Abstract
• Aims and Scope All aerobic organisms require molecular di-oxygen (O2) for efficient production of ATP though oxidative phosphorylation. Cellular depletion of oxygen results in rapid molecular and physiological acclimation. The purpose of this review is to consider the processes of low oxygen sensing and response in diverse organisms, with special consideration of plant cells.
• Conclusions The sensing of oxygen deprivation in bacteria, fungi, metazoa and plants involves multiple sensors and signal transduction pathways. Cellular responses result in a reprogramming of gene expression and metabolic processes that enhance transient survival and can enable long-term tolerance to sub-optimal oxygen levels. The mechanism of sensing can involve molecules that directly bind or react with oxygen (direct sensing), or recognition of altered cellular homeostasis (indirect sensing). The growing knowledge of the activation of genes in response to oxygen deprivation has provided additional information on the response and acclimation processes. Conservation of calcium fluxes and reactive oxygen species as second messengers in signal transduction pathways in metazoa and plants may reflect the elemental importance of rapid sensing of cellular restriction in oxygen by aerobic organisms.
Keywords: Oxygen sensing, gene expression, hypoxia, anoxia, alcohol dehydrogenase, reactive oxygen species, cytosolic calcium, second messenger, G-protein, ethylene
INTRODUCTION
Molecular di-oxygen (O2) is an absolute requirement for efficient production of ATP though oxidative phosphorylation in aerobic organisms. In eukaryotes, oxygen is the final electron acceptor in the mitochondrial electron transport chain. A depletion of oxygen has rapid and profound consequences on cell physiology. This condition alters gene expression, energy consumption, cellular metabolism, growth and development. Plant cells are frequently challenged with limited levels of oxygen due to changes in the external environment or high rates of cellular metabolism (reviewed by Drew, 1997; Geigenberger, 2003; Gibbs and Greenway, 2003; Greenway and Gibbs, 2003). Natural conditions such as spring floods, excess rainfall, winter ice encasement, submergence, soil compaction and microorganism activity can lead to oxygen deficiency. During seed imbibtion and germination, microspore production or fruit development, the availability of oxygen for energy production can become limited. This condition can also be due to restricted diffusion of oxygen into internal tissues or high rates of cellular metabolism, as in actively dividing cells of meristems.
Cells of aerobic organisms have evolved adaptive responses to compensate for the energy crisis caused by oxygen deprivation. The responses at the cellular to whole plant level are varied and include alterations in metabolism and development that in some cases confer long-term tolerance. The mechanisms that underlie the sensing and response to oxygen deprivation have not been fully unravelled in any aerobic organism, let alone in higher plants. Progress has been made toward the understanding of the role of activation or de-repression of transcription factors and alterations in oxygen-regulated gene expression in several model prokaryotes and eukaryotes. The processes that determine these responses include multiple direct or indirect sensors and signal transduction pathways that are independent or interacting (Fig. 1). Direct sensing mechanisms might involve proteins or ligands that bind or react with oxygen. Perturbations in cellular homeostasis, such as altered energy levels, redox status or calcium levels, may underlie indirect sensing mechanisms. Paradoxically, eukaryotic responses to oxygen deprivation appear to involve the formation of reactive oxygen species (ROS) including the superoxide anion (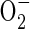 ), hydrogen peroxide (H2O2) and nitric oxide (NO). This review summarizes mechanisms of oxygen sensing in model bacterial and eukaryotic systems and discusses our nascent understanding of low oxygen sensing and response mechanisms in plants.
), hydrogen peroxide (H2O2) and nitric oxide (NO). This review summarizes mechanisms of oxygen sensing in model bacterial and eukaryotic systems and discusses our nascent understanding of low oxygen sensing and response mechanisms in plants.
Fig. 1.
Sensing of oxygen deprivation through direct and indirect mechanisms. Oxygen deprivation can result in rapid changes in cell physiology, transient changes in gene transcription or long-term alterations in physiology and development. These adaptive responses are promoted by rapid alteration in the accumulation, location or activity of transcription factors or activation of signal transduction pathways. The sensing of oxygen deprivation involves molecules which bind or consume oxygen or that are altered by oxidation state. Indirect sensing occurs as a result of a change in cellular homeostasis, possibly driven by flux in cytosolic calcium levels, adenylate charge, ratio of reduced to oxidized glutathione and carbohydrate availability. Diversity in responses may result from cross-talk between one or more sensing and signalling pathways.
OXYGEN SENSING MECHANISMS IN BACTERIA
The most efficient pathway for the production of ATP in Escherichia coli is the aerobic respiratory pathway that utilizes cytochrome bo oxidase as the terminal electron acceptor. The activity of the cytochrome bd oxidase, which has a higher affinity for oxygen, increases when oxygen levels are limiting. Under anaerobic conditions, less energy-efficient alternative oxidoreductases are utilized for ATP production. Escherichia coli adjusts gene regulation as a consequence of oxygen deprivation through multiple direct or indirect sensing mechanisms. These adjustments modulate the transcription of genes that encode the various oxidoreductases and other machinery of cellular metabolism. One of these oxygen sensing regulatory systems is the fumarate and nitrate reduction (FNR) transcriptional regulator. FNR is regulated directly by molecular oxygen concentration. Under fully aerobic conditions, FNR is inactive, whereas under low oxygen conditions FNR forms homodimers that repress the transcription of genes required for aerobic metabolism and promote expression of genes involved in anaerobic electron transport and metabolism (Kiley and Beinert, 2003). The underlying mechanism of FNR regulation is the presence of a structurally dynamic cluster of iron molecules, the [4Fe–4S] cluster. The active homodimeric factor has a [4Fe–4S]2+ cluster in each subunit. In the presence of oxygen, the redox state of this cluster is rapidly converted to [2Fe–2S]2+, promoting a change in conformation that disrupts FNR dimerization and DNA binding activity.
A second system in E. coli that controls gene expression under conditions of reduced oxygen availability is the ArcAB system. This two-component system includes the membrane-bound histidine kinase ArcB and its phosphorylation target ArcA. Oxygen deficiency promotes the auto-phosphorylation of ArcB that activates phosphorylation of ArcA and results in regulation of numerous operons that provide control of carbon catabolism and cellular redox status (Unden et al., 1997; Alexeeva et al., 2003). ArcB auto-phosphorylation is not regulated by direct oxygen sensing but is a response to a reduction in electron flow through the respiratory chain (Alexeeva et al., 2000). Thus, the ArcAB system mediates fine-tuned responses to the reduction in cellular ATP levels that occur as oxygen availability diminishes (Alexeeva et al., 2003).
Root nodule-forming Rhizobia and Bradyrhizobium species adjust metabolism in response to oxygen levels by use of another two-component system (Fischer, 1994). The direct sensing of oxygen tension occurs through an oxygen- and haem-binding histidine kinase, FixL. When oxygen tension is severely reduced during root nodule formation, oxygen is released from the haem-binding site of membrane-bound FixL and triggers its auto-phosphorylation. The kinase then transfers a phosphate to the transcription factor FixJ, causing a change in conformation that results in activation of transcription of genes required for symbiotic nitrogenase reactions (Fischer, 1994; Gong et al., 2000). The activity of FixJ regulation increases as oxygen levels fall, providing tight environmental regulation of gene expression during symbiont invasion (Sciotti et al., 2003).
OXYGEN SENSORS AND SIGNALLING PATHWAYS IN MODEL METAZOANS AND FUNGI
Depletion of cellular oxygen levels promotes changes in gene regulation in yeast and metazoans through varying direct or indirect sensing mechanisms. There is considerable knowledge of the transcription factors that mediate alterations in gene expression. In contrast, the signal transduction pathways activated in response to hypoxia in model eukaryotes are complex and wrought with opposing models, especially those that propose modulation of ROS as a second messenger (Lopez-Borneo et al., 2001; Bruick, 2003; Giaccia et al., 2004; Waypa and Schumaker, 2005). Interest in oxygen sensing in mammals is intense due to its importance in erythropoiesis, tissue vascularization, trachea development, closure of the ductus arterious in newborns, progression of tumour development, and control of blood oxygen levels by the carotid and aortic bodies of the lung, heart and vascular smooth muscle cells. The emerging view is that a cellular oxygen crisis promotes multiple sensing and physiological response pathways.
The HIF1α story: transcriptional regulation by direct oxygen sensing
A key regulator of transcription in response to hypoxia in animals is the hypoxia-inducible heterodimeric transcription factor (HIF) (reviewed by Bruick, 2003; Acker and Acker, 2004; Giaccia et al., 2004; Semenza, 2004). This complex is highly conserved in metazoa including mammals, Drosophila melanogaster and Caenorhabditis elegans. HIF is composed of the constitutively synthesized HIF1α and HIF1β subunits, and its activation promotes transcription of genes required for anaerobic metabolism, iron homeostasis, vascularization and erythropoiesis in mammals. HIF activity also indirectly inhibits the expression of genes through the activation of a transcriptional repressor, Dec1 (Yun et al., 2002). HIF is controlled by multiple parameters, including mRNA accumulation and alternative splicing, protein turnover, nuclear localization and transactivation. Primary control is through turnover of the HIF1α subunit when oxygen is available. Oxygen-dependent HIF prolyl hydroxylases modify specific proline residues within HIF1α in a reaction that is dependent upon 2-oxogluturate and ascorbate. This proline hydroxylation targets HIF1α for rapid ubiquitination and proteosomal degradation (Ivan et al., 2001; Jaakkola, 2001). The three human HIF1α prolyl hydroxylases have a high Km for O2 (230–250 μm) (Hirsila et al., 2003). Therefore, their activity is proportional to oxygen availability. This oxygen concentration-dependent activity qualifies the prolyl hydroxylases as a bone fide oxygen sensor (Bruick, 2003). An additional level of HIF regulation is mediated by a second 2-oxyglutarate-dependent hydroxylase, an asparaginyl hydroxylase. This enzyme modifies an asparagine residue within the N-terminal trans-activation domain of HIF1α (Bruick, 2003). This change reduces the interaction of HIF with transcriptional co-activators, providing another level of control through direct oxygen sensing. Additional enhancement of HIF activity, via indirect sensing of oxygen deprivation, involves elevation of HIF1α mRNA via production of ROS that promotes a G-protein signalling cascade (Turcotte et al., 2003, 2004).
Transcriptional regulation by haem-binding proteins in yeast
Saccharomyces cerevisiae can grow anaerobically if provided with a fermentable carbon source. Yeast cells respond to oxygen deprivation via multiple low oxygen sensing and transduction pathways (Kwast et al., 1998; Poyton, 1999). A predominant pathway involves haem, an oxygen-binding molecule, in the adjustment of transcription by haem-containing factors. Unlike the direct oxygen sensing mediated by haem-containing transcription factors in bacteria, transcriptional regulation by the yeast factors is governed by rates of de novo synthesis of haem, in a redox-insensitive manner. The activity of the haem biosynthesis pathway is proportional to oxygen concentration at levels above 0·1 μm O2, due to properties of several haem biosynthesis enzymes within the mitochondrion (Hon et al., 2003). Consequentially, oxygen depletion dramatically influences the production of haem, which is necessary for the transcriptional activator Hap1 to drive expression of genes required for aerobic metabolism (Poyton, 1999; Zhang and Hach, 1999). The reduction in free haem levels under hypoxia also compromises the stability of Hap1 (Hon et al., 2003). In turn, reduced Hap1 activity limits the production of two transcriptional repressors, Rox1 and Mot3, and thereby de-represses transcription of a number of genes required for anaerobic metabolism. Another haem-binding transcription factor, the tetrameric Hap2/3/4/5, is also regulated by haem levels and controls the expression of aerobic genes in a manner similar to Hap1.
The enigmatic role of ROS in oxygen sensing and response mechanisms
There is considerable evidence that ROS production, by either a plasma membrane (PM) NAD(P)H oxidase and/or mitochondria, regulates responses to oxygen deprivation. There are at least four models that propose a role for ROS produced by one or both of these sources (Sham, 2002). One model proposes that 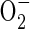 produced by the PM NAD(P)H oxidase functions to constitutively repress low oxygen signalling pathways (Lopez-Barneo et al., 2001, 2004). In this scenario, H2O2 generated by enzymatic or spontaneous dismutation of
produced by the PM NAD(P)H oxidase functions to constitutively repress low oxygen signalling pathways (Lopez-Barneo et al., 2001, 2004). In this scenario, H2O2 generated by enzymatic or spontaneous dismutation of 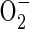 maintains a cellular oxidization state that inhibits signal transduction in cell types such as arterial and airway chemoreceptors. Hypoxia reduces this ROS production leading to a cascade of events: alteration of cytosolic redox state, closure of oxygen-sensitive voltage-dependent potassium channels, activation of voltage-gated calcium channels and an elevation of cytosolic calcium (Archer et al., 2000; Lopez-Barneo et al., 2001, 2003). Although there is considerable support for this PM NAD(P)H oxidase model, it is unlikely to reflect oxygen sensing in all cell types. Examination of PM NAD(P)H oxidase-deficient mice revealed that pulmonary vascular and neuroepithelial cells differ in the requirement for this enzyme in the response to hypoxia (Archer et al., 1999; Fu et al., 2000). Moreover, diphenyl iodonium (DPI), a non-specific inhibitor of flavin-binding proteins including the PM NAD(P)H oxidase, nitric oxide synthase (NOS) and mitochondrial NADH dehydrogenase (complex I), is an effective inhibitor of hypoxia responses in mammalian cells, leading to the suggestion that ROS evolution may actually be promoted under conditions of oxygen deficiency (Chandel et al., 2000).
maintains a cellular oxidization state that inhibits signal transduction in cell types such as arterial and airway chemoreceptors. Hypoxia reduces this ROS production leading to a cascade of events: alteration of cytosolic redox state, closure of oxygen-sensitive voltage-dependent potassium channels, activation of voltage-gated calcium channels and an elevation of cytosolic calcium (Archer et al., 2000; Lopez-Barneo et al., 2001, 2003). Although there is considerable support for this PM NAD(P)H oxidase model, it is unlikely to reflect oxygen sensing in all cell types. Examination of PM NAD(P)H oxidase-deficient mice revealed that pulmonary vascular and neuroepithelial cells differ in the requirement for this enzyme in the response to hypoxia (Archer et al., 1999; Fu et al., 2000). Moreover, diphenyl iodonium (DPI), a non-specific inhibitor of flavin-binding proteins including the PM NAD(P)H oxidase, nitric oxide synthase (NOS) and mitochondrial NADH dehydrogenase (complex I), is an effective inhibitor of hypoxia responses in mammalian cells, leading to the suggestion that ROS evolution may actually be promoted under conditions of oxygen deficiency (Chandel et al., 2000).
Another model proposes that the mitochondrion, the major consumer of cellular oxygen, is a sensor of oxygen availability (Chandel et al., 1998, 2000; Chandel and Schumaker, 2000; Schumaker, 2003). In this scenario, hypoxic conditions promote production of ROS within mitochondria, leading to a release of calcium and other molecules, such as cytochrome c. In support of the mitochondrial sensor hypothesis, mammalian and yeast cell lines deficient in mitochondrial electron transport chain components or treated with compounds that block electron transport upstream of centre N of complex III are impaired in hypoxia responses (Chandel et al., 1998, 2000; Chandel and Schumaker, 2000; Dirmeier et al., 2002; Schroedl et al., 2002; Waypa et al., 2002; Waypa and Schumaker, 2005). In contrast, antimycin A, which blocks electron transport at centre N of complex III, mimics hypoxia responses including the elevation of HIF1α mRNA. These studies attribute mitochondrial ROS production to the elevation of ubisemiquinone ion, which donates an electron to oxygen to produce 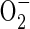 . The effective production of
. The effective production of 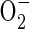 is purportedly stimulated by a decrease in the Vmax of cytochrome c oxidase (Chandel and Schumaker, 2000). Arguments against the mitochondrial sensor model cite the failure of cytochrome c oxidase inhibitors to mimic the low oxygen response in mammals and the inconsistency in the effect of mitochondrial inhibitors on different cell types. However, cytochrome c oxidase-deficient strains of yeast display altered expression of a sub-set of the hypoxia-induced genes (Kwast et al., 1999). A current challenge is to determine if the proposed oxygen concentration sensing by mitochondria is mediated directly by oxygen (i.e. through regulation of haem) or a change in cellular homeostasis (i.e. ATP levels or adenylate charge ratio ([ATP] + 0·5[ADP])/([ATP] + [ADP] + [AMP]), [NAD(P)+]/[NAD(P)H], [GSSG]/[GSH]). If the latter is correct, the mitochondrial response may be similar to the indirect sensing paradigm of ArcAB in E. coli.
is purportedly stimulated by a decrease in the Vmax of cytochrome c oxidase (Chandel and Schumaker, 2000). Arguments against the mitochondrial sensor model cite the failure of cytochrome c oxidase inhibitors to mimic the low oxygen response in mammals and the inconsistency in the effect of mitochondrial inhibitors on different cell types. However, cytochrome c oxidase-deficient strains of yeast display altered expression of a sub-set of the hypoxia-induced genes (Kwast et al., 1999). A current challenge is to determine if the proposed oxygen concentration sensing by mitochondria is mediated directly by oxygen (i.e. through regulation of haem) or a change in cellular homeostasis (i.e. ATP levels or adenylate charge ratio ([ATP] + 0·5[ADP])/([ATP] + [ADP] + [AMP]), [NAD(P)+]/[NAD(P)H], [GSSG]/[GSH]). If the latter is correct, the mitochondrial response may be similar to the indirect sensing paradigm of ArcAB in E. coli.
NO, a gaseous second messenger, is implicated in oxygen sensing and the responses leading to vasodilatation in mammals (Shiva et al., 2005). In hypoxic cells, NO binds competitively to the haem group of cytochrome c oxidase and inhibits its activity (Hagen et al., 2003). This interaction was proposed to enhance the maintenance of HIF1α prolyl hydroxylase activity, thereby delaying the increase in HIF activity. It was also suggested that NO controls the production of 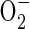 by mitochondria and allows establishment of oxygen gradients within organs (Shiva et al., 2005). Confirmation of a role for NO in the response to hypoxia in Drosophila embryos indicates that this ROS may be involved in evolutionarily conserved response mechanisms.
by mitochondria and allows establishment of oxygen gradients within organs (Shiva et al., 2005). Confirmation of a role for NO in the response to hypoxia in Drosophila embryos indicates that this ROS may be involved in evolutionarily conserved response mechanisms.
There may be cross-talk between ROS produced at the PM and within mitochondria in low oxygen response mechanisms. Both the PM NADPH oxidase and mitochondrial sensor models involve the modulation of production of ROS and flux in cytosolic calcium. It can be predicted that these processes involve positive and negative feedback systems that are controlled by the spatial and temporal location of these second messengers. It seems likely that the presence of multiple interacting sensory circuits would enhance the diversity and fine-tuning of the response to oxygen deprivation (Fig. 1).
LOW OXYGEN SENSING AND SIGNAL TRANSDUCTION MECHANISMS IN PLANT CELLS
Evidence for indirect low oxygen sensing mechanisms in plants
To date, there is no clear understanding of the sensor(s) of oxygen deprivation in plant cells (Drew, 1997; Geigenberger, 2003; Gibbs and Greenway, 2003). Analyses of the molecular responses of maize (Zea mays), rice (Oryza sativa) and arabidopsis (Arabidopsis thaliana) to oxygen deprivation have focused on the regulation of expression of genes and activation of enzymes involved in acclimation of metabolism and alteration of development. These include enzymes involved in the breakdown of starch, catabolism of soluble sugars and fermentation, aerenchyma formation, cell and organ elongation and adventitious root formation. As in other multicellular eukaryotes, the plant response depends upon the severity of the stress, cell type and developmental stage. Cells may endure different degrees of oxygen deficiency within a plant tissue or organ. As the external oxygen concentration declines, the rate of oxygen uptake into internal cells must keep up with the rate of oxygen consumption. A zone of anoxia may occur in internal tissues, as observed in the stele of maize roots or the centre of fruits or tubers, even when surrounding cells have sufficient oxygen to maintain aerobic respiration (Geigenberger, 2003; Gibbs and Greenway, 2003). It is well established that adaptive responses, such as increased alcohol dehydrogenase (ADH) gene expression and fermentative metabolism, are initiated under conditions of hypoxia, as well as anoxia (Paul and Ferl, 1991; Drew, 1997; Koch et al., 2000). Plant cells have evolved adaptive mechanisms that allow for avoidance of anoxia by reducing aerobic respiration and energetic processes as ATP levels decline (Geigenberger, 2003; van Dongen et al., 2004). The sensing and signalling that lead to modification of gene expression may be triggered by a change in cellular homeostasis and not necessarily by a direct sensing of oxygen concentration (Figs 1, 2). As revealed by the ArcAB system of E. coli and indicated by the mitochondrial sensing model of metazoa, a universal indirect response to hypoxia may be curtailment of the consumption of limited ATP and other limited resources such as carbohydrates and lipids. It remains to be determined if the plant response is mediated exclusively by indirect sensing mechanisms or also involves direct oxygen sensing.
Fig. 2.
Sensing and signalling in response to oxygen deprivation in plant cells. Depression of cellular oxygen concentration leads to changes in the cellular milieu that promote altered gene expression, metabolism and development. To date, there is no known mechanism of direct oxygen sensing in plant cells. Indirect sensing may be regulated by the changes in cytosolic pH, local and temporal fluxes in calcium concentration, reduction in adenylate charge or production of ROS, including NO and H2O2. The accumulation of the active form of the ROP GTPase, ROP-GTP, stimulates the induction of ADH expression and ethanolic fermentation, at least in Arabidopsis. The increase in cytosolic calcium, released from the mitochondria and influx from the apoplast, contributes to this gene regulation. Paradoxically, the activation of ROP signalling is associated with increased levels of H2O2; genotypes that are unable to regulate ROP signalling negatively are sensitive to oxygen deprivation despite strong induction of ADH gene expression. ROP GTPase signalling is attenuated by a ROPGAP, which promotes hydrolysis of GTP that is bound to ROP. This negative regulation limits ROS production and most probably conserves carbohydrates. H2O2 is a signalling molecule in plant cells as well as a damaging agent; antioxidants and antioxidant enzymes ameliorate its accumulation. NO is also a signalling molecule; its levels may be regulated by increases in haemoglobin in response to oxygen deprivation. The growth regulators ethylene, gibberellin, auxin and ABA control developmental adaptations including aerenchyma formation, cell, stem and petiole elongation, petiole hyponasty and adventitious root formation. There is evidence of cross-talk between the response pathways.
An oxygen sensor is capable of directly detecting oxygen availability and subsequently triggering a signalling cascade. It is still unclear if such sensors exist in plants. The plant oxygen-binding protein, haemoglobin, has been ruled out as a potential oxygen sensor or carrier because of its extremely low dissociation constant for oxygen (Dordas et al., 2003). Although haem-containing transcription factors have not been reported in plants, there is evidence of redox-sensitive transcription factors, at least one of which might be involved in the adaptive response to low oxygen. ZAT12, a putative zinc finger-containing transcription factor, is recognized as a component in the oxidative stress response signalling network of arabidopsis (Rizhsky et al., 2004). During oxidative stress, ZAT12 promotes expression of other transcription factors and the upregulation of cytosolic ascorbate peroxidase 1, a key enzyme in the removal of H2O2. Accumulation of ROS is a common consequence of biotic and abiotic stresses, including oxygen deprivation and re-oxygenation (discussed below). ZAT12 transcript levels were significantly elevated in response to hypoxia and anoxia in several independent analyses (Branco-Price et al., 2005), indicating involvement of this potentially redox-regulated factor.
Another mechanism of indirect oxygen sensing in plant cells may be manifested through the rapid reduction in cytosolic pH (Fig. 2). A 0·2–0·6 unit decline in cytoplasmic pH occurs within 15 min of oxygen deprivation and is associated with changes in cell physiology (reviewed by Wilkinson, 1999; Greenway and Gibbs, 2003). For example, the decrease in cytosolic pH is proposed to inhibit water transport by water channel proteins (aquaporins) of the PM intrinsic protein subgroup under anoxia (Tournaire-Roux et al., 2003). Greenway and Gibbs (2003) suggested that the decrease in cytosolic pH, typically from about 7·5 to 7·1 in anoxia-tolerant tissues, aids in the acclimation to the cellular energy crisis. This drop in pH may contribute to the reduction in the energy consumptive process of protein synthesis and stimulation of ethanolic fermentation (Roberts et al., 1984a, b; Webster et al., 1991).
Davies' biochemical pH-stat hypothesis predicts that anaerobic metabolism is modulated by the activities of pH-sensitive enzymes, but has been highly debated (Davies, 1986; Drew, 1997; Tadege et al., 1999; Geigenberger, 2003; Greenway and Gibbs, 2003). Both lactate- and ethanol-producing fermentations yield NAD+. Under conditions of oxygen deprivation, the pyruvate produced by glycolysis may initially be converted to lactate in a reaction catalysed by lactate dehydrogenase (LDH). Lactate accumulation, however, contributes to cytosolic acidosis and may ultimately cause cell death if not controlled. As the cytosolic pH falls near to 7·0, pyruvate decarboxylase (PDC) activity increases, promoting the conversion of pyruvate to acetaldehyde, which is reduced to ethanol by ADH. Ethanol, an uncharged molecule, can cross the PM, whereas lactate efflux can require ATP consumption. The root tips of maize seedlings that are deficient in ADH specific activity succumb more rapidly to oxygen deprivation due to an inability to limit lactate production and the decline in cytosolic pH (Roberts et al., 1984b), whereas those root tips acclimated to low oxygen conditions are better equipped to extrude protons from the cytosol (Xia and Roberts, 1996). Thus, avoidance of cytosolic acidosis is likely to involve the mode of fermentation as well as the activity of PM H+ ATPases. Tadege et al. (1999) challenged the hypothesis that lactate-induced acidification triggers the onset of ethanol production, citing that the reduction in cytosolic pH and onset of lactate fermentation were not well correlated in studies of several species. These authors proposed that flux of pyruvate to ethanol is not regulated by lactate production but rather by pyruvate availability, noting that the Km for pyruvate of pyruvate dehydrogenase (PDH) is in the μm range whereas that of PDC is in the mm range. An important observation is that fermentation can occur under aerobic conditions and may not necessarily be driven by a decrease in cytosolic pH. Nonetheless, alteration of cytosolic pH is likely to impact specific metabolic pathways and could play a role in indirect oxygen sensing.
Evaluation of oxygen deprivation-induced changes in mRNA accumulation and recognition of signalling machinery and response mechanisms
It is well established that gene regulation is altered in response to oxygen deprivation in plants. Both early molecular investigations and recent mRNA profiling studies have demonstrated that regulation occurs at the level of mRNA accumulation and translation in A. thaliana and other model species (Sachs et al., 1980; Bailey-Serres, 1999; Klok et al., 2002; Paul et al., 2003; Branco-Price et al., 2005; Liu et al., 2005; Loreti et al., 2005). Profiling of the mRNAs in large polyribosome complexes confirmed that selective mRNA translation is a significant regulatory mechanism under hypoxia (Branco-Price et al., 2005). The strong impairment of protein synthesis is most probably a mechanism of energy conservation since a large proportion of cellular mRNAs show no reduction in steady-state abundance but are poorly translated under hypoxia.
Alterations in mRNA accumulation in response to oxygen deprivation are regulated in a temporal manner and dependent upon multiple parameters including the severity of the stress, light and nutrient availability, tissue, organ and developmental age. The genes that are induced by anoxia and hypoxia in arabidopsis are extremely diverse and include proteins involved in carbohydrate catabolism, glycolysis, ethanolic and other fermentation pathways, lipid metabolism, ethylene synthesis, auxin-mediated processes, amelioration of ROS, calcium and ROS-mediated signal transduction and gene transcription (Klok et al., 2002; Paul et al., 2003; Branco-Price et al., 2005; Liu et al., 2005; Loreti et al., 2005). A comparison of the results from several of these experiments is presented by Branco-Price et al. (2005). Although these experiments involved different organs, developmental ages and treatment conditions, increases in a number of gene transcripts were observed in multiple studies and are consistent with known physiological and developmental processes that are affected by low oxygen stress. Interestingly, a significant proportion of the hypoxia-induced genes encode proteins of unknown function, providing a challenge for future investigations. Despite the complex nature of RNA profiling data, such data are useful for the development of hypotheses on signal transduction events because genes that encode signalling components are frequently upregulated when the pathway is active (Leonhardt et al., 2004; Davletova et al., 2005).
The DNA microarray reports have identified transcription factor/activator mRNAs that increase in response to various regimes of oxygen deprivation in arabidopsis (Klok et al., 2002; Branco-Price et al., 2005; Liu et al., 2005; Loreti et al., 2005). These include heat shock factors, ethylene response-binding proteins, MADS-box proteins, AP2 domain, leucine zipper, zinc finger and WRKY factors. Plants lack orthologues of mammalian HIF1α or yeast Hap1, Rox1 or Mot3; other factors must control the transcriptional reprogramming that occurs. A transcription factor shown to regulate gene expression under hypoxia in plants is the MYB-class transcription factor AtMYB2 of arabidopsis (Hoeren et al., 1998; Dolferus et al., 2003). This factor binds a GT-motif sequence element present in the 5′-flanking region of ADH1 and a number of hypoxia-induced genes of diverse species (Hoeren et al., 1998; Klok et al., 2002; Dolferus et al., 2003; Liu et al., 2005). Other sequence motifs present at a significant frequency in hypoxia-induced genes include a SURE-a-like element that interacts with WRKY factors and a G-box-like element that interacts with bZIP factors (Liu et al., 2005).
The regulation of AtMYB2 appears to be governed by differential turnover of AtMYB2 mRNA (Hoeren et al., 1998; Dolferus et al., 2003). Treatment of cultured arabidopsis seedlings with cycloheximide, an effective inhibitor of protein synthesis, promoted an increase in AtMYB2 mRNA under non-stress conditions (Hoeren et al., 1998). A change in mRNA stability in response to this drug is typical of mRNAs that are destabilized by constitutively synthesized proteins (Gutierrez et al., 1999). AtMYB2 mRNA may be stabilized under hypoxia as a consequence of the depletion of a factor with a short half-life (Dolferus et al., 2003). This could be regulated through direct or indirect oxygen sensing by proteins involved in mRNA degradation. Alternatively, AtMYB2 mRNA stability may be regulated by differential mRNA translation when oxygen or nucleotide triphosphate levels are limiting. AtMYB2 mRNA accumulation is promoted by cold temperature, dehydration stress and wounding (Dolferus et al., 2003), all of which reduce global levels of protein synthesis and increase differential mRNA translation (Bailey-Serres, 1999; Kawaguchi and Bailey-Serres, 2002; Kawaguchi et al., 2004).
The independent DNA microarray analyses have identified a number of genes that encode putative components of signal transduction pathways (Klok et al., 2002; Branco-Price et al., 2005; Liu et al., 2005; Loreti et al., 2005). These include calcium-binding proteins, protein-modifying enzymes [i.e. receptor-like kinases and mitogen-activated protein (MAP) kinase], and known signalling pathway components such as the gp91-phox subunit of the respiratory burst oxidase NAD(P)H oxidase. Several of these RNA profiling studies reported the significant induction of the non-symbiotic haemoglobin mRNA, which participates in regulation of cellular redox and energy status under hypoxia in an NO- and nitrate-dependent manner and has been shown to enhance survival of hypoxia (Dordas et al., 2003; Igamberdiev and Hill, 2004). It is of particular interest to know whether the low oxygen sensing mechanisms and signalling processes in plants and other eukaryotes are evolutionarily conserved, as implicated for NO in diverse eukaryotes.
Calcium flux is necessary for ADH induction under oxygen deprivation
Hypoxia promotes an activation of voltage-gated PM channels and influx of calcium as well as flux in mitochondrial calcium levels in mammalian cells (Archer et al., 2000; Lopez-Barneo, 2001, 2004). In plants, including maize, rice and arabidopsis, movement of calcium is a prerequisite for certain alterations in gene expression in response to anoxia and hypoxia (Subbaiah and Sachs, 2003) (Fig. 2). Maize cells require an increase in cytosolic calcium for the induction of expression of Adh1 and other genes (Subbaiah et al., 1994a, b; He et al., 1996). Fluorescent imaging analyses demonstrated that a reversible increase in cytosolic calcium occurs immediately following transfer of maize cells to anoxia (Subbaiah et al., 1994a, 1998). Treatment of cells with caffeine, which also promotes an increase in cytosolic calcium, was sufficient to induce Adh1 (Subbaiah et al., 1994a). However, anoxia further stimulated an increase in cytosolic calcium in caffeine-treated cells, suggesting that calcium is released from both caffeine-sensitive and -insensitive stores in response to anoxia. Reversible changes in calcium levels inside and on the periphery of mitochondria were observed upon transfer to anoxia and following re-oxygenation, leading to the conclusion that anoxia stimulates calcium release from mitochondria (Subbaiah et al., 1998). The increase in cytosolic calcium and release of calcium from mitochondria under anoxia were dramatically inhibited by ruthenium red, which blocks mitochondrial and endoplasmic reticulum calcium channels (Subbaiah et al., 1994a, 1998). Further evidence for a role for mitochondria in the induction of Adh1 was revealed in a study of gene regulation in the T-cytoplasm mitochondrial genotype of maize. Methomyl, which uncouples the mitochondrial inner membrane potential and promotes calcium release from the organelle, engendered a strong induction in Adh1 transcript accumulation in the absence of oxygen deprivation (Kuzmin et al., 2004). These data suggest that dynamic alterations in mitochondrial and cytosolic calcium, which are likely to play a role as second messengers, are important in the response of oxygen-deprived maize cells.
Calcium modulation is also necessary for responses to hypoxia in arabidopsis and rice. Sedbrook et al. (1996) monitored arabidopsis seedlings expressing a calcium-sensitive luminescent protein, jellyfish AEQUORIN, and confirmed three oscillations in cytosolic calcium in response to anoxia. Transfer to anoxia caused a rapid transient spike (10 min) in calcium followed by a prolonged increase (1·5–4 h), whereas re-oxygenation also elicited a transient spike. The initial spike was less prominent in seedlings repeatedly exposed to 10 min of anoxia or pre-treated with ruthenium red, the calcium chelator EGTA or the PM calcium channel blockers lanthanum and gadolinium chloride. In contrast, the prolonged increase in cytosolic calcium was greatly enhanced in the presence of gadolinium or lanthanum chloride. These observations led to the proposal that the initial calcium increase results from an influx of calcium from the apoplast and release of calcium from an internal store(s). The secondary calcium transient most probably involves the activity of PM channels.
Curiously, the calcium signatures recorded in these seedlings were primarily the response of the stems and cotyledons; roots showed no reproducible increase in cytosolic calcium (Sedbrook et al., 1996). These investigators concluded that either root and aerial tissues have distinct responses or the AEQUORIN of their system lacked sensitivity to record calcium modulation in roots. Most probably, the latter is correct since increased cytosolic calcium was required to drive expression of the ADH1 promoter in the roots and shoot apex of arabidopsis (Chung and Ferl, 1999). The presence of ruthenium red, EGTA and gadolinium chloride effectively blocked the increase in reporter enzyme activity observed in the absence of these compounds. In rice, treatment of seedlings with ruthenium red inhibited the induction of ADH1 and alternative oxidase1a (AOX1a) mRNA, but did not influence the decrease in abundance of several transcripts (Tsuji et al., 2000). Together, these studies indicate that low oxygen-induced alterations in gene expression in plants can be dependent upon calcium flux(es). Further research is needed to resolve the biological relevance of the spatial and temporal distinctions in calcium transients and to determine the specific role(s) of calcium in signal transduction under oxygen deprivation.
A GTPase rheostat regulates ADH1 induction in response to hypoxia in arabidopsis
A screen for transposon insertions into genes induced by hypoxia that influence the induction of ADH1 expression revealed that the RHO-like GTPases of plants (ROP) monomeric GTPase modulates the induction of ADH1 in arabidopsis seedlings (Baxter-Burrell et al., 2002, 2003). Based on findings with arabidopsis, it was proposed that a ROP GTPase rheostat promotes ethanolic fermentation when active and conserves carbohydrates when inactive (Fukao and Bailey-Serres, 2004) (Fig. 2). The ROP family of proteins activates and represses signalling cascades that control diverse mechanisms in plant cells (Gu et al., 2004). Constitutive active and dominant-negative mutants of ROP2, one of the ten ROP proteins of arabidopsis, showed increased sensitivity to hypoxia and altered induction of ADH1 at the seedling stage. The importance of regulation of ROP-GTP and ROP-GTP levels in ADH induction was implicated further by the phenotype of a loss-of-function mutant of a negative regulator of ROP signalling, ROPGAP4. ropgap4-1 seedlings showed increased sensitivity to hypoxia and uncontrolled induction of ADH1. The examination of these mutants led to the finding that the levels of the active form of ROP, ROP-GTP, increased within 1·5 h of oxygen deprivation and declined after prolonged stress (>12 h oxygen deprivation under low light). These observations indicate that ROP activation is necessary to promote ADH1 induction, but moderation and reversibility of ROP signalling is an additional prerequisite for survival of transient hypoxia.
Further studies revealed that a consequence of ROP activation was the induction of H2O2 accumulation. The elevation of H2O2 in crude cell extracts was inhibited by DPI, an inhibitor of flavin-binding proteins. DPI also dramatically inhibited increases in ADH activity, leading to the suggestion that ROS production is a necessary component of a low oxygen signalling pathway. However, the inability of the ropgap4-1 mutant to control the elevation in H2O2 indicated that negative regulation of the signalling is necessary to avoid oxidative stress (Baxter-Burrell et al., 2002; Fukao and Bailey-Serres, 2004). These findings support the hypothesis that rheostat-like regulation of ROP activity mediates temporal activation of an H2O2-dependent signalling pathway that leads to ADH1 expression. The importance of the production and amelioration of ROS in the response of plant cells to oxygen deprivation was recognized prior to the elucidation of the ROP rheostat (Blokhina et al., 2003). A DPI-sensitive increase in H2O2 was reported in response to hypoxia in rhizomes of two iris species (Iris pseudoacorus and Iris germanica). By use of electron microscopy, these studies detected H2O2 accumulation in the apoplast and in association with the PM in hypoxic tissues. An intriguing possibility is that the source of this ROS is a PM NAD(P)H oxidase.
There may be a connection between ROP-promoted H2O2 production and the hypoxia-induced increase in cytosolic calcium. Caffeine treatment mimics anoxia in maize, at least in part, by increasing cytosolic calcium (Subbaiah et al., 1994a, 1998). When arabidopsis seedlings were transferred to solid medium that contained caffeine, significant increases in ROP-GTP levels and ADH specific activity were observed (Baxter-Burrell et al., 2002). An interaction between ROP signalling and calcium is supported by the demonstration that EDTA and the calcium channel antagonists ruthenium red and lanthanum chloride affect the accumulation of ROP-GTP in response to hypoxia (A. Baxter-Burrell and J. Bailey-Serres, unpubl. res.). Interestingly, an increase in cytosolic calcium is a likely prerequisite for the activation of apoplastic H2O2 production by the calcium-dependent NAD(P)H oxidase of plants (Keller et al., 1998; Sagi and Fluhr, 1999). In arabidopsis roots, analysis of mutants of the gene family encoding the gp91-phox subunit of the NAD(P)H oxidase revealed that ROS produced by this oxidase activates calcium channels and facilitates calcium flux(es) necessary for root growth (Foreman et al., 2003; Mori et al., 2004). Moreover, arabidopsis ROP1 is responsible for an intracellular calcium gradient at the tip of pollen tubes (Gu et al., 2004) and root hairs (Jones et al., 2002). These observations lead to the speculation that ROP may further promote an increase in cytosolic calcium under hypoxic conditions, which could be mediated by an NAD(P)H oxidase, possibly located at the PM. Thus, calcium dynamics may provide a balance between production of H2O2 as a signalling molecule and the damage it can cause as an ROS. This is consistent with the finding that DPI treatment prolonged the tolerance of ropgap4-1 seedlings (Baxter-Burrell et al., 2002). Moreover, it is of interest to note that members of the gp91-phox subunit of the PM NAD(P)H oxidase gene family were identified as significantly induced by low oxygen stress in several DNA microarray analyses (Klok et al., 2002; Branco-Price et al., 2005). Additional experimentation is needed to determine if a PM NAD(P)H oxidase, and calcium channels it may regulate, plays a role in low oxygen signal transduction and whether this mechanism is evolutionarily related to the roles during hypoxia of the PM NAD(P)H oxidase and G-proteins in mammalian cells.
Ethylene perception enhances responses to oxygen deprivation
One growth regulator that has received significant attention in the studies of low oxygen responses is ethylene. Ethylene biosynthesis increases within 4 h of transfer to hypoxic conditions in several species (Drew et al., 1979; Lorbiecke and Sauter, 1999). In arabidopsis, phosphorylation of ACC synthase (ACS) by the stress-responsive MAP kinase (MAPK) 6 leads to the accumulation of ACS protein (Liu and Zhang, 2004). Consequently, levels of cellular ACS activity and ethylene production are elevated. It is not yet known if a MAPK signalling pathway is activated by oxygen deprivation. The biosynthesis of ethylene is not likely to occur under strict cellular anoxia because the conversion of 1-aminocyclopropane-1-carboxylic acid (ACC) to ethylene by ACC oxidase (ACO) requires consumption of oxygen (Yang and Hoffman, 1984; Kende, 1993). Possibly to compensate for the ACC to ethylene rate-limiting step during hypoxia, cellular ACC levels and ACO enzyme activity are elevated in submerged Rumex palustris plants (Banga et al., 1996; Vriezen et al., 1999). Ethylene mediates internode elongation and adventitious root initiation in deepwater rice (Lorbiecke and Sauter, 1999), and petiole elongation and leaf hyponasty in R. palustris (Voesenek et al., 2003). An increase in intracellular calcium is involved in the transduction of the ethylene signal that leads to the formation of aerenchyma in hypoxic maize roots (He et al., 1996). Ethylene also enhanced the hypoxic induction of ADH1 in arabidopsis (Peng et al., 2001).
Several studies have examined the interplay between ethylene and other hormones during acclimation of plants to flooding stress. When gibberellic acid (GA) inhibitors are applied to seedlings of deepwater rice, the growth induced by ethylene and submergence is inhibited (Raskin and Kende, 1984). Abscisic acid (ABA) is a potent inhibitor of GA action in rice, but ethylene application can reduce endogenous ABA levels (Hoffmann-Benning and Kende, 1992). Ethylene, GA and indole-3-acetic acid (IAA) act in consort to promote differential petiole elongation and hyponastic upward curvature in submerged R. palustris (Voesenek et al., 2003; Cox et al., 2004). In contrast, ABA appears to be responsible for the negative regulation of the submergence-induced hyponastic growth. In addition, there appears to be a synergism between ethylene and IAA during adventitious root formation in submerged R. palustris (Visser et al., 1996).
Differential responses of aerial organs and roots to oxygen deprivation
There is clear evidence that aerial organs and roots respond differently to oxygen deprivation. In a pioneering characterization of the molecular response, Okimoto et al. (1980) observed that anaerobic polypeptides (ANPs) were synthesized in anaerobic maize roots, but leaves exhibited no detectable protein synthesis and died after a short anaerobic treatment. In arabidopsis, the induction of ADH1 by hypoxia was reported to occur primarily in the roots, and only at low levels in the aerial portion of the plant (Dolferus et al., 1994). Ellis et al. (1999) found that ethanol fermentation was essential for tolerance to hypoxia in roots but not in shoots of arabidopsis. Moreover, ABA treatment promoted tolerance to oxygen deprivation only in the roots of arabidopsis. These observations, as well as organ-specific differences in calcium signatures mentioned earlier (Sedbrook et al., 1996), indicate that shoots and roots differ in mechanisms of sensing and response to oxygen deprivation.
Another aspect of whole plant response is long-distance transport of oxygen, mobilization of carbohydrate reserves and long-distance signalling. Survival of oxygen deprivation is extended by the transport of oxygen from aerial organs through aerenchyma to roots (Drew, 1997). On the other hand, roots of flooded plants transmit signals to aerial organs. For example, in flooded tomato plants, ACC is rapidly synthesized in the roots and transported to the shoot (Shiu et al., 1998). There, in the presence of oxygen, ACC is converted to ethylene, resulting in leaf curvature. Chung and Ferl (1999) observed that the growth of arabidopsis seedling roots through an agar medium triggered ADH1 expression in the root as well as in the shoot apical meristem. The signal transmission to aerial parts of the plant was mediated by a mechanism that uses calcium as a second messenger. These observations emphasize that response to oxygen deprivation occurs at the whole plant level.
Oxygen sensing mechanisms in Chlamydomonas reinhardtii and responses involving chloroplast function
Results from the genetic dissection of sensing and response to hypoxia in the single-celled algae Chlamydomonas reinhardtii provide additional insights. It was noted that a sub-set of hypoxia-induced genes were activated in cells deficient in copper and in cells that were oxygen deprived in the presence of mercuric chloride, an antagonist of the copper-deficiency response (Quinn et al., 2002). The co-regulation of these responses appears to involve the common use of a cis-acting GTAC core, present in one copy for copper-regulated expression and in two copies for hypoxic induction, and a trans-acting factor, copper resistance response 1 (CRR1). Gene induction under hypoxia is regulated by both CRR1-dependent and -independent pathways. The CRR1-dependent pathway governs enzymes involved in plastid function, indicating that plastids contribute to the acclimative response to oxygen deprivation. The importance of this organelle may be reflected by the differential response to oxygen deprivation observed in root and aerial tissue of arabidopsis (Sedbrook et al., 1996; Ellis et al., 1999). The observation that light levels affect submergence tolerance in rice and gene induction in oxygen-deprived arabidopsis and Nicotiana tabacum is also consistent with a role for chloroplasts in the whole plant response programme (Hansch et al., 2003; Mohanty and Ong, 2003). The influence of chloroplasts may be complex due to the role of this organelle in oxygen evolution, carbon dioxide consumption, sucrose production, redox regulation, and ROS generation and amelioration.
CONCLUSIONS AND PERSPECTIVES
Investigation of sensory mechanisms and signal transduction cascades responsible for triggering responses to sub-optimal oxygen levels in plants cells is an exciting area of research. The sensing and signalling mechanisms that are known to contribute to responses to oxygen deprivation in plant cells are generally integrated into a model in Fig. 2. Advances in genome biology, genetic resources and high throughput technologies provide excellent resources for the exploration of oxygen sensing mechanisms in plant cells. Of particular interest is whether a sensing mechanism, perhaps initiated by mitochondria and/or involving PM NAD(P)H oxidases, is present in plants and evolutionarily conserved in eukaryotes. It is imperative to identify sensors and dissect the signalling pathways that occur at the cellular, tissue, organ and whole plant level. Comparative analyses of near isogenic genotypes that differ in the adaptive response to oxygen deprivation are likely to yield critical information on regulatory mechanisms. It is anticipated that distinctions in the location, timing, magnitude, duration and frequency of signalling cascades will contribute to the responses of individual cells. It is also likely that alterations in cytosolic pH and calcium are involved in the signalling processes. Although paradoxical, ROS may prove to be second messengers in the response mechanism. The importance of changes in adenylate charge, redox status and carbohydrate levels must also be considered. Many questions remain to be answered about the response of individual cells. How do cellular signalling and response mechanisms differ between stress-tolerant and intolerant organs and species? What signalling transduction pathway(s) are activated or inhibited? How do multiple and interacting pathways control adaptive responses? Another inviting area for research is the consideration of cell–cell and long-distance signalling mechanisms that determine the organ and whole plant response to oxygen deprivation, such as regulation of leaf and internode elongation, petiole curvature, aerenchyma formation and adventitious root growth. It is likely that these responses involve growth regulators such as ethylene, auxin, gibberellins and ABA. At the organ and whole plant level, there are also many pertinent questions. How might cells that surround an oxygen-deficient core, such as the stele of a root, respond to benefit the survival of the organ or the whole plant? How are the energetic needs of meristematic cells safeguarded and how is programmed cell death promoted or avoided? How do cells in roots and aerial organs communicate over a long distance when there is an oxygen crisis in the roots? Success at answering these questions will be of relevance to agriculture and will provide knowledge of the fundamental nature of aerobic life.
Acknowledgments
This work was supported by grants from the National Science Foundation (MCB-0-131486) and USDA Cooperative State Research, Education and Extension Service (2003-35100-13359). We are indebted to members of the Bailey-Serres lab for many productive discussions, especially Takeshi Fukao.
LITERATURE CITED
- Acker T, Acker H. 2004. Cellular oxygen sensing need in CNS function: physiological and pathological implications. Journal of Experimental Botany 207: 3171–3188. [DOI] [PubMed] [Google Scholar]
- Alexeeva S, de Kort B, Sawers G, Hellingwerf KJ, de Mattos MJ. 2000. Effects of limited aeration and of the ArcAB system on intermediary pyruvate catabolism in Escherichia coli Journal of Bacteriology 182: 4934–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeeva S, Hellingwerf KJ, Teixeira de Mattos MJ. 2003. Requirement of ArcA for redox regulation in Escherichia coli under microaerobic but not anaerobic or aerobic conditions. Journal of Bacteriology 185: 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weir EK. 1999. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proceedings of the National Academy of Sciences of the USA 96: 7944–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, Weir EK, Reeve HL, Michelakis E. 2000. Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation. Advances in Experimental Medical Biology 475: 219–2140. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J. 1999. Selective translation of cytoplasmic mRNAs in plants. Trends in Plant Science 4: 142–148. [DOI] [PubMed] [Google Scholar]
- Banga M, Slaa EJ, Blom CWPM, Voesenek LACJ. 1996. Ethylene biosynthesis and accumulation under drained and submerged conditions. Plant Physiology 112: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Burrell A, Chang R, Springer P, Bailey-Serres J. 2003. Gene and enhancer trap transposable elements reveal oxygen deprivation-regulated genes and their complex patterns of expression in Arabidopsis. Annals of Botany 91: 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J. 2002. ROPGAP4-dependent Rop GTPase rheostat controls of Arabidopsis oxygen deprivation tolerance. Science 296: 2026–2028. [DOI] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany 91: 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco-Price C, Kawaguchi R, Ferreira R, Bailey-Serres J. 2005. Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Annals of Botany 96: 647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK. 2003. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes and Development 17: 2614–2623. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Schumacker PT. 2000. Cellular oxygen sensing by mitochondria: old questions, new insight. Journal of Applied Physiology 88: 1880–1889. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. 1998. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proceedings of the National Academy of Sciences of the USA 95: 11715–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. 2000. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia. Journal of Biological Chemistry 275: 25130–25138. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Ferl RJ. 1999. Arabidopsis alcohol dehydrogenase expression in both shoots and roots is conditioned by root growth environment. Plant Physiology 121: 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MC, Benschop JJ, Vreeburg RA, Wagemaker CA, Moritz T, Peeters AJ, Voesenek LA. 2004. The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiology 136: 2948–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DD. 1986. The fine control of cytosolic pH. Physiologia Plantarum 67: 702–706. [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. 2005. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirmeier R, O'Brien KM, Engle M, Dodd A, Spears E, Poyton RO. 2002. Exposure of yeast cells to anoxia induces transient oxidative stress. Implications for the induction of hypoxic genes. Journal of Biological Chemistry 277: 34773–34784. [DOI] [PubMed] [Google Scholar]
- Dolferus R, de Bruxelles GL, Dennis ES, Peacock WJ. 1994. Regulation of the Arabidopsis Adh gene by anaerobic and other environmental stresses. Annals of Botany 74: 301–308. [Google Scholar]
- Dolferus R, Klok EJ, Delessert C, Wilson S, Ismond KP, Good AG, Peacock WJ, Dennis ES. 2003. Enhancing the anaerobic response. Annals of Botany 91: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Roeb GW, Dautzenberg M, Froehlich A, Vigeolas H, Minchin PE, Geigenberger P. 2004. Phloem import and storage metabolism are highly coordinated by the low oxygen concentrations within developing wheat seeds. Plant Physiology 135: 1809–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordas C, Rivoal J, Hill RD. 2003. Plant haemoglobins, nitric oxide and hypoxic stress. Annals of Botany 91: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC. 1997. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Plant Molecular Biology 48: 223–250. [DOI] [PubMed] [Google Scholar]
- Drew MC, Jackson MB, Giffard S. 1979. Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta 147: 83–88. [DOI] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW. 2000. Programmed cell death and aerenchyma formation in roots. Trends in Plant Science 5: 123–127. [DOI] [PubMed] [Google Scholar]
- Ellis MH, Dennis ES, Peacock WJ. 1999. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiology 119: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennoy SL, Bailey-Serres J. 1995. Post-transcriptional regulation of gene expression in oxygen-deprived roots of maize. Plant Journal 7: 287–295. [DOI] [PubMed] [Google Scholar]
- Fennoy SL, Nong T, Bailey-Serres J. 1998. Transcriptional and post-transcriptional processes regulate gene expression in oxygen-deprived roots of maize. Plant Journal 15: 727–735. [DOI] [PubMed] [Google Scholar]
- Fischer HM. 1994. Genetic regulation of nitrogen fixation in rhizobia. Microbiology Review 58: 352–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, et al. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446. [DOI] [PubMed] [Google Scholar]
- Fu XW, Wang D, Nurse CA, Dinauer MC, Cutz E. 2000. NADPH oxidase is an O2 sensor in airway chemoreceptors: evidence from K+ current modulation in wild-type and oxidase-deficient mice. Proceedings of the National Academy of Sciences of the USA 97: 4374–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. 2004. Hypoxia responses in plants—a balancing act? Trends in Plant Science 9: 449–456. [DOI] [PubMed] [Google Scholar]
- Geigenberger P. 2003. Response of plant metabolism to too little oxygen. Current Opinion in Plant Biology 6: 247–256. [DOI] [PubMed] [Google Scholar]
- Giaccia AJ, Simon MC, Johnson R. 2004. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes and Development 18: 2183–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. 2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30: 1–37. [DOI] [PubMed] [Google Scholar]
- Gong W, Hao B, Chan MK. 2000. New mechanistic insights from structural studies of the oxygen-sensing domain of Bradyrhizobium japonicum FixL. Biochemistry 39: 3955–3962. [DOI] [PubMed] [Google Scholar]
- Greenway H, Gibbs J. 2003. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology 30: 999-1036. [DOI] [PubMed] [Google Scholar]
- Gu Y, Wang Z, Yang Z. 2004. RAC GTPase: an old new master regulator for plant signaling. Current Opinion in Plant Biology 67: 527–536. [DOI] [PubMed] [Google Scholar]
- Gutierrez RA, MacIntosh GC, Green PJ. 1999. Current perspectives on mRNA stability in plants: multiple levels and mechanisms of control. Trends in Plant Science 4: 429–438. [DOI] [PubMed] [Google Scholar]
- Hagen T, Taylor CT, Lam F, Moncada S. 2003. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science 302: 1975–1978. [DOI] [PubMed] [Google Scholar]
- Hansch R, Mendel RR, Cerff R, Hehl R. 2003. Light-dependent anaerobic induction of the maize glyceraldehyde-3-phosphate dehydrogenase 4 (GapC4) promoter in Arabidopsis thaliana and Nicotiana tabacum Annals of Botany 91: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Morgan PW, Drew MC. 1996. Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiology 112: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsila M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. 2003. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. Journal of Biological Chemistry 278: 30772–30780. [DOI] [PubMed] [Google Scholar]
- Hoeren FU, Dolferus R, Wu Y, Peacock WJ, Dennis ES. 1998. Evidence for a role for AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (ADH1) by low oxygen. Genetics 149: 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman-Benning S, Kende H. 1992. On the role of abscisic acid and gibberellin in the regulation of growth of rice. Plant Physiology 99: 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon T, Dodd A, Dirmeier R, Gorman N, Sinclair PR, Zhang L, Poyton RO. 2003. A mechanism of oxygen sensing in yeast. Multiple oxygen-responsive steps in the heme biosynthetic pathway affect Hap1 activity. Journal of Biological Chemistry 278: 50771–50780. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Hill RD. 2004. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. Journal of Experimental Botany 55: 2473–2482. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. 2001. HIF-alpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. 2001. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472. [DOI] [PubMed] [Google Scholar]
- Jones M, Shen JJ, Fu Y, Li H, Yang Z, Grierson CS. 2002. The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14: 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Bailey-Serres J. 2002. Regulation of translational initiation in plants. Current Opinion in Plant Biology 6: 460–465. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Girke T, Bray EA, Bailey-Serres J. 2004. Differential mRNA translation contributes to gene regulation under non-stress and dehydration stress conditions in Arabidopsis thaliana Plant Journal 38: 823–839. [DOI] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. 1998. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. 1993. Ethylene biosynthesis. Annual Review of Plant Physiology 44: 283–307. [Google Scholar]
- Kiley PJ, Beinert H. 2003. The role of Fe–S proteins in sensing and regulation in bacteria. Current Opinion in Microbiology 66: 181–185. [DOI] [PubMed] [Google Scholar]
- Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES. 2002. Expression profile analysis of the low-oxygen response in Arabidopsis root culture. Plant Cell 14: 2481–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE, Ying Z, Wu Y, Avigne W. 2000. Multiple paths of sugar-sensing and a sugar/oxygen overlap for genes of sucrose and ethanol metabolism. Journal of Experimental Botany 51: 417–427. [DOI] [PubMed] [Google Scholar]
- Kuzmin EV, Karpova OV, Elthon TE, Newton KJ. 2004. Mitochondrial respiratory deficiencies signal up-regulation of genes for heat shock proteins. Journal of Biological Chemistry 279: 20672–20677. [DOI] [PubMed] [Google Scholar]
- Kwast KE, Burke PV, Poyton RO. 1998. Oxygen sensing and the transcriptional regulation of oxygen-responsive genes in yeast. Journal of Experimental Botany 201: 1177–1195. [DOI] [PubMed] [Google Scholar]
- Kwast KE, Burke PV, Staahl BT, Poyton RO. 1999. Oxygen sensing in yeast: evidence for the involvement of the respiratory chain in regulating the transcription of a subset of hypoxic genes. Proceedings of the National Academy of Sciences of the USA 96: 5446–5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI. 2004. Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16: 596–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Vantoai T, Moy L, Bock G, Linford LD, Quackenbush J. 2005. Global transcription profiling reveals novel insights into hypoxic response in Arabidopsis Plant Physiology 137: 1115–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang S. 2004. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16: 3386–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Saenz P. 2001. Cellular mechanism of oxygen sensing. Annual Review of Physiology 63: 259–287. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, del Toro R, Levitsky KL, Chiara MD, Ortega-Saenz P. 2004. Regulation of oxygen sensing by ion channels. Journal of Applied Physiology 96: 1187–1195. [DOI] [PubMed] [Google Scholar]
- Loreti E, Poggi A, Novi G, Alpi A, Perata P. 2005. Genome-wide analysis of gene expression in Arabidopsis seedlings under anoxia. Plant Physiology 137: 1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorbiecke R, Sauter M. 1999. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiology 119: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty B, Ong BL. 2003. Contrasting effects of submergence in light and dark on pyruvate decarboxylase activity in roots of rice lines differing in submergence tolerance. Annals of Botany 91: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Schroeder JI. 2004. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiology 135: 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto R, Sachs MM, Porter EK, Freeling M. 1980. Patterns of polypeptide synthesis in various maize organs under anaerobiosis. Planta 150: 89–94. [DOI] [PubMed] [Google Scholar]
- Paul AL, Ferl RJ. 1991.Adh1 and Adh2 regulation. Maydica 36: 129–134. [Google Scholar]
- Paul AL, Schuerger AC, Popp MP, Richards JT, Manak MS, Ferl RJ. 2003. Hypobaric biology: Arabidopsis gene expression at low atmospheric pressure. Plant Physiology 134: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HP, Chan CS, Shih MC, Yang SF. 2001. Signaling events in the hypoxic induction of alcohol dehydrogenase gene in Arabidopsis Plant Physiology 126: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton RO. 1999. Models for oxygen sensing in yeast: implications for oxygen-regulated gene expression in higher eucaryotes. Respiration Physiology 115: 119–133. [DOI] [PubMed] [Google Scholar]
- Quinn JM, Eriksson M, Moseley JL, Merchant S. 2002. Oxygen deficiency responsive gene expression in Chlamydomonas reinhardtii through a copper-sensing signal transduction pathway. Plant Physiology 128: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Kende H. 1984. Regulation of growth in stem sections of deep-water rice. Planta 160: 66–92. [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Davletova S, Liang H, Mittler R. 2004. The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. Journal of Biological Chemistry 279: 11736–11743. [DOI] [PubMed] [Google Scholar]
- Roberts JKM, Callis J, Jardetzky O, Walbot V, Freeling M. 1984. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proceedings of the National Academy of Sciences of the USA 81: 6029–6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Callis J, Wemmer D, Walbot V, Jardetzky O. 1984. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under anoxia. Proceedings of the National Academy of Sciences of the USA 81: 3368–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MM, Freeling M, Okimoto R. 1980. The anaerobic proteins of maize. Cell 20: 761–767. [DOI] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. 2001. Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiology 126: 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroedl C, McClintock DS, Budinger GR, Chandel NS. 2002. Hypoxic but not anoxic stabilization of HIF-1alpha requires mitochondrial reactive oxygen species. American Journal of Physiology Lung Cell and Molecular Physiology 283: L922–L931. [DOI] [PubMed] [Google Scholar]
- Schumacker PT. 2002. Hypoxia, anoxia, and O2 sensing: the search continues. American Journal of Physiology Lung Cell and Molecular Physiology 283: L918–L921. [DOI] [PubMed] [Google Scholar]
- Schumacker PT. 2003. Current paradigms in cellular oxygen sensing. Advances in Experimental Medical Biology 2543: 57–71. [DOI] [PubMed] [Google Scholar]
- Sciotti MA, Chanfon A, Hennecke H, Fischer HM. 2003. Disparate oxygen responsiveness of two regulatory cascades that control expression of symbiotic genes in Bradyrhizobium japonicum Journal of Bacteriology 185: 5639–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham JSK. 2002. Hypoxic pulmonary vasoconstriction. Ups and downs of reactive oxygen species. Circulation Research 91: 649–651. [DOI] [PubMed] [Google Scholar]
- Shiu OY, Oetiker JH, Yip WK, Yang SF. 1998. The promoter of LE-ACS7, an early flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of the tomato, is tagged by a Sol3 transposon. Proceedings of the National Academy of Sciences of the USA 95: 10334–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook JC, Kronebusch PJ, Borisy GG, Trewavas AJ, Masson PH. 1996. Transgenic AEQUORIN reveals organ specific cytosolic Ca2+ responses to anoxia in Arabidopisis thaliana seedlings. Plant Physiology 111: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. 2004. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology 19: 176–182. [DOI] [PubMed] [Google Scholar]
- Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, Darley-Usmar VM. 2005. Nitroxia: the pathological consequence of dysfunction in the nitric oxide–cytochrome c oxidase signaling pathway. Free Radical Biology and Medicine 38: 297–306. [DOI] [PubMed] [Google Scholar]
- Subbaiah CC, Sachs MM. 2003. Molecular and cellular adaptations of maize to flooding stress. Annals of Botany 91: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. 1994. Elevation of cytosolic calcium precedes anoxia gene expression in maize suspension-cultured cells. Plant Cell 6: 1747–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Zhang J, Sachs MM. 1994. Involvement of intracellular calcium in anaerobic gene expression and survival of maize seedlings. Plant Physiology 105: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. 1998. Mitochondrial contribution to the anoxic Ca2+ signal suspension cultured cells. Plant Physiology 118: 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadege M, Dupuis II, Kuhlemeier C. 1999. Ethanolic fermentation: new functions for an old pathway. Trends in Plant Science 4: 320–325. [DOI] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D-T, Bligny R, Maurel C. 2003. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425: 393–397. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Nakazono M, Saisho D, Tsutsumi N, Hirai A. 2000. Transcript levels of the nuclear-encoded respiratory genes in rice decrease by oxygen deprivation: evidence for involvement of calcium in expression of the alternative oxidase 1a gene. FEBS Letters 471: 201–204. [DOI] [PubMed] [Google Scholar]
- Turcotte S, Desrosiers RR, Beliveau R. 2004. Hypoxia upregulates von Hippel–Lindau tumor-suppressor protein through RhoA-dependent activity in renal cell carcinoma. American Journal of Physiology Renal Physiology 286: 338–348. [DOI] [PubMed] [Google Scholar]
- Turcotte S, Desrosiers RR, Beliveau R. 2003. HIF-1alpha mRNA and protein upregulation involves Rho GTPase expression during hypoxia in renal cell carcinoma. Journal of Cell Science 116: 2247–2260. [DOI] [PubMed] [Google Scholar]
- Unden G, Bongaerts J. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochimica et Biophysica Acta 1320: 217–234. [DOI] [PubMed] [Google Scholar]
- Visser E, Cohen JD, Barendse G, Blom C, Voesnek LACJ. 1996. An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physioogy 112: 1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LA, Benschop JJ, Bou J, Cox MC, Groeneveld HW, Millenaar FF, Vreeburg RA, Peeters AJ. 2003. Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris Annals of Botany 91: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriezen WH, Hulzink R, Mariani C, Voesenek LACJ. 1999. 1-Aminocyclopropane-1-carboxylate oxidase activity limits ethylene biosynthesis in Rumex palustris during submergence. Plant Physiology 121: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waypa GB, Schumacker PT. 2005. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. Journal Applied Physiology 98: 404–414. [DOI] [PubMed] [Google Scholar]
- Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. 2002. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circulation Research 91: 719–726. [DOI] [PubMed] [Google Scholar]
- Webster C, Kim C-Y, Roberts JKM. 1991. Elongation and termination reactions of proteins synthesis on maize root tip polyribosomes studied in a homologous cell-free system. Plant Physiology 96: 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. 1999. pH as a stress signal. Plant Growth Regulation 29: 87–99. [Google Scholar]
- Xia J-H, Roberts JKM. 1996. Regulation of H+ extrusion and cytoplasmic pH in maize root tips acclimated to a low-oxygen environment. Plant Physiology 111: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. 1984. Ethylene biosynthesis and its regulation in higher plants. Annual Review of Plant Physiology 35: 155–189. [Google Scholar]
- Yun Z, Maecker HL, Johnson RS, Giaccia AJ. 2002. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Developmental Cell 2: 331–341. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hach A. 1999. Molecular mechanism of heme signaling in yeast: the transcriptional activator Hap1 serves as the key mediator. Cell and Molecular Life Science 56: 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]




