Summary
Physiological and proteome insights were determined into the alleviating effects of pre-anthesis drought priming on drought stress during post-anthesis, which will be of importance for future drought-tolerance studies in cereals.
Key words: Drought tolerance, leaf proteome, photosynthesis, priming, wheat.
Abstract
Drought stress occurring during the reproductive growth stage leads to considerable reductions in crop production and has become an important limiting factor for food security globally. In order to explore the possible role of drought priming (pre-exposure of the plants to mild drought stress) on the alleviation of a severe drought stress event later in development, wheat plants were subjected to single or double mild drought episodes (soil relative water content around 35–40%) before anthesis and/or to a severe drought stress event (soil relative water content around 20–25%) 15 d after anthesis. Here, single or double drought priming before anthesis resulted in higher grain yield than in non-primed plants under drought stress during grain filling. The photosynthesis rate and ascorbate peroxidase activity were higher while malondialdehyde content was lower in primed plants than in the non-primed plants under drought stress during grain filling. Proteins in flag leaves differently expressed by the priming and drought stress were mainly related to photosynthesis, stress defence, metabolism, molecular chaperone, and cell structure. Furthermore, the protein abundance of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) small subunit, Rubisco activase and ascorbate peroxidase were upregulated in primed plants compared with non-primed plants under drought stress during grain filling. In conclusion, the altered protein expression and upregulated activities of photosynthesis and ascorbate peroxidase in primed plants may indicate their potential roles in alleviating a later-occurring drought stress episode, thereby contributing to higher wheat grain yield under drought stress during grain filling.
Introduction
It is known that the frequency and duration of extreme climate episodes are increasing (Ciais et al., 2005). Drought events are limiting crop production, and particularly those occurring during the reproductive growth stage can lead to significant reductions in yield and quality (Barnabas et al., 2008; Farooq et al., 2009).
The mechanisms of plant drought tolerance are complex and involve diverse and multiple physiological and molecular mechanisms (Shinozaki and Yamaguchi-Shinozaki, 2007; Farooq et al., 2009). It has been reported that the downregulation of photosynthesis due to mild drought stress is mainly the result of a reduction in stomatal conductance, while the photosynthetic apparatus is not significantly affected (Cornic and Fresneau, 2002; Harb et al., 2010). As a consequence of severe drought stress events, both stomatal and non-stomatal limitations lead to a decline in photosynthesis (Bota et al., 2004; Flexas et al., 2008), For example, electron transport from photosystem II (PSII) to PSI and enzymes of carbon metabolism [e.g. ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and enzymes related to ribulose-1,5-bisphosphate synthesis] accounted for the lower photosynthesis rate under these conditions (Medrano et al., 2002; Tezara et al., 1999).
The electron transport chain in the chloroplastic thylakoid membrane is the major source of reactive oxygen species (ROS) under stress conditions (Apel and Hirt, 2004). Damage to the PSII oxygen-evolving complex (Canaani et al., 1986) and to the reaction centre (He et al., 1995; Reddy et al., 2004) may lead to the imbalance of electron generation and utilization, resulting in the generation of ROS (Reddy et al., 2004), and consequently cause lipid peroxidation of cell membranes (Murata et al., 2007). Antioxidant enzymes, such as superoxide dismutase, glutathione reductase and ascorbate peroxidase, play important roles in scavenging excess ROS, which are generated by abiotic stress (Jiang and Zhang, 2002; Hernández et al., 2012).
It has been shown that priming—the pre-exposure of plants to an eliciting factor—enables plants to become more tolerant to later-occurring biotic or abiotic stress events (Bruce et al., 2007). To date, many studies have focused on biotic-induced priming and the mechanisms, which include, among others, the accumulation of mitogen-activated protein kinases (MPKs), epigenetic changes, and regulation of primary metabolism (Uwe et al., 2006; Beckers and Conrath, 2007; Bruce et al., 2007; Conrath, 2011). Attention has also been given to chemical-induced priming, such as by nitric oxide (Tanou et al., 2009; Molassiotis et al., 2010), β-aminobutyric acid (Tsai et al., 2011), hydrogen sulfide (Christou et al., 2013; Shi et al., 2013), and acclimation mainly through regulation of defence-related genes/proteins as well as induction of antioxidant mechanisms (Jakab et al., 2005; Filippou et al., 2012; Tanou et al., 2012).
However, fewer studies investigated abiotic stress-induced priming. It was found in Arabidopsis that multiple pre-exposures to mild drought stress episodes increased the flexibility of the plant to cope with a recurring drought stress event, and that a stalled RNA polymerase II was involved in the transcriptional drought memory (Ding et al., 2012). However, the duration between priming and the reoccurring stress was very short (several hours or days) (Bruce et al., 2007; Walter et al., 2011). Whether plants are able to conserve the ‘memory’ of a previous stress episode to a subsequent stress event later in development, as well as the underlying mechanisms, is far from clear. Our previous studies have shown that priming with high temperatures (Wang et al., 2011, 2012) or waterlogging (Li C et al., 2011) before anthesis could alleviate the negative effects of the same stress occurring after anthesis, as exemplified by improved grain yields in primed plants compared with non-primed plants in wheat. Walter et al. (2011) found that plants experiencing an early drought episode showed a higher percentage of biomass and improved photo-protection than non-primed plants under a second drought event in Arrhenatherum elatius. However, Zavalloni et al. (2008) found that elevated temperature and mild drought applied early in development did not enhance tolerance to a later drought stress event in several grass species.
Proteomics is an important tool both for understanding the mechanisms of plants in response to abiotic stress (Chen and Harmon, 2006; Caruso et al., 2009; Kottapalli et al., 2009; Kamal et al., 2013) and for gaining insight into possible priming mechanisms (Tanou et al., 2012). Thus, it has been shown that salicylic acid induced drought tolerance in wheat seedlings through regulation of the proteins related to signal transduction, stress defence, photosynthesis and metabolism (Kang et al., 2012). However, to the best of our knowledge, proteome analysis has not been applied for revealing the mechanisms of drought priming in response to a later-occurring drought stress event.
In the present study, we first subjected wheat plants to single and/or multiple mild drought priming events before anthesis, and then to a severe drought stress event during grain filling. Our hypothesis was that: (i) drought priming before anthesis would affect the synthesis and/or activities of enzymes related to photosynthesis and stress defence as mechanisms to enhance tolerance to drought stress occurring during the grain-filling stage; and (ii) there is no difference between drought priming once or twice on the alleviating effect of drought stress during grain filling.
Materials and methods
Experimental design
A pot experiment was performed outdoors under field conditions at the Research Centre Flakkebjerg, University of Aarhus, Denmark, in 2011. Each pot (height 18cm and diameter 23cm; 90 pots in total) was filled with 800g of a mixture of soil and peat (v/v, 1:3). Six grains of commercial spring wheat (Triticum aestivum L. cv. Vinjett) were sown in each pot and thinned to three plants at the three-leaf stage (growth stage 13, according to Lancashire et al., 1991).
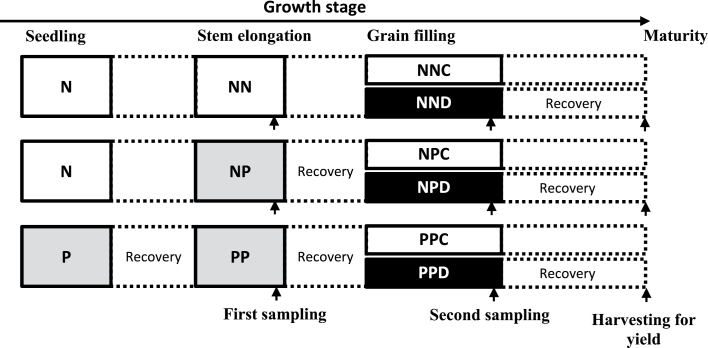
The experimental design is shown in Fig. 1. Drought priming was applied either once, during the stem elongation stages (37 d after sowing, growth stage 39; see Lancashire et al., 1991) or twice, during both seedling (27 d after sowing, growth stage 17) and stem elongation stages. The soil relative water content (SRWC) for the well-watered plants was set at 80–90%. Drought priming was applied by withholding watering for 5–7 d until the SRWC reached approx. 35–40% and maintained for 2 d. Drought stress was applied 15 d after anthesis by withholding watering for 5 d until the SRWC dropped to 20–25% and maintained for 3 d. The pots were weighed every day to monitor the soil water content at the target level. To avoid the heat stress and drought stress interaction, during drought priming and drought stress treatment, both the control plants and the drought plants were moved to a growth chamber (PGV36; Conviron, Montreal, Canada), at temperatures of 24/16 °C (day/night), a light intensity of 400 μmol photons m–2 s–1 and a humidity of 60%. After treatment, the pots were rewatered and moved outdoors until harvest maturity. There were no significant differences in phenological development of the plants among the treatments. Sampling and measurements were done at the end of drought priming at stem elongation and drought stress during grain filling. The last fully expanded leaves were used for physiological and proteome analysis during drought priming. Three treatments were included: non-primed plants (NN), drought priming applied at stem elongation stage (NP), and drought priming at both seedling and stem elongation stages (PP). The flag leaves were used for physiological and proteome analysis under drought stress during grain filling. Six treatments were included: control (NNC), no priming+drought stress during grain filling (NND), priming at stem elongation stage+non-stress during grain filling (NPC), priming at stem elongation stage+drought stress during grain filling (NPD), priming twice+non-stress during grain filling (PPC), and priming twice+drought stress during grain filling (PPD). All primed or drought-stressed plants were immediately rewatered after the sampling and measurements.
Fig. 1.
Diagram of the experimental design. Drought priming was applied either once during the stem elongation stage or twice during seedling and stem elongation. Drought priming was induced by withholding watering for 5–7 d until the soil water content reached approx. 35–40% for 2 d. Drought stress was applied 15 d after anthesis by withholding watering for 5 d until the soil water content dropped to 20–25% for 3 d. N, non-priming at the respective growth stage; P, priming at respective stage; NN, non-primed plants; NP, drought priming only at stem elongation stage; PP, drought priming at both seedling and stem elongation stages; NNC, control; NND, no priming+drought stress during grain filling; NPC, priming at stem elongation stage+non-stress during grain filling; NPD, priming at stem elongation stage+drought stress during grain filling; PPC, priming twice+non-stress during grain filling; PPD, priming twice+drought stress during grain filling. The small up arrows indicate time of sampling/measurement or harvesting. The primed plants and the drought-stressed plants during grain filling were rewatered immediately after the sampling and measurement.
SRWC and leaf relative water content (LRWC)
SRWC was calculated using the formula:
LRWC was measured according to Jensen et al. (2000) and determined as follows:
Three biological replicates were analysed.
Leaf gas exchange
Leaf gas exchange was measured as reported previously (Wang et al., 2011) using an LI- 6400 portable photosynthesis system (LI-COR Biosciences, Lincoln, NE, USA) from 9:00 a.m. to 11:30 a.m. A standard 2×3cm chamber and light-emitting diode light source were used to support a constant photosynthetically active radiation level of 1000mol m–2 s–1. All measurements were taken at a constant flow rate of 500ml min–1 and a CO2 concentration of 400 μmol mol–1. Three biological replicates were analysed.
Determination of malondialdehyde (MDA) content and ascorbate peroxidase (APX) activity
The extract for determination of the membrane lipid peroxidation was prepared according to Tan et al. (2008) and Jiang and Zhang (2002). Leaf sample (0.1g) was homogenized with a cold mortar and pestle in 3ml of extraction solution [50mM PBS (pH 7.0), 0.4% (w/v) polyvinylpyrrolidone] and with the addition of 1mM ascorbic acid for the APX assay. The homogenate was centrifuged at 12 000g for 20min at 4 °C, and the supernatant was collected and for further analysis.
A mixture of 1ml of supernatant and 4ml of reaction solution (thiobarbituric acid reactive substances (with 0.5% in 20% trichloroacetic acid)] were heated by incubating at 95 °C for 25min and immediately cooled in ice bath. The mixture was centrifuged at 12 000 g for 10min, and supernatant was used to determined MDA content at 532nm and 600nm. APX (EC 1.11.1.11) was assayed according to Nakano and Asada (1981). In brief, 1ml of reaction solution contained 50mM PBS (pH 7.0), 0.5mM ascorbic acid, 0.1mM H2O2, and 100 μl of extraction supernatant. APX activity was observed by recording the decreased rate of ascorbic acid oxidized at 290nm for 1min. Absorbance was measured with a UV/visible spectrophotometer (UltrospecTM 2100 pro; Amersham Biosciences). Three biological replicates were analysed.
Protein extraction and quantification
Leaf protein extraction was performed according to Rinalducci et al. (2011), with minor modifications. Three biological replicates of fresh wheat leaves (0.5g) were finely ground in liquid nitrogen. The powder was suspended in 5ml of cold (–20 °C) 10% (w/v) trichloroacetic acid in acetone containing 0.07% (w/v) dithiothreitol (DTT) and one tablet per 50ml of extraction solution Protease Inhibitor Cocktail tablets (Roche), vortexed, and incubated at –20 °C overnight. The extraction was centrifuged at 35 000g for 1h at 4 °C. The collected pellet was washed with 5ml of chilled (–20 °C) acetone containing 0.07% (w/v) DTT, precipitated for 2h at –20 °C, and then centrifuged at 20 000g at 4 °C for 30min; the procedure was repeated three times to make sure the pellet was colourless. The supernatant was removed and the pellet was dried at 4 °C. The pellet was solubilized in a freshly prepared buffer containing 9M urea, 4% (w/v) CHAPS, 1% (w/v) DTT, and 1% (v/v) pH 4–7 ampholytes (GE Healthcare, Freiburg, Germany), 35mM Tris (Sigma) via incubation at 30 °C overnight with continuous stirring (Thermo mixer). The mixture was centrifuged at 12 000g at room temperature for 20min and the supernatant was analysed by two-dimensional gel electrophoresis. A small aliquot was used to determine protein concentration by a modified Bradford assay (Ramagli, 1999). Three biological replicates were analysed.
Two-dimensional gel electrophoresis
Isoelectric focusing was performed with the EttanTM IPGphor (GE Healthcare) using IPG strips (linear pH 4–7, 18cm; GE Healthcare). Protein samples of 400 μg were added to a total volume of 350 μl of same solubilization solution containing 1% ampholyte pH 4–7 (GE Healthcare) and a trace of orange G. The solution was thoroughly vortexed and loaded on the strip. Isoelectric focusing was performed at 20 °C at a total of 67 kVh. IPG strips were subsequently equilibrated in 5ml of equilibration buffer [50mM Tris/HCl (pH 8.8), 6M urea, 30% (v/v) glycerol, 2% (w/v) SDS, 0.01% (w/v) bromophenol blue] with 1% (w/v) DTT (15min), followed by 5ml of equilibration buffer with 2.5% (w/v) iodoacetamide (15min). Separation in the second dimension was performed in 12.5% acrylamide (40% T, 3% C) gels using an EttanTM Daltsix Electrophoresis Unit (GE Healthcare) according to the manufacturer’s protocol. Strips together with a molecular marker (Mark 12TM; Invitrogen, Denmark) were placed on the gels and overlaid with 0.5% molten agarose. Separation was performed at 2W per gel (45min), followed by 12W per gel (4h) (until the dye front reached the gel bottom). Gels were stained overnight by colloidal Coomassie Brilliant Blue G-250 (Candiano et al., 2004). Three biological replicates were analysed.
Image analysis
Two-dimensional gels were scanned using a ScanMaker 9800XL (Microtek) at 300 dpi resolution in both colour and greyscale (16 bits). Spot detection and gel comparison were analysed using Progenesis SameSpots v.4.1 software (Nonlinear Dynamics, UK). The gel image from the control was chosen as a reference template, and spots in other gels were automated to match the reference gel and then edited manually to correct mismatched and unmatched spots. From the software, the average normalized spot quantity value was determined. The threshold of analysis of variance (ANOVA) was carried out at P≤0.05, power ≥0.8 and 1.5-fold change in average spot volume between treatments and the corresponding control was used to select the different spots for further mass spectrometry (MS) analysis. Spots had to be present on three replicate gels to be considered as present in a reproducible way.
In-gel digestion and protein identification
Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS and MS/MS are commonly used for the protein identification of spots separated by two-dimensional electrophoresis gels (Jensen et al., 1997; Rinalducci et al., 2011; Huang et al., 2012; Kausar et al., 2013). The spots analysed by Progenesis SameSpots software were excised manually and subjected to in-gel trypsin digestion according to Yang et al. (2010). Aliquots (1 μl) of trypsin spot digests were applied to an Anchor ChipTM target plate (Bruker-Daltonics, Bremen, Germany), covered by 1 μl of matrix solution (0.5g l–1 of α-cyano-4-hydroxycinnamic acid in 70% acetonitrile, 0.1% trifluoroacetic acid) and washed in 1 μl of 0.5% trifluoroacetic acid. Spectra were calibrated externally and internally using a trypic digest of β-lactoglobulin (5 pmol μl–1) and porcine trypsin autolysis products, respectively. The trypsin autolysis products (m/z 842.51 and 2211.10, respectively) were used as internal spectra calibration. Peptide mass fingerprinting and MS/MS data were acquired with Flex analysis 3.0 software (Bruker- Daltonics). Protein identification was performed by searching the NCBInr (National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/) database using an in-house Mascot server (http://www.matrixscience.com) integrated with BioTools v3.1 software (Bruker-Daltonics). The following parameters were applied: taxonomic category: Green plant, allowed global modification, carbamidomethyl cysteine; variable modification, oxidation of methionine; missed cleavages, 1; peptide tolerance, 80 ppm; and MS/MS tolerance ±0.5Da. To be considered as a positive identification, the score of results had to be over the significance threshold level (P<0.05), and at least five matched independent peptides for peptide mass mapping were required. Sequences encoding proteins of unknown function were subjected to a BLAST (Basic Local Alignment Search Tool; http://www.ncbi.nlm.nih.gov/BLAST/) search in NCBI (Altschul et al., 1990). Functional classifications of identified proteins were based on Bevan et al. (1998).
Statistics
One-way ANOVA was applied to analyse the difference between treatments. Significant differences at P<0.05 among all treatments was determined by Duncan’s multiple range test (Sigmaplot 11.0; Systat Software).
Results
SRWC and LRWC
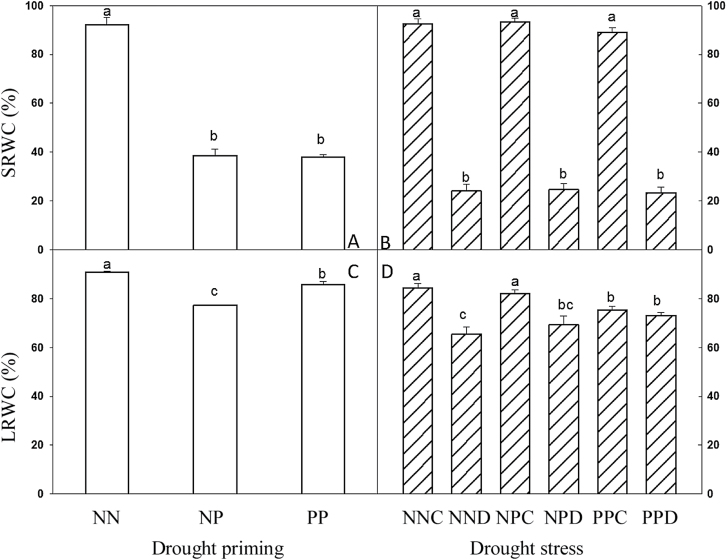
SRWC was maintained at around 37% during drought priming and at 25% under drought stress during grain filling (Fig. 2A, B). After drought priming, LRWC was lower in NP and PP compared with NN, and NP had a lower LRWC than PP (Fig. 2C). Under drought stress during grain filling, LRWC was lower in NND, NPD, PPC, and PPD, in relation to NNC (Fig. 2D).
Fig. 2.
SRWC and LRWC under drought priming and drought stress. (A, B) SRWC under drought priming (A) and drought stress (B). (C, D) LRWC under drought priming (C) and under drought stress (D). See Fig. 1 legend for abbreviations. Different letters indicate significant differences at P<0.05 among all treatments as determined by Duncan’s multiple range test.
Grain yield and leaf photosynthesis
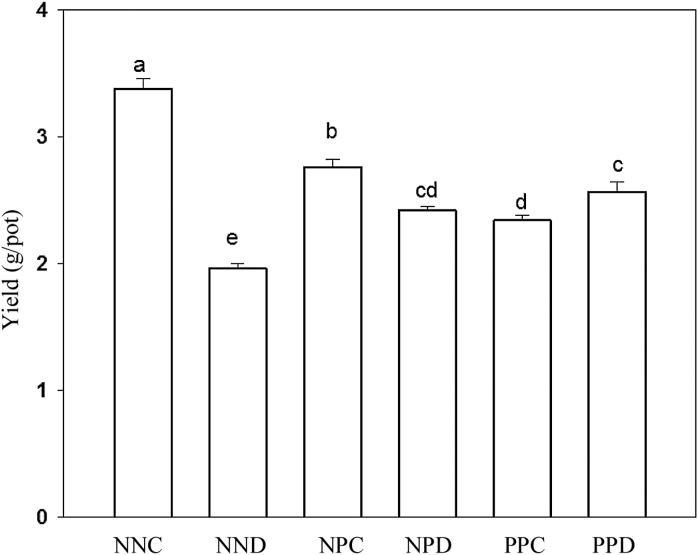
Drought stress during grain filling (NND, NPD, and PPD) significantly decreased grain yield, in relation to NNC (Fig. 3). However, the primed plants (NPD and PPD) showed less yield reduction than non-primed plants (NND). In relation to NNC, grain yield was downregulated by 45, 30, and 31% in NND, NPD, and PPD, respectively. In addition, drought priming before anthesis treatments (NPC and PPC) significantly downregulated grain yield compared with NNC.
Fig. 3.
Effect of drought priming on grain yield in wheat experiencing drought stress after anthesis. See Fig. 1 legend for abbreviations. Three biological replicates were performed. Different letters indicated significant differences at P<0.05 among all treatments as determined by Duncan’s multiple range test.
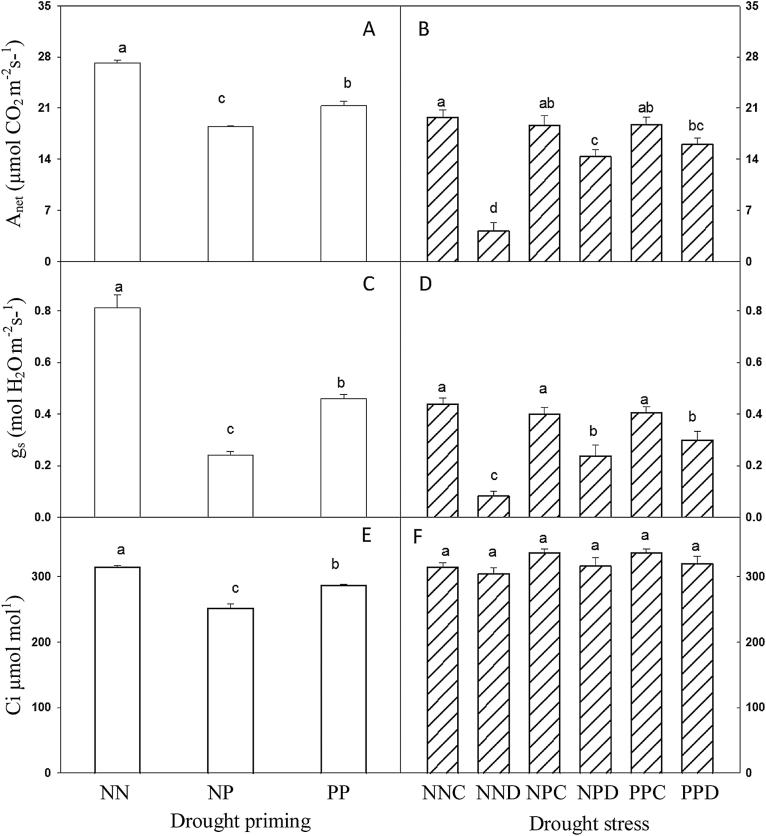
After drought priming, the photosynthetic assimilation rate (A net) was downregulated by 32 and 15% in NP and PP, respectively, in relation to NN (Fig. 4A). Under drought stress during grain filling, in relation to NNC, A net decreased by 78, 26, and 17% in NND, NPD, and PPD, respectively. Thus, the drought-primed plants (NPD and PPD) showed significantly higher A net than the non-primed plants (NND) under drought stress during grain filling (Fig. 4B). Stomatal conductance (g s) in NP and PP was downregulated by 70 and 47%, respectively, compared with NN (Fig. 4C). Drought stress also significantly reduced g s in relation to NNC, while NPD and PPD showed significantly higher g s than NND (Fig. 4D). Internal CO2 concentration (C i) was downregulated in NP and PP in relation to NN (Fig. 4E), while no significant differences were found under drought stress (Fig. 4F). There were no differences of A net and g s among NNC, NPC, and PPC.
Fig. 4.
Effect of drought priming on net photosynthetic assimilation rate (A net), stomatal conductance (g s), and internal CO2 concentration (C i) of wheat leaves under drought priming and drought stress. (A, B) A net under drought priming (A) and drought stress (B). (C, D) g s under drought priming (C) and drought stress (D). (E, F) C i under drought priming (E) and drought stress (F). See Fig. 1 legend for abbreviations. Three biological replicates were analysed. Different letters indicated significant differences at P<0.05 among all treatments as determined by Duncan’s multiple range test.
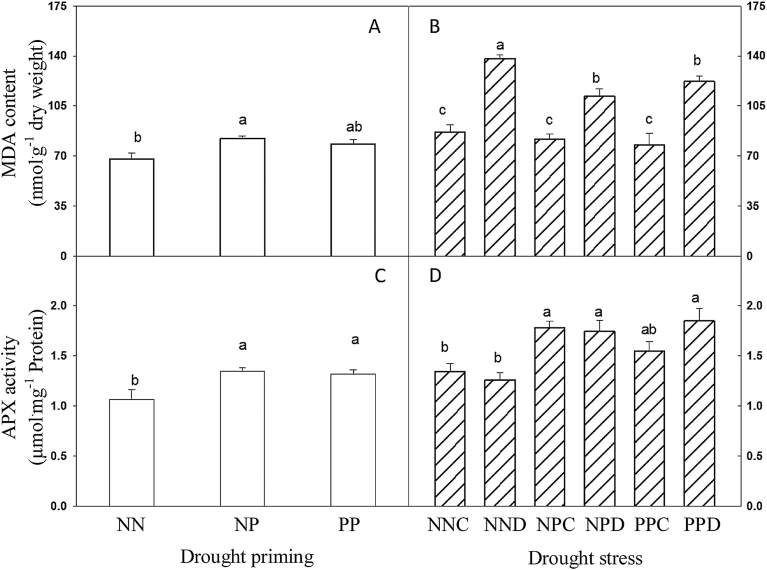
Leaf MDA content and APX activities
Leaf MDA content was upregulated by 33 and 30% in NP and PP, respectively, in relation to NN. Drought stress upregulated the MDA content significantly, while the MDA content in NND, NPD, and PPD was upregulated by 59, 28, and 42% respectively, relative to NNC. There were no significantly differences of MDA content among NNC, NPC, and PPC (Fig. 5A, B).
Fig. 5.
Effect of pre-anthesis drought priming on MDA content and APX activity in wheat leaves under drought priming and drought stress. (A, B) MDA content under drought priming (A) and drought stress (B). (C, D) APX activity under drought priming (C) and drought stress (D). See Fig. 1 legend for abbreviations. Three biological replicates were performed. Different letters indicated significant differences at P<0.05 among all treatments as determined by Duncan’s multiple range test.
After drought priming, APX activities were upregulated by 23 and 19% in NP and PP compared with NN. Under drought stress during grain filling, compared with NNC, no significant difference of the APX activity in NND was observed, while APX activity was upregulated by 30 and 38% in NPD and PPD, respectively (Fig. 5C, D). There were no significant differences in NPD and PPD compared with the corresponding control NPC and PPC. NPC and PPC showed higher APX activity in relation to NNC.
Proteome profiles
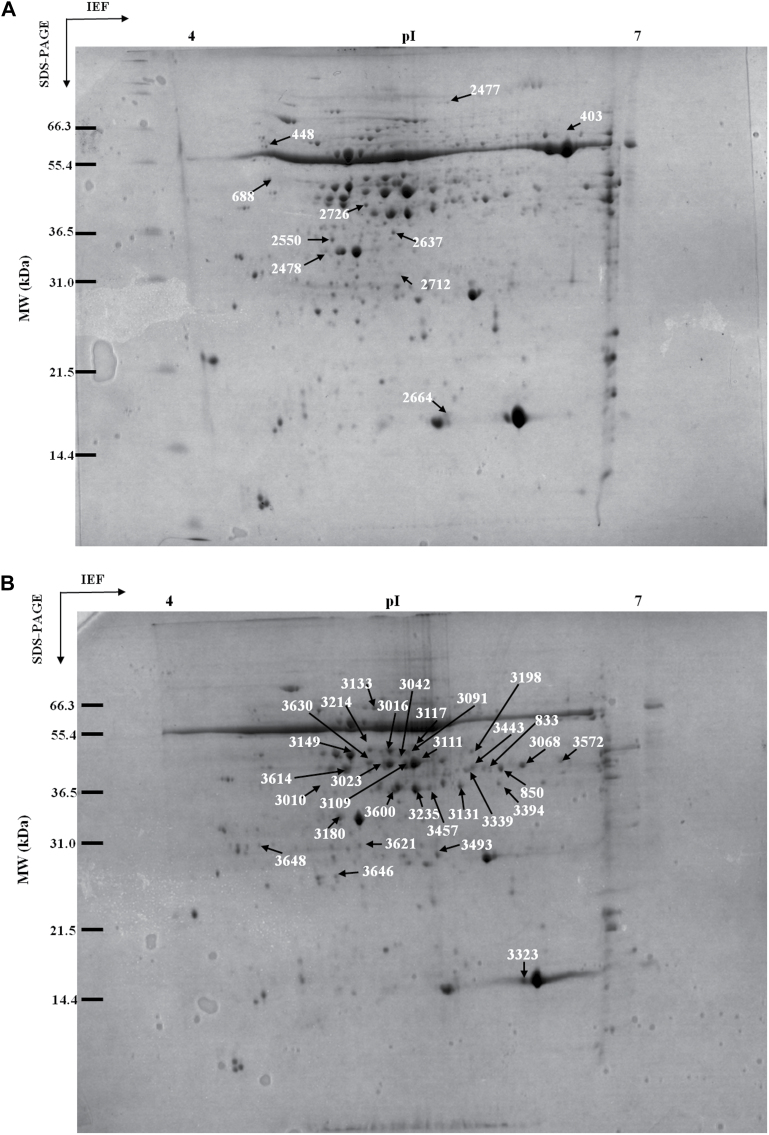
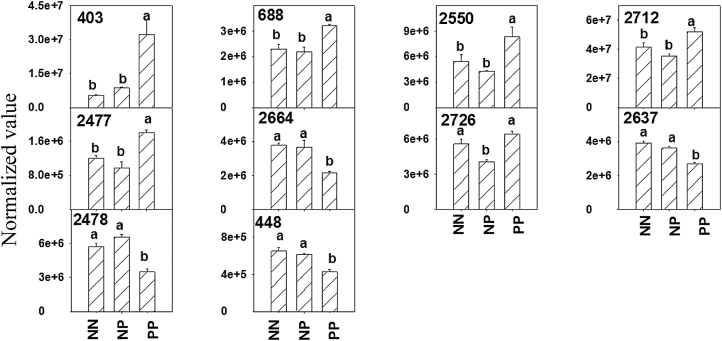
Representative gels of protein expression profiles in wheat leaves after priming before anthesis and drought stress during grain filling are shown in Fig. 6. In total, approximately 600 and 400 protein spots were detected in samples after priming and drought stress, respectively, which corresponds well with previous studies (Budak et al., 2013; Donnelly et al., 2005; Fulda et al., 2011). Ten protein spots were detected as differentially expressed between the drought priming treatments (NP and PP) and the non-priming treatment (NN) (Fig. 6A), of which seven were successfully identified (Table 1). These identified proteins were classified as being related to photosynthesis, stress defence, cell structure, and unknown function. Compared with NP, six spots (403, 688, 2550, 2712, 2477, and 2726) were upregulated and four spots (2664, 2637, 2478, and 448) were downregulated in PP (Fig. 7). The upregulated proteins included: Rubisco large subunit (RLS, spot 403), peptidyl-prolyl cis-trans isomerase CYP38 (spot 688), 4-nitrophenyl phosphatase (spot 2550), and PSII stability/assembly factor HCF136 (spot 2726). The downregulated proteins included the Rubisco small subunit (RSS, spot 2664), thioredoxin-like protein CDSP32 (spot 2637), and the putative myosin heavy chain (spot 2478).
Fig. 6.
Representative two-dimensional electrophoresis gels of wheat leaf proteins during drought priming and drought stress. (A) Representative gel resulting from Progenesis Samespot software comparing the non-primed plants, stem elongation stage-primed plants, and twice-primed plants. (B) Representative gel resulting from Progenesis Samespot software comparing control plants, non-primed plants under drought stress, stem elongation-primed plants under drought stress, and twice-primed plants under drought stress. Differentially expressed protein spots in the drought priming treatments and drought stress treatments are indicated by arrows and listed in Tables 1 and 2. M r, relative molecular mass; pI, isoelectric point.
Table 1.
List of proteins differently expressed under drought priming analysed by MALDI-MS and MS/MS
| Spot no. | FCa | Protein name | Accession no. | Taxonomy | Theor. M r b/pI | Exp. M r c/ pI | Match no.d | SCe | E-value | Peptides sequence | Function |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Upregulated under drought priming | |||||||||||
| 403 | 6.1 | Rubisco large chain | gi|14017580 | Triticum aestivum | 53.4/6.2 | 63/6.6 | 39 | 63 | 1.3E–09 | Photosynthesis | |
| 688 | 1.5 | Predicted: peptidyl-prolyl cis- trans isomerase CYP38 | gi|357147646 | Brachypodium distachyon | 46.5/4.8 | 46/4.7 | 13 | 30 | 6.5E–07 | Photosynthesis | |
| 2550 | 1.5 | 4-nitrophenylphosphatase | gi|226491816 | Zea mays | 39.5/5.5 | 35/5.1 | 13 | 26 | 4.1E–05 | Unknown | |
| 2712 | 1.8 | No match | |||||||||
| 2477 | 1.5 | No match | |||||||||
| Downregulated under drought priming | |||||||||||
| 2664 | 1.8 | Rubisco small chain PW9 | gi|132099 | Triticum aestivum | 19.8/8.5 | 17/5.8 | 7.0E–03 | EYPDAYVR | Photosynthesis | ||
| 1.7E–05 | EHNSSPGYYDGR | ||||||||||
| 2726 | 1.5 | Photosystem II stability/ assembly factor HCF136 | gi|357117071 | Brachypodium distachyon | 37.0/5.4 | 43/5.3 | 10 | 30 | 3.2E–03 | Photosynthesis | |
| 2637 | 1.5 | Thioredoxin-like protein CDSP32 | gi|15222954 | Arabidopsis thaliana | 34.0/8.7 | 36/5.5 | 3.2E–04 | GELIGEILR | Stress defence | ||
| 2478 | 1.9 | Putative myosin heavy chain | gi|20197623 | Arabidopsis thaliana | 86.2/4.9 | 34/5.1 | 14 | 15 | 1.5E–02 | Cell structure | |
| 448 | 1.5 | No match | |||||||||
aThe value represents the highest fold change of stem elongation-primed plants (NP) and twice-primed plants (PP) compared with non-primed plants (NN).
bTheoretical M r/pI (kDa).
cExperimental M r/pI (kDa) values were calculated using the Progenesis Samespot software.
dMatch no. represents the number of identified peptides that can be matched with protein peptides in the database.
eSequence coverage (%).
Fig. 7.
Quantitative variations (expressed as average normalized volumes) of spots differently expressed by drought priming. See Fig. 1 legend for abbreviations. Numbers are the same as spot numbers shown in Fig. 6A. Different letters indicate significant differences at P<0.05 among all treatments as determined by Duncan’s multiple range test.
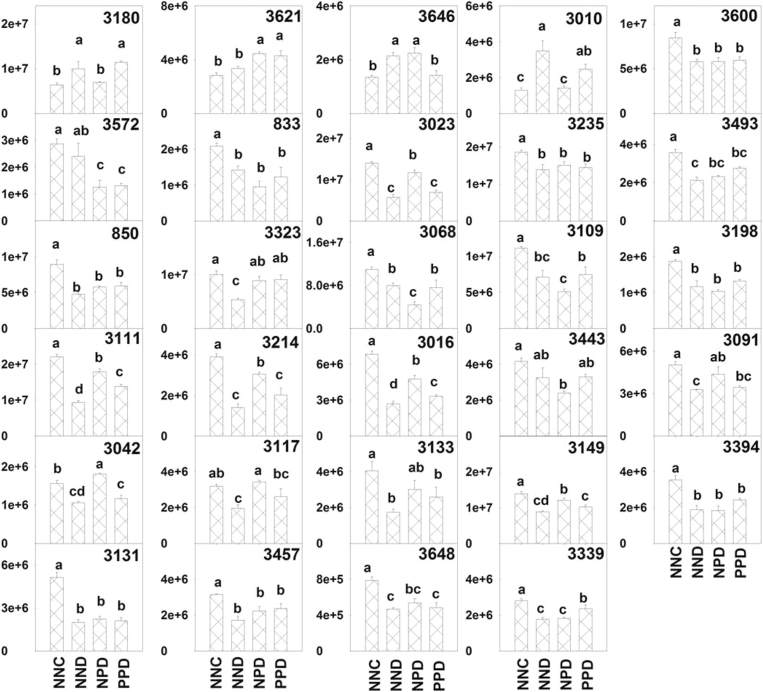
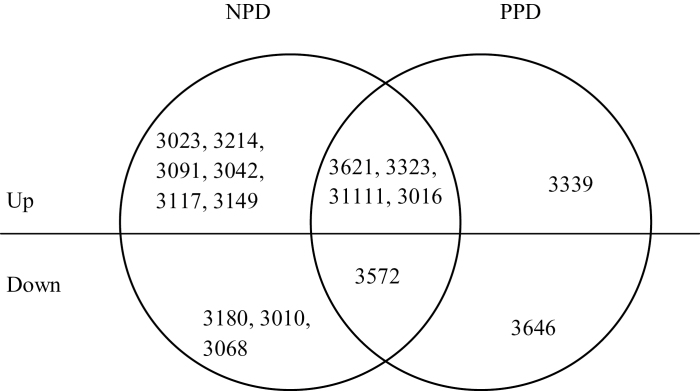
Under drought stress during grain filling (NND, NPD, and PPD), 29 proteins were differently expressed and successfully identified as compared with NNC (Fig. 6B), and of these, 25 proteins spots were downregulated and four were upregulated by drought stress. The differential expression abundances are shown in Fig. 8. The identified proteins were classified as photosynthesis, stress defence, metabolism, molecular chaperone, protein synthesis, cell structure, and unknown function (Table 2). The protein spots identified as Rubisco activase (RCA, spots 3111 and 3016), RSS (spot 3323), and APX (spot 3621) were commonly upregulated, while the spot identified as fructose-bisphosphate aldolase (FBA, spot 3572) was downregulated in primed plants compared with the non-primed plants (Fig. 9). In relation to NPD, spots identified as phosphoglycerate kinase (spot 3042), actin (spot 3117), glutamine synthetase (spot 3149), and 2-Cys peroxiredoxin BAS1 (spot 3646) were downregulated in PPD, while spots identified as glutamine synthetase (spot 3010), oxygen-evolving enhancer proteins (spot 3180), and Hypothetical protein MTR_026s0001 (spot 3339) were upregulated in PPD (Fig. 9). Two spots (3010, 3149) identified as glutamine synthetase showed contrasting expression profiles under drought stress during grain filling. Spot 3149 was upregulated in NPD compared with NND and PPD. Spots 3010 and 3149 had similar pI values (5.1 and 5.3), while the lower molecular weight of spot 3010 (43kDa) compared with spot 3149 (49kDa) might suggest that 3010 is a fragment or another form of glutamine synthetase (spot 3149).
Fig. 8.
Quantitative variations (expressed as average normalized volume) of spots identified as differently expressed between primed plants and non-primed plants under drought stress. See Fig. 1 legend for abbreviations. Numbers are the same as spot numbers shown in Fig. 6B. Different letters indicate significant differences at P<0.05 among all treatments as determined by Duncan’s multiple range test.
Table 2.
List of proteins differently expressed under drought stress analysed by MALDI-MS and MS/MS
| Spot no. | FCa | Protein name | Accession no. | Taxonomy | Theor. M r b/pI | Exp. Mrc/pI | Match no.d | SCe | E-value | Peptides sequence | Function |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Upregulated under drought stress | |||||||||||
| 3180 | 1.8 | Oxygen-evolving enhancer protein 1 | gi|357111487 | Brachypodium distachyon | 34.8/5.7 | 35/5.2 | 10 | 39 | 3.3E–07 | Photosynthesis | |
| 3621 | 1.6 | Ascorbate peroxidase | gi|226897533 | Triticum aestivum | 26.8/5.5 | 32/5.3 | 11 | 38 | 5.2E–05 | Stress defence | |
| 3646 | 1.6 | 2-Cys peroxiredoxin BAS1 | gi|2499477 | Hordeum vulgare | 23.4/5.5 | 28/5.2 | 6 | 32 | 5.1E–04 | Stress defence | |
| 3010 | 2.7 | Plastid glutamine synthetase isoform GS2c | gi|71362640 | Triticum aestivum | 47/5.8 | 43/5.1 | 6 | 17 | 8.4E–03 | Protein synthesis | |
| Downregulated under drought stress | |||||||||||
| 3600 | 1.5 | Fructose-bisphosphate aldolase 2 | gi|326499908 | Hordeum vulgare | 41.8/6.4 | 39/5.6 | 18 | 38 | 5.2E–08 | Metabolism | |
| 3572 | 2.3 | Fructose-bisphosphate aldolase, cytoplasmic isozyme 1-like | gi|326493652 | Hordeum vulgare | 38.1/6.1 | 42/6.7 | 9 | 21 | 2.1E–08 | Metabolism | |
| 833 | 2.2 | Fructose-bisphosphate aldolase, cytoplasmic isozyme 1-like | gi|326523629 | Hordeum vulgare | 41.6/7.1 | 42/6.2 | 7 | 11 | 1.4E–02 | Metabolism | |
| 3023 | 2.5 | Chloroplast fructose-bisphosphate aldolase | gi|223018643 | Triticum aestivum | 42.2/5.9 | 46/5.6 | 17 | 50 | 5.2E–12 | Metabolism | |
| 3235 | 1.3 | Fructose-bisphosphate aldolase, cytoplasmic isozyme 1-like | gi|326499908 | Hordeum vulgare | 41.7/6.4 | 37/5.7 | 17 | 38 | 3.7E–02 | Metabolism | |
| 3493 | 1.7 | Triosephosphate-isomerase | gi|326496613 | Hordeum vulgare | 32.7/7.0 | 30/5.8 | 9 | 28 | 6.4E–03 | Metabolism | |
| 850 | 1.9 | Cytosolic malate dehydrogenase | gi|37928995 | Triticum aestivum | 24.6/6.6 | 41/6.3 | 6 | 23 | 1.3E–02 | Metabolism | |
| 3042 | 1.5 | Phosphoglycerate kinase | gi|129915 | Triticum aestivum | 50/6.6 | 48/5.6 | 4.7E–05 | SVGDLTAADLEGKR | Metabolism | ||
| 3323 | 1.9 | Rubisco small subunit | gi|11990901 | Triticum aestivum | 19.7/8.8 | 16/6.3 | 9 | 50 | 2.1E–05 | Photosynthesis | |
| 3068 | 2.5 | Rubisco activase small isform | gi|313574196 | Hordeum vulgare | 47.3/7.6 | 42/6.4 | 15 | 38 | 5.1E–10 | Photosynthesis | |
| 3109 | 2.2 | Rubisco activase B | gi|7960277 | Triticum aestivum | 48/6.9 | 45/5.7 | 12 | 34 | 2.9E–03 | Photosynthesis | |
| 3198 | 1.8 | Rubisco activase | gi|37783283 | Triticum aestivum | 22.5/5.0 | 47/6.1 | 11 | 51 | 1.3E–10 | Photosynthesis | |
| 3111 | 2.4 | Rubisco activase alpha form precursor | gi|32481061 | Deschampsia antarctica | 51.4/6.0 | 45/5.7 | 23 | 44 | 5.1E–11 | Photosynthesis | |
| 3214 | 2.8 | Rubisco activase alpha form precursor | gi|32481061 | Deschampsia antarctica | 51.4/6.0 | 50/5.4 | 16 | 37 | 1.6E–09 | Photosynthesis | |
| 3016 | 2.5 | Rubisco activase alpha form precursor | gi|32481061 | Deschampsia antarctica | 51.4/6.0 | 50/5.6 | 12 | 21 | 4.1E–07 | Photosynthesis | |
| 3443 | 1.7 | Rubisco activase | gi|37783283 | Triticum aestivum | 22.5/5.0 | 42/6.1 | 7 | 24 | 9.9E–04 | Photosynthesis | |
| 3091 | 1.5 | Rubisco activase small isoform | gi|313574196 | Hordeum vulgare | 47.3/7.6 | 49/5.7 | 16 | 42 | 2.9E–03 | Photosynthesis | |
| 3117 | 1.6 | Actin | gi|168472715 | Lolium temulentum | 41.2/5.5 | 51/5.7 | 12 | 31 | 2.1E–10 | Cell structure | |
| 3133 | 2.3 | Rubisco large subunit-binding protein subunit beta | gi|2493650 | Secale cereale | 53.7/4.9 | 61/5.5 | 12 | 26 | 6.6E–08 | Molecular chaperones | |
| 3149 | 1.6 | Plastid glutamine synthetase isoform GS2c | gi|71362640 | Triticum aestivum | 47/5.8 | 49/5.3 | 19 | 47 | 3.3E–11 | Protein synthesis | |
| 3394 | 1.9 | Cysteine synthase | gi|585032 | Triticum aestivum | 34.2/5.5 | 36/6.3 | 11 | 32 | 5.2E–05 | Protein synthesis | |
| 3131 | 2.6 | Cysteine synthase | gi|585032 | Triticum aestivum | 34.2/5.5 | 37/6.1 | 11 | 31 | 2.6E–06 | Protein synthesis | |
| 3457 | 1.8 | Cysteine synthase | gi|585032 | Triticum aestivum | 34.2/5.5 | 37/5.8 | 13 | 46 | 1.6E–06 | Protein synthesis | |
| 3648 | 1.7 | 29kDa ribonucleo protein | gi|326510421 | Hordeum vulgare | 29.6/4.8 | 33/4.6 | 1.6E–02 | VNSGPPPPRDEFAPR | Unknown | ||
| 3339 | 1.6 | Hypothetical protein MTR_026s0001 | gi|358343995 | Medicago truncatula | 12.7/10 | 41/6.1 | 6 | 54 | 2.1E–03 | Unknown | |
aThe value represents the highest fold change of non-primed plants under drought stress (NND), stem elongation-primed plants under drought stress (NPD) and twice-primed plants under drought stress (PPD) compared with control (NNC).
bTheoretical Mr/pI (kDa).
cExperimental Mr/pI (kDa) values were calculated using the Progenesis Samespot software.
dMatch no. represents the number of identified peptides that can be matched with protein peptides in the database.
eSequence coverage (%).
Fig. 9.
Venn diagrams of changed protein spots in primed plants (NPD and PPD) compared with non-primed plants (NND) under drought stress during grain filling. See Fig. 1 legend for abbreviations. The numbers indicate differently expressed protein spots.
Discussion
It is known that epigenetic modifications are involved in trans-generational stress memory (Molinier et al., 2006; Schmitz et al., 2011). However, it is not clear whether the mechanisms of epigenetic modifications (histone and DNA modifications) are involved in stress memory within the same generation (Chinnusamy and Zhu, 2009). It has been shown that accumulation of mRNA and proteins of MPKs are involved in signalling mechanisms for pathogen priming. In particular, the upregulation of activities of MPK3 and MPK6 were found to enhance the immune response in Arabidopsis (Beckers et al., 2009; Conrath, 2011). However, the mechanisms of abiotic stress-based priming on subsequent abiotic stress events are far from clear. The objective of this study was to study the effect and potential mechanisms of drought priming before anthesis on drought stress episodes occurring during grain filling. Both physiological and proteome studies were performed to elucidate differences between primed and non-primed plants under drought stress during grain filling, which may contribute to improved drought tolerance.
Response of priming on physiological traits in leaves and on grain yield
LRWC is often used as an index of the water status of the plant (Sinclair and Ludlow, 1986; Ali et al., 1999), and can be used as an indicator for drought tolerance in wheat (Loutfy et al., 2012). In this study, primed plants showed higher stomatal conductance and higher leaf water content than non-primed plants. Stomatal control is important for regulation of both water loss and CO2 assimilation in response to drought stress (Jones, 1998; Munns et al., 2010). Here, the higher stomatal conductance in primed than in non-primed plants was in accordance with the higher rates of photosynthesis. It has been shown that abscisic acid (ABA)-based chemical signals may regulate physiological responses to drought stress (Socias et al., 1997; Dodd, 2005), and that increased leaf ABA concentrations would lead to lower stomatal conductance. In this study, under drought priming, ABA and g s showed a negative correlation (Supplementary Fig. S1B at JXB online). However, there was no correlation between ABA and g s under drought stress during grain filling (Supplementary Fig. S2B at JXB online). Thus, the higher ABA concentration under grain filling might not have been responsible for stomatal closure. Instead, the higher leaf water status could have allowed the stomata to remain open in primed plants. In line with this, in drought-stressed soybean, Liu et al. (2004) showed that exogenous ABA application during drought could enhance stomatal conductance and leaf water potential as compared with non-ABA-treated plants.
As alternative functions of ABA in abiotic stress response, ABA can induce genes encoding dehydration proteins (Zhu, 2002) as well as the transcription of heat-shock proteins (Larkindale and Huang, 2004). In addition, it has been shown that accumulation of ABA can also trigger the generation of ROS leading to upregulation of activities of antioxidant enzymes (Jiang and Zhang, 2002; Penfield, 2008).
This was in accordance with our results showing a positive correlation of ABA with activities of APX under drought stress during grain filling (Supplementary Fig. 2A). Primed plants showed higher leaf ABA content, higher APX activity, and higher grain yield compared with non-primed plants. In other experiments, ABA levels in flag leaves during grain filling were higher in tolerant than in sensitive varieties, and have led to higher grain yield through regulation of assimilate partitioning into grains (Guóth et al., 2009).
In this study, the LRWC of primed plants PPD was significantly higher than in non-primed plants (NND), and was higher in primed plants (PPD and NPD). The better maintenance of leaf water status in primed plants than in non-primed plants may have contributed to the better photosynthetic performance. Thus, the better maintenance of leaf water status could be a consequence of the priming effect, contributing to improved drought tolerance under drought stress during grain filling in primed compared with non-primed plants. Furthermore, biochemical mechanisms also contribute to drought tolerance under prolonged drought stress (Costa França et al., 2000).
Photosynthesis is the primary process affected by water deficit and can lead to reductions in crop yield (Chaves, 1991; Flexas et al., 2004; Chaves et al., 2008). Our previous studies found that heat priming alleviated the inhibition of photosynthesis under a subsequent heat-stress episode (Wang et al., 2011, 2014). In this study, there were no significant differences in the photosynthesis rate between primed plants under non-stress (NPC and PPC) and non-primed plants under non-stress during grain filling (NNC). However, primed plants under drought stress (NPD and PPD) showed significantly higher photosynthesis rates than did non-primed plants under drought stress (NND), suggesting that primed plants showed a higher capacity to protect photosynthetic activity in response to a later drought stress rather than the establishment of an altered state. It is known that mild drought stress lowered photosynthetic rate by stomatal limitations, while both stomatal and non-stomatal limitations occur during severe drought stress (Flexas et al., 2004; Lawlor and Tezara, 2009; Sengupta et al., 2011). Non-stomatal limitation is attributed to reduced carboxylation efficiency (Feller et al., 1998), reduced ribulose-1,5-bisphosphate regeneration (Kubien and Sage, 2008) or inhibition of RCA (Bayramov et al., 2010). According to Farquhar and Sharkey (1982), a decrease in g s and C i means that stomatal limitation is responsible for the decrease in photosynthesis, while a decrease in both photosynthesis rates and g s but higher C i would indicate non-stomatal limitation inhibiting the CO2 assimilation. In accordance with this, in our experiment, it seems that the stomatal limitation occurred during drought priming, while under drought stress during grain filling the decrease in both the photosynthesis rate and g s with no difference in C i during drought stress may indicate the non-stomatal limitation of photosynthesis. The higher photosynthesis rates in primed plants under drought stress during grain filling (NPD and PPD) may indicate less limitation of both stomatal and non-stomatal factors. Inhibition of photosynthesis activity usually leads to higher production of ROS, which may cause oxidative damage to the photosynthetic apparatus, proteins, DNA, and lipids (Xiong et al., 2002). MDA content is often used as an indicator of the extent of lipid peroxidation by ROS (Sairam et al., 2000; Wang et al., 2014). We have reported that heat priming through upregulation of both the activities and gene expression of superoxide dismutase, glutathione reductase, and peroxidase could alleviate oxidative damage under subsequent heat stress in wheat (Wang et al., 2011, 2014). To counteract ROS damages, APX plays an important role by catalysing the conversion of H2O2 to H2O (Mittler, 2002). It has been reported that drought-acclimated plants showed a higher APX activity, which enhanced oxidative stress tolerance compared with non-acclimated plants (Selote and Khanna-Chopra, 2006). In accordance with this, our results demonstrated that, with drought stress during grain filling, primed plants showed higher APX activity in relation to non-primed plants, which is in accordance with lower MDA content. These results indicated that priming induced upregulation of APX activity to alleviate oxidative damage under drought stress during grain filling.
Drought stress is one of the limiting factors for the wheat yield production (Li P et al., 2011), especially when it happened during the reproductive growth stage (Barnabas et al., 2008). However, it has been observed that plants that experienced drought stress during vegetative stage showed higher grain yield than non-acclimated plants under drought stress during the flowering stage (Yang et al., 2011; Zhang et al., 2013). In this study, grain yield was significantly downregulated by drought stress, while the higher grain yield in primed plants may have resulted from the higher rates of photosynthesis and lower oxidative damage during grain filling compared with the non-primed plants. The reduction in grain yield (and no difference in rates of photosynthesis) under non-stress during grain filling (NPC and PPC) may be because pre-anthesis drought priming decreased kernel numbers (unpublished data). The higher yield in PPD than PPC might be related to drought stress-induced senescence that enhanced the remobilization of pre-stored assimilates from vegetative organs to gains (Plaut et al., 2004).
Response of priming on the leaf proteome
Drought priming
RSS and RLS are both important components of Rubisco, the key enzyme involved in photosynthetic CO2 assimilation (Caruso et al., 2009). There have been contrasting results in different studies on the regulation of Rubisco in response to drought stress, as some studies found upregulation (Zhou et al., 2011; Budak et al., 2013), some reported downregulation (Ali and Komatsu, 2006; Plomion et al., 2006), and some reported both up- and downregulation (Caruso et al., 2009). In this study, the protein abundances of RSS and RLS were differently expressed in PP compared with NN and NP. Since the photosynthesis rate in PP was higher than in NP, and the lower photosynthesis rate may result from stomatal limitation, it is suggested that the Rubisco activity may have not been significantly changed under mild drought stress during priming. Peptidyl-prolyl cis-trans isomerase CYP38, which is essential for PSII assembly (Fu et al., 2007), and PSII stability/assembly factor HCF136, which is involved in assisting the folding of proteins and is required for the assembly and stabilization of PSII, were upregulated in PP but downregulated in NP in relation to NN. The upregulation of these proteins may indicate a protective role of the photosynthetic apparatus in PP compared with NP, which is in accordance with the higher photosynthesis rate in PP than in NP.
Thioredoxin-like protein CDSP32, which is a chloroplastic drought-induced stress protein of 32kDa, was induced by oxidative stress (Broin et al., 2000). Here, after drought priming, Trx-CDSP32 was downregulated in PP in relation to NP. This may due to the absence of oxidative damage in PP under drought priming, as exemplified by no significant difference in MDA content between PP and NN. Putative myosin heavy chain has been identified in plants in response to heavy metal (Ahsan et al., 2007) and cold stress (Yan et al., 2006). However, the role of this protein is not clear. While the putative myosin heavy chain was downregulated in the PP treatment in this study, we were unable to relate the function of this protein to drought priming. As this protein was expressed in fully expanded leaves, it might be related to actin organization, organelle movement or signal transduction (Sparkes, 2011). Collectively, the different protein expression between PP and NP might be result of the priming effect during the seedling stage, which could be contributing to enhancing tolerance to mild drought stress during the stem elongation stage.
Drought stress during grain filling
The protein spots identified as RCA, RSS, and APX were upregulated, and FBA was downregulated in primed plants (NPD and PPD) compared with non-primed plants (NND), suggesting that these proteins may be involved in drought tolerance by priming. RCA removes inhibitors from the catalytic sites of Rubisco (Portis, 1995) and is reported to be impaired under water-deficit conditions (Tezara et al., 1999). Under drought stress during grain filling, eight proteins spots were identified as RCA, and have also been found in other plant proteome studies (Ciais et al., 2005; Donnelly et al., 2005; Caruso et al., 2009). These additional spots identified as one protein might be due to cleaved isoforms of the same protein (Hurkman et al., 1994) or post-translational modified isoforms (Weiss and Görg, 2007). Here, two spots identified as RCA and one spot identified as RSS were significantly more highly expressed in primed plants (NPD and PPD) than in non-primed plants (NND). The higher RSS and RCA abundance in primed plants (NPD and PPD) indicated the contribution to higher photosynthesis rate compared with non-primed plants (NND) under drought stress during grain filling.
Changes in both amounts and activities of APX have been identified as an indicator of a redox status to counteract ROS damage under water deficit (Mittler, 2002). The upregulated expression of APX protein, higher activities of APX, and lower MDA content in primed plants compared with non-primed plants in the present study indicated a higher capacity for ROS scavenging and lower cell lipid peroxidation. This may indicate that primed plants can activate the antioxidant defence system when subjected to subsequent drought stress episodes.
It has been reported that increased photosynthesis and antioxidative defence-related proteins play important roles in hybrid Bermuda grass to adapt to drought stress (Zhao et al., 2011). In our study, the differences in protein expression between primed plants (NPD and PPD) and non-primed plant (NND) indicated that photosynthesis and antioxidant defence-related proteins may play important roles in priming and drought stress during grain filling.
In our study, we did not find regulation of proteins related to signal transduction may be because the signalling proteins usually are only induced at the very early stage of stress sensing (maximum one day) and may not be related to long-lasting stress tolerance (Harb et al., 2010; Pastor et al., 2012).
FBA exist in two isoforms, a chloroplastic FBA and a cytosolic FBA, and are involved in gluconeogenesis and glycolysis (Schnarrenberger and Krüger, 1986; Michelis and Gepstein, 2000). In this study, two spots were identified as FBA, and one of them was downregulated in primed plants, while the other one was upregulated in NPD compared with PPD and non-primed plants, suggesting that FBA was regulated by drought stress. Several spots identified as FBA showing different expression have also been found in other studies (Xu and Huang, 2008; Zhao et al., 2011). The upregulation of proteins involved in glycolysis, such as FBA and phosphoglycerate kinase (Houston et al., 2009), in NPD compared with PPD indicated the higher energy demand in NPD under drought stress. In accordance, glutamine synthetase, which plays an important role in nitrogen metabolism (Caruso et al., 2009), was expressed more highly in NPD than in PPD. This might indicate a higher ATP demand for nitrogen metabolism in NPD than in PPD.
The 2-Cys peroxiredoxin BAS1 is the target of the Trx-CDSP32, which plays roles in protecting the photosynthetic apparatus against oxidative damage (Broin et al., 2002). Oxygen-evolving enhancer protein is a manganese-stabilizing protein for PSII core stability (Yi et al., 2005). Actin, which is involved in the cell structure, was upregulated in NPD compared with PPD. The higher expression of proteins related to actin and 2-Cys peroxiredoxin BAS1 but lower expression of oxygen-evolving enhancer protein 1 in NPD compared with PPD may be related to the measured higher expression of PSII stability related proteins (peptidyl-prolyl cis-trans isomerase CYP38 and PSII stability/assembly factor HCF136) and cell structure-related protein (putative myosin heavy chain) but lower expression of Trx-CDSP in NP compared with PP under drought priming. These results suggest that these differences may have been ‘conserved’ from drought priming rather than induced by drought stress during grain filling.
Conclusions
The single or double drought priming events before anthesis resulted in higher grain yield under drought stress during grain filling. The primed plants showed higher leaf water status, higher photosynthesis rates, higher APX activity, and lower cell membrane peroxidation than did the non-primed plants. Furthermore, the protein abundances of RSS, RCA, and APX were upregulated in primed plants compared with non-primed plants. Both the upregulated synthesis (expressed as protein abundance) and activities of proteins involved in photosynthesis and stress defence in primed plants could be contributing to the priming effects enabling the plants to cope with the drought stress during grain filling. In addition, proteins involved in general metabolism (glycolysis and nitrogen metabolism) were differently expressed in plants primed once or twice under drought stress, which might indicate that these processes are differently regulated.
Supplementary Material
Acknowledgements
This paper is dedicated to the late Dr Susanne Jacobsen, who suddenly passed away during the final work of this study. The Ministry for Food, Agriculture and Fisheries of Denmark is acknowledged for a research grant to BW. The Chinese Scholarship Council is acknowledged for supporting XW for her Danish PhD. We thank Advanced Food Studies (LMC) for financial support for the Bruker Ultraflex III MALDI-TOF/TOF mass spectrometer. We thank Bettina Viola Hansen and Ulla Andersen for their help in setting up the pot experiment at the Research Centre Flakkebjerg. We are grateful to Anne Blicher, Birgit Andersen, and Louise Helsted for laboratory guidance, and Abida Sultan, Avishek Majumder, Morten Ejby, and Tune Wulff for the proteome results discussion. The authors have declared no conflicts of interest.
Glossary
Abbreviations:
- ABA
abscisic acid
- ANOVA
analysis of variance
- Anet
net photosynthetic assimilation rate
- APX
ascorbate peroxidase
- Ci
internal CO2 concentration
- DTT
dithiothreitol
- FBA
fructose-bisphosphate aldolase
- gs
stomatal conductance
- LRWC
leaf relative water content
- MALDI-TOF MS
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MDA
malondialdehyde
- MPK
mitogen-activated protein kinase
- PS
photosystem
- RCA
Rubisco activase
- RLS
Rubisco large subunit
- ROS
reactive oxygen species
- RSS
Rubisco small subunit
- Rubisco
ribulose-1,5-bisphosphate carboxylase/oxygenase
- SRWC
soil relative water content.
References
- Ahsan N, Lee D, Lee S, Kang K, Bahk J, Choi M, Lee I, Renaut J, Lee B. 2007. A comparative proteomic analysis of tomato leaves in response to waterlogging stress. Physiologia Plantarum 131, 555–570. [DOI] [PubMed] [Google Scholar]
- Ali GM, Komatsu S. 2006. Proteomic analysis of rice leaf sheath during drought stress. Journal of Proteome Research 5, 396–403. [DOI] [PubMed] [Google Scholar]
- Ali M, Jensen C, Mogensen V, Andersen M, Henson I. 1999. Root signalling and osmotic adjustment during intermittent soil drying sustain grain yield of field grown wheat. Field Crops Research 62, 35–52. [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Barnabas B, Jager K, Feher A. 2008. The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell and Environment 31, 11–38. [DOI] [PubMed] [Google Scholar]
- Bayramov SM, Babayev HG, Khaligzade MN, Guliyev NM, Raines CA. 2010. Effect of water stress on protein content of some calvin cycle enzymes in different wheat genotypes. Proceedings of ANAS (Biological Sciences) 65, 106–111. [Google Scholar]
- Beckers G, Conrath U. 2007. Priming for stress resistance: from the lab to the field. Current Opinion in Plant Biology 10, 425–431. [DOI] [PubMed] [Google Scholar]
- Beckers GJ, Jaskiewicz M, Liu Y, Underwood WR, He SY, Zhang S, Conrath U. 2009. Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana . Plant Cell 21, 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M, Bancroft I, Bent E, et al. 1998. Analysis of 1.9Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana . Nature 391, 485–488. [DOI] [PubMed] [Google Scholar]
- Bota J, Medrano H, Flexas J. 2004. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytologist 162, 671–681. [DOI] [PubMed] [Google Scholar]
- Broin M, Cuiné S, Eymery F, Rey P. 2002. The plastidic 2-cysteine peroxiredoxin is a target for a thioredoxin involved in the protection of the photosynthetic apparatus against oxidative damage. Plant Cell 14, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broin M, Cuiné S, Peltier G, Rey P. 2000. Involvement of CDSP 32, a drought-induced thioredoxin, in the response to oxidative stress in potato plants. FEBS Letters 467, 245–248. [DOI] [PubMed] [Google Scholar]
- Bruce TJA, Matthes MC, Napier JA, Pickett JA. 2007. Stressful “memories” of plants: evidence and possible mechanisms. Plant Science 173, 603–608. [Google Scholar]
- Budak H, Akpinar BA, Unver T, Turktas M. 2013. Proteome changes in wild and modern wheat leaves upon drought stress by two-dimensional electrophoresis and nanoLC-ESI-MS/MS. Plant Molecular Biology 83, 89–103. [DOI] [PubMed] [Google Scholar]
- Canaani O, Havaux M, Malkin S. 1986. Hydroxylamine, hydrazine and methylamine donate electrons to the photooxidizing side of photosystem II in leaves inhibited in oxygen evolution due to water stress. Biochimica et Biophysica Acta—Bioenergetics 851, 151–155. [Google Scholar]
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. 2004. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25, 1327–1333. [DOI] [PubMed] [Google Scholar]
- Caruso G, Cavaliere C, Foglia P, Gubbiotti R, Samperi R, Laganà A. 2009. Analysis of drought responsive proteins in wheat (Triticum durum) by 2D-PAGE and MALDI-TOF mass spectrometry. Plant Science 177, 570–576. [Google Scholar]
- Chaves M. 1991. Effects of water deficits on carbon assimilation. Journal of Experimental Botany 42, 1–16. [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. 2008. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany 103, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Harmon AC. 2006. Advances in plant proteomics. Proteomics 6, 5504–5516. [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J-K. 2009. Epigenetic regulation of stress responses in plants. Current Opinion in Plant Biology 12, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou A, Manganaris GA, Papadopoulos I, Fotopoulos V. 2013. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. Journal of Experimental Botany 64, 1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciais P, Reichstein M, Viovy N, Granier A, Ogee J, Allard V, Aubinet M, Buchmann N, Bernhofer C, Carrara A. 2005. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437, 529–533. [DOI] [PubMed] [Google Scholar]
- Conrath U. 2011. Molecular aspects of defence priming. Trends in Plant Science 16, 524–531. [DOI] [PubMed] [Google Scholar]
- Cornic G, Fresneau C. 2002. Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Annals of Botany 89, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Franca MG, Pham Thi AT, Pimentel C, Pereyra Rossiello RO, Zuily-Fodil Y, Laffray D. 2000. Differences in growth and water relations among Phaseolus vulgaris cultivars in response to induced drought stress. Environmental and Experimental Botany 43, 227–237. [DOI] [PubMed] [Google Scholar]
- Ding Y, Fromm M, Avramova Z. 2012. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis . Nature Communications 3, 740. [DOI] [PubMed] [Google Scholar]
- Dodd IC. 2005. Root-to-shoot signalling: assessing the roles of ‘up’in the up and down world of long-distance signalling in planta. In: Root physiology: from gene to function, Dordrecht: Springer, 251–270. [Google Scholar]
- Donnelly BE, Madden RD, Ayoubi P, Porter DR, Dillwith JW. 2005. The wheat (Triticum aestivum L.) leaf proteome. Proteomics 5, 1624–1633. [DOI] [PubMed] [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. 2009. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development 29, 185–212. [Google Scholar]
- Farquhar GD, Sharkey TD. 1982. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33, 317–345. [Google Scholar]
- Feller U, Crafts-Brandner S, Salvucci M. 1998. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiology 116, 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippou P, Tanou G, Molassiotis A, Fotopoulos V. 2012. Plant acclimation to environmental stress using priming agents. In: Tuteja N, Gill SS, eds. Plant acclimation to environmental stress, NY: Springer, 1–28. [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey T. 2004. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology 6, 269–279. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas Carbó M, Diaz Espejo A, Galmes J, Medrano H. 2008. Mesophyll conductance to CO2: current knowledge and future prospects. Plant,Cell and Environment 31, 602–621. [DOI] [PubMed] [Google Scholar]
- Fu A, He Z, Cho HS, Lima A, Buchanan BB, Luan S. 2007. A chloroplast cyclophilin functions in the assembly and maintenance of photosystem II in Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 104, 15947–15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Mikkat S, Stegmann H, Horn R. 2011. Physiology and proteomics of drought stress acclimation in sunflower (Helianthus annuus L.). Plant Biology 13, 632–642. [DOI] [PubMed] [Google Scholar]
- Guóth A, Tari I, Gallé Á, Csiszár J, Pécsváradi A, Cseuz L, Erdei L. 2009. Comparison of the drought stress responses of tolerant and sensitive wheat cultivars during grain filling: changes in flag leaf photosynthetic activity, ABA levels, and grain yield. Journal of Plant Growth Regulation 28, 167–176. [Google Scholar]
- Harb A, Krishnan A, Ambavaram MM, Pereira A. 2010. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiology 154, 1254–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JX, Wang J, Liang HG. 1995. Effects of water stress on photochemical function and protein metabolism of photosystem II in wheat leaves. Physiologia Plantarum 93, 771–777. [Google Scholar]
- Hernández I, Cela J, Alegre L, Munné-Bosch S. 2012. Antioxidant defenses against drought stress. In: Aroca R, ed. Plant responses to drought stress, Heidelberg: Springer, 231–258. [Google Scholar]
- Houston NL, Hajduch M, Thelen JJ. 2009. Quantitative proteomics of seed filling in castor: comparison with soybean and rapeseed reveals differences between photosynthetic and nonphotosynthetic seed metabolism. Plant Physiology 151, 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Møller IM, Song S-Q. 2012. Proteomics of desiccation tolerance during development and germination of maize embryos. Journal of Proteomics 75, 1247–1262. [DOI] [PubMed] [Google Scholar]
- Hurkman WJ, Lane BG, Tanaka CK. 1994. Nucleotide sequence of a transcript encoding a germin-like protein that is present in salt-stressed barley (Hordeum vulgare L.) roots. Plant Physiology 104, 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G, Ton J, Flors V, Zimmerli L, Métraux J-P, Mauch-Mani B. 2005. Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiology 139, 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C, Jacobsen S-E, Andersen MN, Nunez N, Andersen S, Rasmussen L, Mogensen V. 2000. Leaf gas exchange and water relation characteristics of field quinoa (Chenopodium quinoa Willd.) during soil drying. European Journal of Agronomy 13, 11–25. [Google Scholar]
- Jensen ON, Mortensen P, Vorm O, Mann M. 1997. Automation of matrix-assisted laser desorption/ionization mass spectrometry using fuzzy logic feedback control. Analytical Chemistry 69, 1706–1714. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. 2002. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. Journal of Experimental Botany 53, 2401–2410. [DOI] [PubMed] [Google Scholar]
- Jones H. 1998. Stomatal control of photosynthesis and transpiration. Journal of Experimental Botany 49, 387–398. [Google Scholar]
- Kamal AHM, Cho K, Choi J-S, Bae K-H, Komatsu S, Uozumi N, Woo SH. 2013. The wheat chloroplastic proteome. Journal of Proteomics 93, 326–342. [DOI] [PubMed] [Google Scholar]
- Kang G, Li G, Xu W, Peng X, Han Q, Zhu Y, Guo T. 2012. Proteomics reveals the effects of salicylic acid on growth and tolerance to subsequent drought stress in wheat. Journal of Proteome Research 11, 6066–6079. [DOI] [PubMed] [Google Scholar]
- Kausar R, Arshad M, Shahzad A, Komatsu S. 2013. Proteomics analysis of sensitive and tolerant barley genotypes under drought stress. Amino Acids 44, 345–359. [DOI] [PubMed] [Google Scholar]
- Kottapalli KRAO, Rakwal R, Shibato J, Burow G, Tissue D, Burke J, Puppala N, Burow M, Payton P. 2009. Physiology and proteomics of the water-deficit stress response in three contrasting peanut genotypes. Plant, Cell and Environment 32, 380–407. [DOI] [PubMed] [Google Scholar]
- Kubien DS, Sage RF. 2008. The temperature response of photosynthesis in tobacco with reduced amounts of Rubisco. Plant, Cell and Environment 31, 407–418. [DOI] [PubMed] [Google Scholar]
- Lancashire PD, Bleiholder H, Boom T, Langelüddeke P, Stauss R, Weber E, Witzenberger A. 1991. A uniform decimal code for growth stages of crops and weeds. Annals of Applied Biology 119, 561–601. [Google Scholar]
- Larkindale J, Huang B. 2004. Thermotolerance and antioxidant systems in Agrostis stolonifera: Involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. Journal of Plant Physiology 161, 405–413. [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. 2009. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany 103, 561–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Jiang D, Wollenweber B, Li Y, Dai T, Cao W. 2011. Waterlogging pretreatment during vegetative growth improves tolerance to waterlogging after anthesis in wheat. Plant Science 5, 672–678. [DOI] [PubMed] [Google Scholar]
- Li P, Chen J, Wu P. 2011. Agronomic characteristics and grain yield of 30 spring wheat genotypes under drought stress and nonstress conditions. Agronomy Journal 103, 1619–1628. [Google Scholar]
- Liu F, Jensen CR, Andersen MN. 2004. Pod set related to photosynthetic rate and endogenous ABA in soybeans subjected to different water regimes and exogenous ABA and BA at early reproductive stages. Annals of Botany 94, 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutfy N, El-Tayeb MA, Hassanen AM, Moustafa MF, Sakuma Y, Inouhe M. 2012. Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). Journal of Plant Research 125, 173–184. [DOI] [PubMed] [Google Scholar]
- Medrano H, Escalona J, Bota J, Gulias J, Flexas J. 2002. Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Annals of Botany 89, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelis R, Gepstein S. 2000. Identification and characterization of a heat-induced isoform of aldolase in oat chloroplast. Plant Molecular Biology 44, 487–498. [DOI] [PubMed] [Google Scholar]
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7, 405–410. [DOI] [PubMed] [Google Scholar]
- Molassiotis A, Tanou G, Diamantidis G. 2010. NO says more than ‘YES’ to salt tolerance: salt priming and systemic nitric oxide signaling in plants. Plant Signaling and Behavior 5, 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinier J, Ries G, Zipfel C, Hohn B. 2006. Transgeneration memory of stress in plants. Nature 442, 1046–1049. [DOI] [PubMed] [Google Scholar]
- Munns R, James RA, Sirault XRR, Furbank RT, Jones HG. 2010. New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. Journal of Experimental Botany 61, 3499–3507. [DOI] [PubMed] [Google Scholar]
- Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. 2007. Photoinhibition of photosystem II under environmental stress. Biochimica et Biophysica Acta—Bioenergetics 1767, 414–421. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22, 867–880. [Google Scholar]
- Pastor V, Luna E, Mauch-Mani B, Ton J, Flors V. 2012. Primed plants do not forget. Environmental and Experimental Botany 94, 46–56. [Google Scholar]
- Penfield S. 2008. Temperature perception and signal transduction in plants. New Phytologist 179, 615–628. [DOI] [PubMed] [Google Scholar]
- Plaut Z, Butow B, Blumenthal C, Wrigley C. 2004. Transport of dry matter into developing wheat kernels and its contribution to grain yield under post-anthesis water deficit and elevated temperature. Field Crops Research 86, 185–198. [Google Scholar]
- Plomion C, Lalanne C, Claverol S, et al. 2006. Mapping the proteome of poplar and application to the discovery of drought-stress responsive proteins. Proteomics 6, 6509–6527. [DOI] [PubMed] [Google Scholar]
- Portis AR., Jr 1995. The regulation of Rubisco by Rubisco activase. Journal of Experimental Botany 46, 1285–1291. [DOI] [PubMed] [Google Scholar]
- Ramagli LS. 1999. Quantifying protein in 2-D PAGE solubilization buffers. Methods in Molecular Biology 112, 99–103. [DOI] [PubMed] [Google Scholar]
- Reddy AR, Chaitanya KV, Vivekanandan M. 2004. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology 161, 1189–1202. [DOI] [PubMed] [Google Scholar]
- Rinalducci S, Egidi MG, Mahfoozi S, Jahanbakhsh Godehkahriz S, Zolla L. 2011. The influence of temperature on plant development in a vernalization-requiring winter wheat: a 2-DE based proteomic investigation. Journal of Proteomics 74, 643–659. [DOI] [PubMed] [Google Scholar]
- Sairam R, Srivastava G, Saxena D. 2000. Increased antioxidant activity under elevated temperatures: a mechanism of heat stress tolerance in wheat genotypes. Biologia Plantarum 43, 245–251. [Google Scholar]
- Schmitz RJ, Schultz MD, Lewsey MG, O’Malley RC, Urich MA, Libiger O, Schork NJ, Ecker JR. 2011. Transgenerational epigenetic instability is a source of novel methylation variants. Science 334, 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C, Krüger I. 1986. Distinction between cytosol and chloroplast fructose-bisphosphate aldolases from pea, wheat, and corn leaves. Plant Physiology 80, 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selote DS, Khanna-Chopra R. 2006. Drought acclimation confers oxidative stress tolerance by inducing co-ordinated antioxidant defense at cellular and subcellular level in leaves of wheat seedlings. Physiologia Plantarum 127, 494–506. [Google Scholar]
- Sengupta D, Kannan M, Reddy AR. 2011. A root proteomics-based insight reveals dynamic regulation of root proteins under progressive drought stress and recovery in Vigna radiata (L.) Wilczek. Planta 233, 1111–1127. [DOI] [PubMed] [Google Scholar]
- Shi H, Ye T, Chan Z. 2013. Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiology and Biochemistry 71, 226–234. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. 2007. Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany 58, 221–227. [DOI] [PubMed] [Google Scholar]
- Sinclair T, Ludlow M. 1986. Influence of soil water supply on the plant water balance of four tropical grain legumes. Functional Plant Biology 13, 329–341. [Google Scholar]
- Socias X, Correia M, Chaves M, Medrano H. 1997. The role of abscisic acid and water relations in drought responses of subterranean clover. Journal of Experimental Botany 48, 1281–1288. [Google Scholar]
- Sparkes I. 2011. Recent advances in understanding plant myosin function: life in the fast lane. Molecular Plant 4, 805–812. [DOI] [PubMed] [Google Scholar]
- Tan W, Liu J, Dai T, Jing Q, Cao W, Jiang D. 2008. Alterations in photosynthesis and antioxidant enzyme activity in winter wheat subjected to post-anthesis water-logging. Photosynthetica 46, 21–27. [Google Scholar]
- Tanou G, Fotopoulos V, Molassiotis A. 2012. Priming against environmental challenges and proteomics in plants: update and agricultural perspectives. Frontiers in Plant Science 3, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanou G, Job C, Rajjou L, Arc E, Belghazi M, Diamantidis G, Molassiotis A, Job D. 2009. Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. The Plant Journal 60, 795–804. [DOI] [PubMed] [Google Scholar]
- Tezara W, Mitchell V, Driscoll S, Lawlor D. 1999. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401, 914–917. [Google Scholar]
- Tsai CH, Singh P, Chen CW, Thomas J, Weber J, Mauch Mani B, Zimmerli L. 2011. Priming for enhanced defence responses by specific inhibition of the Arabidopsis response to coronatine. The Plant Journal 65, 469–479. [DOI] [PubMed] [Google Scholar]
- Uwe CGJMB, Victor F, Pilar GA, et al. 2006. Priming: getting ready for battle. 19, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Walter J, Nagy L, Hein R, Rascher U, Beierkuhnlein C, Willner E, Jentsch A. 2011. Do plants remember drought? Hints towards a drought-memory in grasses. Environmental and Experimental Botany 71, 34–40. [Google Scholar]
- Wang X, Cai J, Jiang D, Liu F, Dai T, Cao W. 2011. Pre-anthesis high-temperature acclimation alleviates damage to the flag leaf caused by post-anthesis heat stress in wheat. Journal of Plant Physiology 168, 585–593. [DOI] [PubMed] [Google Scholar]
- Wang X, Cai J, Liu F, Dai T, Cao W, Wollenweber B, Jiang D. 2014. Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiology and Biochemistry 74, 185–192. [DOI] [PubMed] [Google Scholar]
- Wang X, Cai J, Liu F, Jin M, Yu H, Jiang D, Wollenweber B, Dai T, Cao W. 2012. Pre-anthesis high temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat. Journal of Cereal Science 55, 331–336. [Google Scholar]
- Weiss W, Görg A. 2007. T wo-dimensional electrophoresis for plant proteomics. Methods in Molecular Biology 355, 121–143. [DOI] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu J-K. 2002. Cell signaling during cold, drought, and salt stress. Plant Cell 14, S165–S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Huang B. 2008. Root proteomic responses to heat stress in two Agrostis grass species contrasting in heat tolerance. Journal of Experimental Botany 59, 4183–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S-P, Zhang Q-Y, Tang Z-C, Su W-A, Sun W-N. 2006. Comparative proteomic analysis provides new insights into chilling stress responses in rice. Molecular & Cellular Proteomics 5, 484–496. [DOI] [PubMed] [Google Scholar]
- Yang F, Jensen JD, Spliid NH, Svensson B, Jacobsen S, Jørgensen LN, Jørgensen HJL, Collinge DB, Finnie C. 2010. Investigation of the effect of nitrogen on severity of Fusarium head blight in barley. Journal of Proteomics 73, 743–752. [DOI] [PubMed] [Google Scholar]
- Yang F, Jørgensen AD, Li H, Søndergaard I, Finnie C, Svensson B, Jiang D, Wollenweber B, Jacobsen S. 2011. Implications of high-temperature events and water deficits on protein profiles in wheat (Triticum aestivum L. cv. Vinjett) grain. Proteomics 11, 1684–1695. [DOI] [PubMed] [Google Scholar]
- Yi X, McChargue M, Laborde S, Frankel LK, Bricker TM. 2005. The manganese-stabilizing protein is required for photosystem II assembly/stability and photoautotrophy in higher plants. Journal of Biological Chemistry 280, 16170–16174. [DOI] [PubMed] [Google Scholar]
- Zavalloni C, Gielen B, Lemmens C, De Boeck H, Blasi S, Van den Bergh S, Nijs I, Ceulemans R. 2008. Does a warmer climate with frequent mild water shortages protect grassland communities against a prolonged drought? Plant and Soil 308, 119–130. [Google Scholar]
- Zhang X, Cai J, Wollenweber B, Liu F, Dai T, Cao W, Jiang D. 2013. Multiple heat and drought events affect grain yield and accumulations of high molecular weight glutenin subunits and glutenin macropolymers in wheat. Journal of Cereal Science 57, 134–140. [Google Scholar]
- Zhao Y, Du H, Wang Z, Huang B. 2011. Identification of proteins associated with water deficit tolerance in C4 perennial grass species, Cynodon dactylon×Cynodon transvaalensis and Cynodon dactylon . Physiologia Plantarum 141, 40–55. [DOI] [PubMed] [Google Scholar]
- Zhou G, Yang L-T, Li Y-R, Zou C-L, Huang L-P, Qiu L-H, Huang X, Srivastava MK. 2011. Proteomic analysis of osmotic stress-responsive proteins in sugarcane leaves. Plant Molecular Biology Reporter 30, 349–359. [Google Scholar]
- Zhu J-K. 2002. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology 53, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.