Abstract
Postural orthostatic tachycardia syndrome (POTS) is characterized by chronic fatigue and dizziness and affected individuals by definition have orthostatic intolerance and tachycardia. There is considerable overlap of symptoms in patients with POTS and chronic fatigue syndrome (CFS), prompting speculation that POTS is akin to a deconditioned state. We previously showed that adolescents with postural orthostatic tachycardia syndrome (POTS) have excessive heart rate (HR) during, and slower HR recovery after, exercise – hallmarks of deconditioning. We also noted exaggerated cardiac output during exercise which led us to hypothesize that tachycardia could be a manifestation of a high output state rather than a consequence of deconditioning. We audited records of adolescents presenting with long‐standing history of any mix of fatigue, dizziness, nausea, who underwent both head‐up tilt table test and maximal exercise testing with measurement of cardiac output at rest plus 2–3 levels of exercise, and determined the cardiac output (![]() ) versus oxygen uptake (
) versus oxygen uptake (![]() ) relationship. Subjects with chronic fatigue were diagnosed with POTS if their HR rose ≥40 beat·min−1 with head‐up tilt. Among 107 POTS patients the distribution of slopes for the
) relationship. Subjects with chronic fatigue were diagnosed with POTS if their HR rose ≥40 beat·min−1 with head‐up tilt. Among 107 POTS patients the distribution of slopes for the ![]() , relationship was skewed toward higher slopes but showed two peaks with a split at ~7.0 L·min−1 per L·min−1, designated as normal (5.08 ± 1.17, N = 66) and hyperkinetic (8.99 ± 1.31, N = 41) subgroups. In contrast, cardiac output rose appropriately with
, relationship was skewed toward higher slopes but showed two peaks with a split at ~7.0 L·min−1 per L·min−1, designated as normal (5.08 ± 1.17, N = 66) and hyperkinetic (8.99 ± 1.31, N = 41) subgroups. In contrast, cardiac output rose appropriately with ![]() in 141 patients with chronic fatigue but without POTS, exhibiting a normal distribution and an average slope of 6.10 ± 2.09 L·min−1
in 141 patients with chronic fatigue but without POTS, exhibiting a normal distribution and an average slope of 6.10 ± 2.09 L·min−1
![]() per L·min−1
per L·min−1![]() . Mean arterial blood pressure and pulse pressure from rest to exercise rose similarly in both groups. We conclude that 40% of POTS adolescents demonstrate a hyperkinetic circulation during exercise. We attribute this to failure of normal regional vasoconstriction during exercise, such that patients must increase flow through an inappropriately vasodilated systemic circulation to maintain perfusion pressure.
. Mean arterial blood pressure and pulse pressure from rest to exercise rose similarly in both groups. We conclude that 40% of POTS adolescents demonstrate a hyperkinetic circulation during exercise. We attribute this to failure of normal regional vasoconstriction during exercise, such that patients must increase flow through an inappropriately vasodilated systemic circulation to maintain perfusion pressure.
Keywords: Cardiac output, exercise, hyperkinetic circulation, orthostatic intolerance, sympathetic nervous system
e12122
Forty percent of postural orthostatic tachycardia syndrome (POTS) adolescents who, by definition have abnormal sympathetic control of HR and BP, demonstrate a hyperkinetic circulation during exercise. We attribute this to failure of normal regional vasoconstriction during exercise, such that patients must increase flow through an inappropriately vasodilated systemic circulation to maintain perfusion pressure.
Introduction
Postural tachycardia syndrome (POTS) is defined by symptoms of orthostatic intolerance associated with a physical sign: an excessive increase in heart rate (HR) on orthostatic challenge (Medow and Stewart 2007). It was initially described in adults as a syndrome of orthostatic intolerance characterized by dizziness – sometimes syncope – fatigue, abdominal discomfort, and headache or other somatic pain. POTS is a heterogeneous disorder of unknown etiology with neuropathic, neurohumoral, or circulatory variants (Garland et al. 2007; Medow and Stewart 2007; Benarroch 2012; Raj 2013). Many patients with POTS limit physical activity whether due to pervasive fatigue and malaise or difficulty from orthostatic intolerance, presumably the reason why the majority of patients with POTS are deconditioned. Parsaik et al. (2012) found peak oxygen uptake (![]() ) <85% predicted value in 90% of patients with POTS. Observations such as these prompt speculation that POTS is akin to a deconditioned state (Joyner and Masuki 2008; Lewis et al. 2013). Whereas these reports invariably studied adult patients and it is now recognized that diagnostic criteria for POTS in adolescents differ from adult (Johnson et al. 2010; Singer et al. 2012), deconditioning ought to have similar consequences or manifestations in adults or adolescents.
) <85% predicted value in 90% of patients with POTS. Observations such as these prompt speculation that POTS is akin to a deconditioned state (Joyner and Masuki 2008; Lewis et al. 2013). Whereas these reports invariably studied adult patients and it is now recognized that diagnostic criteria for POTS in adolescents differ from adult (Johnson et al. 2010; Singer et al. 2012), deconditioning ought to have similar consequences or manifestations in adults or adolescents.
We previously reported excessive tachycardia during and after exercise in adolescents with POTS, compatible with deconditioning, but also noted that cardiac output rose more steeply than expected (Burkhardt et al. 2011). The heart functions as demand pump during exercise controlled principally by the tissues' need for oxygen and by the capacitance of the circulatory system. Blood flow through tissues is regulated by local metabolic requirement, and therefore must increase with exercise, leading to greater venous return to the heart. Distribution of blood flow is modulated as vessels dilate and constrict thereby increasing and decreasing resistance to flow depending on local need, orchestrated by the sympathetic nervous system (Charkoudian and Wallin 2014). Furlan et al. found that patients with chronic orthostatic intolerance exhibit discordant cardiac and vascular sympathetic control such that they had greater tachycardia but blunted rise in muscle sympathetic nerve activity accompanied by lesser vasoconstrictor response during head‐up tilt compared with controls (Furlan et al. 1998). It follows that disturbances in sympathetic nervous system control of circulation such as one sees in POTS could influence the HR and stroke volume (SV) responses to exercise.
Stewart and Montgomery (Stewart and Montgomery 2004) proposed a model of high‐, normal‐, and low‐flow POTS based on measurements of limb blood flow. If there were indeed high limb blood flow there must be high cardiac output, otherwise patients would present with swollen lower extremities. Some patients with POTS report vasoactive changes of cutaneous blood flow such as acrocyanosis or mottling rather than edema (Medow and Stewart 2007; Raj 2013). Our prior observations on cardiovascular responses to exercise in adolescents with POTS (Burkhardt et al. 2011) led us to postulate that some POTS patients may have a high‐flow state wherein ![]() rises more steeply than expected with respect to
rises more steeply than expected with respect to ![]() , termed a hyperkinetic circulation (Haller et al. 1983). We hypothesized that there were at least two groups of adolescents with POTS – normal and high output or hyperkinetic – with unique cardiovascular responses to exercise such that a subgroup of adolescents whose head‐up tilt table test was positive would also show an amplified cardiac output response to exercise. We compare adolescents with POTS (nearly all of whom complained of fatigue) to chronically fatigued adolescents without POTS whose head‐up tilt table test was negative. Moreover, deconditioned patients – whether or not they had POTS – would show classical features of rapid HR and low SV during exercise. In contrast, patients with POTS who demonstrated a hyperkinetic circulation during exercise might achieve this high‐output state with relative tachycardia‐like normal‐flow POTS (consistent with abnormal head‐up tilt test) but with a normal SV. Therefore, a corollary hypothesis is that not all POTS patients with high exercise HR are de facto deconditioned.
, termed a hyperkinetic circulation (Haller et al. 1983). We hypothesized that there were at least two groups of adolescents with POTS – normal and high output or hyperkinetic – with unique cardiovascular responses to exercise such that a subgroup of adolescents whose head‐up tilt table test was positive would also show an amplified cardiac output response to exercise. We compare adolescents with POTS (nearly all of whom complained of fatigue) to chronically fatigued adolescents without POTS whose head‐up tilt table test was negative. Moreover, deconditioned patients – whether or not they had POTS – would show classical features of rapid HR and low SV during exercise. In contrast, patients with POTS who demonstrated a hyperkinetic circulation during exercise might achieve this high‐output state with relative tachycardia‐like normal‐flow POTS (consistent with abnormal head‐up tilt test) but with a normal SV. Therefore, a corollary hypothesis is that not all POTS patients with high exercise HR are de facto deconditioned.
Methods
Patient populations
We reviewed medical records of adolescents (12–19 years of age) seen in the Mayo Pediatric Diagnostic Referral Clinic from January 2010 to April 2012 with any mix of: chronic fatigue, dizziness, abdominal discomfort (nausea or pain), or other pain (headache, myalgia or arthralgia); who underwent both autonomic reflex and maximal exercise tests. We did not employ any particular set of inclusion criteria either when patients were triaged in clinic or in data abstraction, other than symptoms of at least 6 months duration. Thus, patients may or may not have fit the case definition (Fukuda et al. 1994) of chronic fatigue syndrome (CFS) but we did exclude those with alternative medical diagnoses explaining their symptoms. Patients were diagnosed with POTS if their ΔHR was ≥40 beats·min−1 during a 10‐min head‐up tilt table test performed according to standard‐of‐care methods at Mayo Clinic (Thieben et al. 2007). Patients whose ΔHR fell below that threshold and whose chief complaint or reason for seeking medical evaluation was chronic fatigue were assigned to the chronic fatigue group to serve as a comparison group specifically to address issues and differences in exercise cardiac output measurement and results. We anticipated this group would maintain a normal ![]() response since deconditioning has not been shown to alter this function, and because autonomic regulation of the cardiovascular system ought to be intact. The breakdown of patients triaged, inclusions, and exclusions, are shown in Figure 1. Testing was conducted for clinical indications and informed consent was, therefore, not required. Mayo Clinic Institutional Review Board approved the study.
response since deconditioning has not been shown to alter this function, and because autonomic regulation of the cardiovascular system ought to be intact. The breakdown of patients triaged, inclusions, and exclusions, are shown in Figure 1. Testing was conducted for clinical indications and informed consent was, therefore, not required. Mayo Clinic Institutional Review Board approved the study.
Figure 1.
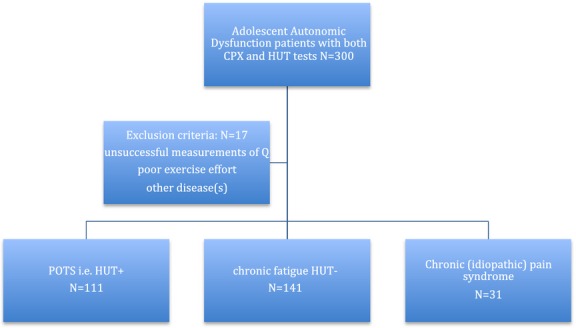
Flow diagram showing triage of patients with POTS, chronic fatigue, and exclusions.
Measurements
Subjects performed a maximal cardiopulmonary exercise test on a cycle ergometer according to the Godfrey protocol of 1‐min incremental workloads (Godfrey 1974). Ventilation and gas exchange was measured breath‐by‐breath at rest and throughout exercise using MedGraphics CPX/D (Breeze software; MGC Diagnostics, St. Paul, MN) which employs a Pitot tube to measure flow, electronically integrated to give minute volume (![]() ), yielding values for
), yielding values for ![]() , carbon dioxide output (
, carbon dioxide output (![]() ) computed as means over the final 15–20 sec of each workload. Cardiac output was measured using a closed circuit, acetylene–helium rebreathe technique (Triebwasser et al. 1977). Exhaled gases were measured by mass spectrometry (Perkin‐Elmer, Pomona, CA).
) computed as means over the final 15–20 sec of each workload. Cardiac output was measured using a closed circuit, acetylene–helium rebreathe technique (Triebwasser et al. 1977). Exhaled gases were measured by mass spectrometry (Perkin‐Elmer, Pomona, CA). ![]() and
and ![]() were measured at rest with the subject seated on the cycle ergometer;
were measured at rest with the subject seated on the cycle ergometer; ![]() was also measured during the first workload then at alternate workloads at the discretion of the supervising physician in order to obtain at least 2–3 measures of
was also measured during the first workload then at alternate workloads at the discretion of the supervising physician in order to obtain at least 2–3 measures of ![]() during exercise without causing the patient undue distress from rebreathing. Experience from previous testing had taught us that some patients from this population became visibly upset or simply came off the mouthpiece if rebreathing was done during heavy exercise. Heart rate was monitored with a 12‐lead continuous ECG (Quinton Cardiology Systems, Inc., Bothell, WA). Blood pressure was measured by auscultation prior to each
during exercise without causing the patient undue distress from rebreathing. Experience from previous testing had taught us that some patients from this population became visibly upset or simply came off the mouthpiece if rebreathing was done during heavy exercise. Heart rate was monitored with a 12‐lead continuous ECG (Quinton Cardiology Systems, Inc., Bothell, WA). Blood pressure was measured by auscultation prior to each ![]() determination plus every other subsequent work increment. Oxygen saturation was continuously monitored by pulse oximetry (OxiMax N‐600X Nellcor, Hayward, CA). Patients were strongly encouraged to exercise to voluntary exhaustion but given their chronic fatigue state, a minority of patients failed to achieve the usual, accepted criteria (e.g., respiratory quotient >1.1 or HR >180 beats·min−1) for a maximal test. Their data were excluded from peak
determination plus every other subsequent work increment. Oxygen saturation was continuously monitored by pulse oximetry (OxiMax N‐600X Nellcor, Hayward, CA). Patients were strongly encouraged to exercise to voluntary exhaustion but given their chronic fatigue state, a minority of patients failed to achieve the usual, accepted criteria (e.g., respiratory quotient >1.1 or HR >180 beats·min−1) for a maximal test. Their data were excluded from peak ![]() analysis but included in cardiac output analysis. Peak
analysis but included in cardiac output analysis. Peak ![]() was determined as the highest achieved
was determined as the highest achieved ![]() sustained over 20 sec and peak oxygen pulse was calculated as
sustained over 20 sec and peak oxygen pulse was calculated as ![]() /HR at peak exercise. Peak
/HR at peak exercise. Peak ![]() results were compared with normal, age‐appropriate, values (NHANES) from a US adolescent population (Eisenmann et al. 2011). Deconditioning was defined as peak
results were compared with normal, age‐appropriate, values (NHANES) from a US adolescent population (Eisenmann et al. 2011). Deconditioning was defined as peak ![]() less than sex‐specific 10‰. Stroke volume was computed as
less than sex‐specific 10‰. Stroke volume was computed as ![]() divided by HR and normalized for body surface area to give stroke volume index (SVI) in mL·m−2). The preponderance of females precluded separate analyses by sex. Some patients with POTS hyperventilate (Stewart et al. 2006) which may contribute to dizziness. Therefore, ventilatory response was examined and characterized by intercept and slope: end‐tidal PCO2 at the ventilatory anaerobic threshold (Beaver et al. 1986); and change in ventilation versus change in CO2 output during subthreshold exercise (
divided by HR and normalized for body surface area to give stroke volume index (SVI) in mL·m−2). The preponderance of females precluded separate analyses by sex. Some patients with POTS hyperventilate (Stewart et al. 2006) which may contribute to dizziness. Therefore, ventilatory response was examined and characterized by intercept and slope: end‐tidal PCO2 at the ventilatory anaerobic threshold (Beaver et al. 1986); and change in ventilation versus change in CO2 output during subthreshold exercise (![]() ), respectively.
), respectively.
Statistical methods
Values are reported as mean ± SD, or median (IQR) as appropriate. Linear regression was used to compute ![]() and
and ![]() versus
versus ![]() . Correlations were assessed using the Pearson correlation coefficient (r) or Spearman correlation coefficient (rS) for nonnormal data (e.g., ∆HR during head‐up tilt. Comparisons between group means were tested using two‐sample t‐test, whereas proportions were compared with χ2; P‐values of <0.05 were considered significant.
. Correlations were assessed using the Pearson correlation coefficient (r) or Spearman correlation coefficient (rS) for nonnormal data (e.g., ∆HR during head‐up tilt. Comparisons between group means were tested using two‐sample t‐test, whereas proportions were compared with χ2; P‐values of <0.05 were considered significant.
Results
POTS patients
Patient anthropometric data are shown in Table 1. None were anemic. Presenting complaints included dizziness (84%), fatigue (71%), headache (74%), nausea (46%), abdominal pain (45%), and syncope (26%). Median (IQR) duration of symptoms was 22 (DeLorenzo et al. 1998; Stewart and Montgomery 2004) months. All 111 patients with POTS, by definition, exhibited ≥40 beats·min−1 rise in HR during head‐up tilt. Median (IQR) ∆HR was 46 (Trevisani et al. 1999; Rowland and Whatley Blum 2000) beats·min−1; ∆BPsys with head‐up tilt was −12 (−6, −18) mmHg, but 18% experienced a drop ≥20 mmHg. Two‐thirds of patients were symptomatic during head‐up tilt, causing premature test termination in 10%, but only one had syncope.
Table 1.
Selected anthropometric, and resting circulatory data, split according to diagnosis, sex, and slope of the cardiac output – oxygen uptake relationship during exercise (POTS only)
| POTS | Chronic fatigue | |||||
|---|---|---|---|---|---|---|
| Normal flow | Hyperkinetic | |||||
| M | F | M | F | M | F | |
| N | 16 | 50 | 7 | 34 | 38 | 103 |
| Age (years) | 15 ± 3 | 16 ± 2 | 16 ± 1 | 15 ± 2 | 14.5 ± 1.7 | 15.8 ± 1.5 |
| Height (cm) | 174 ± 14 | 167 ± 8 | 183 ± 12 | 165 ± 7 | 171 ± 11 | 163 ± 7 |
| Weight (kg) | 63.2 ± 13.5 | 60.1 ± 12.5 | 76.5 ± 16.8 | 58.4 ± 11.0 | 70.2 ± 18.7 | 61.3 ± 13.0 |
| BSA (m2) | 1.76 ± 0.25 | 1.67 ± 0.18 | 1.98 ± 0.25 | 1.64 ± 0.17 | 23.7 ± 4.7 | 1.65 ± 0.17 |
| BMI (kg·m−2) | 20.7 ± 2.7 | 21.5 ± 3.7 | 22.8 ± 4.3 | 21.2 ± 3.4 | 1.81 ± 0.27 | 23.1 ± 4.1 |
| HR (beat·min−1) | 96 ± 17 | 90 ± 17 | 91 ± 11 | 99 ± 18 | 90 ± 14 | 94 ± 18 |
| CI (L·min−1·m−2) | 3.75 ± 0.89 | 3.65 ± 1.15 | 3.93 ± 0.85 | 3.34 ± 0.84 | 3.67 ± 0.76 | 3.77 ± 1.14 |
| Stroke volume index (mL·m−2) | 41 ± 12 | 37 ± 11 | 41 ± 9 | 36 ± 10 | 40 ± 11 | 39 ± 11 |
| Hgb (g·dL−1) | 14.6 ± 1.1 | 13.2 ± 0.6 | 14.9 ± 0.8 | 13.0 ± 0.7 | 14.5 ± 1.1 | 13.0 ± 0.9 |
Maximal exercise data are shown in Table 2. There was no correlation between peak ![]() (mL·kg−1·min−1) and ∆HR during head‐up tilt. Peak
(mL·kg−1·min−1) and ∆HR during head‐up tilt. Peak ![]() was <10‰ in 56% of females and in 65% of males (χ2 = 0.6791, P = 0.41). The hyperkinetic POTS group had lower peak
was <10‰ in 56% of females and in 65% of males (χ2 = 0.6791, P = 0.41). The hyperkinetic POTS group had lower peak ![]() (Table 2) and there was weak (P = 0.05) correlation between peak
(Table 2) and there was weak (P = 0.05) correlation between peak ![]() (mL·kg−1·min−1) and
(mL·kg−1·min−1) and ![]() slope. The principal reason for termination of the exercise test was (N): generalized fatigue (Rowland and Whatley Blum 2000), sore legs (Higginbotham et al. 1986), dizziness (Fukuda et al. 1994), dyspnea (di Prampero 2003), none specified (Charkoudian and Wallin 2014). End‐tidal PCO2 at the ventilatory anaerobic threshold was the lowest in the patients who ceased exercise complaining of dizziness, averaging 37.9, 38.0, 34.4, 37.4, 37.5 mmHg, respectively (P =0.01). The slope
slope. The principal reason for termination of the exercise test was (N): generalized fatigue (Rowland and Whatley Blum 2000), sore legs (Higginbotham et al. 1986), dizziness (Fukuda et al. 1994), dyspnea (di Prampero 2003), none specified (Charkoudian and Wallin 2014). End‐tidal PCO2 at the ventilatory anaerobic threshold was the lowest in the patients who ceased exercise complaining of dizziness, averaging 37.9, 38.0, 34.4, 37.4, 37.5 mmHg, respectively (P =0.01). The slope ![]() during subthreshold exercise averaged 30.7 ± 4.5 and correlated inversely end‐tidal PCO2 at the ventilatory anaerobic threshold (r = −0.63, P <0.0001). Furthermore,
during subthreshold exercise averaged 30.7 ± 4.5 and correlated inversely end‐tidal PCO2 at the ventilatory anaerobic threshold (r = −0.63, P <0.0001). Furthermore, ![]() correlated with (P < 0.0001), and explained 17% of the variance of, peak
correlated with (P < 0.0001), and explained 17% of the variance of, peak ![]() .
.
Table 2.
Selected peak exercise data in patients, split according to diagnosis, sex, and slope of the cardiac output–oxygen uptake relationship during exercise (in POTS patients only)
| POTS | Chronic fatigue | |||||
|---|---|---|---|---|---|---|
| Normal |
Hyperkinetic | |||||
| M | F | M | F | M | F | |
|
| ||||||
| mL·kg−1·min−1 | 33.8 ± 8.2 | 31.3 ± 6.2* | 34.0 ± 7.6 | 27.9 ± 6.5* | 36.1 ± 8.2 | 27.4 ± 6.0 |
| L·min−1 | 2.11 ± 0.60 | 1.86 ± 0.44 | 2.51 ± 0.38 | 1.63 ± 0.41 | 2.46 ± 0.57 | 1.64 ± 0.32 |
| Work | ||||||
| Watts | 150 ± 50 | 138 ± 30 | 184 ± 315 | 125 ± 31 | 170 ± 46 | 127 ± 26 |
| % predicted | 60 ± 23 | 77 ± 17 | 59 ± 14 | 73 ± 23 | 71 ± 19% | 76 ± 25% |
| HR | ||||||
| Beats·min−1 | 186 ± 17 | 187 ± 11 | 190 ± 11 | 189 ± 8 | 189 ± 10 | 187 ± 13 |
| O2 pulse | ||||||
| mL·beat−1 | 11.3 ± 3.0 | 9.3 ± 2.3 | 11.7 ± 3.8 | 8.7 ± 2.2 | 13.1 ± 3.2 | 8.8 ± 1.6 |
| VAT | ||||||
| % peak |
58 ± 9 | 58 ± 8 | 53 ± 6 | 58 ± 7 | 55 ± 12% | 61 ± 8% |
P < 0.02 girls with hyperkinetic circulation versus normal cardiac output.
Data to determine slope of the ![]() versus
versus ![]() relationship during light to moderate exercise were obtained in 107 patients: rest plus one exercise
relationship during light to moderate exercise were obtained in 107 patients: rest plus one exercise ![]() in 17% of subjects (
in 17% of subjects (![]() in one patient measured successfully only at 25 and 75 watts); rest plus two measurements of exercise
in one patient measured successfully only at 25 and 75 watts); rest plus two measurements of exercise ![]() in 65%; and rest plus three or four measurements of exercise
in 65%; and rest plus three or four measurements of exercise ![]() in the remaining 18%. Mean r value of linear regression analyses was 0.90. Figure 2 shows the frequency distribution for
in the remaining 18%. Mean r value of linear regression analyses was 0.90. Figure 2 shows the frequency distribution for ![]() versus
versus ![]() slopes for all subjects. The distribution obtained from the POTS patients (lower panel) was skewed with a prominent tail reaching supranormal values. Skewness was verified statistically because the 95% confidence interval for a difference between mean (6.69) and median (6.07) values should fall within ± 0.4 for a sample of N = 100 (Doane and Seward 2011). There appeared to be two clusters in the distribution from our POTS population, with a split at ~7.0. On average, Q rose by 5.06 ± 1.17 L·min−1 versus 9.61 ± 1.31 L·min−1 for each L·min−1 rise in
slopes for all subjects. The distribution obtained from the POTS patients (lower panel) was skewed with a prominent tail reaching supranormal values. Skewness was verified statistically because the 95% confidence interval for a difference between mean (6.69) and median (6.07) values should fall within ± 0.4 for a sample of N = 100 (Doane and Seward 2011). There appeared to be two clusters in the distribution from our POTS population, with a split at ~7.0. On average, Q rose by 5.06 ± 1.17 L·min−1 versus 9.61 ± 1.31 L·min−1 for each L·min−1 rise in ![]() for each cluster‐mode, which we label normal (N = 66) and hyperkinetic (N = 41) POTS subgroups. We were unable to find a correlation between individual slope for
for each cluster‐mode, which we label normal (N = 66) and hyperkinetic (N = 41) POTS subgroups. We were unable to find a correlation between individual slope for ![]() and ∆HR during head‐up tilt table testing.
and ∆HR during head‐up tilt table testing.
Figure 2.
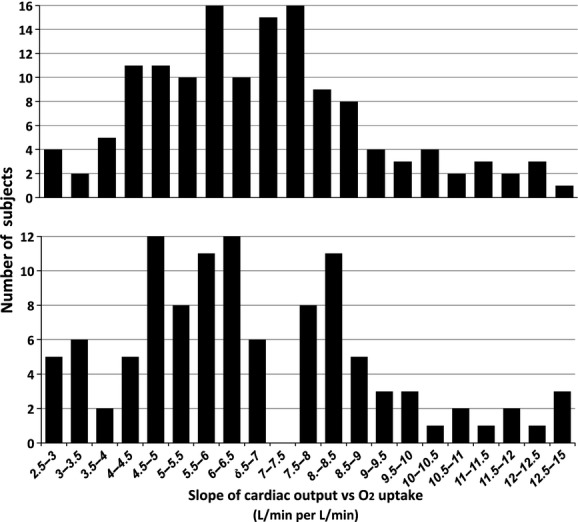
Frequency distribution of slopes of cardiac output versus oxygen uptake from rest to exercise in patients with POTS (lower panel) or chronic fatigue (upper panel).
Mean resting SVI for all POTS patients was 36 ± 11 mL·m−2 and rose significantly in light exercise to 46 ± 11 mL·m−2 before leveling off in moderate exercise at 47.5 ± 11.5 mL·m−2. Both normal output and hyperkinetic groups showed the expected rise in SVI with light exercise and moderate exercise but mean SVI was lower (P =0.02) during heavy (~⅔ peak ![]() ) exercise in the normal flow (47 ± 11 mL·m−2) versus the hyperkinetic (56 ± 7 mL·m−2) POTS subgroup (Fig. 3). Exercise HR trajectories were similar (Fig. 3). Mean blood and pulse pressures also followed similar trajectories during exercise in normal output and hyperkinetic POTS patients (Fig. 4).
) exercise in the normal flow (47 ± 11 mL·m−2) versus the hyperkinetic (56 ± 7 mL·m−2) POTS subgroup (Fig. 3). Exercise HR trajectories were similar (Fig. 3). Mean blood and pulse pressures also followed similar trajectories during exercise in normal output and hyperkinetic POTS patients (Fig. 4).
Figure 3.
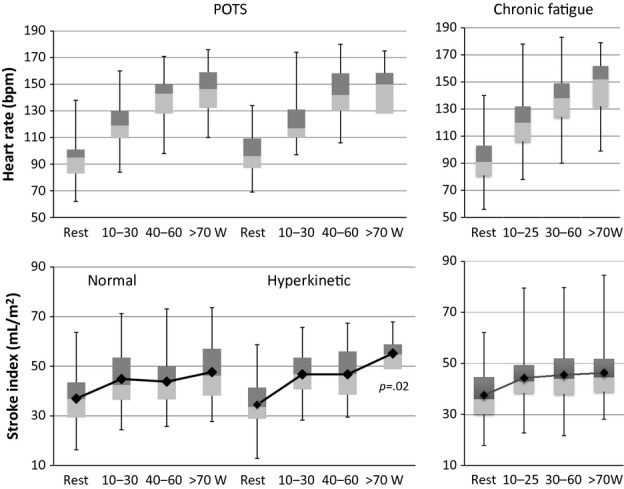
Box and whisker plots of HR (above) and stroke volume index (below) at rest, and during progressive exercise shown separately for normal and hyperkinetic cardiac output groups of POTS patients; and for patients with chronic fatigue.
Figure 4.
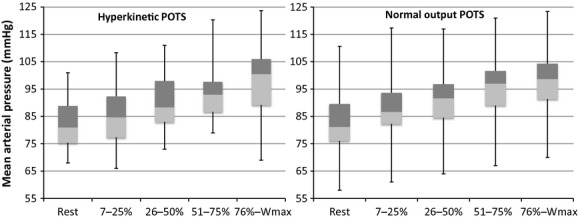
Box and whisker plots of mean arterial blood pressure at rest and during progressive exercise (abscissa is percent maximum work capacity) shown separately for normal cardiac output and hyperkinetic groups of POTS patients.
Chronic fatigue patients
Patient anthropometric data are shown in Table 1. There were 141 patients in the chronic fatigue group, all of whom exhibited <40 min−1 rise in HR during HUT: median (IQR) ∆HR was 28 (Haller et al. 1983; Lewis et al. 2013) beats·min−1. Reported symptoms included fatigue (100%), dizziness or “brain fog” (85%), headache (70%), nausea or abdominal pain (48%), and other pain for example, chest, muscles, joints (50%); and median (IQR) symptom duration was 29.5 (Stewart and Montgomery 2004; Fu et al. 2010) months. Maximal exercise data are shown in Table 2. Only 39% of males had peak ![]() >10‰ (~36.8 mL·kg−1·min−1) compared to 32% of females who exceeded 30.8 mL·kg−1·min−1 threshold (χ2 = 0.31, P = NS).
>10‰ (~36.8 mL·kg−1·min−1) compared to 32% of females who exceeded 30.8 mL·kg−1·min−1 threshold (χ2 = 0.31, P = NS).
Slope of the ![]() relationship from rest to moderate exercise was determined in 137 patients: two measurements of
relationship from rest to moderate exercise was determined in 137 patients: two measurements of ![]() in 23%; three in 55% of subjects; four measurements of
in 23%; three in 55% of subjects; four measurements of ![]() in the remaining 22%. The frequency distribution of
in the remaining 22%. The frequency distribution of ![]() versus
versus ![]() (Fig. 2, upper panel) was normal with a mean slope of 6.10 ± 2.09 L·min−1
(Fig. 2, upper panel) was normal with a mean slope of 6.10 ± 2.09 L·min−1
![]() per L·min−1 rise of
per L·min−1 rise of ![]() , significantly less than the mean slope for POTS patients (P =0.039). Mean r of individual linear regression analyses was 0.92. Exercise HR trajectory is shown in (Fig. 3) and was very similar to that in POTS. SVI in chronic fatigue patients rose with increasing work (Fig. 3) but reached a plateau no different than normal output POTS patients, exceeding 50 mL·m−2 in only 46 (33%) subjects.
, significantly less than the mean slope for POTS patients (P =0.039). Mean r of individual linear regression analyses was 0.92. Exercise HR trajectory is shown in (Fig. 3) and was very similar to that in POTS. SVI in chronic fatigue patients rose with increasing work (Fig. 3) but reached a plateau no different than normal output POTS patients, exceeding 50 mL·m−2 in only 46 (33%) subjects.
Discussion
This study on the cardiac output response to exercise in adolescents with POTS supports the hypothesis that there are at least two distinct populations based on blood flow characteristics: one that maintains the normal coupling between ![]() and
and ![]() plus another in whom
plus another in whom ![]() rises more than expected with respect to
rises more than expected with respect to ![]() . This hyperkinetic state was achieved by relative tachycardia but with either low or normal SV response to exercise. Deconditioning is typically inferred from an incremental exercise test to voluntary exhaustion when a subject fails to achieve a reference peak
. This hyperkinetic state was achieved by relative tachycardia but with either low or normal SV response to exercise. Deconditioning is typically inferred from an incremental exercise test to voluntary exhaustion when a subject fails to achieve a reference peak ![]() , has a low ventilatory anaerobic threshold, plus an exaggerated HR response such that O2 pulse is low (Ross 2003). The underlying presumption is that
, has a low ventilatory anaerobic threshold, plus an exaggerated HR response such that O2 pulse is low (Ross 2003). The underlying presumption is that ![]() rises normally with
rises normally with ![]() , and therefore, if HR is high then SV must be low. Our results show this reasoning does not apply to at least one‐third of adolescents with POTS. We also found that patients who hyperventilated were more likely to cite dizziness as a limiting exercise, which contributed to lower peak
, and therefore, if HR is high then SV must be low. Our results show this reasoning does not apply to at least one‐third of adolescents with POTS. We also found that patients who hyperventilated were more likely to cite dizziness as a limiting exercise, which contributed to lower peak ![]() .
.
In their review of circulatory control during exercise, Rowell and O'Leary (1990) posed the fundamental question of whether the primary error corrected by the (normally functioning) sympathetic nervous system during exercise is a mismatch between blood flow and metabolism – a flow error – or a mismatch between vascular conductance and cardiac output – a pressure error. Inasmuch as blood pressure was very similar between normal flow and hyperkinetic POTS, our results imply that blood pressure is indeed the regulated variable during exercise, even at the expense of cardiac output. We demonstrated a linear ![]() relationship but the slope of that function was abnormally high in 40% of our patients.
relationship but the slope of that function was abnormally high in 40% of our patients. ![]() is related to both the degree of muscle activation and to
is related to both the degree of muscle activation and to ![]() during leg exercise (Dufour et al. 2007), rising at an average rate of ~5–6 L·min−1
during leg exercise (Dufour et al. 2007), rising at an average rate of ~5–6 L·min−1
![]() per L·min−1 rise in
per L·min−1 rise in ![]() at least during submaximal exercise in adults (Astrand and Rodahl 1970) and children (Godfrey 1974). Slopes for the
at least during submaximal exercise in adults (Astrand and Rodahl 1970) and children (Godfrey 1974). Slopes for the ![]() relationship in healthy adults vary in a normal distribution from 5.5 to 10.3 (Yamaguchi et al. 1986). Intersubject variability in the slope of the
relationship in healthy adults vary in a normal distribution from 5.5 to 10.3 (Yamaguchi et al. 1986). Intersubject variability in the slope of the ![]() relationship is due to unique variations in resting or exercise hemodynamics and to arteriovenous O2 content differences, particularly during exercise. The circulatory response to exercise in our “control” adolescents with chronic fatigue was similar to that reported in normal individuals, and different from POTS adolescents by virtue of its normal distribution with a normal mean value. In contrast, patients with POTS comprised a skewed distribution with statistically significant differences between mean and median, and with a higher group mean value than a large sample of chronically fatigued adolescents with negative head‐up tilt table test. Hyperkinetic circulation has been described in a few other disorders (Lonsdorfer et al. 1983; Trevisani et al. 1999) but none related to POTS. High
relationship is due to unique variations in resting or exercise hemodynamics and to arteriovenous O2 content differences, particularly during exercise. The circulatory response to exercise in our “control” adolescents with chronic fatigue was similar to that reported in normal individuals, and different from POTS adolescents by virtue of its normal distribution with a normal mean value. In contrast, patients with POTS comprised a skewed distribution with statistically significant differences between mean and median, and with a higher group mean value than a large sample of chronically fatigued adolescents with negative head‐up tilt table test. Hyperkinetic circulation has been described in a few other disorders (Lonsdorfer et al. 1983; Trevisani et al. 1999) but none related to POTS. High ![]() for
for ![]() implies reduced O2 extraction at the level of exercising muscle, such as one might see if a deficiency in metabolic capacity existed (Haller et al. 1983). Indeed, Holmgren et al. (1957) showed precisely this (see Fig. 5) in adults with “vasoregulatory asthenia,” likely patients who would now be diagnosed as POTS. Exercise may not be limited by O2 delivery but rather by its utilization in working muscle in such patients. This impairment of peripheral O2 transfer and uptake explains decrements in supine peak
implies reduced O2 extraction at the level of exercising muscle, such as one might see if a deficiency in metabolic capacity existed (Haller et al. 1983). Indeed, Holmgren et al. (1957) showed precisely this (see Fig. 5) in adults with “vasoregulatory asthenia,” likely patients who would now be diagnosed as POTS. Exercise may not be limited by O2 delivery but rather by its utilization in working muscle in such patients. This impairment of peripheral O2 transfer and uptake explains decrements in supine peak ![]() and that portion of upright peak
and that portion of upright peak ![]() not explained by classical deconditioning (Bringard et al. 2010).
not explained by classical deconditioning (Bringard et al. 2010).
Bicycle exercise performed in this study utilizes large muscle groups, and more than 70% of ![]() may go to contracting muscles during maximal exertion (Calbet et al. 2007). In order to achieve this, blood must be diverted from nonessential vascular beds to preserve flow to essential tissues and organs, failure of which would deprive the working muscles of vital blood supply when needed most. The diaphragm requires relatively rich blood supply at high levels of ventilation. Hyperventilation may thus deprive working leg muscles of blood flow and limit performance (Harms et al. 1997). Moreover, changes in local perfusion produce the same effects proportionately on muscle contractility such that muscle fatigue would occur more rapidly without an adequate (local) exercise pressor response (Luu and Fitzpatrick 2013). Hyperkinetic circulation could in essence become a “steal” phenomenon that, coupled with hyperventilation, explained almost 20% of the variance in low peak
may go to contracting muscles during maximal exertion (Calbet et al. 2007). In order to achieve this, blood must be diverted from nonessential vascular beds to preserve flow to essential tissues and organs, failure of which would deprive the working muscles of vital blood supply when needed most. The diaphragm requires relatively rich blood supply at high levels of ventilation. Hyperventilation may thus deprive working leg muscles of blood flow and limit performance (Harms et al. 1997). Moreover, changes in local perfusion produce the same effects proportionately on muscle contractility such that muscle fatigue would occur more rapidly without an adequate (local) exercise pressor response (Luu and Fitzpatrick 2013). Hyperkinetic circulation could in essence become a “steal” phenomenon that, coupled with hyperventilation, explained almost 20% of the variance in low peak ![]() in a multivariate regression model. Stroke volume is the principal determinant of maximum cardiac output, in turn a major determinant of peak
in a multivariate regression model. Stroke volume is the principal determinant of maximum cardiac output, in turn a major determinant of peak ![]() (Bassett and Howley 2000). If one subscribes to the paradigm that maximum
(Bassett and Howley 2000). If one subscribes to the paradigm that maximum ![]() is ultimately limited by O2 transport (di Prampero 2003), it follows that one reason for low peak
is ultimately limited by O2 transport (di Prampero 2003), it follows that one reason for low peak ![]() in some POTS patients is not that they are unable to muster sufficient
in some POTS patients is not that they are unable to muster sufficient ![]() but rather they are unable to direct flow to where it is most needed. Affected patients comprise a heterogeneous population who share certain abnormalities of blood flow regulation (Garland et al. 2007; Medow and Stewart 2007; Benarroch 2012; Raj 2013). Stewart (Stewart and Montgomery 2004) described a model of POTS with “low‐flow,” “normal‐flow,” and “high‐flow” state. Hyperkinetic circulation could be a manifestation of their high‐flow group, and it is conceivable that the low‐flow patients were buried in subjects whose
but rather they are unable to direct flow to where it is most needed. Affected patients comprise a heterogeneous population who share certain abnormalities of blood flow regulation (Garland et al. 2007; Medow and Stewart 2007; Benarroch 2012; Raj 2013). Stewart (Stewart and Montgomery 2004) described a model of POTS with “low‐flow,” “normal‐flow,” and “high‐flow” state. Hyperkinetic circulation could be a manifestation of their high‐flow group, and it is conceivable that the low‐flow patients were buried in subjects whose ![]() rose slightly less than expected for
rose slightly less than expected for ![]() (left tail of Fig. 2 lower panel). Our novel observation elaborates current models of POTS and injects a cautionary note on invocation of deconditioning in this population.
(left tail of Fig. 2 lower panel). Our novel observation elaborates current models of POTS and injects a cautionary note on invocation of deconditioning in this population.
Relative tachycardia and low SV during exercise are considered hallmarks of cardiovascular deconditioning, yet many of our POTS patients achieved normal exercise SV. There are few normal reference values for exercise SVI in adolescents (Rowland and Whatley Blum 2000; Pianosi 2004) but peak values approximate 50 mL·m−2, close to values found in adults (Higginbotham et al. 1986). Conventional wisdom is that SV rises at onset of exercise, reaches a plateau in moderate exercise, but may fall during heavy exercise in adults (Gonzalez‐Alonso 2008; Warburton and Gledhill 2008). There is a dearth of such data in pediatric subjects though SV may continue to increase right up to peak exercise in healthy children and adolescents (Pianosi 2004). Rowland et al. found no differences in inotropic or lusitropic responses to progressive exercise despite higher SVI in adolescent athletes versus nonathletes, and concluded that greater aerobic fitness in trained subjects reflected volume expansion of the circulation rather than enhanced ventricular function (Rowland et al. 2009). Volume depletion is a cardinal feature of the deconditioned state (Coyle et al. 1986) and POTS (Benarroch 2012; Raj 2013) and may account for much of the observed reduction in heart size in patients with CFS (Miwa and Fujita 2008; Hurwitz et al. 2010). We reported slower HR recovery post‐exercise – ostensibly a marker of deconditioning – in patients with POTS (Burkhardt et al. 2011), but HR recovery immediately after exercise is a function of a decline of sympathetic drive coupled with reactivation of parasympathetic drive (Imai et al. 1994). Though we did not measure intravascular volume in our patients, one could propose, an alternative, cogent, and coherent, interpretation of these observations. Development of the POTS phenotype begins with abnormal sympathetic regulation of volume status via renin‐angiotensin system (Garland et al. 2007) leading to volume depletion. At this point, little or no effect on inotropy results and SV is preserved but altered sympathetic modulation of the metaboreflex results in hyperkinetic circulation with normal SV in those with positive head‐up tilt table test. Low peak ![]() can supervene at any time, depending on premorbid peak
can supervene at any time, depending on premorbid peak ![]() but will be exacerbated should a given individual develop a hyperkinetic circulation. Once the deconditioning spiral has begun, fatigue and dizziness perpetuate it culminating in the small heart associated with POTS (Fu et al. 2010; Parsaik et al. 2012).
but will be exacerbated should a given individual develop a hyperkinetic circulation. Once the deconditioning spiral has begun, fatigue and dizziness perpetuate it culminating in the small heart associated with POTS (Fu et al. 2010; Parsaik et al. 2012).
There is much overlap between morbidity due to POTS and CFS (Freeman and Komaroff 1997; Schondorf et al. 1999), and some consider POTS to be a subset of CFS (Lewis et al. 2013). Cardinal symptoms of CFS are increased malaise or extreme exhaustion following physical exertion or mental activity, hypersomnolence, difficulties with memory and concentration, persistent muscle pain, joint pain, and headache, for which no cause can be found (http://www.cdc.gov/cfs/symptoms/). The overall prevalence of CFS was estimated at 235/100,000 persons (Reyes 2003), higher among women (373/100,000) than among men (83/100,000). The prevalence of CFS among adolescents is lower (Jones et al. 2004) though it does occur in the pediatric population (http://www.cdc.gov/cfs/symptoms/ accessed June 21, 2014). POTS typically develops between 15 and 25 years of age with a striking female predilection (Johnson et al. 2010; Benarroch 2012). There are >500,000 patients in the United States with POTS (Garland et al. 2007), with one estimate of the prevalence of POTS is 170/100,000 based on the investigators' finding that ~40% of patients with CFS have POTS. The degree of deconditioning was similar in patients with POTS as in patients with chronic fatigue (Table 2), yet this amplified circulatory response to exercise was seen almost exclusively in patients who had a ΔHR >40 beats·min−1 with head‐up tilt. This test threshold differentiated those with POTS and distinguished them from chronically fatigued adolescents with simple deconditioning whose regulation of circulation remained intact. Left ventricular mass is lower and pulse higher in CFS patients than in healthy controls, changes consistent with physical deconditioning and/or altered sympathetic‐parasympathetic balance (DeLorenzo et al. 1998; Miwa and Fujita 2008). Yet, the circulatory response to exercise should be no different in patients with POTS versus those with chronic fatigue if POTS were merely a consequence or signature of deconditioning as some have argued (Fu et al. 2010; Parsaik et al. 2012). In contradistinction, we submit that whereas most patients with POTS suffer from chronic fatigue and have a similar circulatory response to exercise – that of cardiovascular deconditioning – many do not fit this bill and are not merely one end of a chronic fatigue spectrum.
Limitations of our study include the fact that ![]() measurements were restricted to light or moderate exercise. There is some evidence that the
measurements were restricted to light or moderate exercise. There is some evidence that the ![]() relationship is curvilinear, leveling off in heavy exercise in more fit individuals, implying additional O2 consumption is achieved by greater O2 extraction, as suggested by Beck et al. (2006). Their subjects in the nonlinear group had higher SV and lower HR at rest, consistent with data they obtained in more fit individuals. While we may have overestimated the change in
relationship is curvilinear, leveling off in heavy exercise in more fit individuals, implying additional O2 consumption is achieved by greater O2 extraction, as suggested by Beck et al. (2006). Their subjects in the nonlinear group had higher SV and lower HR at rest, consistent with data they obtained in more fit individuals. While we may have overestimated the change in ![]() for
for ![]() by limiting observations to subthreshold exercise, even Beck et al. concur that in all their subjects, the mean slope for
by limiting observations to subthreshold exercise, even Beck et al. concur that in all their subjects, the mean slope for ![]() was 5.2 during light to moderate exercise. Different fitness levels do not explain the divergent slopes for the
was 5.2 during light to moderate exercise. Different fitness levels do not explain the divergent slopes for the ![]() relationship for the following reasons: (1) patients in each cluster exhibited different trajectories for
relationship for the following reasons: (1) patients in each cluster exhibited different trajectories for ![]() during exercise at low‐moderate intensities; and (2) differences in HR and SV were not apparent at rest in our groups of patients, unlike Beck et al. The normal relationship between
during exercise at low‐moderate intensities; and (2) differences in HR and SV were not apparent at rest in our groups of patients, unlike Beck et al. The normal relationship between ![]() and
and ![]() during exercise is well established (Astrand and Rodahl 1970), though it may become curvilinear and asymptotic near maximal exercise (Beck et al. 2006). Rebreathing methods become intolerable for subjects breathing at high levels of exercise, which limit observations to subthreshold exercise. Designation of deconditioning relied on a large national reference (NHANES) sample but was justified as it truly reflected the make‐up of our study population. We did not have a true, “healthy” control population but instead used patients with chronic fatigue. We submit this is an appropriate control population in that they exhibited nearly identical symptoms and differed only inasmuch as their head‐up tilt table test was normal/negative.
during exercise is well established (Astrand and Rodahl 1970), though it may become curvilinear and asymptotic near maximal exercise (Beck et al. 2006). Rebreathing methods become intolerable for subjects breathing at high levels of exercise, which limit observations to subthreshold exercise. Designation of deconditioning relied on a large national reference (NHANES) sample but was justified as it truly reflected the make‐up of our study population. We did not have a true, “healthy” control population but instead used patients with chronic fatigue. We submit this is an appropriate control population in that they exhibited nearly identical symptoms and differed only inasmuch as their head‐up tilt table test was normal/negative.
In conclusion, most adolescents with POTS or chronic fatigue are deconditioned, and show the expected cardiovascular manifestations of low SV coupled with high HR. Measurement of cardiac output during exercise provided additional data regarding hemodynamic changes by differentiating CFS from some POTS patients and allowed better characterization of their pathophysiology and fitness level. This should lead to phenotyping this heterogeneous population and may assist in tailoring therapy, for example, β‐blockade versus α‐agonist, and offering appropriate suggestions for exercise prescription. Physical inactivity leading to deconditioning appears to be a final common pathway for many disorders. It follows that exercise training ought to be an effective remedy for, and become incorporated into the management of, POTS and CFS. That said, an intriguing possibility is that simply exercising in the supine posture might restore a normal ![]() relationship in patients with hyperkinetic POTS.
relationship in patients with hyperkinetic POTS.
Acknowledgments
Authors are grateful to A. L. Weaver MSc, for providing statistical consultation services.
Conflict of Interest
None declared.
Footnotes
Funding Information
This study was supported by the American Dysautonomia Institute and The Hohmann Foundation.
References
- Astrand P. O., Rodahl K. 1970. 178-180inTextbook of work physiology 3rd edNew York, NY: McGraw‐Hill [Google Scholar]
- Bassett D. R., Howley E. T. 2000. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc.; 32:70-84. [DOI] [PubMed] [Google Scholar]
- Beaver W. L., Wasserman K., Whipp B. J. 1986. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol.; 60:2020-2027. [DOI] [PubMed] [Google Scholar]
- Beck K. C., Randolph L. N., Bailey K. R., Wood C. M., Snyder E. M., Johnson B. D. 2006. Relationship between cardiac output and oxygen consumption during upright cycle exercise in healthy humans. J. Appl. Physiol.; 101:1474-1480. [DOI] [PubMed] [Google Scholar]
- Benarroch E. E. 2012. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin. Proc.; 87:1214-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringard A., Pogliaghi S., Adami A., De Roia G., Lador F., Lucini D. 2010. Cardiovascular determinants of maximal oxygen consumption in upright and supine posture at the end of prolonged bed rest in humans. Respir. Physiol. Neurobiol.; 172:53-62. [DOI] [PubMed] [Google Scholar]
- Burkhardt B. E., Fischer P. R., Brands C. K., Porter C. J., Weaver A. L., Yim P. J. 2011. Exercise performance in adolescents with autonomic dysfunction. J. Pediatr.; 158:15-19. [DOI] [PubMed] [Google Scholar]
- Calbet J. A., Gonzalez‐Alonso J., Helge J. W., Søndergaard H., Munch‐Andersen T., Boushel R. 2007. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J. Appl. Physiol.; 103:969-978. [DOI] [PubMed] [Google Scholar]
- Charkoudian N., Wallin B. G. 2014. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr. Physiol.; 4:827-850. [DOI] [PubMed] [Google Scholar]
- Coyle E. F., Hemmert M. K., Coggan A. R. 1986. Effects of detraining on cardiovascular responses to exercise: role of blood volume. J. Appl. Physiol.; 60:95-99. [DOI] [PubMed] [Google Scholar]
- DeLorenzo F., Xiao H., Mukherjee M., Harcup J., Suleiman S., Kadziola Z. 1998. Chronic fatigue syndrome: physical and cardiovascular deconditioning. Q. J. Med.; 91:475-481. [DOI] [PubMed] [Google Scholar]
- Doane D. P., Seward L. E. 2011. Measuring skewness: a forgotten statistic? J. Stat. Educ.; 19:1-17. [Google Scholar]
- Dufour S. P., Doutreleau S., Lonsdorfer‐Wolf E., Lampert E., Hirth C., Piquard F. 2007. Deciphering the metabolic and mechanical contributions to the exercise‐induced circulatory response: insights from eccentric cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol.; 292:R1641-R1648. [DOI] [PubMed] [Google Scholar]
- Eisenmann J. C., Laurson K. R., Welk G. J. 2011. Aerobic fitness percentiles for US adolescents. Am. J. Prev. Med.; 41:S106-S110. [DOI] [PubMed] [Google Scholar]
- Freeman R., Komaroff A. L. 1997. Does the chronic fatigue syndrome involve the autonomic nervous system? Am. J. Med.; 102:357-364. [DOI] [PubMed] [Google Scholar]
- Fu Q., VanGundy T. B., Galbreath M. M., Shibata S., Jain M., Hastings J. L. 2010. Cardiac origins of the postural orthostatic tachycardia syndrome. J. Am. Coll. Cardiol.; 55:2858-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Straus S. E., Hickie I., Sharpe M. C., Dobbins J. G., Komaroff A. 1994. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann. Intern. Med.; 121:953-959. [DOI] [PubMed] [Google Scholar]
- Furlan R., Jacob G., Snell M., Robertson D., Porta A., Harris P. 1998. Chronic orthostatic intolerance. A disorder with discordant cardiac and vascular sympathetic control. Circulation; 98:2154-2159. [DOI] [PubMed] [Google Scholar]
- Garland E. M., Raj S. R., Black B. K., Harris P. A., Robertson D. 2007. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology; 69:790-798. [DOI] [PubMed] [Google Scholar]
- Godfrey S. 1974. 97-100inExercise testing in children London: WB Saunders [Google Scholar]
- Gonzalez‐Alonso J. 2008. Point: stroke volume does/does not decline during exercise at maximal effort in healthy individuals. J. Appl. Physiol.; 104:275-276. [DOI] [PubMed] [Google Scholar]
- Haller R. G., Lewis S. F., Cook J. D., Blomqvist C. G. 1983. Hyperkinetic circulation during exercise in neuromuscular disease. Neurology; 33:1283. [DOI] [PubMed] [Google Scholar]
- Harms C. A., Babcock M. A., McClaran S. R., Pegelow D. F., Nickele G. A., Nelson W. B. 1997. Respiratory muscle work compromises leg blood flow during maximal exercise. J. Appl. Physiol.; 82:1573-1583. [DOI] [PubMed] [Google Scholar]
- Higginbotham M. B., Morris K. G., Williams R. S., McHale P. A., Coleman R. E., Cobb F. R. 1986. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ. Res.; 58:281-291. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Jonsson B., Levander M., Linderholm H., Sjöstrand T., Ström G. 1957. Low physical working capacity in suspected heart cases due to inadequate adjustment of peripheral blood flow (Vasoregulatory Asthenia) 1. Acta Med. Scand.; 158:413-436. [DOI] [PubMed] [Google Scholar]
- Hurwitz B., Coryell V., Parker M., Martin P., LaPerriere A., Klimas N. 2010. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin. Sci.; 118:125-135. [DOI] [PubMed] [Google Scholar]
- Imai K., Sato H., Hori M., Kusuoka H., Ozaki H., Yokoyama H. 1994. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J. Am. Coll. Cardiol.; 24:1529-1535. [DOI] [PubMed] [Google Scholar]
- Johnson J. N., Mack K. J., Kuntz N. L., Brands C. K., Porter C. J., Fischer P. R. 2010. Postural orthostatic tachycardia syndrome: a clinical review. Pediatr. Neurol.; 42:77-85. [DOI] [PubMed] [Google Scholar]
- Jones J. F., Nisenbaum R., Solomon L., Reyes M., Reeves W. C. 2004. Chronic fatigue syndrome and other fatiguing illnesses in adolescents: a population‐based study. J. Adolesc. Health; 35:34-40. [DOI] [PubMed] [Google Scholar]
- Joyner M. J., Masuki S. 2008. POTS versus deconditioning: the same or different. Clin. Auton. Res.; 18:300-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis I., Pairman J., Spickett G., Newton J. L. 2013. Clinical characteristics of a novel subgroup of chronic fatigue syndrome patients with postural orthostatic tachycardia syndrome. J. Intern. Med.; 273:501-510. [DOI] [PubMed] [Google Scholar]
- Lonsdorfer J., Bogui P., Otayeck A., Bursaux E., Poyart C., Cabannes R. 1983. Cardiorespiratory adjustments in chronic sickle cell anemia. Bull. Eur. Physiopathol. Respir.; 19:339-344. [PubMed] [Google Scholar]
- Luu B. L., Fitzpatrick R. C. 2013. Blood pressure and the contractility of human leg muscle. J. Physiol.; 591:5401-5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medow M. S., Stewart J. M. 2007. The postural tachycardia syndrome. Cardiol. Rev.; 15:67-75. [DOI] [PubMed] [Google Scholar]
- Miwa K., Fujita M. 2008. Small heart syndrome in patients with chronic fatigue syndrome. Clin. Cardiol.; 31:328-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsaik A., Allison T. G., Singer W., Sletten D. M., Joyner M. J., Benarroch E. E. 2012. Deconditioning in patients with orthostatic intolerance. Neurology; 79:1435-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianosi P. 2004. Measurement of exercise cardiac output by thoracic impedance in healthy children. Eur. J. Appl. Physiol.; 92:425-430. [DOI] [PubMed] [Google Scholar]
- di Prampero P. E. 2003. Factors limiting maximal performance in humans. Eur. J. Appl. Physiol.; 90:420-429. [DOI] [PubMed] [Google Scholar]
- Raj S. R. 2013. Postural tachycardia syndrome (POTS). Circulation; 127:2336-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M., Nisenbaum R., Hoaglin D. C., Unger E. R., Emmons C., Randall B. 2003. Prevalence and incidence of chronic fatigue syndrome in Wichita, KS. Arch. Intern. Med.; 163:1530-1536. [DOI] [PubMed] [Google Scholar]
- Ross R. M. 2003. ATS/ACCP statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med.; 167:145110.1164/ajrccm.167.10.950 [DOI] [PubMed] [Google Scholar]
- Rowell L. B., O'Leary D. S. 1990. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J. Appl. Physiol.; 69:407-418. [DOI] [PubMed] [Google Scholar]
- Rowland T., Whatley Blum J. 2000. Cardiac dynamics during upright cycle exercise in boys. Am. J. Hum. Biol.; 12:749-757. [DOI] [PubMed] [Google Scholar]
- Rowland T. W., Garrard M., Marwood S., Guerra M. E., Roche D., Unnithan V. B. 2009. Myocardial performance during progressive exercise in athletic adolescent males. Med. Sci. Sports Exerc.; 41:1721-1728. [DOI] [PubMed] [Google Scholar]
- Schondorf R., Benoit J., Wein T., Phaneuf D. 1999. Orthostatic intolerance in the chronic fatigue syndrome. J. Auton. Nerv. Syst.; 75:192-201. [DOI] [PubMed] [Google Scholar]
- Singer W., Sletten D. M., Opfer‐Gehrking T. L., Brands C. K., Fischer P. R., Low P. A. 2012. Postural tachycardia in children and adolescents: what is abnormal? J. Pediatr.; 160:222-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. M., Montgomery L. D. 2004. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol.; 287:H1319-H1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. M., Medow M. S., Cherniack N. S., Natelson B. H. 2006. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am. J. Physiol. Heart Circ. Physiol.; 291:H904-H913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieben M. J., Sandroni P., Sletten D. M., Benrud‐Larson L. M., Fealey R. D., Vernino S. 2007. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin. Proc.; 82:308-313. [DOI] [PubMed] [Google Scholar]
- Trevisani F., Sica G., Mainqua P., Santese G., De Notariis S., Caraceni P. 1999. Autonomic dysfunction and hyperkinetic circulation in cirrhosis with ascites. Hepatology; 30:1387-1392. [DOI] [PubMed] [Google Scholar]
- Triebwasser J. H., Johnson R. L., Burpo R. P., Campbell J. C., Reardon W. C., Blomquist G. C. 1977. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat. Space Environ. Med.; 48:203-209. [PubMed] [Google Scholar]
- Warburton D. E., Gledhill N. 2008. Counterpoint: stroke volume does not decline during exercise at maximal effort in healthy individuals. J. Appl. Physiol.; 104:276-278. [DOI] [PubMed] [Google Scholar]
- Yamaguchi I., Komatsu E., Miyazawa K. 1986. Intersubject variability in cardiac output‐O2 uptake relation of men during exercise. J. Appl. Physiol.; 61:2168-2174. [DOI] [PubMed] [Google Scholar]


