Significance
Decision-making involves integrating different pieces of sensory information over time. Looking at how sensory information is weighted and integrated during a natural behavior can yield insight into the evolutionary forces shaping that behavior. Here, we investigated how female grasshoppers of the species Chorthippus biguttulus integrate information provided by male calling songs. Fitting a drift-diffusion model to behavioral data, we find that integration is highly asymmetrical: an unattractive song subunit far outweighs an attractive subunit. This asymmetrical integration is consistent with theories of sexual selection because it helps females avoid potentially costly interactions with unsuitable mating partners if the song belongs to another species or indicates a low-quality male; moreover, it suggests that song-based decision-making in grasshoppers is optimized by evolution.
Keywords: acoustic communication, insects, decision-making, courtship, drift-diffusion model
Abstract
Decision-making processes, like all traits of an organism, are shaped by evolution; they thus carry a signature of the selection pressures associated with choice behaviors. The way sexual communication signals are integrated during courtship likely reflects the costs and benefits associated with mate choice. Here, we study the evaluation of male song by females during acoustic courtship in grasshoppers. Using playback experiments and computational modeling we find that information of different valence (attractive vs. nonattractive) is weighted asymmetrically: while information associated with nonattractive features has large weight, attractive features add little to the decision to mate. Accordingly, nonattractive features effectively veto female responses. Because attractive features have so little weight, the model suggests that female responses are frequently driven by integration noise. Asymmetrical weighting of negative and positive information may reflect the fitness costs associated with mating with a nonattractive over an attractive singer, which are also highly asymmetrical. In addition, nonattractive cues tend to be more salient and therefore more reliable. Hence, information provided by them should be weighted more heavily. Our findings suggest that characterizing the integration of sensory information during a natural behavior has the potential to provide valuable insights into the selective pressures shaping decision-making during evolution.
One crucial decision in the lifetime of an animal is the decision with whom to mate. The males of many animals have evolved elaborate traits to attract females (1). These traits often involve the production of calling or courtship songs (2–4). Females base their decision to engage in courtship and to mate on the properties of these signals. Though many different factors drive and constrain the evolution of behavioral decisions in general (5, 6), the way information is integrated in the context of mate selection may reflect the fitness costs and benefits associated with mate choice. These costs depend on the mating system, e.g., the abundance of potential mating partners, the likelihood and costs of multiple matings, direct benefits of mating, and the costs of assessing a potential mate (7–10).
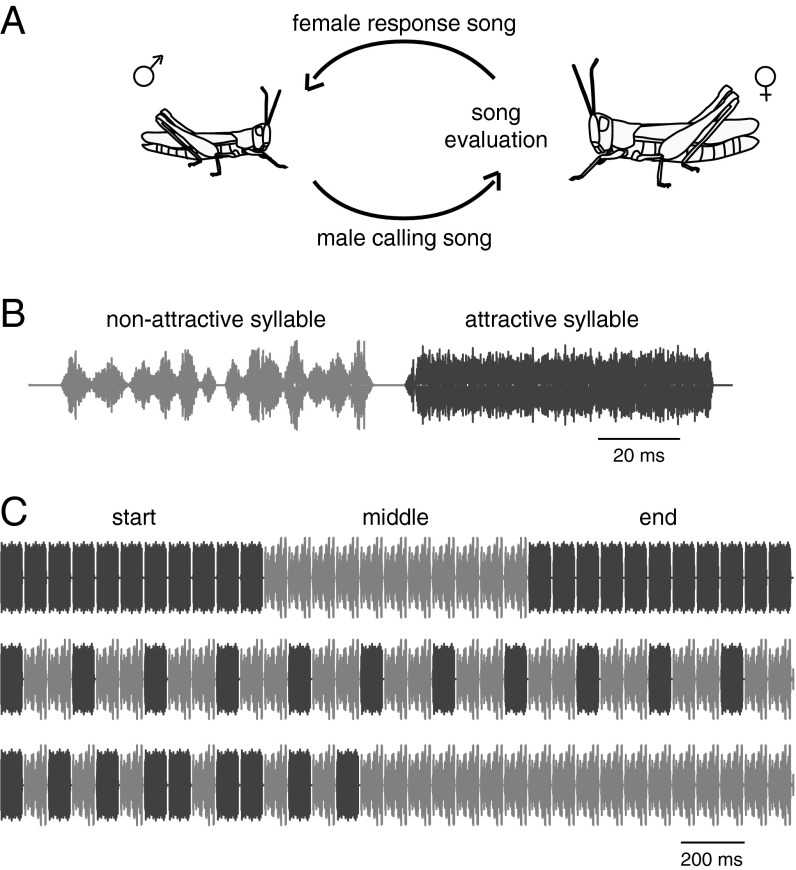
Here, we investigate the implementation of a decision-making strategy by studying the integration of song features during courtship in grasshoppers. Acoustic courtship in the species Chorthippus biguttulus involves bidirectional communication. Males produce calling songs consisting of a sequence of simple stereotyped subunits—30–50 syllable–pause pairs. The female waits until the end of this song and indicates her readiness to further engage in a courtship ritual by producing a response song that allows the male to localize and approach the female (11) (Fig. 1A). This phase of courtship constitutes a first, important preselection step before females make their final assessment of an approaching mating partner. Not responding or responding to a male calling from a distance is the first step in this sequence, which likely reduces the female’s costs of assessing potential mates (10).
Fig. 1.
Courtship behavior in grasshoppers and stimuli used. (A) During natural courtship behavior, male grasshoppers produce a calling song. Based on temporal features of this song, the female decides whether to respond with her own song to initiate further courtship. In playback experiments, songs were broadcast using a speaker and the female response was recorded by a microphone. (B) Songs used in playback experiments were sequences of attractive and nonattractive syllables (dark and light gray, respectively). Details of an attractive (black) and a nonattractive (gray) syllable are shown. (C) Three of the songs used in the playback experiments. The full stimulus set is shown in Fig. S1.
Though many of the stimulus features influencing a female’s decision during the preselection phase have been identified (12–14), little is known about how this information is dynamically integrated over the course of the song. In the mating system of C. biguttulus, direct mating benefits do not exist and multiple mating is rare, hence much is at stake with the decision for a mate (15). Females should thus be selective and exploit all information provided by the song—i.e., they should not be impulsive decision-makers, which respond after hearing a few attractive song subunits. In addition, because mating with a male of a different species greatly reduces female fitness (9), nonattractive song features associated with other species should be weighted heavily to avoid such mistakes.
We used playback experiments with mixed sequences of two types of song subunits to determine the dynamics of integration: subunits that exhibit attractive, species-specific features and nonattractive subunits with negative features as found in songs of other species. Attractor networks and drift-diffusion models are successful in uncovering the dynamics of information integration during decision-making (16). Here, we chose drift-diffusion models, because this model class allows a direct mapping between model parameters and the phenomenology of decision-making in terms of weights for sensory information, integration noise, and decision thresholds (17).
Fitting such a model to our behavioral data reveals that attractive and nonattractive subunits are integrated in a strongly asymmetrical manner. The model suggests that the weight of attractive subunits in the decision is negligible relative to that of nonattractive subunits; this leads to nonattractive subunits effectively vetoing female responses. Actual female responses are often driven by noise in the decision process. Our results suggest that these dynamics were acquired during evolution to optimize the decision for a mating partner and likely reflect the reliability of information and the costs of (mis)decisions during courtship.
Results
Integration of Information Is Biased Toward the Beginning of Song.
Natural calling songs produced by healthy male grasshoppers of the species C. biguttulus consist of relatively smooth sound pulses (syllables) separated by pauses. A model song consisting exclusively of these attractive syllables elicited female responses with high probability (presponse = 0.86), confirming that this syllable type is a highly attractive pattern that matches the attractiveness of natural songs (18). By contrast, sound pulses with interrupted plateaus produced by males of other species or by crippled conspecifics are largely rejected by females (presponse = 0.16). Males producing such songs are thus far less likely to localize a female and to be accepted as a mate, in particular in the presence of other, more attractive singers (19).
To reveal how song properties are integrated during courtship, we generated mixed sequences of attractive and nonattractive syllables (Fig. 1B). Sequences were chosen such that different parts of the song contained varying proportions of these two syllable types (Fig. 1C; see Fig. S1 for all 32 sequences used in the study). Female response probabilities for these stimuli were determined using playback experiments (see Methods for details).
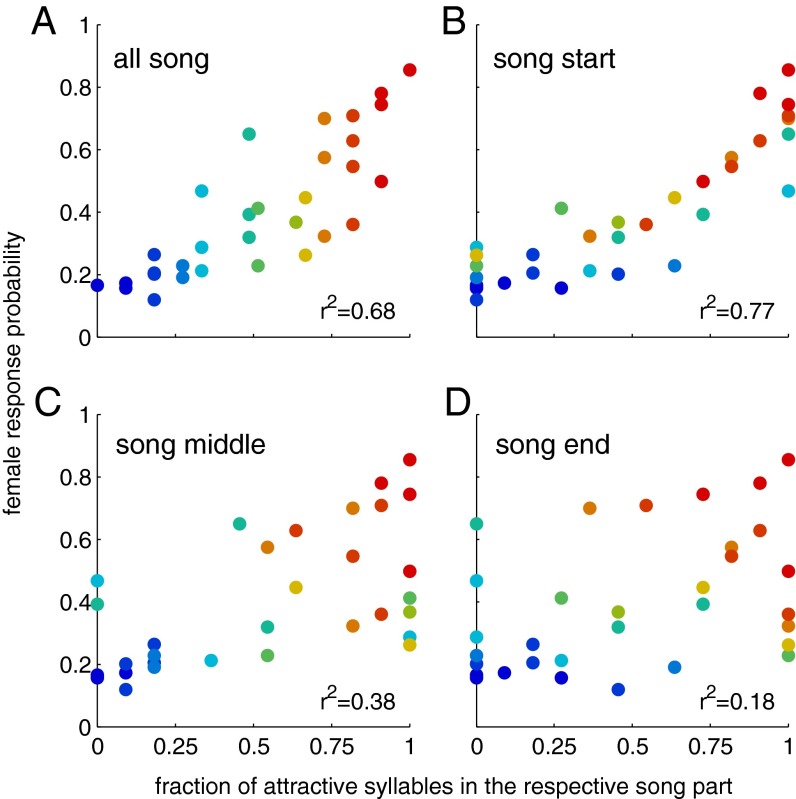
To get a first impression of how sequences of attractive and nonattractive syllables are integrated, we looked at the correlation between the female response probability at the song’s end and the amount of attractive elements in different song segments (Figs. 2 and 3B, blue bars). Response probabilities correlated well with the fraction of attractive syllables in the entire song (r2 = 0.68). Interestingly, the beginning of a song—its first third—determined female response probabilities even more strongly (r2 = 0.77), whereas a song’s middle and end parts had much less effect on the females’ decisions (middle: r2 = 0.38; end: r2 = 0.18).
Fig. 2.
Dependence of behavior on the structure of song sequences. Dependence of female response probability on the fraction of attractive and nonattractive syllables for (A) the entire song, (B) the song start (syllables 1–11), (C) the middle of song (syllables 12–22), and (D) song end (syllables 23–33; Fig. 1C). The color code corresponds to fraction of attractive syllables in the full song ranging from 1.0 (red) to 0.0 (blue). Shown is the average response probability based on n = 27 animals.
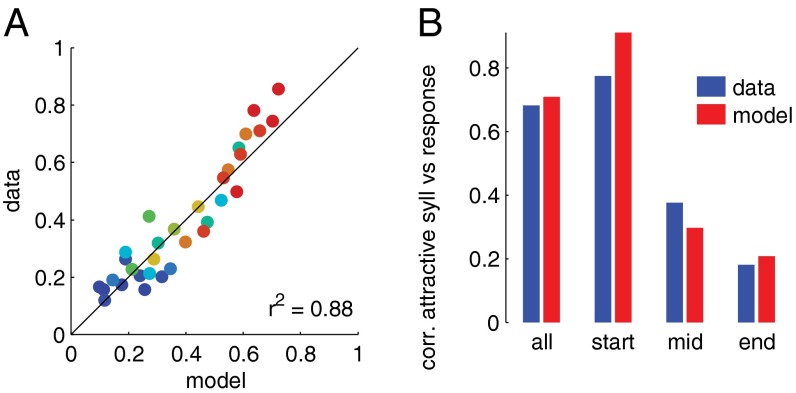
Fig. 3.
A drift-diffusion model predicts behavioral choices. (A) Predictions of a drift-diffusion model and actual behavior. The color code corresponds to fraction of attractive syllables in the full song ranging from 1.0 (red) to 0.0 (blue). (B) Correlation (r2) between measured/predicted responses and the fraction of attractive syllables in different parts of the song [data, blue (Fig. 2); model, red].
Females thus appear to weight the beginning of the song most strongly and seem to largely ignore the remainder of the song. Females responding after hearing only a few syllables could indicate low decision thresholds combined with large weights of sensory information leading to early commitment to a decision—features commonly associated with impulsive decision-making. However, since C. biguttulus females rarely perform multiple matings, the decision to accept a mate is crucial for her reproductive success (9, 19). Hence, the assumption of impulsive decisions is at odds with theories of sexual selection. Females should not be too easily coerced by a few attractive subunits of a male song. Rather, their response thresholds should be high to increase selectivity and ensure that information provided by the whole song can be used to reliably assess male quality.
A Decision-Making Model Reproduces Female Response Probabilities.
To determine in more detail how female decisions depend on sensory information, we chose to assess the parameters influencing integration of song by fitting a drift-diffusion model to our behavioral data (17).
In drift-diffusion models, positive and negative sensory information is integrated in a noisy manner. In our case, an attractive syllable constitutes a piece of positive information, a nonattractive syllable a piece of negative information, each associated with a weight w+ and w−. Within this framework, negative information corresponds to the presence of nonattractive features or the lack of attractive features (Fig. S2). Along with sensory information, decision noise with SD σ is added at each time step. The forgetfulness of the integrator is set by the integration time constant τ. When the decision variable crosses a positive or negative threshold (θ+ and θ−, respectively), the female commits to a decision and stops integrating further. If neither threshold is crossed after the song ends, the decision is based on the sign of the information accumulated thus far. A positive value leads to the female producing a response song that stimulates the male’s approach. A negative value is associated with a rejection of the male; in this case, no response song is produced. Without loss of generality, we set the weight for positive information w+ to 1. The other five parameters (w−, τ, σ, θ+, and θ−) were free to vary and were fitted to the behavioral data using a genetic algorithm (Fig. S3).
The model predicts behavioral responses very well (r2 = 0.88; Fig. 3A); moreover, it reproduces the relation between the fraction of attractive syllables in different parts of the song and the response, e.g., the beginning of the song correlates most strongly with predicted behavior (Fig. 3B, compare blue and red bars).
Model Parameters Reveal Strongly Asymmetrical Integration.
The data reliably constrained the model parameters (Fig. S3 and Table 1, Upper), which in turn allowed inference about how female grasshoppers integrate song quality information during courtship. The model parameters are easily interpreted as to their contribution to the decision dynamics: The time constant corresponds to the number of syllables that potentially influence the decision. The weight for each type of information and the decision thresholds determine the susceptibility of each type of decision to sensory information and noise. In this modeling framework, the strong influence of a song’s beginning on the female grasshopper’s response probability could indicate a large weight for positive information and a low threshold for positive decisions (10).
Table 1.
Parameter values for leaky and nonleaky drift-diffusion models
| Model | r2 | w+ | w− | τ | σ | θ+ | θ− |
| Leaky | 0.88 ± 0.02 | 1 | −46.5 ± 4.3 | 213 ± 29 | 97.6 ± 8.8 | 175.7 ± 5.8 | −795.7 ± 98.7 |
| Perfect | 0.88 ± 0.02 | 1 | −46.5 ± 1.9 | ∞ | 102.7 ± 3.8 | 187.4 ± 7.0 | −880.4 ± 78.6 |
r2, cross-validated correlation coefficient between model prediction and behavior. w+, w−, weight for positive and negative evidence. τ, integration time constant in units of syllables; perfect integration corresponds to τ = ∞. σ, SD of the noise added at each integration step. θ+, θ−, threshold for positive and negative decisions.
Perfect integration of information.
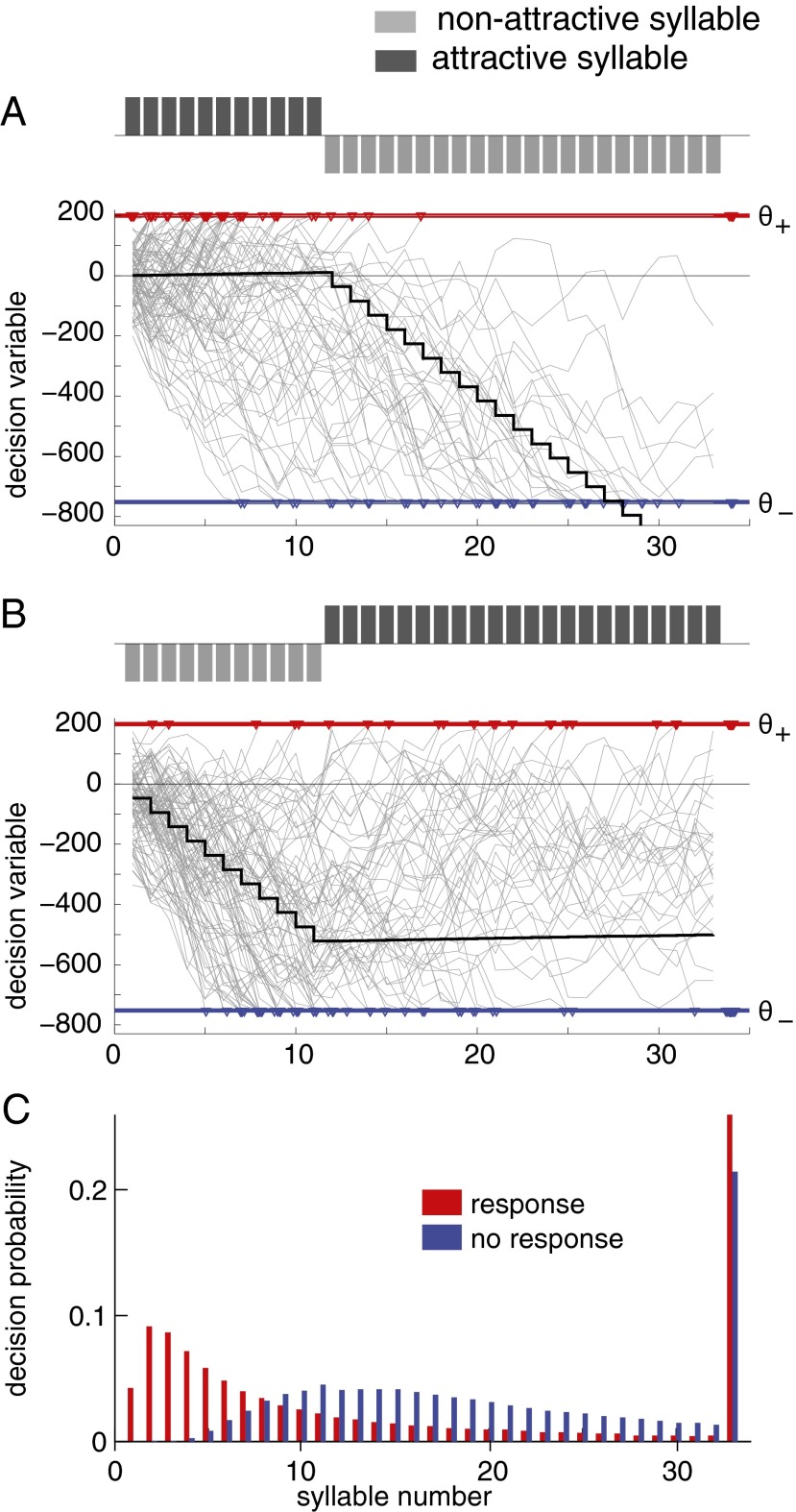
The time constant of integration found by the genetic algorithm is much longer than the duration of the songs (τ = 213 ± 29 syllables vs. a song duration of 33 syllables), which means that the decision variable barely drops over the course of the song. Given that natural songs rarely contain more than 50 syllables, the model indicates that females can potentially assess the full male song for making a decision (Fig. 4 A and B). Indeed, a model with perfect integration exhibits virtually identical performance and similar parameter values (Table 1, Lower), which is well in accord with theories of optimal decision-making, since it allows utilization of all information provided by the sensory input (20).
Fig. 4.
Model traces and decision times. (A and B) Example traces of the drift-diffusion model for two stimuli; bars show the stimulus (positive bars, attractive syllables; negative bars, nonattractive syllables). Gray traces show integration of information for 100 runs of the model with independent noise. The thick black trace shows the accumulated noiseless information—each syllable constitutes a certain amount of information according to its weight. Red and blue correspond to the positive and negative thresholds, respectively. Colored triangles mark decisions, after threshold crossings or given by the sign of the integrated information if no threshold crossing occurred (rightmost triangles). Due to the small weight of positive information, the integrated information is barely affected by the occurrence of attractive syllables. In contrast, nonattractive syllables exhibit a much greater impact on the decision variable. (C) Distribution of decision times for either response type for all songs. Rightmost bars indicate decision after song end based on the sign of the integrated information (without a threshold crossing). Red, positive responses; blue, negative responses.
Positive and negative information is weighted asymmetrically.
The model’s weight of negative information was much higher than that of positive information (w− = −46.5 ± 4.3 vs. w+ = 1.0). Thus, the impact of each syllable type on the integrated information is highly asymmetrical—a single nonattractive syllable strongly reduces the decision variable, whereas attractive syllables have comparatively small effects (Fig. 4 A and B).
Positive information only negligibly impacts the decision.
The small weight of positive information seems inconsistent with the interpretation of an impulsive decision provided above. Nonetheless, impulsive decisions could occur if the low weight of positive information were compensated by a decision threshold that was equally low. However, the asymmetry in decision thresholds is much weaker than that in weights, differing only 4.5-fold instead of 47-fold for the weights (θ+ = 175.7 ± 5.8, θ− = −795.7 ± 98.7; Fig. 4 A and B and Table 1).
Indeed, attractive syllables have only a negligible impact on the modeled female decision; this is revealed by quantifying the sensitivity of each type of decision to the amount of sensory information. Sensitivity is given by the ratio between the threshold and weight and measures the amount of information needed for a perfect, noiseless integrator to cross threshold. For negative decisions, 18 nonattractive syllables drive a noiseless perfect integrator to the decision threshold (θ−/w− = 17.1). In contrast, for a positive decision, 176 attractive syllables are needed to reach threshold (θ+/w+ = 175.7). For naturally occurring songs, whose duration rarely exceeds 50 syllables, the positive threshold will thus never be reached by positive information alone.
The model thus challenges our initial interpretation of impulsivity in female choice behavior. Given the inability of positive information alone to drive decisions, we then asked (i) What drives positive responses? and (ii) Why do decisions depend largely on the beginning of songs (Figs. 2 and 3B)?
Integration noise often drives female responses.
In addition to quantifying the sensitivity of a decision to sensory information, we examined the impact of decision noise on each decision. The integration noise level σ found by the genetic algorithm was 97.6 ± 8.8. Noise sensitivity corresponds to the number of noise steps of size σ to reach the decision threshold and shows that positive responses are highly susceptible to integration noise: two noise steps are sufficient to drive a positive response (θ+/σ = 1.8). In contrast, negative information is much less sensitive to noise: nine noise steps are needed for a purely noise-driven negative response (θ−/σ = −8.2). This high noise sensitivity in combination with the negligible impact of positive sensory information suggests that it frequently is integration noise that induces positive threshold crossings (Fig. 4 A and C).
Nonattractive syllables veto female responses early in song.
Taken together, this aspect of the model helps to explain why the beginning of song is the strongest determinant of the female decision to respond (Figs. 2 and 3B); it is not due to a strong influence of positive information that leads to threshold crossings after few syllables. As shown above, the model suggests that positive information alone is incapable of inducing threshold crossings. If a song starts with a sequence of attractive syllables, the decision variable stays close to zero, which either allows a threshold crossing with the help of integration noise or leads to a positive value of the integrated information at the end of the songs (Fig. 4 A and C).
However, a single intervening nonattractive syllable at the beginning of a song greatly moves the integrated information away from the positive decision threshold (Fig. 4B). To compensate for this shift, 47 attractive syllables would be necessary. Moreover, given the natural range of song durations, there is little chance for noise to induce positive decisions after a few nonattractive syllables (Fig. 4B). Hence, a few nonattractive syllables early in a song effectively veto positive responses, and this is consistent with the times when the positive threshold is crossed in the model—most positive decisions are either made within the first 10 syllables through threshold crossing with the help of noise or after the end of song without an actual threshold crossing (Fig. 4C).
Discussion
In this study, we have determined the dynamics of integration of sensory information during an innate, sexual communication behavior. A drift-diffusion model reproduced our behavioral data very well (r2 = 0.88), indicating that this framework is well-suited to describe the major aspects of information integration in grasshoppers. In the drift-diffusion model, integration is highly asymmetrical: nonattractive song elements exhibit a strong weight on the decision and effectively veto behavioral responses. By contrast, attractive information has only small weight. Furthermore, the model suggests that threshold crossings leading to female responses are often elicited by integration noise. Before considering these results in the context of the selective pressures shaping acoustic sexual communication, we will discuss how the decision-making process described here could be implemented physiologically.
Neural Implementation.
Perceptual decision-making is usually conceptualized as a two-step progress. First, task-relevant information is extracted from sensory input. Second, this sensory information is integrated to yield a decision. The drift-diffusion model sheds light on the second step of information integration. In a previous study, we were concerned with the first step and had identified the stimulus features driving female responses (14). We could show that the number of offsets in a song strongly determines its attractiveness; this is consistent with the results of the present study, because the nonattractive syllables exhibit disrupted plateaus and can thus be discriminated on the basis of a high number of offsets.
This finding does not imply that our approach relies on the existence of dedicated feature detectors for all possible forms of negative information; in most communication systems this would be unrealistic because many signal patterns constitute negative information and hence an unreasonably large number of detectors would be necessary. However, negative information could simply correspond to a lack of attractive features in a signal (SI Methods).
Conceivably, there could exist dedicated feature detectors also for specific, nonattractive features, if these occur frequently and confer reliable information. Such feature detectors could be the basis for reinforcement and reproductive character displacement (3, 21). In C. biguttulus, syllables with interrupted plateaus as used in our study are consistently produced by males with developmental abnormalities or by males of other sympatric species (19, 22). Thus, interrupted plateaus indicate poor or incompatible genetic quality and hence their detection by a dedicated feature detector would be beneficial. There indeed exists an auditory neuron (AN4) that is strongly inhibited by interrupted sound pulses, possibly constituting a detector of a negative song feature (23).
Neurons in the grasshopper’s early auditory system—located in the metathoracic ganglion—are likely involved in feature extraction (23, 24). Several neurons that project to the brain—so-called “ascending neurons”—are sensitive to the number of offsets in a syllable and thereby exhibit differential responses to positive and negative information; this is achieved through the interaction of differently timed excitatory and inhibitory inputs (25).
By contrast, very little is known about the circuits that further process and integrate this sensory information. The results in this study provide a starting point for future electrophysiological studies in the grasshopper brain to identify the neural circuits involved in decision-making. The parameters of the drift-diffusion model can be easily mapped to properties of neural circuits: weights for positive and negative information could correspond to the efficacy of synapses feeding positive and negative information to the integrator. Alternatively, the vetoing of female response could be implemented by proper placement of inhibitory inputs in a dendritic tree (26). The near-perfect integration of information (Table 1) could be implemented as a recurrent network or through synaptic plasticity (27, 28). Otherwise, the slow accumulation of intracellular calcium or of extracellular signaling molecules has also been implied in information integration (29–31).
Note that we have used only two syllable types in this study; however, there likely exists a diversity of different syllable types, ranging from strongly repellent to neutral (weight close to zero) to highly attractive. It will be interesting to see whether the weight of a syllable depends on the values of other syllables recently heard by the animal, i.e., whether the value of a syllable is absolute or context dependent (32); the latter could adapt the weighting of syllables to maintain mating preferences in varying acoustic environments and social contexts.
Noise in the model is implemented as internal noise and could be located either in the feature detector neurons or in the integrator neurons themselves. In natural conditions, external noise is another source of variability that can either distort attractive stimulus features or mask unattractive ones (18, 33, 34)—future studies will explore how both noise sources interact during feature detection and integration.
Asymmetrical Integration as a Consequence of Sexual Selection.
The parameters determining song integration during courtship likely reflect the costs and benefits associated with mate choice, and this is true even though the evolutionary optimization of decision-making during courtship is likely constrained in several ways. For instance, song evaluation in grasshoppers is shaped by selective pressures exerted on the auditory system by demands not directly associated with mate selection, such as the detection of predators (35). Also, traits that have evolved in contexts other than mate choice can induce perceptual biases that affect female decisions (5, 6, 36). Still, the highly asymmetrical integration is consistent with predictions made by theories of optimal decision-making and of sexual selection.
Asymmetrical weights reflect reliability of sensory cues.
In Bayes-optimal integration of information, cues are weighted by their reliability (37). In our case, nonattractive syllables exhibit salient features that reliably indicate “wrong male”—a large weight should therefore be assigned to negative information. In contrast, the differences between the attractive syllables of conspecifics of different quality rely on more subtle features of the song that are prone to be distorted by environmental noise (33, 38, 39). Hence, positive information tends to be less reliable and should therefore be weighted less. The distribution of weights found in the model is thus consistent with optimal cue integration (37).
Asymmetrical weights reflect fitness costs of misdecisions.
Differential weighting leads to a consistent rejection of bad singers—be they heterospecifics or conspecifics of inferior quality—at the cost of enabling only poor discrimination between different grades of good singers. The weights thus reflect the differential costs associated with a (mis)decision: it is much more costly to engage in courtship and to mate with a poor singer than to miss a good one. A poor singer is likely to be a heterospecific mating with whom produces hybrid offspring with developmental defects and high mortality rates (21). In addition, hybrid males are often behaviorally sterile, because their hybrid song is not attractive to females of either parental species, thus further increasing the fitness costs for the female (9); to a lesser extent, this also applies to mating with a conspecific low-quality singer. Songs with interrupted syllables are often produced by males that have crippled wings or have lost a hindleg. These song deficits thus point to problems during molt, which are likely transmitted to the offspring, thereby reducing the female’s fitness. In contrast, mating with a conspecific good singer will normally produce healthy and fertile offspring. The additional fitness gained by a long search for a better singer is relatively small compared with the severe costs of mating with a poor singer that may belong to a different species or provides inferior genetic material. Hence to reliably avoid the high hybridization costs in the presence of noisy information integration, nonattractive syllables should be assigned a large weight (8).
More generally, our results suggest that the dynamics of decision-making may influence how a behavioral pattern is interpreted. Usually a mate choice behavior is interpreted as “females prefer attractive traits.” However, different weightings can underlie the same mate choice pattern and these weightings can reflect the costs and benefits of the choice behavior as well the reliability of the traits involved (10): (i) attractive traits could be weighted strongly positively in the case of sensory biases or “honest” traits (1, 6, 7); (ii) attractive traits could be weighted less than nonattractive ones if mating occurs rarely and errors are costly (9); and (iii) attractive traits could be ignored altogether if mating is costly to the female as in the case of sexual conflict (40). In our experiments, nonattractive syllables dominated mate choice (case ii above)—it might thus be more appropriate to label the nonattractive syllables repellent and to interpret the choice behavior as “females are repelled by nonattractive syllables.”
Conclusion
Our behavioral data in combination with the drift-diffusion model suggest that the dynamics of decision-making reflect the selective pressures that have shaped choice behavior. In the model system used here—song evaluation in the grasshopper C. biguttulus—asymmetrical integration of information is consistent with optimal cue integration and with predictions from theories of sexual selection and sexual conflict. Mating systems influenced by different selective pressures are expected to exhibit other modes of information integration. For example, in species where females directly benefit from copulation through nuptial gifts (41, 42), females should weight information more symmetrically. In addition, the relative costs and benefits are expected to change with the status of the female, e.g., sensory experience or age (43, 44). Hence, the dynamics of integration processes are expected to change in an adaptive manner over the lifetime of an animal. Our approach thus provides a rich framework to study the adaptation of decision-making processes on evolutionary and individual time scales.
Methods
Thirty-two sequences consisting of an attractive and a nonattractive syllable (Fig. 1 B and C) were presented in pseudorandom order to virgin female C. biguttulus. Female response songs were detected and song attractiveness was quantified as the average female response probability over 18 trials per animal and 27 animals. We then fitted a drift-diffusion model to these responses using a genetic algorithm. See SI Methods for details.
Supplementary Material
Acknowledgments
The authors thank Arnulf Köhncke and Matthias Hennig for fruitful discussions. This work was funded by German Federal Ministry of Education and Research Grants 01GQ0410 and 01GQ1001A; Deutsche Forschungsgemeinschaft Grants SFB618, RO 547/12-1,GK1589/1; and the German Academic Exchange Service.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412741111/-/DCSupplemental.
References
- 1.Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol Evol. 2006;21(6):296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Kroodsma DE, Miller EH. Ecology and Evolution of Acoustic Communication in Birds. Cornell Univ Press; Ithaca, NY: 1996. [Google Scholar]
- 3.Gerhardt CH, Huber F. Acoustic Communication in Insects and Anurans. Univ of Chicago Press; Chicago: 2002. [Google Scholar]
- 4. Hedwig B, ed (2013) Insect Hearing and Acoustic Communication (Springer, Berlin)
- 5.Bateson M, Healy SD. Comparative evaluation and its implications for mate choice. Trends Ecol Evol. 2005;20(12):659–664. doi: 10.1016/j.tree.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Ryan MJ, Cummings ME. Perceptual biases and mate choice. Annu Rev Ecol Evol Syst. 2013;44:437–459. [Google Scholar]
- 7.Ryan MJ, Rand AS. Species recognition and sexual selection as a unitary problem in animal communication. Evolution. 1993;47(2):647–657. doi: 10.1111/j.1558-5646.1993.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 8.Safi, et al. Species recognition influences female mate preferences in the common European grasshopper (Chorthippus biguttulus Linnaeus, 1758) Ethology. 2006;112(12):1225–1230. [Google Scholar]
- 9.Gottsberger B, Mayer F. Behavioral sterility of hybrid males in acoustically communicating grasshoppers (Acrididae, Gomphocerinae) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193(7):703–714. doi: 10.1007/s00359-007-0225-y. [DOI] [PubMed] [Google Scholar]
- 10.Jennions MD, Petrie M. Variation in mate choice and mating preferences: A review of causes and consequences. Biol Rev Camb Philos Soc. 1997;72(2):283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- 11.von Helversen D, von Helversen O. Recognition of sex in the acoustic communication of the grasshopper Chorthippus biguttulus (Orthoptera, Acrididae) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1997;180(4):373–386. [Google Scholar]
- 12.von Helversen D, von Helversen O. Acoustic pattern recognition in a grasshopper: Processing in the time or frequency domain? Biol Cybern. 1998;79(6):467–476. [Google Scholar]
- 13.Schmidt A, Ronacher B, Hennig RM. The role of frequency, phase and time for processing of amplitude modulated signals by grasshoppers. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194(3):221–233. doi: 10.1007/s00359-007-0295-x. [DOI] [PubMed] [Google Scholar]
- 14.Clemens J, Ronacher B. Feature extraction and integration underlying perceptual decision making during courtship behavior. J Neurosci. 2013;33(29):12136–12145. doi: 10.1523/JNEUROSCI.0724-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirmer A, Faustmann M, Heinrich R. Reproductive behaviour of female Chorthippus biguttulus grasshoppers. J Insect Physiol. 2010;56(7):745–753. doi: 10.1016/j.jinsphys.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Wang XJ. Decision making in recurrent neuronal circuits. Neuron. 2008;60(2):215–234. doi: 10.1016/j.neuron.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunton BW, Botvinick MM, Brody CD. Rats and humans can optimally accumulate evidence for decision-making. Science. 2013;340(6128):95–98. doi: 10.1126/science.1233912. [DOI] [PubMed] [Google Scholar]
- 18.Einhäupl A, Stange N, Hennig RM, Ronacher B. Attractiveness of grasshopper songs correlates with their robustness against noise. Behav Ecol. 2011;22(4):791–799. [Google Scholar]
- 19.Kriegbaum H. Female choice in the grasshopper Chorthippus biguttulus. Naturwissenschaften. 1989;76:81–82. [Google Scholar]
- 20.Bogacz R. Optimal decision-making theories: Linking neurobiology with behaviour. Trends Cogn Sci. 2007;11(3):118–125. doi: 10.1016/j.tics.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: Invited review. Mol Ecol. 2000;9(8):1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- 22.von Helversen O, von Helversen D. Forces driving coevolution of song and song recognition in grasshoppers. Fortschr Zool. 1994;39:253–284. [Google Scholar]
- 23.Stumpner A, Ronacher B. Neurophysiological aspects of song pattern recognition and sound localization in grasshoppers. Am Zool. 1994;34:696–705. [Google Scholar]
- 24.Clemens J, Kutzki O, Ronacher B, Schreiber S, Wohlgemuth S. Efficient transformation of an auditory population code in a small sensory system. Proc Natl Acad Sci USA. 2011;108(33):13812–13817. doi: 10.1073/pnas.1104506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemens J, Wohlgemuth S, Ronacher B. Nonlinear computations underlying temporal and population sparseness in the auditory system of the grasshopper. J Neurosci. 2012;32(29):10053–10062. doi: 10.1523/JNEUROSCI.5911-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gidon A, Segev I. Principles governing the operation of synaptic inhibition in dendrites. Neuron. 2012;75(2):330–341. doi: 10.1016/j.neuron.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat Neurosci. 2000;3(Suppl):1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- 28.Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319(5869):1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- 29.Major G, Tank D. Persistent neural activity: Prevalence and mechanisms. Curr Opin Neurobiol. 2004;14(6):675–684. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Flavell SW, et al. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell. 2013;154(5):1023–1035. doi: 10.1016/j.cell.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinrich R, Kunst M, Wirmer A. Reproduction-related sound production of grasshoppers regulated by internal state and actual sensory environment. Front Neurosci. 2012;6:89. doi: 10.3389/fnins.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2012;13(1):51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuhofer D, Stemmler M, Ronacher B. Neuronal precision and the limits for acoustic signal recognition in a small neuronal network. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2011;197(3):251–265. doi: 10.1007/s00359-010-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt AK, Riede K, Römer H. High background noise shapes selective auditory filters in a tropical cricket. J Exp Biol. 2011;214(Pt 10):1754–1762. doi: 10.1242/jeb.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuhofer D, Wohlgemuth S, Stumpner A, Ronacher B. Evolutionarily conserved coding properties of auditory neurons across grasshopper species. Proc Biol Sci. 2008;275(1646):1965–1974. doi: 10.1098/rspb.2008.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnqvist G. Sensory exploitation and sexual conflict. Philos Trans R Soc Lond B Biol Sci. 2006;361(1466):375–386. doi: 10.1098/rstb.2005.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fetsch CR, DeAngelis GC, Angelaki DE. Bridging the gap between theories of sensory cue integration and the physiology of multisensory neurons. Nat Rev Neurosci. 2013;14(6):429–442. doi: 10.1038/nrn3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Römer H. Ecological constraints for sound communication: From grasshoppers to elephants. In: Barth F, Schmid A, editors. Ecology of Sensing. Springer; Berlin: 2001. pp. 59–77. [Google Scholar]
- 39.Römer H, Lewald J. High-frequency sound transmission in natural habitats: Implications for the evolution of insect acoustic communication. Behav Ecol Sociobiol. 1992;29(6):437–444. [Google Scholar]
- 40.Rice WR. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381(6579):232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 41.Reinhold K, Heller K-G. The ultimate function of nuptial feeding in the bushcricket Poecilimon veluchianus (Orthoptera: Tettigoniidae: Phaneropterinae) Behav Ecol Sociobiol. 1993;32(1):55–60. [Google Scholar]
- 42.Lehmann GUC. Weighing costs and benefits of mating in bushcrickets (Insecta: Orthoptera: Tettigoniidae), with an emphasis on nuptial gifts, protandry and mate density. Front Zool. 2012;9(1):19. doi: 10.1186/1742-9994-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey NW, Zuk M. Acoustic experience shapes female mate choice in field crickets. Proc Biol Sci. 2008;275(1651):2645–2650. doi: 10.1098/rspb.2008.0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qvarnström A, Pärt T, Sheldon BC. Adaptive plasticity in mate preference linked to differences in reproductive effort. Nature. 2000;405(6784):344–347. doi: 10.1038/35012605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.