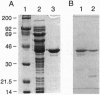
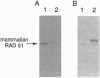
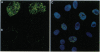
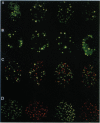
Abstract
Rad51 protein of Saccharomyces cerevisiae is a structural homolog of the Escherichia coli recombination enzyme RecA. In yeast, the Rad51 protein is required for mitotic and meiotic recombination and for repair of double-strand breaks in DNA. We have used antibodies raised against the homologous human protein, HsRad51, expressed in E. coli, to visualize the spatial distribution of the protein in mammalian somatic and meiotic cells. In cultured human cells, the HsRad51 protein is concentrated in multiple discrete foci in the nucleoplasm; it is largely absent from cytoplasm and nucleoli. After treatment of cells with methyl methanesulfonate, ultraviolet irradiation, or 137Cs irradiation, the percentage of cells with HsRad51 protein immunofluorescence increases; the same cells show unscheduled DNA synthesis. Induction of Rad51 foci is blocked by inhibitors of transcription. In mouse pachytene spermatocytes, the mouse homolog of Rad51 protein is highly enriched in synaptonemal complexes that are formed between the paired homologous chromosomes during meiotic prophase. We conclude that the mammalian proteins homologous to yeast Rad51 are involved in repair of DNA damage and recombinational repair during meiosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. K., Park D., Xu L., Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992 May 1;69(3):439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- Bishop D. K. RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell. 1994 Dec 16;79(6):1081–1092. doi: 10.1016/0092-8674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Brenner S. L., Hall J. M., Tousson A., Balczon R. D., Valdivia M. M. Arrangements of kinetochores in mouse cells during meiosis and spermiogenesis. Chromosoma. 1986;94(4):309–317. doi: 10.1007/BF00290861. [DOI] [PubMed] [Google Scholar]
- Haaf T., Machens A., Schmid M. Immunocytogenetics. II. Human autoantibodies to synaptonemal complexes. Cytogenet Cell Genet. 1989;50(1):6–13. doi: 10.1159/000132710. [DOI] [PubMed] [Google Scholar]
- Haaf T., Schmid M. Chromosome topology in mammalian interphase nuclei. Exp Cell Res. 1991 Feb;192(2):325–332. doi: 10.1016/0014-4827(91)90048-y. [DOI] [PubMed] [Google Scholar]
- Heyer W. D. The search for the right partner: homologous pairing and DNA strand exchange proteins in eukaryotes. Experientia. 1994 Mar 15;50(3):223–233. doi: 10.1007/BF01924005. [DOI] [PubMed] [Google Scholar]
- Heyting C., Dietrich A. J., Moens P. B., Dettmers R. J., Offenberg H. H., Redeker E. J., Vink A. C. Synaptonemal complex proteins. Genome. 1989;31(1):81–87. doi: 10.1139/g89-016. [DOI] [PubMed] [Google Scholar]
- Kowalczykowski S. C., Eggleston A. K. Homologous pairing and DNA strand-exchange proteins. Annu Rev Biochem. 1994;63:991–1043. doi: 10.1146/annurev.bi.63.070194.005015. [DOI] [PubMed] [Google Scholar]
- Loidl J. Cytological aspects of meiotic recombination. Experientia. 1994 Mar 15;50(3):285–294. doi: 10.1007/BF01924012. [DOI] [PubMed] [Google Scholar]
- Meuwissen R. L., Offenberg H. H., Dietrich A. J., Riesewijk A., van Iersel M., Heyting C. A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J. 1992 Dec;11(13):5091–5100. doi: 10.1002/j.1460-2075.1992.tb05616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Yoshimura Y., Yamamoto A., Murata K., Mori M., Yamamoto H., Matsushiro A. A mouse homolog of the Escherichia coli recA and Saccharomyces cerevisiae RAD51 genes. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6577–6580. doi: 10.1073/pnas.90.14.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmore R., Cao L., Kleckner N. Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell. 1991 Sep 20;66(6):1239–1256. doi: 10.1016/0092-8674(91)90046-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Helical interactions in homologous pairing and strand exchange driven by RecA protein. J Biol Chem. 1991 Mar 25;266(9):5355–5358. [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Roeder G. S. Chromosome synapsis and genetic recombination: their roles in meiotic chromosome segregation. Trends Genet. 1990 Dec;6(12):385–389. doi: 10.1016/0168-9525(90)90297-j. [DOI] [PubMed] [Google Scholar]
- Selig S., Okumura K., Ward D. C., Cedar H. Delineation of DNA replication time zones by fluorescence in situ hybridization. EMBO J. 1992 Mar;11(3):1217–1225. doi: 10.1002/j.1460-2075.1992.tb05162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara A., Ogawa H., Matsuda Y., Ushio N., Ikeo K., Ogawa T. Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat Genet. 1993 Jul;4(3):239–243. doi: 10.1038/ng0793-239. [DOI] [PubMed] [Google Scholar]
- Shinohara A., Ogawa H., Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992 May 1;69(3):457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- Tamm I., Hand R., Caliguiri L. A. Action of dichlorobenzimidazole riboside on RNA synthesis in L-929 and HeLa cells. J Cell Biol. 1976 May;69(2):229–240. doi: 10.1083/jcb.69.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C. Enzymes and molecular mechanisms of genetic recombination. Annu Rev Biochem. 1992;61:603–640. doi: 10.1146/annurev.bi.61.070192.003131. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y., Morita T., Yamamoto A., Matsushiro A. Cloning and sequence of the human RecA-like gene cDNA. Nucleic Acids Res. 1993 Apr 11;21(7):1665–1665. doi: 10.1093/nar/21.7.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenvirth D., Arbel T., Sherman A., Goldway M., Klein S., Simchen G. Multiple sites for double-strand breaks in whole meiotic chromosomes of Saccharomyces cerevisiae. EMBO J. 1992 Sep;11(9):3441–3447. doi: 10.1002/j.1460-2075.1992.tb05423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wettstein D., Rasmussen S. W., Holm P. B. The synaptonemal complex in genetic segregation. Annu Rev Genet. 1984;18:331–413. doi: 10.1146/annurev.ge.18.120184.001555. [DOI] [PubMed] [Google Scholar]