Significance
DEET (N,N-diethyl-3-methylbenzamide) has intrigued medical entomologists, neurobiologists, insect physiologists, and chemical ecologists for decades, and hitherto it was not known how and why it works. We have discovered an odorant receptor in the southern house mosquito, which is essential for repellency, thus unravelling how DEET works. Additionally, we have identified a link between this synthetic repellent and methyl jasmonate, thus suggesting that DEET might work by mimicking defensive compound(s) from plants. The discovery of a molecular target may pave the way for the development of better and more affordable insect repellents.
Keywords: picaridin, IR 3535, odorant receptor, Culex quinquefasciatus, southern house mosquito
Abstract
Insect repellents are important prophylactic tools for travelers and populations living in endemic areas of malaria, dengue, encephalitis, and other vector-borne diseases. DEET (N,N-diethyl-3-methylbenzamide) is a 6-decade-old synthetic repellent, which is still considered the gold standard of mosquito repellents. Mosquitoes use their sense of smell to detect DEET, but there are currently two hypotheses regarding its mode of action: activation of ionotropic receptor IR40a vs. odorant receptor(s). Here, we demonstrate that DEET, picaridin, insect repellent 3535, and p-menthan-3,8-diol activate the odorant receptor CquiOR136 of the southern house mosquito, Culex quinquefasciatus. Electrophysiological and behavioral assays showed that CquiIR40a knockdown had no significant effect on DEET detection and repellency. By contrast, reduction of CquiOR136 transcript levels led to a significant decrease in electroantennographic responses to DEET and a complete lack of repellency. Thus, direct activation of an odorant receptor, not an ionotropic receptor, is necessary for DEET reception and repellency in Culex mosquitoes. Interestingly, methyl jasmonate, a repellent derived from the nonvolatile jasmonic acid in the signaling pathway of plant defenses, elicited robust responses in CquiOR136•CquiOrco-expressing Xenopus oocytes, thus suggesting a possible link between natural products with long insect–plant evolutionary history and synthetic repellents.
Insect repellents have been used since ancient times as prophylactic agents against diseases transmitted by mosquitoes and other arthropods, including malaria, dengue fever, and encephalitis. They were developed from plant-based smoke or extracts (essential oils) into formulations with a single active ingredient. DEET (N,N-diethyl-3-methylbenzamide), a synthetic compound developed more than 6 decades ago, is the most widely used substance. Unfortunately, people in endemic areas who need insect repellents the most cannot afford to use DEET daily, whereas a significant proportion of those who need and can afford it, do not use DEET because of undesirable properties such as an unpleasant odor. Molecular modeling led to the development of insect repellent (IR) 3535 (1) and picaridin (2), but progress toward development of better and more affordable repellents has been slow, because DEET receptors in mosquitoes are hitherto unknown.
There are currently two hypotheses regarding DEET reception. One school postulates that the widespread effect of DEET olfactory repellency is mediated by a well-conserved ionotropic receptor, IR40a (3), whereas the other (4, 5) favors a pathway involving odorant receptor(s). Here we report that the southern house mosquito Culex quinquefasciatus uses an odorant receptor, CquiOR136, to detect DEET, picaridin, IR3535, p-menthan-3,8-diol (PMD), and a plant defense-signaling compound, methyl jasmonate.
Results and Discussion
To test whether DEET olfactory repellency in the southern house mosquito is mediated by IR40a (3), we cloned CquiIR40a, the C. quinquefasciatus ortholog of DmelIR40a (3), and expressed this putative receptor along with coreceptor CquiIR8a in the Xenopus oocytes. CquiIR40a•CquiIR8a-expressing oocytes did not generate detectable currents when challenged with DEET, 200 compounds in our panel, or the newly identified mosquito repellents, ethyl anthranilate, butyl anthranilate, and methyl N,N-dimethylanthranilate (3).
To rule out a possible malfunction of the coreceptor CquiIR8a, we first recorded from a known Drosophila IR•coreceptor system and then mismatched receptors and coreceptors. DmelIR84a•DmelIR8a-expressing oocytes generated robust currents (253 ± 26 nA, 1 µM; 1,616 ± 294 nA, 10 µM; and 3,783 ± 159 nA, 100 µM) to phenylacetaldehyde, in agreement with a previous report (6). Although CquiIR40a coexpressed with DmelIR8a remained silent, DmelIR84a coexpressed with CquiIR8a responded to phenylacetaldehyde (717 ± 166 nA, 1 mM), thus suggesting that CquiIR8a was indeed functional. It is worth mentioning, however, that our findings, albeit inconclusive, are not entirely surprising, given that to date only a handful of IRs have been deorphanized (6–8).
We then changed our strategy and next used RNAi to reduce CquiIR40a transcript levels in adult female mosquitoes and examined the phenotypes by electrophysiology and behavior assays.
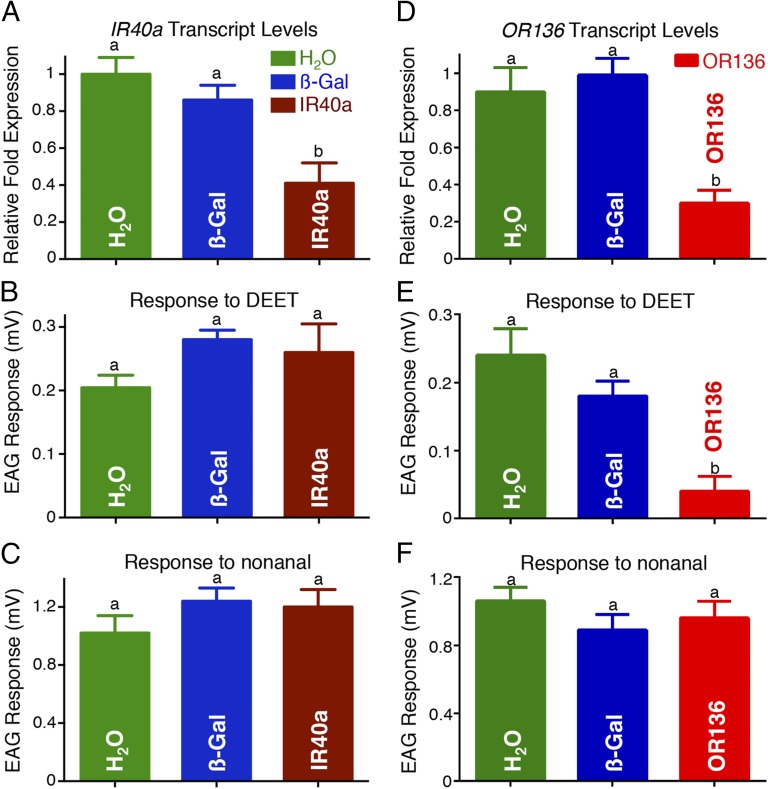
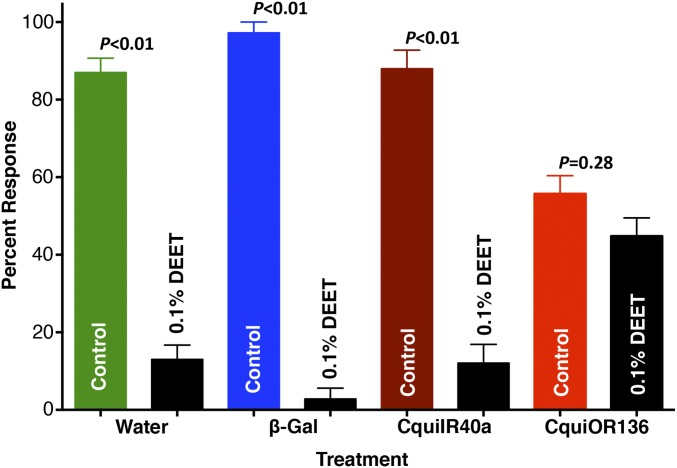
Our RNAi approach has been demonstrated to cause long-lasting transcript-level reductions (9). Thus, we injected three groups of female pupae with CquiIR40a–double-strand RNA (dsRNA), water, or β-galactosidase-dsRNA. As a result, transcript levels of CquiIR40a in adults were significantly reduced in CquiIR40a-dsRNA–injected mosquitoes compared with the two control groups (Fig. 1A). Given that the antennae of C. quinquefasciatus house neurons sensitive to DEET (5), we compared the phenotypes initially by sensory physiology. Electroantennographic (EAG) responses recorded from female antennae of CquiIR40a-dsRNA–injected mosquitoes were not significantly different from those obtained with mosquitoes injected with water or dsRNA of a control gene (Fig. 1B). Likewise, there were no significant differences in EAG responses to a control odorant, nonanal (Fig. 1C). To measure repellency behavior of the phenotype, we modified our previously described surface-landing assay (5) by adding chemical stimuli and a feeding reward, in addition to physical stimuli—heat and color. Responses of water-treated mosquitoes and those injected with β-galactosidase-dsRNA did not differ from those of WT mosquitoes, i.e., they were significantly repelled by DEET (P < 0.01), as more than 80% landed on the control side (Fig. 2). Similarly, CquiIR40a-dsRNA–treated mosquitoes significantly (P < 0.01) avoided the DEET-treated side of the arena. We therefore concluded that, in contrast to what has been reported for the fruit fly (3), DEET repellency in the southern house mosquito is not mediated by IR40a and subsequently tested the alternative hypothesis.
Fig. 1.
Effect of RNAi treatment on electrophysiological responses to DEET and a control odorant, nonanal. (A) CquiIR40a transcript levels in water-treated (green), β-galactosidase-dsRNA–injected (blue), and CquiIR40a-dsRNA–injected mosquitoes (brown). Although CquiIR40a transcript levels were significantly reduced in CquiIR40a-treated mosquitoes (one-way ANOVA, F = 22.63; P = 0.0016; R2 = 0.883), EAG responses to (B) DEET (F = 0.5015, P = 0.6216, R2 = 0.103) and (C) nonanal (F = 2.829, P = 0.1114, R2 = 0.386) from the three groups of treated mosquitoes did not significantly differ. (D) CquiOR136 transcript levels in water-treated (green), β-galactosidase-dsRNA–injected (blue), and CquiOR136-dsRNA–injected mosquitoes (red) (F = 22.63; P = 0.0016; R2 = 0.883). (E) EAG responses to DEET decreased significantly in CquiOR136-dsRNA–injected mosquitoes (F = 13.04, P = 0.0065, R2 = 0.8129), but (F) responses to nonanal were not significantly different from the controls (F = 1.453, P = 0.2537, R2 = 0.108). Data are expressed as mean ± SEM. Bars labeled with the same letters are not significantly different at the 5% level (Tukey’s multiple comparison tests). For quantitative PCR, n = 3 biological samples of 30 mosquitoes each, with three technical replicates for each sample, were replicated three times (total biological samples per test gene, n = 9). For EAG, n = 3–4 female mosquitoes, with 8–12 replicates for DEET from each insect.
Fig. 2.
Two-choice repellent assay results. When tested separately, female mosquitoes injected with water, β-galactosidase-dsRNA, or CquiIR40a-dsRNA landed preferentially on the control side of the arena (P < 0.01), thus clearly avoiding DEET. By contrast, female mosquitoes injected with CquiOR136-dsRNA, albeit actively landing and feeding (Movies S1 and S2), showed no side preference, thus suggesting inability to detect DEET. n = 6–10. Data were arcsin-transformed before paired two-tailed Student t test comparisons. Data are expressed as mean ± SEM.
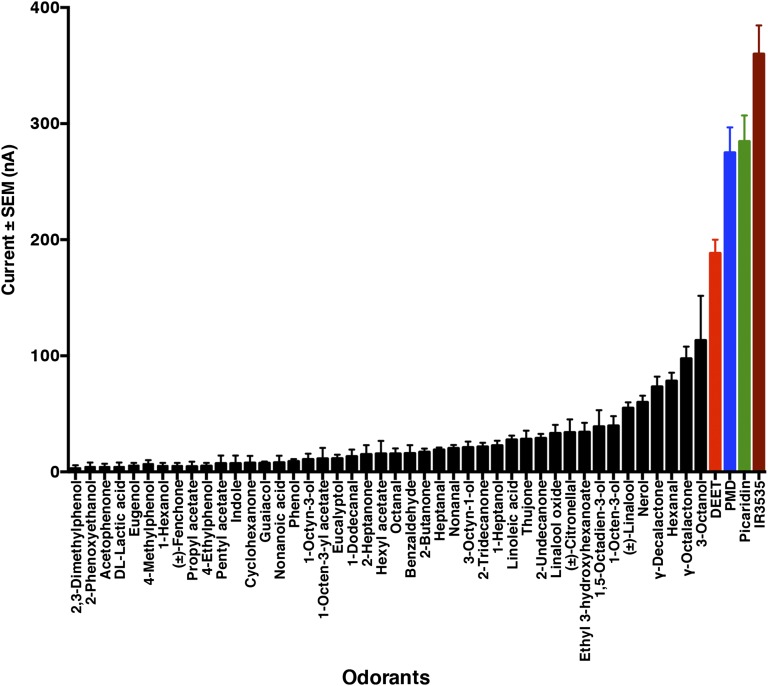
To be a repellent sensu stricto (10, 11), DEET must act as a vapor at some distance from where it evaporated. However, its vapor pressure is very low (5.6 × 10−3 mm Hg at 20 °C; toxnet.nlm.nih.gov). Moreover, DEET olfactory receptor neurons in both C. quinquefasciatus and Aedes aegypti displayed low sensitivity (5, 12). We therefore surmised that DEET must be detected by highly expressed odorant receptors (ORs) given that only a very small fraction of molecules released in the air would reach neurons housed in antennal sensilla (13). Two out of the top 10 putative OR genes enriched in female antennae compared with control tissues (14), CquiOR21 (formerly CquiOR10) (15) and CquiOR95 (14), have already been deorphanized, and one putative OR, CquiOR36, is probably a pseudogene. We cloned the other seven putative ORs, CquiOR55, CquiOR64, CquiOR93, CquiOR125, CquiOR132, CquiOR136, and CquiOR151. We screened them for a DEET receptor by using the Xenopus oocyte recording system, as previously done to deorphanize multiple Culex ORs (9, 14–17). CquiOR136•CquiOrco-expressing oocytes were mainly activated by DEET, PMD, IR3535, picaridin, and two other repellents, γ-octalactone and 3-octanol (Fig. 3 and Fig. S1) in our panel of 200 odorants, which included, in addition to repellents, attractants, oviposition attractants, plant kairomones, mosquito oviposition pheromone (18), and EAG-active compounds. For clarity, in Fig. 3 we omitted compounds that do not elicit responses. By contrast, CquiOR55, CquiOR64, CquiOR93, CquiOR125, CquiOR132, and CquiOR151 did not respond to DEET or other compounds in our panel.
Fig. 3.
Quantification of current responses of CquiOR136•CquiOrco-expressing oocytes. Two hundred compounds were tested at a source dose of 1 mM. For clarity, compounds that did not elicit detectable currents were omitted, and responding compounds were displayed in increasing order of responses. n = 4, mean ± SEM.
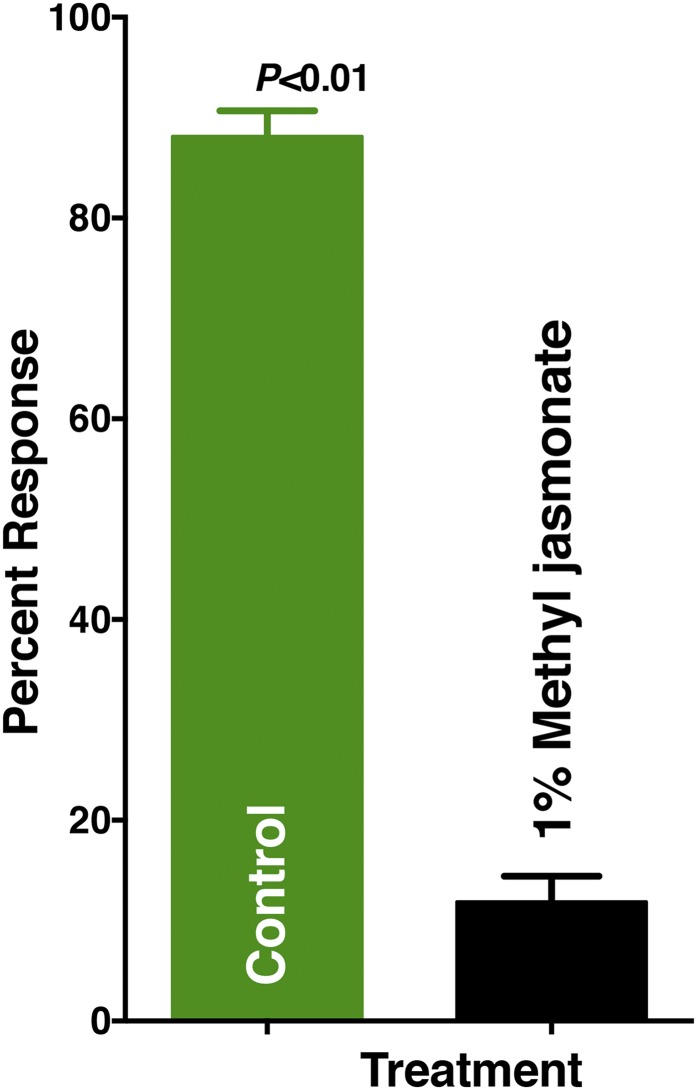
Having identified a DEET-sensitive OR highly expressed in mosquito female antennae (14) we tested whether DEET might mimic plant defense compound(s) with a long insect–plant evolutionary history, such as those in the jasmonate-signaling pathway (19). After all, DEET, a more recent synthetic counterpart developed by molecular modeling (2), picaridin, and methyl jasmonate share similar structural motifs, which are essential for activity (20), and methyl jasmonate is a repellent for the southern house mosquito, as well as ticks (21). CquiOR136•CquiOrco-expressing oocytes challenged with methyl jasmonate generated strong responses, whereas jasmonic acid elicited very low responses (Fig. 4). We then compared dose-dependent responses elicited by these plant-derived compounds and the four major insect repellents. DEET, picaridin, IR3535, and PMD elicited dose-dependent currents from CquiOR136•CquiOrco-expressing oocytes, thus suggesting a possible role of CquiOR136 in mosquito reception of repellents. Jasmonic acid elicited only very small currents at higher doses, whereas methyl jasmonate displayed slightly stronger responses than DEET in a dose-dependent fashion (Fig. 4). Jasmonic acid is a nonvolatile compound and, therefore, unlikely to repel at a distance, whereas methyl jasmonate has the ideal vapor pressure for a repellent (3.37 × 10−4 mmHg at 25 °C; toxnet.nlm.nih.gov), is released by injured plants (19), and has been demonstrated to be a repellent (21). Indeed, we observed in our surface-landing and feeding assay that methyl jasmonate is a repellent for the southern house mosquito (Fig. 5). It is, therefore, conceivable that methyl jasmonate is a natural ligand for CquiOR136.
Fig. 4.
Concentration–response relationships for CquiOR136•CquiOrco-expressing oocytes. Dose-dependence curves were generated for DEET, picaridin, IR3535, PMD, jasmonic acid, and methyl jasmonate. The four repellents and methyl jasmonate showed nearly the same sensitivity (threshold <10 µM), but their responses differed at high concentrations. n = 4 oocytes; all six compounds were tested in the same oocyte at the four tested concentrations, starting from low to high doses.
Fig. 5.
Females of the southern house mosquito are repelled by methyl jasmonate. In the surface-landing and feeding assays, WT females of the southern house mosquito were significantly repelled by methyl jasmonate (1%). n = 20, mean ± SEM.
Last, we attempted to knockdown CquiOR136 using the same approach we used for CquiIR40a. CquiOR136 transcript levels in adult female mosquitoes injected with water or β-galactosidase-dsRNA were not significantly different, but those injected with CquiOR136-dsRNA had significantly reduced transcripts (Fig. 1D). EAG responses recorded from female antennae of CquiOR136-dsRNA–injected mosquitoes had dramatically reduced responses to DEET (Fig. 1E), whereas responses to nonanal (22) were not significantly different from the two controls (Fig. 1F). Similarly, EAG responses to a second control odorant, decanal, recorded from the three groups of mosquitoes did not differ significantly (Fig. S2). Therefore, it is unlikely that CquiOR136-dsRNA injection had off-target effects on the olfactory system, as indicated by EAG responses to both positive control odorants, nonanal and decanal. More importantly, in our behavioral assay, CquiOR136-dsRNA–injected mosquitoes did not discriminate the solvent-treated side from the DEET-treated side and landed equally on both sides of the arena (Fig. 2 and Movies S1 and S2).
In summary, the data reported here suggest that repellency of the southern house mosquito by DEET can be explained by direct activation of CquiOR136, not CquiIR40a. In agreement with loss of activity of orco mutant mosquitoes (4), our findings suggest that direct activation of an OR is necessary for repellency [i.e., noncontact disengagement (10)] of host-seeking mosquitoes. Although our research does not rule out the possible involvement of the gustatory system in contact disengagement (10) [also referred to as contact irritant (23)], our results demonstrate that CquiOR136 is sufficient for repellency at a distance and paves the way toward the development of better and/or more affordable repellents.
Experimental Procedures
Animal Sources.
C. quinquefasciatus mosquitoes used in this study were from a laboratory colony started from adult mosquitoes collected in Merced, California, in the 1950s and maintained by Anthony Cornel in the Kearney Agricultural Center, University of California. In Davis, mosquitoes were maintained in an insect chamber at 27 ± 1 °C, under a photoperiod of 16:8 h (light:dark) for almost 5 y. Xenopus laevis oocytes were purchased from EcoCyte Bioscience.
RNA Extraction and Cloning.
Five hundred pairs of antennae from 4- to 6-d-old blood-fed female Culex mosquitoes were dissected under a stereo microscope (Zeiss, Stemi DR 1663) and collected in 50% (vol/vol) ethanol diluted in diethylpyrocarbonate (DEPC)-treated water on ice. Total RNA was extracted using TRIzol reagent (Invitrogen). cDNA was synthesized from 1 μg of total RNA using an RT-for-PCR kit according to the manufacturer’s instructions (Clontech). To obtain full-length coding sequences, PCRs were performed using gene-specific primers. PCR products were purified by a QIAquick gel extraction kit (Qiagen) and then cloned into pGEM-T vector (Promega). Plasmids were extracted by a QIAprep spin mini prep kit (Qiagen) and sequenced (Davis Sequencing). Using pGEM-T-CquiORs as templates, the sequences were amplified by Pfu II DNA polymerase with primers containing Kozak motif (acc) for subcloning into pGEMHE. PCR products were purified by using a Qiagen gel extraction kit and digested by XmaI and XbaI (BioLabs) before being subcloned into pGEMHE. pGEMHE-CquiIR8a was synthetized by GenScript USA, Inc. After transformation, inserts were verified by DNA sequencing (Davis Sequencing).
In Vitro Transcription and Oocyte Microinjection.
Capped OR cRNAs containing 3′ and 5′ untranslated regions (UTRs) of a Xenopus ß-globin gene were synthesized by using the mMESSAGE mMACHINE T7 Kit (Ambion), as previously described (24). Purified OR cRNAs were resuspended in nuclease-free water at 200 ng/μL and microinjected into X. laevis oocytes on stage V or VI along with the same amount of Orco. Injected oocytes were incubated at 18 °C for 3–7 d in modified Barth’s solution [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, and 10 mM Hepes at pH 7.4], supplemented with 10 μg/mL gentamycin, 10 μg/mL streptomycin, and 1.8 mM sodium pyruvate.
dsRNA Synthesis.
dsRNAs of CquiOR136, CqIR40a, and β-galactosidase were synthesized by in vitro transcription from PCR product using the MEGAscript T7 transcription kit (Ambion). PCR was performed using plasmids containing the target genes as DNA template. The following gene-specific primers that included T7 promoter sequence (underlined); CquiOR136-F: 5′-TAATACGACTCACTATAGGGTTGCTCCGCCTTATCATACTT-3′ and CquiOR136-R: 5′-TAATACGACTCACTATAGGGCTTCAAGATTTGTCTAAGGTTGATTAG -3′; CquiIR40a-F: 5′-TAATACGACTCACTATAGGGAAAACGTTGGTTCTACTGGTCCT -3′ and CquiIR40a-R: 5′- TAATACGACTCACTATAGGGGTGCCACGGAGGTTTATGGATAA -3′; Cquiβ-gal-F: 5′-TAATACGACTCACTATAGGGAATGGTTCAGGTCGAAAACG-3′ and Cquiβ-gal-R: 5′-TAATACGACTCACTATAGGGCCGCCTCGTACAAAACAAGT-3′ were selected. We BLASTed our target nucleotides (525–690 bp) against predicted transcript sequences in CpipJ2.2 geneset in VectorBase to confirm that there were no off-target genes. Large-scale dsRNAs were purified by the MEGAclear kit (Ambion) and precipitated with 5 M ammonium acetate to yield 6–9 µg/µL dsRNA.
dsRNA Microinjection.
Female pupae (0-d-old) were collected in plastic cups filled with distilled water and kept on ice for 15 min. The sharp end of a yellow pipette tip was cut diagonally to make a stage to hold a pupa. Forty-five nanograms of dsRNAs in 9.2 nL volume were injected in the dorsal membrane close to the base of the trumpet using a NanoLiter 2000 inject (World Precision Instruments). The injected pupae were put in new plastic cups with distilled water and kept at 27 °C. Newly emerged adults were supplied with sugar water (10% wt/vol), and newly emerged males (ratio 1:1) were released into the cage for mating.
Quantitative Analysis of Transcription Levels.
Thirty pairs of antennae were dissected and collected in 50% (vol/vol) ethanol diluted in DEPC-treated water on ice using a stereomicroscope for qRT-PCR. Total RNAs were extracted, and cDNAs were synthesized using iScript Reverse Transcription Supermix for RT-qPCR according to the manufacturer’s instructions (Bio-Rad). Real-time quantitative PCR (qPCR) was carried out by using a CFX96 TouchTM Real-Time PCR Detection System (Bio-Rad) and SsoAdvanced SYBR Green Supermix (Bio-Rad). CquiRPS7 gene was used as reference. The following pairs of detection primers were designed with Primer 3 program (frodo.wi.mit.edu/): CquiOR136-F: 5′- CAACGCTCGCAAATATCTCA -3′; R: 5′- TGAGCACTCGCCATTTGTAG -3′;CquiIR40a-F: 5′-TACATCCGGGAAATGGGATA-3′; R: 5′- AGAGCCAAAGCAAAATCGAA-3′; CquiRPS7-F; 5′-ATCCTGGAGCTGGAGATGA -3′; R: 5′-GATGACGATGGCCTTCTTGT -3′. qPCR was performed with three biological replicates, and each of them was replicated three times (three technical replicates per biological replicate); data were analyzed using the 2−∆∆CT method.
Two-Electrode Voltage Clamp Records.
The two-electrode voltage-clamp (TEVC) technique was used to measure odorant-induced currents in Xenopus oocytes at a holding potential of −80 to −70 mV. Signals were amplified with an OC-725C amplifier (Warner Instruments), low-pass–filtered at 50 Hz, and digitized at 1 kHz. Data acquisition and analysis were conducted with Digidata 1440A and software pCLAMP10 (Molecular Devices). Oocytes were challenged with the following compounds, which were acquired from Sigma-Aldrich, unless otherwise indicated: 1-octen-3-yl acetate, 2-butoxylacetone, (E)-2-methyl-2-butenal, 1-butanol, 1-pentanol, 1-hexanol, 1-heptanol, 1-octanol, 1-nonanol, 2,3-butanediol, 2-butoxyethanol, 3-methyl-1-butanol (isoamyl alcohol), (E)-2-hexen-1-ol, (Z)-2-hexen-1-ol, 1-hexen-3-ol, 1-hepten-3-ol, 3-octanol, 1-octen-3-ol, 1-octyn-3-ol, 3-octyn-1-ol, (E)-2-nonen-1-ol, (Z)-2-nonen-1-ol, 4-methylcyclohexanol, (5R,6S)-6-acetoxy-5-hexadecanolide (Bedoukian Research, Inc.), PMD [IUPAC (International Union of Pure and Applied Chemistry) name: 2-(2-hydroxypropan-2-yl)-5-methylcyclohexan-1-ol] (Bedoukian), methyl acetate, ethyl acetate, propyl acetate, butyl acetate, pentyl acetate, hexyl acetate, heptyl acetate, octyl acetate, nonyl acetate, decyl acetate, methyl propionate, ethyl propionate, methyl butyrate, ethyl butanoate, methyl hexanoate, ethyl hexanoate, ethyl 3-hydroxyhexanoate, ethyl 3-hydroxybutanoate, (E)-2-hexenyl acetate, (Z)-3-hexenyl acetate, β-terpinene, α-terpinene, ethyl lactate, methyl salicylate, 1-octen-3-yl acetate, isopentyl acetate, m-tolyl acetate, ethyl phenylacetate, geranyl acetate, octadecyl acetate, propanal, butanal, pentanal, hexanal, heptanal, octanal, nonanal, decanal, undecanal, 1-dodecanal, (E)-2-hexenal, (Z)-8-undecenal, (E)-2-heptenal, (E)-2-nonenal, phenylacetaldehyde, furfural, 2-butanone, 2-heptanone, geranylacetone, 6-methyl-5-hepten-2-one, 5-methyl-2-hexanone, 2,3-butanedione, 3-hydroxy-2-butanone, 2-undecanone, 2-tridecanone, 2-nonanone, 5-isobutyl-2,3-dimethylpyrazine, 2-octanol, (±)-fenchone, cyclohexanone, acetophenone, thujone mix (α+β), isovaleric acid, 1-dodecanol, dodecanoic acid, DL-lactic acid, ethanoic acid, propanoic acid, butanoic acid, isobutyric acid, 2-oxobutyric acid, pentanoic acid, 2-oxovaleric acid, hexanoic acid, (E)-2-hexenoic acid, 5-hexanoic acid, (E)-3-hexenoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, triethylamine, n-tridecanoic acid, linoleic acid, ammonia, trimethylamine, propylamine, butylamine, pentylamine, hexylamine, heptylamine, octylamine, 1,4-diaminobutane, 1,5-diaminopentane, benzaldehyde, phenol, 2-methylphenol (o-cresol), 3-methylphenol (m-cresol), 4-methylphenol (p-cresol), 4-ethylphenol, 3,5-dimethylphenol, 2,3-dimethylphenol, guaiacol, naphthalene, 2-methoxy-4-propylphenol, 2-phenoxyethanol, 1,2-dimethoxybenzene, benzyl alcohol, 2-phenylethanol, 1-phenylethanol, phenylether, isoprene, ethyl linoleate, (R)-(+)-limonene, (S)-(−)-limonene, α-humulene, linalool oxide, geraniol, nerol, 1,5-octadien-3-ol, (±)-linalool, eucalyptol, (E/Z)-citral, eugenol, α-pinene, ocimene, (±)-citronellal, indole, 3-methylindole (skatole), γ-valerolactone, γ-hexalactone, γ-octalactone, γ-decalactone, 4-hydroxy-2,5-dimethyl-3(2H)-furanone, 2,5-dimethyl-4-methoxy-3(2H)-furanone, γ-dodecalactone, pyrrolidine, 2-acetylthiophene, dimethyl phthalate, benzyl formate, phenethyl formate, isovaleraldehyde, ethyl stearate, tetradecanoic acid, methyl myristate, 1-iodohexadecane, 1-hexadecanol, (E)-2-hexenyl acetate, terpinolene, N-methylbenzamide, β-myrcene, palmitoleic acid, phenyl propanoate, phenyl isobutyrate, theophylline, N-phenyl-1-naphthylamine, 2-ethyltoluol, 2,4-thiazolidinedione, 2-methyl-2-thiazoline, phenethyl propionate, ethyl 2-(E)-4-(Z)-decadienoate, α-hexylcinnamaldehyde, 2-pentanone, 2-hexanone, 2-octanone, 4,5-dimethylthiazole, (E,E)-farnesol, (E,E)-farnesyl acetate, (Z)-3-hexen-1-ol, (1R)-(+)-camphor, (1S)-(−)-camphor, acetaldehyde, DEET (IUPAC name: N,N-diethyl-3-methylbenzamide), picaridin (IUPAC name, butan-2-yl 2-(2-hydroxyethyl)piperidine-1-carboxylate), and IR3535 (IUPAC name, ethyl 3-[acetyl(butyl)amino]propanoate). Additionally, we tested ethyl anthranilate, butyl anthranilate (Bedoukian Research, Inc.), methyl N,N-dimethylanthranilate (Tokyo Kasei), jasmonic acid, and methyl jasmonate (Sigma-Aldrich). Stock solutions (1M) of all compounds were prepared in DMSO and subsequently diluted with oocyte Ringer buffer. Clampfit 10 Software was used for data analysis.
Electroantennographic Recordings.
A female adult mosquito (4–6 d after emergence) was chilled on ice for 1 min and then carefully placed into a truncated 200-µL pipette tip with the antennae and eyes fully exposed. Modeling clay was used to secure the position of the mosquito’s head while a soaked piece of paper towel was inserted into the pipette end to secure the position of the abdomen and provide humidity. The preparation was mounted on a Syntech EAG platform equipped with a micromanipulator (MP-12, Syntech) and a high-impedance AC/DC amplifier (Serial Interface, IDAC-232, Syntech). Reference and recording electrodes were composed of chloridized silver wires in drawn-out glass capillaries filled with a solution of 0.1% KCl and 0.5% polyvinylpyrrolidone (PVP). The reference electrode was inserted in one eye, and the tips of the antennae were inserted into the recording electrode. The antennae were kept under a constant stream of purified and humidified air (170 mL/min). EAG signals were processed using EAG2000 software (Syntech). Odorants were dissolved in dichloromethane to make DEET solution (10% wt/vol) and in hexane to make nonanal solution (1 µg/µL). Ten microliters of chemical solution or solvent was loaded onto a 2 × 0.5 cm filter paper (Whatman qualitative, grade #1, Sigma-Aldrich) and allowed to evaporate for 15 s in a fume hood before placement into a 5-mL applicator syringe (5 mL syringe, 0.8 × 40 mm, Becton, Dickinson, and Company). Antennae were exposed to stimulus for 0.3 s, administered at 1-min intervals. EAG responses to tested repellents were corrected to the averaged EAG responses of the solvent for each respective insect.
Surface-Landing and Feeding Assay.
The bioassay arena was modified from our surface-landing assay (5) initially designed to mimic a human arm without odors or humidity. CO2 at 50 mL/min was added to activate female mosquitoes, and blood was provided as both an attractant and a reward. In short, two 50-mL Dudley bubbling tubes, painted internally with black hobby and craft enamel (Krylon, SCB-028), were held in a wooden board (30 × 30 cm), 17 cm apart from each end and 15 cm from the bottom. The board was attached to the frame of an aluminum collapsible field cage (30.5 × 30.5 × 30.5 cm; Bioquip). Two small openings were made 1 cm above each Dudley tube to hold two syringe needles (Sigma-Aldrich, 16-gauge, Z108782) to deliver CO2. To minimize handling of mosquitoes, test females were kept inside collapsible field cages since the latest pupal stage. These female cages had their cover premodified for behavioral studies. A red cardstock (The Country Porch, GX-CF-1) was placed internally at one face of the cage, and openings were made in the cardboard and cage cover so the cage could be attached to the wooden board with the two Dudley tubes and CO2 needles projecting inside the mosquito cage 6 and 3 cm, respectively. Additionally, windows were made on the top and the opposite end of the red cardstock for manipulations during the assays and a video camera connection, respectively. The mosquito cage housing 30–50 test females was connected to the platform holding the Dudley tubes (Movie S1) at least 2 h before bioassays. At least 10 min before the assays, water at 38 °C started to be circulated with a Lauda’s Ecoline water bath, and CO2 at 50 mL/min was delivered from a gas tank just at the time of the behavioral observations. Sample rings were prepared from strips of filter papers 25 cm long and 4 cm wide and hung on the cardstock wall by insect pins to make a circle around the Dudley tubes. Cotton rolls (iDental, 1 × 3 cm) were loaded with 100 μL of defibrinated sheep blood purchased from University of California, Davis, VetMed shop and placed between a Dudley tube and a CO2 needle. For each run one paper ring was loaded with 200 μL of hexane (control) and the other with 200 μL DEET 0.1% in hexane. Solvent was evaporated for 1–2 min, blood-impregnated cotton plugs and filter paper rings were placed in the arena, CO2 was started, and the assays were recorded with a camcorder equipped with Super NightShot Plus infrared system (Sony Digital Handycan, DCR-DVD 810). During the assay the arena was inspected with a flashlight whose lens was covered with a red filter. After 5 min, the number of females that landed and continued to feed on each side of the arena was recorded. Insects were gently removed from the cotton rolls, and the assays were reinitiated after rotation of sample and control. Thus, repellency for each set of test mosquitoes was measured with the filter paper impregnated with the same sample at least once on the left and once on the right side of the arena. After three runs, filter paper strips and cotton plugs were disposed of, and new loads were prepared.
Supplementary Material
Acknowledgments
We thank Dr. Julien Pelletier (Keele University), Dr. Ken Haynes (University of Kentucky), and members of the W.S.L. laboratory for comments on the manuscript, Dr. Anthon Cornel (University of California) for providing mosquitoes that allowed us to duplicate his colony at the Davis campus, and Dr. Richard Benton (University of Lausanne) for sharing Drosophila IR clones. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award R01AI095514.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession no. KM229531-8).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417244111/-/DCSupplemental.
References
- 1.Klier M, Kuhlow F. Novel insect repellents: Nitrogen-substituted β-Alanine derivatives. J Soc Cosmet Chem. 1976;27:141–153. [Google Scholar]
- 2.Boeckh J, et al. Acylated 1,3-aminopropanols as repellents against bloodsucking arthropods. Pestic Sci. 1996;48(4):359–373. [Google Scholar]
- 3.Kain P, et al. Odour receptors and neurons for DEET and new insect repellents. Nature. 2013;502(7472):507–512. doi: 10.1038/nature12594. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.DeGennaro M, et al. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498(7455):487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syed Z, Leal WS. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci USA. 2008;105(36):13598–13603. doi: 10.1073/pnas.0805312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abuin L, et al. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69(1):44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136(1):149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang YV, Ni J, Montell C. The molecular basis for attractive salt-taste coding in Drosophila. Science. 2013;340(6138):1334–1338. doi: 10.1126/science.1234133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu F, Xu P, Barbosa RM, Choo YM, Leal WS. RNAi-based demonstration of direct link between specific odorant receptors and mosquito oviposition behavior. Insect Biochem Mol Biol. 2013;43(10):916–923. doi: 10.1016/j.ibmb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JR, Siegert PY, Amimo FA, Walker ED. Designation of chemicals in terms of the locomotor responses they elicit from insects: An update of Dethier et al. (1960) J Econ Entomol. 2009;102(6):2056–2060. doi: 10.1603/029.102.0606. [DOI] [PubMed] [Google Scholar]
- 11.Dethier VG, Browne LB, Smith CN. The designation of chemicals in terms of the responses they elicit from insects. J Econ Entomol. 1960;53(1):134–136. doi: 10.1603/029.102.0606. [DOI] [PubMed] [Google Scholar]
- 12.Stanczyk NM, Brookfield JF, Ignell R, Logan JG, Field LM. Behavioral insensitivity to DEET in Aedes aegypti is a genetically determined trait residing in changes in sensillum function. Proc Natl Acad Sci USA. 2010;107(19):8575–8580. doi: 10.1073/pnas.1001313107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaissling KE. In: Pheromone Reception in Insects: The Example of Silk Moths. Neurobiology of Chemical Communication, Frontiers in Neuroscience. Mucignat-Caretta C, editor. CRC Press, Boca Raton; FL: 2014. [PubMed] [Google Scholar]
- 14.Leal WS, Choo YM, Xu P, da Silva CS, Ueira-Vieira C. Differential expression of olfactory genes in the southern house mosquito and insights into unique odorant receptor gene isoforms. Proc Natl Acad Sci USA. 2013;110(46):18704–18709. doi: 10.1073/pnas.1316059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes DT, Pelletier J, Luetje CW, Leal WS. Odorant receptor from the southern house mosquito narrowly tuned to the oviposition attractant skatole. J Chem Ecol. 2010;36(8):797–800. doi: 10.1007/s10886-010-9828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu P, et al. Silent, generic and plant kairomone sensitive odorant receptors from the southern house mosquito. J Insect Physiol. 2013;59(9):961–966. doi: 10.1016/j.jinsphys.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelletier J, Hughes DT, Luetje CW, Leal WS. An odorant receptor from the southern house mosquito Culex pipiens quinquefasciatus sensitive to oviposition attractants. PLoS ONE. 2010;5(4):e10090. doi: 10.1371/journal.pone.0010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurence BB, Pickett JA. erythro-6-Acetoxy-5-hexadecanolide, the major component of a mosquito attractant pheromone. J Chem Soc Chem Commun. 1982;1982(1):59–60. [Google Scholar]
- 19.Gfeller A, Dubugnon L, Liechti R, Farmer EE. Jasmonate biochemical pathway. Sci Signal. 2010;3(109):cm3. doi: 10.1126/scisignal.3109cm3. [DOI] [PubMed] [Google Scholar]
- 20.Gupta RK, Bhattacharjee AK. Discovery and design of new arthropod/insect repellents by computer-aided molecular modeling. In: Debboun M, Frances SP, Strickmann D, editors. Insect Repellents: Principles, Methods, and Uses. CRC Press; Boca Raton, FL: 2007. pp. 195–228. [Google Scholar]
- 21.Garboui SS, Jaenson TG, Borg-Karlson AK, Pålsson K. Repellency of methyl jasmonate to Ixodes ricinus nymphs (Acari: Ixodidae) Exp Appl Acarol. 2007;42(3):209–215. doi: 10.1007/s10493-007-9066-1. [DOI] [PubMed] [Google Scholar]
- 22.Syed Z, Leal WS. Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proc Natl Acad Sci USA. 2009;106(44):18803–18808. doi: 10.1073/pnas.0906932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grieco JP, et al. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS ONE. 2007;2(8):e716. doi: 10.1371/journal.pone.0000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu P, et al. Moth sex pheromone receptors and deceitful parapheromones. PLoS ONE. 2012;7(7):e41653. doi: 10.1371/journal.pone.0041653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.