Table 3). Upon further adjustment for several established CHD risk factors (including smoking status, blood pressure and lipid levels, model 4), the HR was attenuated but a positive association persisted (HR = 1.32, 95% CI: 1.14 – 1.53, p-trend<0.0001). There was a 6% (95% CI 3% to 10%) increase in CHD events associated with a per quintile increase in baseline Lp(a), with an HR of 1.06 (95%CI: 1.03-1.10) per quintile (model 4). Associations were similar in men and women, with no evidence for interaction by sex (p=0.97). There was also no evidence of interaction of Lp(a) concentration with prevalent diabetes status at baseline on future CHD (p=0.48) or with LDL-C levels (p = 0.83).
Table 3. Association of Lp(a) levels with incident coronary heart disease in the EPIC-Norfolk study.
| Q1 (n=3,583) | Q2 (n=3,579) | Q3 (n=3,575) | Q4 (n=3,594) | Q5 (n=3,577) | Per quintile effect | P for trend | |
|---|---|---|---|---|---|---|---|
| Lp(a) (mg/dL): median (range) | 3.9 (2.0-5.3) | 7.1 (5.3-9.0) | 11.6 (9.0-15.4) | 21.8 (15.4-35.4) | 53.5 (35.4-175.0) | ||
| No. of cases | 324 | 345 | 384 | 432 | 493 | ||
| No. of non-cases | 3287 | 3241 | 3199 | 3150 | 3044 | ||
| Model 1 (n=17,903) | 1 | 1.04 (0.90-1.21) | 1.08 (0.94-1.26) | 1.16 (1.01-1.34) | 1.45 (1.26-1.67) | 1.10 (1.07-1.13) | <0.0001 |
| Model 2 (n=17,903) | 1 | 1.06 (0.91-1.23) | 1.13 (0.97-1.31) | 1.20 (1.04-1.38) | 1.55 (1.35-1.79) | 1.12 (1.08-1.15) | <0.0001 |
| Model 3 (n=17,574) | 1 | 1.05 (0.90-1.23) | 1.10 (0.94-1.27) | 1.17 (1.01-1.36) | 1.53 (1.33-1.76) | 1.11 (1.08-1.15) | <0.0001 |
| Model 4 (n=16,847) | 1 | 1.01 (0.87-1.19) | 1.01 (0.86-1.18) | 1.05 (0.91-1.23) | 1.32 (1.14-1.53) | 1.06 (1.03-1.10) | <0.0001 |
Data show hazard ratio (HR) and 95% confidence intervals (95% CIs). P for trend is from Cox regression models with quintiles
Model 1: adjusted for age as underlying timescale
Model 2: model 1 additionally adjusted for sex and BMI
Model 3: model 2 additionally adjusted for alcohol, smoking status, systolic and diastolic blood pressure, family history of coronary disease, physical activity, and education level
Model 4: model 3 additionally adjusted for total cholesterol, LDL-C, prevalent diabetes, prevalent cancer, antihypertensive medication, lipid lowering drugs, and C-reactive protein (CRP)
Sensitivity analyses were performed using model 3:
1. If individuals with prevalent diabetes and prevalent cancer were excluded (n=1435), the HRs were 1.02 (0.86-1.21), 1.11 (0.94-1.31), 1.20 (1.03-1.41), 1.51 (1.29-1.76) for Q2 to Q5, respectively.
2. If incident CHD diagnosed < 5 years of follow-up were excluded (n=434), the HRs were 1.02 (0.86-1.21), 1.09 (0.93-1.29), 1.10 (0.93-1.29), 1.39 (1.18-1.63) for Q2 to Q5, respectively.
Comparison of association of Lp(a) with type 2 diabetes and with coronary heart disease
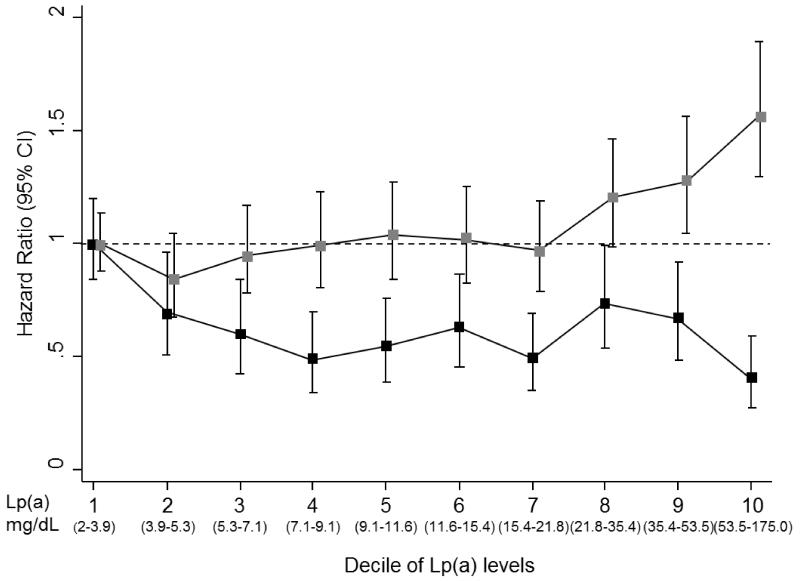
Figure 1 shows the nature of the association of Lp(a) levels with T2D and with CHD, with Lp(a) concentrations categorised into deciles. For the overall associations of Lp(a) levels with T2D and CHD, statistical analyses with a linear versus a quadratic model suggested a better fit with a curvilinear shape for both T2D (p = 0.0025) and CHD (p = 0.003). For T2D, in a comparison of individuals in the top decile (90th percentile) of Lp(a) versus the lowest decile (10th percentile), the HR for T2D was 0.40 (95%CI: 0.27-0.59). For CHD, in a similar comparison, the HR for CHD was 1.58 (95%CI: 1.32-1.90).
Figure 1.
The association between deciles of baseline Lp(a) distribution and incident type 2 diabetes and coronary heart disease in the EPIC-Norfolk study
Lp(a): lipoprotein(a); Confidence intervals (CIs) were calculated using a floating absolute risk technique; Hazard ratios were adjusted for age as underlying timescale, sex, BMI, alcohol, smoking status, systolic and diastolic blood pressure, physical activity, education level, and family history of diabetes (only for T2D) or family history of CHD (only for CHD);  : CHD; ■: T2D
: CHD; ■: T2D
Association of rs10455872 with Lp(a) levels and diabetes risk factors
The baseline characteristics among non-diabetic participants by the rs10455872 genotype (n=14,536) in the EPIC-Norfolk study are summarized in Supplementary Table. The variant rs10455872 was significantly associated with Lp(a) in age, sex, and BMI adjusted analyses, with an additive effect of 1.32 SD increase in logLp(a) per allele (p<0.0001). No association was observed with other diabetes risk factors including BMI, smoking, alcohol, blood pressure, triglyceride, CRP, and physical activity.
Association of genetically elevated Lp(a) with T2D
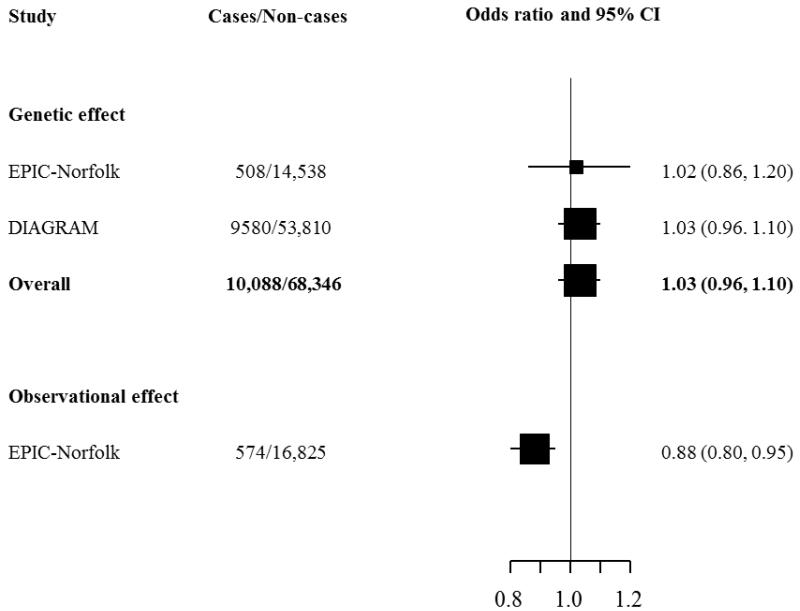
Figure 2 shows that the observed effect estimate in the EPIC-Norfolk study was an odds ratio of 0.88 (95%CI: 0.80-0.95) per 1-SD change in circulating logLp(a) levels for the risk of T2D in analyses adjusted for several confounding factors. However, the association between the genetic instrumental variable (rs10455872) and T2D was not significant, either in EPIC-Norfolk alone (1.02, 0.86-1.20) or in a combined analysis with DIAGRAM data (1.03, 0.96-1.10, p = 0.41).
Figure 2.
Instrumental variable analysis estimate of the association of genetically elevated circulating Lp(a) and risk of T2D using rs10455872 as an instrument
Discussion
In a prospective analysis of 18,490 participants from a European population, we observed a strong inverse association between baseline Lp(a) levels and the onset of incident T2D, with a per Lp(a) quintile decrease in incident T2D of 7% (95%CI: 1 to 13%). However, in Mendelian randomisation analyses, genetically elevated Lp(a) levels were not associated with a lower risk of T2D, with a discordance between the observed and genetic effects, suggesting that this association is not causal.
The inverse association between Lp(a) levels and risk of T2D observed in our study is consistent with but stronger than recent findings in women from the WHS [9], with a significant inverse association across the entire range of the Lp(a) spectrum in our study. We found no clear evidence for a threshold effect within the lowest quintile, or in the overall sample. Notably the distribution of Lp(a) levels in each quintile was broadly similar in our UK based study that included men and women, and the US based study in women (9), except the lowest quintile cut-point was higher (5.3 vs 3.9 mg/dL respectively) and conversely the highest cut-point was lower in EPIC-Norfolk study (35.4 vs. 45.3 respectively).
Unlike previous Mendelian randomisation studies indicating a causal role of Lp(a) levels in CHD (15, 16), present analyses that used rs10455872, a genetic determinant of Lp(a) levels as an instrument suggest that Lp(a) may be merely a marker rather than a determinant of T2D as our observed inverse association of Lp(a) levels with risk of T2D was inconsistent with our genetic analysis. Several explanations could be advanced to explain the discrepancy in results between the observed and genetic association: residual confounding due to inability to adjust the model for all possible confounders appropriately; reverse association bias, leading to reverse associations due to subclinical disease in individuals at baseline; and survivor bias due to individuals with high Lp(a) levels dropping out of the cohort early in follow up, and surviving individuals in the highest quintile of Lp(a) being much healthier than those in the lowest quintile. The time varying hazard ratio described for the Lp(a)-CVD association from EPIC-Norfolk previously is consistent with the possibility of survivor bias (7). Adjusting for lifestyle factors in our study also showed attenuation of the association, which is also consistent with this explanation; however, we were unable to substantiate this in our sensitivity analyses. We were unable to find evidence for reverse association bias, as exclusion of individuals with prevalent and chronic disease did not have any effect on observed estimates. Our results highlight the need for Mendelian randomisation studies to supplement analyses from longitudinal and cross-sectional studies, in order to assess causality in associations, where appropriate genetic markers are readily available. Our investigation of the causal relationship between Lp(a) levels and T2D is novel, and extends past understanding of the association between Lp(a) and T2D, suggesting that despite epidemiological findings, any interventions to elevate the levels of Lp(a) are not warranted as there is likely no causal association with T2D, while higher Lp(a) levels are adversely causally related to CHD risk.
To our knowledge, ours is the first study to investigate the associations of Lp(a) levels with incident T2D as well as incident CHD within the same longitudinal study, which allows a better understanding of the nature of the association. In contrast to our findings for T2D, there was a positive (direct) association between Lp(a) levels and CHD, with a HR for CHD of 1.32 (95%CI 1.14, 1.53), equating to a per quintile increase in incident CHD of 6% (95%CI 3% to 10%). This finding for CHD is in line with previous findings from a large scale individual participant meta-analysis (5). The reasons for the opposing directions of association of Lp(a) with T2D and CHD are currently not clear, For CHD, several mechanisms have been suggested to explain the association with Lp(a) levels. Given that Lp(a) consists of an LDL particle and apo(a) shares high homology with plasminogen, Lp(a) contributes to atherosclerosis. Previous studies have shown that Lp(a) can enter the human arterial wall (25) and bind pro-inflammatory oxidized phospholipids (26), which are strongly associated with future CHD events (27, 28). Also, given the high homology between apo(a) and plasminogen, Lp(a) may potentially interfere with fibrinolysis and increase the risk of thrombosis, although this has not been supported by recent studies on venous thrombosis (29, 30). The potential reasons for an inverse association between Lp(a) and incident diabetes are unknown. However, human and animal studies suggest an effect of insulin in reducing Lp(a) levels. For example, Rainwater et al found an inverse correlation of Lp(a) levels with fasting insulin and 2-hour glucose concentrations in both diabetic and non-diabetic participants (31). Neele et al showed that insulin suppressed apolipoprotein(a) synthesis by primary cultures of cynomolgus monkey hepatocytes (32). These data suggest that insulin resistance may be manifested by a lowering of Lp(a) levels that precedes overt diagnosis of T2D. However, the EPIC-Norfolk study did not have fasting samples for glucose and insulin levels at baseline, thus other prospective studies with serial Lp(a) measurements and fasting glucose and insulin levels would be informative in testing this hypothesis.
The present study has several strengths. The study used a population-based prospective design, which is unlikely to be susceptible to recall bias and included many incident T2D and CHD cases, which were identified through self-report of diagnosis by a physician, as well as from their general practice and local hospital registers, through hospital admissions data, and through death certificates using ICD-10 codes. This was an important strength of this study since new cases of incident diabetes were identified through record linkage with sources of data that were not from a follow-up questionnaire from participants or from a follow-up health check-up, and hence not dependent on participation or loss to follow-up. In our study, we have performed more detailed analyses than in the WHS by adjusting for a wide range of covariates such as physical activity, education levels, prevalent diseases (CHD, stroke, and cancer), and lipid lowering drugs. Because the data of prevalent and incident diseases (e.g. T2D, CHD) are available in this cohort study, we were thus able to carry out comprehensive sensitivity analyses to exclude the possible effect of residual confounding, reverse causality, and co-morbidities. Since people may have T2D or CHD for many years before diagnosis, we performed sensitivity analyses to minimize these potential biases by excluding those with potentially undiagnosed diseases during the first 5 years of follow-up. This cohort study also has a long follow-up, and the availability of both T2D and CHD outcomes, which enabled us to directly compare the differential association of Lp(a) with each of them. Our ability to conduct a Mendelian randomisation study of the Lp(a)/T2D association enabled us to investigate the causal inference of the observed inverse association, and we found no evidence of causality. Including large-scale genetic data from DIAGRAM enabled us to increase the power to reliably assess the association of the genetic variant with T2D risk.
Our study has some limitations. Lp(a) measurement was only available at a single-time-point. However, previous evidence indicated that Lp(a) values are highly consistent from decade to decade and not affected by many external factors (33). Therefore, unlike many other biomarkers, effects of Lp(a) on future disease risk can be reliably assessed by a single measurement. A previous meta-analysis indicated that Lp(a) particles with smaller apo(a) isoforms could increase the risk of CHD by 2-fold (34). However, as the Lp(a) assay used in this study was insensitive to apo(a) isoforms, we were unable to provide specific information about smaller-sized Lp(a) particles associated with T2D and CHD. Our findings are limited to T2D as we did not ascertain cases of type 1 diabetes in this cohort because the EPIC Norfolk study recruited middle-aged and elderly participants at baseline (aged 40-79 years in 1993-97). Since we studied only white European population in the present study, results from this study may not apply to other ethnic groups, particularly African and South Asian origin populations, who have different profiles of Lp(a) concentrations (35, 36). Thus, replication of our findings in other populations would further increase our understanding of the relationships of Lp(a) with T2D and CHD. Although the frequency of rs10455872 deviated from Hardy-Weinberg equilibrium in the control population, such deviation was not due to genotyping error. Several previous studies have identified similar genotyping frequency (~7 - 8%) in their populations using different genotyping platforms (e.g. illumina CVD BeadChip) (15, 37-41). Since we studied only white European population in the present study, we also excluded the possibility of population stratification. It has been speculated that higher concentrations of Lp(a) may be advantageous for survival due to its role as a pro-clotting factor and scavenger of oxidized phospholipids (26-28). Therefore, such deviation could be due to survivor bias with the individuals who are homozygotes for the Lp(a)-raising variant being more common than expected under Hardy-Weinberg equilibrium. This implies that the deviation from HWE may be due to underlying biology. The plasma Lp(a) levels are largely determined by the LPA gene that encodes apolipoprotein(a). A common copy-number variant within LPA gene (kringle IV-type 2 (KIV-2) repeat polymorphism) influences the isoform size of apolipoprotein(a). It is known that the rare allele of rs10455872 in the intron 25 of LPA gene was inversely correlated with KIV-2 repeats (15). The association between rs10455872 and Lp(a) levels is largely dependent on KIV-2 repeats. Therefore, the possibility that the deviation of HWE for rs10455872 is influenced by KIV-2 repeats could be important and should be further considered in disease causation. We are reassured that rs10455872 explained 26.8% of the variability in Lp(a) levels, which make it a good instrument to explore the possible causal association between Lp(a) levels and T2D, and the MR assumptions were satisfied.
In conclusion, our prospective findings suggest that there is a strong inverse association between Lp(a) levels and new onset T2D. However, a genetic variant in the LPA locus that elevated Lp(a) levels was not associated with risk of T2D, suggesting that elevated Lp(a) levels are not causally associated with a lower risk of T2D. Our findings do not lend support to any differential management of Lp(a) levels for the prevention of T2D and CHD, and current clinical guidelines should be followed unchanged by clinicians. The opposing directions of association of Lp(a) levels with T2D (inverse) and CHD (positive) in our study warrant further investigation including the identification of factors that confound the observed association between Lp(a) and T2D.
Supplementary Material
Acknowledgements
We thank all study participants, the general practitioners and the EPIC-Norfolk study team for their helpful input. We would like to thank Quotient Bioresearch for the measurement of lipoprotein(a). We would also like to thank the DIAGRAM consortium for use of the publicly available DIAGRAM data. The EPIC-Norfolk study is supported by programme grants from the Medical Research Council and Cancer Research UK.
Abbreviations
- T2D
type 2 diabetes
- CHD
coronary heart disease
- CRP
C-reactive protein
- CI
confidence interval
- CVD
cardiovascular disease
- SD
standard deviation
- BMI
body mass index
- LDL-C
low-density lipoprotein cholesterol
- HDL
high-density lipoprotein
- Lp(a)
lipoprotein (a)
Footnotes
Duality of interest: The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Tsimikas S, Hall JH. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: A rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60:716–721. doi: 10.1016/j.jacc.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Dube JB, Boffa MB, Hegele RA, Koschinsky ML. Lipoprotein(a): more interesting than ever after 50 years. Curr Opin Lipidol. 2012;23:133–140. doi: 10.1097/MOL.0b013e32835111d8. [DOI] [PubMed] [Google Scholar]
- 3.Craig WY, Neveux LM, Palomaki GE, Cleveland MM, Haddow JE. Lipoprotein(a) as a risk factor for ischemic heart disease: metaanalysis of prospective studies. Clin Chem. 1998;44:2301–2306. [PubMed] [Google Scholar]
- 4.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102(5):1082–1085. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 5.The Emerging Risk Factors Collaboration Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genser B, Dias KC, Siekmeier R, Stojakovic T, Grammer T, Maerz W. Lipoprotein (a) and risk of cardiovascular disease--a systematic review and meta-analysis of prospective studies. Clin Lab. 2011;57:143–156. [PubMed] [Google Scholar]
- 7.Gurdasani D, Sjouke B, Tsimikas S, Hovingh GK, Luben RN, Wainwright NWJ, et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2012;32:3058–3065. doi: 10.1161/ATVBAHA.112.255521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995;44:369–374. doi: 10.2337/diab.44.4.369. [DOI] [PubMed] [Google Scholar]
- 9.Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein(a) and risk of type 2 diabetes. Clin Chem. 2010;56:1252–1260. doi: 10.1373/clinchem.2010.146779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koschinsky ML, Marcovina SM. The relationship between lipoprotein(a) and the complications of diabetes mellitus. Acta Diabetol. 2003;40:65–76. doi: 10.1007/s005920300007. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins AJ, Steele JS, Janus ED, Santamaria JD, Best JD. Plasma apolipoprotein(a) is increased in type 2 (non-insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1992;35:1055–1059. doi: 10.1007/BF02221681. [DOI] [PubMed] [Google Scholar]
- 12.Rainwater DL, MacCluer JW, Stern MP, VandeBerg JL, Haffner SM. Effects of NIDDM on lipoprotein(a) concentration and apolipoprotein(a) size. Diabetes. 1994;43:942–946. doi: 10.2337/diab.43.7.942. [DOI] [PubMed] [Google Scholar]
- 13.Haffner SM, Morales PA, Stern MP, Gruber MK. Lp(a) concentrations in NIDDM. Diabetes. 1992;41:1267–1272. doi: 10.2337/diab.41.10.1267. [DOI] [PubMed] [Google Scholar]
- 14.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 15.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 16.Kamstrup PR, Tybjarg-Hansen A, Nordestgaard BG. Lipoprotein(a) and risk of myocardial infarction – genetic epidemiologic evidence of causality. Scandinavian J Clin & Lab Investigation. 2011;71:87–93. doi: 10.3109/00365513.2010.550311. [DOI] [PubMed] [Google Scholar]
- 17.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 18.Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, et al. EPIC-Norfolk: study design and characteristics of the cohort. Br J Cancer. 1999;80(suppl 1):95–103. [PubMed] [Google Scholar]
- 19.Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6:407–13. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a) JAMA. 1995;274:1771–1774. [PubMed] [Google Scholar]
- 22.Saely CH, Koch L, Schmid F, Marte T, Aczel S, Langer P, et al. Lipoprotein(a), type 2 diabetes and vascular risk in coronary patients. Eur J Clin Invest. 2006;36:91–97. doi: 10.1111/j.1365-2362.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- 23.Qi Q, Workalemahu T, Zhang C, Hu FB, Qi L. Genetic variations, plasma lipoprotein(a) levels, and risk of cardiovascular morbidity and mortality among two prospective cohorts of type 2 diabetes. Eur Heart J. 2012;33:325–34. doi: 10.1093/eurheartj/ehr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Silva NM, Freathy RM, Palmer TM, Donnelly LA, Luan J, Gaunt T, et al. Mendelian randomization studies do not support a role for raised circulating triglyceride levels influence type 2 diabetes, glucose levels, or insulin resistance. Diabetes. 2011;60:1008–18. doi: 10.2337/db10-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen LB, Nordestgaard BG, Stender S, Niendorf A, Kjeldsen K. Transfer of lipoprotein(a) and LDL into aortic intima in normal and in cholesterol-fed rabbits. Arteroscler Thromb Vasc Biol. 1995;15:1492–1502. doi: 10.1161/01.atv.15.9.1492. [DOI] [PubMed] [Google Scholar]
- 26.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 27.Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–29. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 28.Tsimikas S, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Sandhu MS, et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56:946–955. doi: 10.1016/j.jacc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 29.Helgadottir A, Gretarsdottir S, Thorleifsson G, Holm H, Patel RS, Gudnason T, et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol. 2012;60:722–729. doi: 10.1016/j.jacc.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 30.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1732–1741. doi: 10.1161/ATVBAHA.112.248765. [DOI] [PubMed] [Google Scholar]
- 31.Rainwater DL, Haffner SM. Insulin and 2-hour glucose levels are inversely related to Lp(a) concentrations controlled for LPA genotype. Arterioscler Thromb Vasc Biol. 1998;18:1335–1341. doi: 10.1161/01.atv.18.8.1335. [DOI] [PubMed] [Google Scholar]
- 32.Neele DM, deWit EC, Princen HM. Insulin suppresses apolipoprotein(a) synthesis by primary cultures of cynomolgus monkey hepatocytes. Diabetologia. 1999;42:41–44. doi: 10.1007/s001250051110. [DOI] [PubMed] [Google Scholar]
- 33.Bennet A, Di Angelantonio E, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, et al. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med. 2008;168:598–608. doi: 10.1001/archinte.168.6.598. [DOI] [PubMed] [Google Scholar]
- 34.Erqou S, Thompson A, Di Angelantonio E, Saleheen D, Kaptoge S, Marcovina S, et al. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55:2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 35.Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) Lancet. 2000;356:279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 36.Paultre F, Pearson TA, Weil HF, Weil HF, Tuck CH, Myerson M, et al. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arterioscler Thromb Vasc Biol. 2000;20:2619–2624. doi: 10.1161/01.atv.20.12.2619. [DOI] [PubMed] [Google Scholar]
- 37.Lanktree MB, Anand SS, Yusuf S, Hegele RA. Comprehensive analysis of genomic variation in the LPA locus and its relationship to plasma lipoprotein(a) in South Asians, Chinese, and European Caucasians. Circ Cardiovasc Genet. 2010;3:39–46. doi: 10.1161/CIRCGENETICS.109.907642. [DOI] [PubMed] [Google Scholar]
- 38.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hopewell JC, Clarke R, Parish S, Armitage J, Lathrop M, Hager J, et al. Lipoprotein(a) genetic variants associated with coronary and peripheral vascular disease but not with stroke risk in the Heart Protection Study. Circ Cardiovasc Genet. 2011;4:68–73. doi: 10.1161/CIRCGENETICS.110.958371. [DOI] [PubMed] [Google Scholar]
- 40.Anderson JL, Knight S, May HT, Horne BD, Bair TL, Huntinghouse JA, et al. Validation and Quantification of Genetic Determinants of Lipoprotein-a Levels and Predictive Value for Angiographic Coronary Artery Disease. Am J Cardiol. 2013;112:799–804. doi: 10.1016/j.amjcard.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Qi Q, Workalemahu T, Zhang C, Hu FB, Qi L. Genetic variants, plasma lipoprotein(a) levels, and risk of cardiovascular morbidity and mortality among two prospective cohorts of type 2 diabetes. Eur Heart J. 2012;33:325–334. doi: 10.1093/eurheartj/ehr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.