Abstract
• Background and Aims Growth and development of plant organs, including leaves, depend on cell division and expansion. Leaf size is increased by greater cell ploidy, but the mechanism of this effect is poorly understood. Therefore, in this study, the role of cell division and expansion in the increase of leaf size caused by polyploidy was examined by comparing various cell parameters of the mesophyll layer of developing leaves of diploid and autotetraploid cultivars of two grass species, Lolium perenne and L. multiflorum.
• Methods Three cultivars of each ploidy level of both species were grown under pot conditions in a controlled growth chamber, and leaf elongation rate and the cell length profile at the leaf base were measured on six plants in each cultivar. Cell parameters related to division and elongation activities were calculated by a kinematic method.
• Key Results Tetraploid cultivars had faster leaf elongation rates than did diploid cultivars in both species, resulting in longer leaves, mainly due to their longer mature cells. Epidermal and mesophyll cells differed 20-fold in length, but were both greater in the tetraploid cultivars of both species. The increase in cell length of the tetraploid cultivars was caused by a faster cell elongation rate, not by a longer period of cell elongation. There were no significant differences between cell division parameters, such as cell production rate and cell cycle time, in the diploid and tetraploid cultivars.
• Conclusion The results demonstrated clearly that polyploidy increases leaf size mainly by increasing the cell elongation rate, but not the duration of the period of elongation, and thus increases final cell size.
Keywords: Cell division, cell elongation, kinematic method, leaf elongation rate, leaf size, Lolium, mesophyll cells, tetraploid
INTRODUCTION
Cell division and cell expansion are fundamental processes for growth and development of plant organs. It is well known that polyploidy leads to an increase in organ size (Stebbins, 1971; Tal, 1980) which may be caused by changes in activities of cell division and expansion, resulting from duplication of gene loci and the increase in nuclear DNA content. However, the relative importance of cell division and of expansion in changing organ size as a consequence of polyploidy is unclear. The aim of this study was to determine how polyploidy affects cellular mechanisms and thereby changes leaf size, by comparing various cell parameters in diploid and autotetraploid cultivars of two species of the graminaceous genus, Lolium.
Lolium multiflorum (annual) and L. perenne (perennial) are grass species that grow widely in temperate regions. Although the two species are genetically closely related (Bulinska-Radomska and Lester, 1985), their leaf anatomical structures are distinctly different (Sant, 1969). Furthermore, many autotetraploid cultivars have been produced in these two species by colchicine treatment of diploid individuals (Wilkins, 1991). Since their original genetic composition has been maintained by controlled pollination, phenotypic traits of tetraploid cultivars are influenced by the direct effects of polyploidization as well as by the difference in genetic composition of a population. To evaluate the relative contribution of species, polyploidization and genetic composition of a population, three cultivars in each ploidy of the two species were used.
To understand the cellular basis of leaf growth and how polyploidy affects it via cell division and expansion processes, and to assess their relative importance, quantification of division and expansion is essential. Currently, the only approach capable of estimating cell division and elongation parameters is a kinematic method. Under the assumption of steady-state cell production in the leaf meristem, a kinematic method quantifies cell parameters, such as cell production rate, cell cycle duration and residence time of cells in the division and elongation zone, by measuring leaf elongation rate and spatial distribution of cell length along the growth axis (Gandar, 1980; Silk, 1984; Beemster et al., 1996). This method is particularly suitable for analysis of grass leaves for which expansion is mostly unidirectional and in which cell division and elongation occur in two distinct segments near the leaf base (Fiorani et al., 2000; Masle, 2000).
Epidermal and mesophyll cells are major cell types constituting leaf tissues. Epidermal cells have been used in most kinematic studies for measuring cellular parameters of leaf growth (Beemster et al., 1996; Fiorani et al., 2000; Masle, 2000). However, epidermal and mesophyll cells differ greatly in size and shape (MacAdam et al., 1989; Tardieu and Granier, 2000), and thus cell division and elongation activities of the epidermal layer may not necessarily reflect those of mesophyll cells, which occupy a large proportion of leaf tissues. In this study, cellular activities of epidermal and mesophyll layers in the leaf growth zone were compared. Since division parameters of the mesophyll layer were more consistent than those of the epidermal layer, the influence of ploidy on leaf size, including length and width, and the role of cell division and elongation were analysed using parameters of the mesophyll cell layer in the two species of Lolium.
MATERIALS AND METHODS
Materials and growth conditions
Three diploid and three tetraploid cultivars in each of L. multiflorum L. and L. perenne L. were used in this study. The diploid cultivars of L. perenne were ‘Manhattan II’, ‘Competitor’ and ‘Yorktown’, and the tetraploid cultivars were ‘Tove’, ‘Tonga’ and ‘Fantom’. The diploid cultivars of L. multiflorum were ‘Yama-aoba’, ‘Wase-aoba’ and ‘Excelent’, and the tetraploid cultivars were ‘Hitachi-aoba’, ‘Futaharu’ and ‘Giant’. All seeds of these cultivars were provided by a commercial seed company (Hokuren Co. Ltd, Sapporo).
Plants were grown in pots in a controlled growth chamber with day/night temperature of 22/16 °C and a photon flux of 250 µmol m−2 s−1 for a 16 h photoperiod (4 : 00 to 20 : 00 h); relative humidity was 70 % during the day and night. Seeds of all cultivars were germinated on wetted filter paper in plastic trays in the same growth chamber. When the coleoptile was at least 2 cm long, two seedlings were transplanted into a pot 10 cm in diameter and 8 cm in depth filled with sandy loam containing 0·35 g of N, 0·35 g of P2O5 and 0·35 g of K2O per 1 kg of soil. When leaf 2 appeared, seedlings were thinned to one per pot. The experiment was a randomized block design with six replications.
Leaf growth and cell parameters
The length of leaf 4 was measured on eight successive days between 12 : 00 and 13 : 00 h using a ruler, and taking the ligule of the preceding leaf as a reference point. After elongation had ceased, final leaf length and width at the midpoint were recorded. Measurements were made on one leaf per plant on six plants per cultivar. A linear regression of leaf length against the number of days after leaf appearance was calculated for each leaf, and the slope of the regression gave the leaf elongation rate (LER). The duration of leaf extension was determined for each individual leaf as the time interval between the day at which the leaf appeared above the ligule and the day at which 95 % of the final leaf length was attained.
When leaf 4 stopped its elongation (30 d after transplanting), the main stem was excised from the root, and leaf tissue surrounding the newly developing leaf (leaf 6) was carefully dissected away. The lowermost 50 mm of a developing leaf was removed with a razor blade from the shoot apex and placed in FAA fixative [formalin, acetic acid and 70 % ethanol in a ratio of 5 : 5 : 90 (v/v/v)] for storage and clearing of tissues. The leaf segment was mounted on an objective slide and covered with lactic acid.
The mesophyll in Lolium leaves consists of two layers: a single palisade-like layer under the upper (adaxial) epidermis, with tightly packed cells, and a spongy layer under the lower (abaxial) epidermis with cells arranged around the air spaces beneath the numerous stomata in the epidermis. Microscopic observation revealed that cells in the palisade-like layer were less variable in size and shape than those in the spongy layer. Therefore, cell files of the palisade layer, located between two adjacent vascular bundles, were used for measurement. Mesophyll cells are sometimes highly lobed in monocots such as wheat (Parker and Ford, 1982). Since, for a leaf consisting of lobed cells, it is difficult to distinguish lobes from cells by microscopic observation from the leaf surface, the shape of isolated mesophyll cells was examined after incubation of a mature leaf section of the fourth leaf of L. perenne (ManhattanII) and L. multiflorum (Wase-aoba) with polygalaturonase (Pectlyase Y-23) solution containing 0·7 m d-mannitol and 0·5 % dextran sulfate sodium adjusted to pH 5·8 for 24 h. Microscopic observation of isolated mesophyll cells did not reveal any lobed structure.
Cell lengths were measured at a magnification of ×400 using a light microscope (BX40 Olympus, Tokyo) equipped with Nomarsky interference contrast. Microscopic images were displayed on a 15 inch video screen connected to a CCD camera (DP11 Olympus, Tokyo). Cell length on the display was measured with a ruler and the actual size was calculated by comparison with an objective micrometer. A total of 25 cells in different fields were measured at intervals of 0·5 mm in the division zone and at intervals of 1·0 mm in the elongation-only zone from the leaf base to a point 40 mm distal along the longitudinal axis.
Cell length profiles of the epidermal layer were measured in ‘Wase-aoba’ for diploid and ‘Futaharu’ for tetraploid in L. multiflorum, and ‘ManhattanII’ for diploid and ‘Tonga’ for tetraploid in L. perenne. Measurements were made on the adaxial epidermis from the leaf base to a point 30 m distal from the leaf base at which cells had stopped elongating. Cell lengths were measured at intervals of 1·0 mm between 0 and 16 mm and 2·0 mm between 16 and 30 mm.
Widths of the leaf and of cell files along the transverse axis in the leaf growth zone were measured in ‘Wase-aoba’ for diploid and ‘Futaharu’ for tetraploid in L. multiflorum, and ‘ManhattanII’ for diploid and ‘Tonga’ for tetraploid in L. perenne. A total of 25 cell files in different fields were measured at every 2 mm from the leaf base. The number of cell files was calculated by dividing the leaf width by the average file width. Measurements of cell length and cell file width were made on six plants per cultivar.
Calculation of cell parameters
Original cell length data were smoothed using a 1 : 2 : 1 running mean (Schnyder et al., 1990) and cell division and elongation were calculated by the method of Fiorani et al. (2000). The cell length profile along the longitudinal axis reflects activities of cell division and expansion in the leaf growth zone (Fig. 1). Since the number of cells entering the elongation-only zone per unit time under steady-state growth conditions is equal to the number of cells leaving the zone, the cell production rate in the growth zone (P) is:
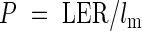 |
where lm is mature cell length. The length of the division zone (Ldiv) was calculated as the distance between the leaf base and the point of minimum cell length, while the length of the elongation-only zone (Lel) was calculated as the distance from the end of the division zone to the position at which the cell length reached 95 % of the mature length. The lengths of mature cells (lm) were calculated by averaging all data points distal to the position where the increases in cell length between two successive points were 0 µm. The numbers of cells in the division zone (Ndiv) and the elongation-only zone (Nel) were calculated from the reciprocal of cell length at each position by:
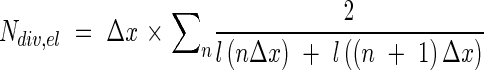 |
where l(x) is the cell length at each position, n = 1, 2, 3…, and Δx is a step of 500 µm for the division zone and 1000 µm for the elongation-only zone. Under steady-state growth conditions, the duration of the cell cycle can be considered as the time necessary for a cell population to double in number (Tardieu and Granier, 2000). Considering the exponential nature of the increase in cell number, the average duration of the cell cycle (Tc, in hours) was calculated (Ivanov and Dubrovsky, 1997) as:
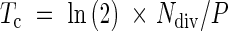 |
Fig. 1.
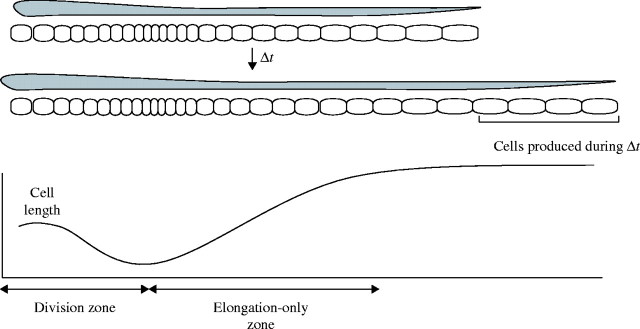
Diagram representing leaf elongation and changes in cell length profile in the leaf growth zone during a given time interval. The division and elongation-only zones of the growth zone can be identified by the spatial distribution of cell length. Under steady-state condition of cell production, the number of cells produced in the division zone during a given time (Δt) is equal to the number of cells leaving the elongation-only zone. Under this assumption, a kinematic method quantifies various cell parameters in the division and elongation-only zone (see Materials and Methods).
The residence time of the division zone (Tdiv) is represented by the product of the time per cell cycle and the number of cell cycles in the division zone. This relationship is represented as follows (Beemster et al., 1996):
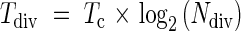 |
On the other hand, since the number of cells entering the elongation-only zone under steady-state growth conditions is equal to the number of cells leaving the zone, the residence time of cells in the elongation-only zone is given by
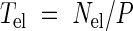 |
The cell elongation rate per unit cell length, also called the strain rate, at each position has been used as a parameter of cell elongation in most kinematic studies. However, since cell elongation is one-dimensional growth, as is the leaf elongation rate, the mean cell elongation rate per unit time (µm h−1, CER) was used in this study. CER averaged over the whole elongation-only zone can be obtained by dividing the elongated length (lm − ld) by the residence time of the elongation-only zone:
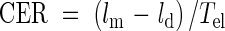 |
where ld is cell length at the end of the division zone.
Statistical analysis
In this study, 72 plants were examined for leaf elongation and cell parameters of the mesophyll (6 plants × 3 cultivars × 2 ploidy × 2 species). In order to assess the relative contribution of species, ploidy and cultivar, two-way analysis of variance (ANOVA) was applied. Total sum of square (SS) between 72 plants was partitioned into SS due to species, SS due to ploidy and SS due to cultivars within ploidy, and their relative proportions to the total SS were calculated. The least significant difference (LSD) was calculated by the Tukey–Kramer HSD test. Statistical analysis was performed by JMP (SAS Institute, USA).
RESULTS
Leaf length variation
Ploidy, species and cultivar explained 41·0, 7·3 and 10·5 % of the total variation in final leaf length, respectively, and hence ploidy had a predominant effect on leaf size determination compared with species and cultivar. The tetraploid cultivars had significantly longer leaves than did diploid cultivars in both species (Table 1). The final leaf lengths averaged over the three cultivars were 22·7 ± 0·81 cm for the diploid and 25·2 ± 0·79 cm for the tetraploid in L. multiflorum, and 16·7 ± 0·54 cm for the diploid and 26·1 ± 0·71 cm for the tetraploid in L. perenne.
Table 1.
Mean values for leaf growth and its cellular parameters in each ploidy of each species
|
L. multiflorum |
L. perenne |
% of SS |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2n |
4n |
2n |
4n |
Species |
Ploidy |
Cultivar |
||||||||
| Leaf growth | ||||||||||||||
| Leaf length (LL, cm) | 22·7b | 25·2ab | 16·7c | 26·1a | 7·3*** | 41·0*** | 10·5* | |||||||
| Leaf elongation rate (LER, mm h−1) | 1·82a | 1·95a | 1·37b | 1·97a | 10·9*** | 31·1*** | 12·9* | |||||||
| Duration of leaf extension (day) | 5·73a | 5·82a | 5·7a | 6·34 | 4·4* | 14·3** | 8·2* | |||||||
| Cell parameters | ||||||||||||||
| Mature epidermal cell length (le, µm) | 482·5b | 677·3a | 503·5b | 701·0a | 1·1 | 34·3*** | 3·5 | |||||||
| Mature mesophyll cell length (lm, µm) | 21·4b | 25·5a | 17·7c | 24·5a | 9·5*** | 48·7*** | 4·9 | |||||||
| Minimum cell length (ld, µm) | 6·74b | 7·82a | 6·17ab | 7·17c | 9·4** | 27·1*** | 4·7 | |||||||
| Cell elongation rate (CER, µm h−1) | 0·95bc | 1·23a | 0·80c | 1·05ab | 8·1** | 22·3*** | 7·3 | |||||||
| Cell production rate (P, h−1) | 86a | 78a | 77a | 81a | 0·3 | 0·1 | 17·5 | |||||||
| Cell cycle duration (Tc, h) | 7·3a | 7·5a | 7·4a | 7·6a | 0·2 | 0·1 | 32·1** | |||||||
| Length of the division zone (Ldiv, mm) | 9·1a | 9·3a | 7·9b | 9·9a | 0·4 | 7·8* | 28·6** | |||||||
| Length of the elongation zone (Lel, mm) | 16·3a | 17·3a | 12·6b | 19·0a | 1·8 | 11·9** | 11·5 | |||||||
| No. of cells in the division zone (Ndiv) | 923a | 890a | 827a | 883a | 1·2 | 0·1 | 19·6 | |||||||
| No. of cells in the elongation zone (Nel) | 1388a | 1127a | 1152a | 1420a | 0·7 | 4·1 | 7·1 | |||||||
| Residence time of the division zone (Tdiv, h−1) | 72·3a | 77·5a | 71·7a | 74·0a | 1·0 | 0·4 | 19·9 | |||||||
| Residence time of the elongation zone (Tel, h−1) | 16·3a | 14·8a | 15·0a | 17·6a | 0·4 | 2·5 | 13·9 | |||||||
The relative proportions of the sum of square (SS) due to species (d.f. = 1), ploidy (d.f. = 1) and cultivars within ploidy (d.f. = 8) to the total SS were shown.
*, ** and *** represent significance at the 5, 1 and 0·1 % level, respectively.
Different letters show significant differences by the Tukey–Kramer HSD test.
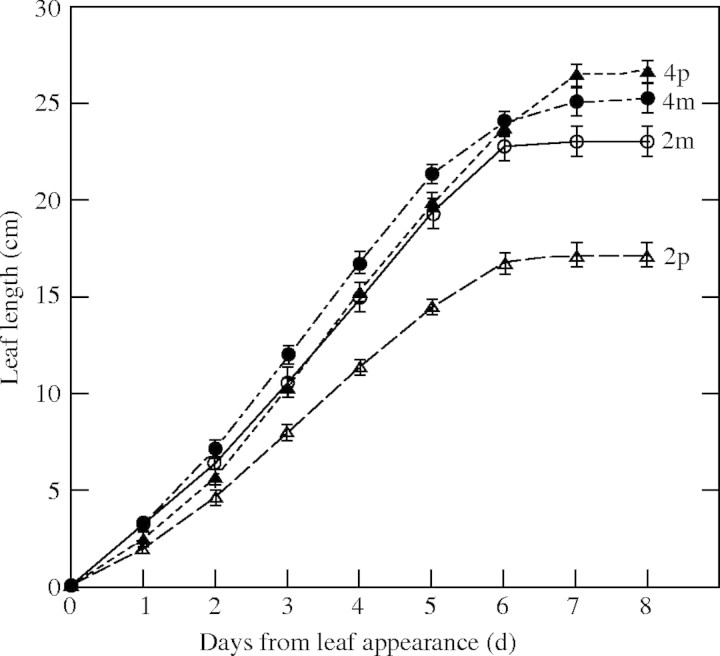
Final leaf length is determined by the rate and duration of leaf elongation. Ploidy explained 31·1 % of the variation in leaf elongation rate (LER) and 14·3 % for duration of leaf elongation, while cultivars explained 12·9 and 8·2 % of the variation, respectively. The relative contribution of species was 10·9 % for LER and 4·4 % for duration of leaf elongation. The tetraploid cultivars had significantly longer duration and higher LER than the diploid cultivars (Fig. 2). In particular, diploid L. perenne had a significantly lower LER than tetraploid cultivars. Correlations of leaf length with LER and with the duration of leaf elongation among 72 plants were r = 0·842 and r = 0·371, respectively.
Fig. 2.
Increase in blade length of leaf 4 as a function of time from leaf appearance for diploid and tetraploid cultivars of Lolium multiflorum and L. perenne. The mean leaf length averaged over three cultivars (n = 18) was shown in each ploidy of the two species. p2, p4, m2 and m4 represent the diploid and tetraploid of L. perenne and of L. multiflorum, respectively. Bars are ± s.e.
Cell length profile
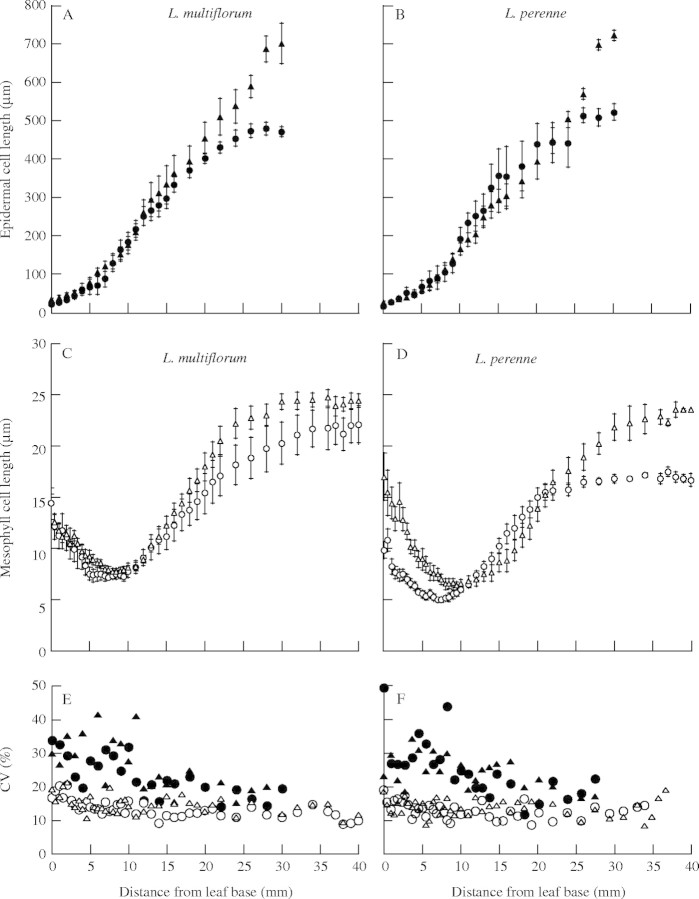
The length of cells in the epidermal and mesophyll layers of the leaf growth zone of diploid and tetraploid in the two species were markedly different (Fig. 3). Epidermal cell length, which was approx. 40 µm at the leaf base, increased progressively to approx. 500 µm at a point approx. 25 mm distal from the leaf base (Fig. 3A and B). In contrast, mesophyll cell length decreased from 1 to 10 mm from the base and then increased to 20–25 µm approx. 30 mm distal from the leaf base (Fig. 3C and D). The final length of the epidermal cells was 20-fold larger than that of mesophyll cells, while cell division was much greater for the mesophyll layer than for the epidermal layer, as shown by the length of the division zone. Tetraploid cultivars of both species had longer mature cells in both epidermal and mesophyll layers than did diploid cultivars (Table 1), so the effects of ploidy on cell length were consistent in the two cell types.
Fig. 3.
Cell length profiles of epidermal cells (A and B), cell length profile of mesophyll cells (C and D) and profile of CVs of cell length (E and F) in diploid (circles) and tetraploid (triangles) cultivars of L. multiflorum and L. perenne. Open symbols are mesophyll cells and closed symbols are epidermal cells. Data of a representative cultivar of each group are presented (n = 6). Bars are ± s.e.
Since cell division reduces cell length by half, if cell division does not occur synchronously between neighbouring cells, cell length in the division zone is generally more variable than in the elongation-only zone. The coefficient of variation (CV) in cell length differed greatly between epidermal and mesophyll cells (Fig. 3E and F). CVs of cell length in the epidermal layer were larger at the base of the leaf from 0 to 10 mm, where both cell division and elongation seem to occur, and decreased toward the distal position. On the other hand, CVs of the mesophyll layer were consistently smaller even for the basal zone in which active cell division occurs, suggesting synchronous cell division in the mesophyll layer.
Cellular basis of leaf length variation
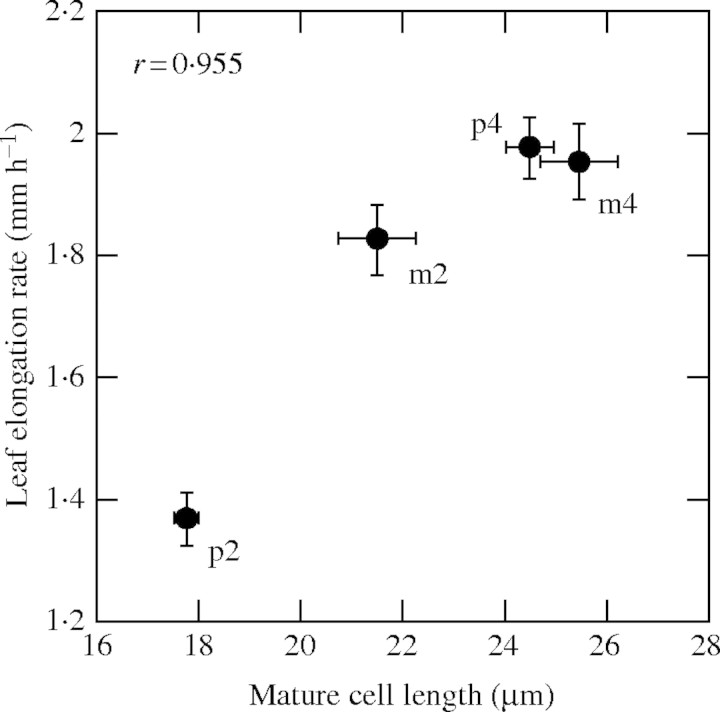
LER, which is a major component determining final leaf length, is determined by the two parameters, cell production rate and mature cell length. Mature cell length showed significant differences between species and with ploidy. Species, ploidy and cultivars explained 9·5, 48·7 and 4·9 % of the total variation of mature cell length, respectively. Ploidy had greater effects on mature cell length than did species and cultivars. The mean values of mature cell length averaged over the three cultivars were 25·5 ± 0·72 µm for tetraploid and 21·4 ± 0·77 µm for diploid of L. multiflorum, and 24·5 ± 0·48 µm for tetraploid and 17·7 ± 0·25 µm for diploid of L. perenne. On the other hand, there were no significant differences in cell production rate between species and with ploidy. The correlation between LER and mature cell length was significantly positive (r = 0·955) as shown in Fig. 4 and, hence, the difference in LER was due to the difference in mature cell length.
Fig. 4.
Relationship between leaf elongation rate and mature cell length. p2, p4, m2 and m4 represent diploid and tetraploid of L. perenne and of L. multiflorum, respectively (n = 18). Bars are ± s.e.
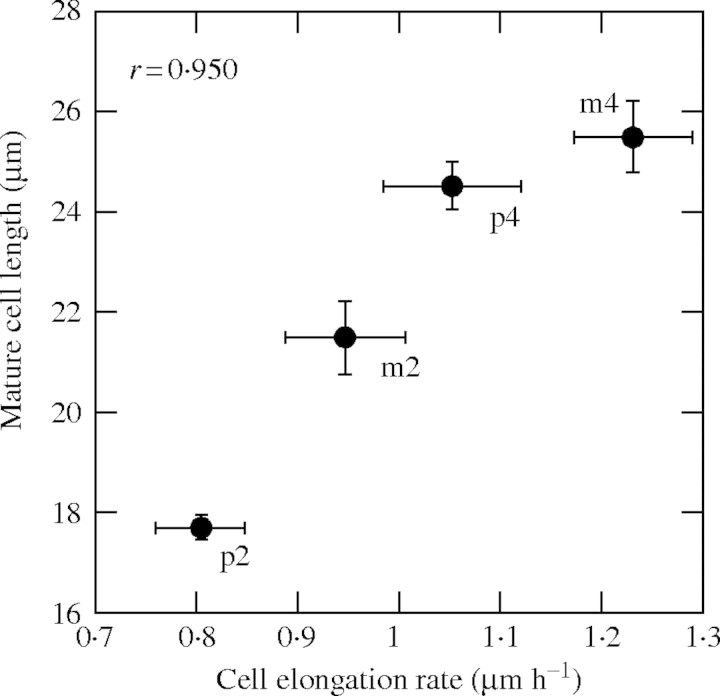
Mature cell length is determined by three parameters, cell length at the end of the division zone (minimum cell length), mean CER and residence time in the elongation-only zone. Although ANOVA showed a significant difference in minimum cell length between ploidy, the significant difference was found only in L. perenne (Table 1 and Fig. 3). There was also no significant difference between the residence time in the elongation-only zone in both species. However, tetraploid cultivars had significantly larger CER than diploid cultivars in both species. Ploidy explained 22·3 % of the variation in CER, while the proportions of the variation explained by species and cultivars were 8·1 and 7·3 %, respectively. Furthermore, mature cell length was very positively correlated with CER (r = 0·950; Fig. 5). These results demonstrate that the longer cells of the tetraploid cultivars were due to their faster cell elongation rate.
Fig. 5.
Relationship between mature cell length and cell elongation rate (n = 18). p2, p4, m2 and m4 represent the diploid and tetraploid of L. perenne and of L. multiflorum, respectively.
Other cell parameters such as cell production rate, number of cells and residence time in the division and elongation-only zones did not show any significant differences between species and with ploidy (Table 1). The cell cycle duration was significantly different between cultivars, but was not significantly different with ploidy.
Leaf width
Changes in leaf width, file width and the number of files along the transverse axis in the growth zone with species and ploidy are shown in Fig. 6. The tetraploid cultivas had wider leaves than did the diploid cultivars in both species (Fig. 6A), because the former had a wider cell file than the latter (Fig. 6B). However, L. multiflorum had consistently wider leaves than did L. perenne throughout the growth zone. The difference between leaf widths in the two species was much larger than the difference in the leaf width between ploidy. The wider leaves of L. multiflorum resulted from the increase in the number of files, not from the increase in file width (Fig. 6C). The difference in the numbers of files between the two species was already established at the leaf base (Fig. 6B).
Fig. 6.
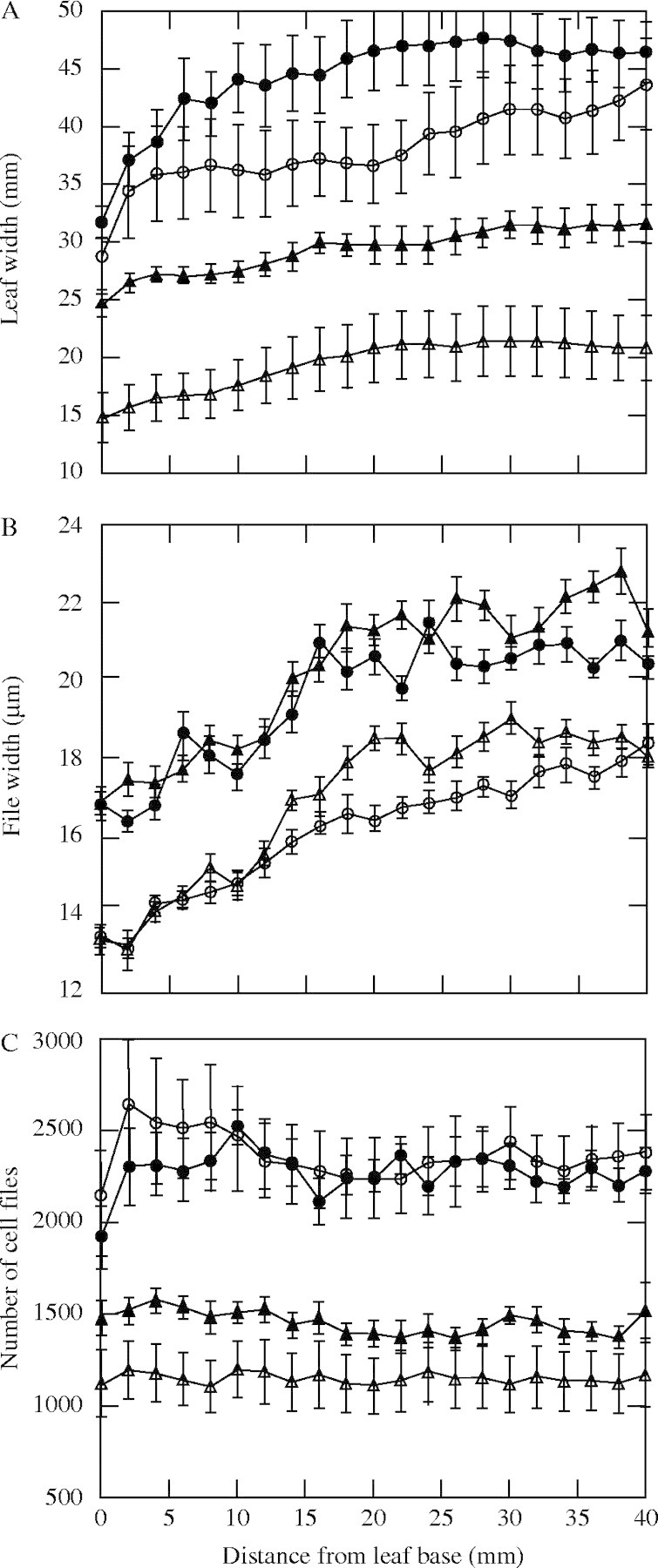
Profiles of leaf width (A), file width (B) and number of files (C) along the leaf growth zone in the diploid (open circles) and tetraploid (closed circles) of L. multiflorum and the diploid (open triangles) and tetraploid (closed triangles) of L. perenne. Bars are ± 1 s.e.
DISCUSSION
Leaf size variation
It is well known that polyploidy increases organ size in many species of dicots and monocots (Stebbins, 1971). In this study on closely related species of grasses, L. multiflorum and L. perenne, the tetraploid cultivars had significantly longer leaves than the diploid cultivars. The difference due to ploidy was particularly evident for L. perenne. The increase in leaf length can be brought about by an increase in LER and/or by the increase in the duration of leaf elongation. Although the tetraploid cultivars in the two species had both a faster rate and longer duration of leaf elongation than the diploid cultivars, the increase in leaf elongation rate was chiefly responsible for the increase in the leaf length.
LER is determined by two cell parameters, cell production rate and mature cell length. The present study shows that ploidy determined differences in LER by affecting cell length and not cell production rate. However, contrasting results on the relative importance of cell number and cell size in determining leaf size variation have been reported. Interspecific difference in LER among four Poa species was determined mainly by the difference in cell production rate (Fiorani et al., 2000). Cell division in the leaf meristem was responsible for changes in LER when plants were grown with different soil nutrients (MacAdam et al., 1989; Fricke et al., 1997) or different CO2 concentrations (Masle, 2000). However, a genotypic difference in the LER of Festuca arundinacea was due mostly to the difference in cell length (MacAdam et al., 1989). For mutants of barley differing in leaf length, there was no consistent relationships between leaf size and cell size or cell number (Wenzel et al., 1997).
Leaf width is another important component determining leaf area, and it was significantly different between the species and with ploidy. The tetraploid cultivars had wider leaves than the diploid cultivars in both species, because they had wider cell files than the latter. However, the difference between the species, which was determined by the difference in the file number, was larger than the ploidy difference. The differences in leaf width were already established at the leaf base (Fig. 6), mostly due to the difference in cell division and elongation of the shoot apical meristem during development of the leaf primordium (Beemster and Masle, 1996).
Cell mechanisms of leaf size variation
The longer leaves of the tetraploid cultivars were ascribed to longer mature cells in both species of Lolium. The proportional increase in epidermal cell size with ploidy in endoreduplication of Arabidopsis thaliana indicates the importance of polyploidy in cell size determination (Melaragno et al., 1993). The proportional increase in cell volume with increase in DNA content seems to contribute to balanced cell growth through maintenance of a constant ratio between the nuclear volume devoted to transcription and the cytoplasmic volume devoted to protein synthesis (Cavalier-Smith, 1985). The changes in cell size caused by polyploidy can be derived from two genetically different factors, duplication of gene loci and an increase in nuclear DNA content. Increasing ploidy alters expression of some genes in proportion, and this may involve determination of cell size (Galitski et al., 1999; Kondorosi et al., 2000; Lee and Chen, 2001). On the other hand, it has been suggested that the amount of DNA per cell has marked effects on cell size in a direct way that does not depend on the action of specific genes, which is known as the nucleotype effect (Bennett, 1972; Cavalier-Smith, 1985).
The difference in cell length due to ploidy becomes clear during cell elongation after mitosis (Fig. 3C and D). Cell growth of the leaf in the elongation zone is determined by the rate and duration of elongation. The strong positive correlation between mature cell length and elongation rate (Fig. 5) indicates that tetraploids attained longer cells through a faster rate of cell elongation which may involve cell wall loosening followed by a turgor-driven expansion of the wall matrix and deposition of cellulose microfibrils and other wall components on the cell surface (Nicol and Höfte, 1998; Cosgrove, 2003).
It has been suggested that greater DNA content leads to a slower cell cycle, because more time is required for DNA replication in S phase (Bennett, 1972; Dale, 1992). However, there was no significant difference between the cell cycle duration in the diploid and tetraploid cultivars, and thus the tetraploid (with duplicated chromosomes) does not necessarily need more time for DNA replication. No clear correlation between DNA content and cell cycle duration has been reported in Dactyis glomerata (Kinsman et al., 1996) or Poa (Fiorani et al., 2000). Also there were no significant effects of polyploidy on cell division parameters such as cell production rate, number of dividing cells and residence time in the division zone in this study. These results demonstrate that polyploidy has great effects on cell elongation processes and not on the cell division processes.
Epidermal and mesophyll cells
This study showed that tetraploid cultivars have consistently larger cell length than diploid cultivars in both epidermal and mesophyll cells, although the two cell types showed a 20-fold difference in mature cell length. Thus the effects of polyploidy on the mechanisms of cell elongation processes in the two cell types are similar. Most studies on cellular mechanisms of organ growth using a kinematic method applied to epidermal cells. However, the epidermal layer comprises different cell types, such as stomatal guard cells and trichromes, which show division and elongation patterns different from those of usual epidermal cells: this study showed that the cell length profile of the epidermal layer was more variable than that of the mesophyll layer (Fig. 3). Since a kinematic method assumes a steady-state cell production rate over the growth zone, small CVs of cell length in the mesophyll, which suggest synchronous cell division between neighbouring cells, make it possible to estimate consistent cell division parameters.
In conclusion, although it is well known that polyploidization leads to an increase in leaf size, the cellular mechanisms underlying leaf size change by ploidy have been unclear. The present study, which analysed cell division and expansion activities of mesophyll cells by a kinematic method, clearly showed that polyploidy increases leaf size mainly by increasing the cell elongation rate, but not the duration of the period of elongation, and that polyploidy does not necessarily lead to changes in cell division activities, such as cell production rate and cell cycle time. The present study also demonstrates the effectiveness of a kinematic method for analysis of cellular processes of organ growth.
Supplementary Material
Acknowledgments
This study was supported by the Japanese Ministry of Education, Culture, Science, Sport and Technology.
LITERATURE CITED
- Beemster GT, Masle J. 1996. The role of apical development around the time of leaf initiation in determining leaf width at maturity in wheat seedlings (Triticum aestivum L.) with impeded roots. Journal of Experimental Botany 4: 1679–1688. [Google Scholar]
- Beemster GT, Masle J, Williamson R, Farquhar G. 1996. Effects of soil resistance to root penetration on leaf expansion in wheat (Tritivum aestivum L.): kinematic analysis of leaf elongation. Journal of Experimental Botany 47: 1663–1678. [Google Scholar]
- Bennett M. 1972. Nuclear DNA content and minimum generation time in herbaceous plants. Proceedings of the Royal Society of London Series B: Biological Sciences 181: 109–135. [DOI] [PubMed] [Google Scholar]
- Bulinska-Radomska Z, Lester RN. 1985. Relationships between five species of Lolium (Poaceae). Plant Systematics and Evolution 148: 169–175. [Google Scholar]
- Cavalier-Smith T. 1985. Cell volume and the evolution of eukaryotic genome size. In: Cavalier-Smith T. eds. The evolution of genome size. Chichester. UK: John Wiley & Sons, 105–184. [Google Scholar]
- Cosgrove D. 2003. Expansion of plant cell wall. In: Rose JKC, ed. The plant cell wall. Oxford: Blackwell Publishing, 237–263. [Google Scholar]
- Dale J. 1992. How do leaves grow? Bioscience 42: 423–432. [Google Scholar]
- Fiorani F, Beemster GT, Bultynck L, Lambers H. 2000. Can meristem activity determine variation in leaf size and elongation rate among four poa species? A kinematic study. Plant Physiology 124: 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke W, McDonald JS, Mattson-Djos L. 1997. Why do leaves and leaf cells of Nnn-limited barley elongate at reduced rates? Planta 202: 522–530. [Google Scholar]
- Galitski T, Saldanha AJ, Styles CA, Lander ESS, Fink CR. 1999. Ploidy regulation of gene expression. Science 285: 251–254. [DOI] [PubMed] [Google Scholar]
- Gandar P. 1980. The analysis of growth and cell production in root apices. Botanical Gazette 141: 131–138. [Google Scholar]
- Ivanov VB, Dubrovsky JG. 1997. Estimation of the cell-cycle duration in the root apical meristem: a model of linkage between cell-cycle duration, rate of cell production, and rate of root growth. International Journal of Plant Science 158: 757–763. [Google Scholar]
- Kinsman EA, Lewis C, Davies MS, Young JE, Francis D, Thomas ID, Chorlton KH, Ougham H. 1996. Effects of temperature and elevated CO2 on cell division in shoot meristems: differential responses of two natural populations of Dactylis glomerata Plant, Cell and Environment 19: 775–780. [Google Scholar]
- Kondorosi E, Roudier F, Gendreau E. 2000. Plant cell-size control: growing by ploidy? Current Opinion in Plant Biology 3: 488–492. [DOI] [PubMed] [Google Scholar]
- Lee H, Chen ZJ. 2001. Protein-coding genes are epigenetically regulated in Arabidopsis polyploids. Proceedings of National Academy of Sciences of the USA 98: 6753–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAdam JW, Volence JJ, Nelson CJ. 1989. Effects of nitrogen on mesophyll cell division and epidermal cell elongation in tall fescue leaf blades. Plant Physiology 89: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masle J. 2000. The effects of elevated CO2 concentrations on cell division rates, growth patterns, and blade anatomy in young wheat plants are modulated by factors related to leaf position, vernalization, and genotype. Plant Physiology 122: 1399–14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaragno JE, Mehrotra B. Coleman AW. 1993. Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis Plant Cell 5: 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol F, Höfte H. 1998. Plant cell expansion: scaling the wall. Curent Opinion in Plant Biology 1: 12–17. [DOI] [PubMed] [Google Scholar]
- Parker ML, Ford MA. 1982. The structure of the mesophyll of flag leaves in three Triticum species. Annals of Botany 49: 165–176. [Google Scholar]
- Sant FI. 1969. A comparison of the morphology and anatomy of seedling leaves of Lolium multiflorum Lam. and L. perenne L. Annals of Botany 33: 303–313. [Google Scholar]
- Schnyder H, Seo S, Rademcher IF, Kuhbauch W. 1990. Spatial distribution of growth rates and of epidermal cell lengths in the elongation zone during leaf development in Lolium perenne L. Planta 181: 423–431. [DOI] [PubMed] [Google Scholar]
- Silk W. 1984. Quantitative descriptions of development. Annual Review of Plant Physiology 35: 479–518. [Google Scholar]
- Stebbins G. 1971. Chromosome evolution in higher plants. London: Arnold. [Google Scholar]
- Tal M. 1980. Physiology of polyploids. In: Lewis W, ed. Polyploidy—biological relevance. New York: Plenum Press, 61–75. [Google Scholar]
- Tardieu F, Granier C. 2000. Quantitative analysis of cell division in leaves: methods, developmental patterns and effects of environmental conditions. Plant Molecular Biology 43: 555–567. [DOI] [PubMed] [Google Scholar]
- Wenzel CL, Chandler PM, Cunningham RB, Passioura JB. 1997. Comparative leaf epidermal anatomy of mutants of barley (Hordeum vulgare L. ‘Himalaya’ which differ in leaf length. Annals of Botany 79: 47–52. [Google Scholar]
- Wilkins P. 1991. Breeding perennial ryegrass for agricutlure. Euphytica 52: 201–214. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.