Abstract
This review describes how a cascade of associative relationships involving the sensory properties of foods, the nutritional consequences of their consumption and perceived internal states may play an important role in the learned control of energy intake and body weight regulation. In addition, we describe ways in which dietary factors in the current environment can promote excess energy intake and body weight gain by degrading these relationships or by interfering with the neural substrates that underlie the ability of animals to use them to predict the nutritive or energetic consequences of intake. We propose that an expanded appreciation of the diversity of orosensory, gastrointestinal, and energy state signals about which animals learn, combined with a greater understanding of predictive relationships in which these cues are embedded, will help generate new information and novel approaches to addressing the current global problems of obesity and metabolic disease.
A peculiarity about the current widespread problem of obesity (Kimokoti & Millen, 2011; B. A. Swinburn et al., 2011) is that almost everyone knows how to stop and even reverse it. There is little doubt that “Eat right and exercise”, a health profession mantra for more than 2000 years (Hippocrates, 460-377 BC)1, describes a virtually foolproof method to not only manage body weight but to maintain overall good health. We are frequently reminded to follow this advice by celebrities, political figures, government and private advertising campaigns, and by our parents, loved ones, and friends. While it may be good advice, statistically most adults in the United States (e.g., Kapetanakis et al., 2012; Majer, Mackenbach, & van Baal, 2013; Sturm & Hattori, 2013), along with an increasing proportion of the global population (e.g., Finucane et al., 2011; Malik, Willett, & Hu, 2013) appear unable to follow it.
Why has this seemingly sound advice proven futile? One explanation that has become popular during the past 30 years is that physiological mechanisms that evolved to prevent weight gain by closely matching energy intake with energy expenditure are being overwhelmed by our current “obesogenic” environment (e.g., Corsica & Hood, 2011; King, 2013). The term obesogenic environment” refers to “the sum of influences that the surroundings, opportunities “or conditions of life have on promoting obesity in individuals or populations’ (Corsica & Hood, 2011; King, 2013; B. Swinburn & Egger, 2002). It is true that many of us live in places where energy dense, highly palatable foods and beverages are abundant and available at relatively low cost. Widespread advertising and inescapable sophisticated marketing techniques are designed to keep thoughts of these foods and beverages almost constantly in mind. It is now a commonly-held view that these types of food-related stimuli contribute to an “obesogenic”, or obesity-promoting, environment as a result of becoming conditioned to powerful rewards produced by eating (Birch & Anzman, 2010; Chaput, Klingenberg, Astrup, & Sjodin, 2011; Cohen & Babey, 2012). Because these kinds of cues are now so prevalent and because the conditioning is so strong, both food approach and food consumption continue to be elicited even after energy homeostasis has been achieved and further caloric intake is in excess of energy needs.
Consistent with this idea are a number of early studies of “resistance to satiation” that took advantage of the extensive experimental control provided by animal models to probe aspects of learning related to food intake (e.g., Capaldi, Davidson, & Myers, 1981; Capaldi & Myers, 1979; Morgan, 1974). In those studies, both appetitive and consummatory responses that had been strongly conditioned to food rewards in animals when they were food restricted (i.e. “hungry”) continued to be evoked by environmental cues even after animals were food sated. Weingarten (1983) recapitulated these findings within a Pavlovian framework, in a very influential paper that showed that a cue that was trained as conditioned stimulus (CS) for a food unconditioned stimulus (US) when rats were hungry continued to evoke conditioned food cup approach responses after the rats had been food sated. This study has been widely cited as evidence for the idea that environmental cues conditioned to food rewards can contribute to obesity by overriding physiological controls that normally inhibit intake (Berthoud, 2011; Epstein, Lin, Carr, & Fletcher, 2012; Petrovich, 2011).
While the findings of Weingarten and earlier researchers demonstrate that previously conditioned environmental cues can evoke appetitive and consummatory responses even when rats are sated, there are reasons to question whether this simple type of conditioning is an important contributor to obesity. For example, while food-related conditioned cues can evoke eating behavior acutely, these cues are apparently unable to evoke enough eating to promote weight gain over the long-term. The results of two recent studies support this point. Boggiano, Dorsey, Thomas, & Murdaugh (2009) and Reppucci & Petrovich (2012) both reported body weight data for rats that had been trained to exhibit cue-induced eating. In neither study did rats that exhibited extensive cue-induced feeding also exhibit increased weight gain relative to control rats that did not receive cue-exposure training. Berthoud (2012) also reviewed this literature and found no animal or human studies which directly established that long-term exposure to conditioned food cues leads to obesity. Thus, it seems that overeating evoked by environmental conditioned stimuli at one time point may be compensated for by reduced eating later on. Furthermore, other recent analyses of human data question a direct link between exposure to obesogenic environments and excess energy intake. For example, a meta-analysis by Giskes, van Lenthe, Avendano-Pabon, & Brug (2011) reported that there was little relationship between the prevalence of obesogenic environments (e.g., replete with energy dense, palatable foods) and the actual dietary behavior of people who live in those environments. And while living in an obesogenic environment is strongly correlated with body mass index (BMI), this correlation is by no means perfect. Many people who live in obesogenic environments maintain healthy body weights without apparent difficulty. Thus, it appears that the view of obesity as a consequence of simple excitatory conditioning of environmental food-related cues is incomplete on both empirical and theoretical grounds.
The purpose of the present paper is to summarize our account of the learning and memory mechanisms that underlie energy and body weight regulation and to review many of our experimental findings which show that dietary factors prominent in the obesogenic environment can alter, on associative grounds, the operation of those mechanisms. However, instead of identifying the obesogenic environment with learning so strong that it overwhelms the body’s normal regulatory controls, we propose that it is a weakening of the learned controls of intake that underlies excess food intake and body weight gain. From our perspective, these factors that act to compromise the learning mechanisms that contribute to energy and body weight regulation are, in fact, what make the current environment obesogenic. Thus, within our model, the learned control of intake and body weight involves more than the formation of simple excitatory associations between a CS and a US or between a stimulus and a response. The control of feeding behavior also depends on the formation of inhibitory associations and on learning to use other types of stimuli that enable animals to predict when food–related CSs will and will not be followed by appetitive post-ingestive outcomes. Including a role for these stimuli and complex associative relationships generates some novel answers to the question, “What makes the current environment obesogenic”? In recent papers, we have addressed other implications of this model, including the effects of these environmental factors on brain substrates for learning and memory (Davidson, Sample, & Swithers, 2014) and on cognitive processes in humans that may contribute to the regulation of energy intake and body weight (Martin & Davidson, 2014).
Learning and energy regulation—cephalic phase responses
In his foundational work on conditioning, Pavlov referred to salivary responses as “psychic secretions” (see Todes, 1997) in reference to the fact that they could be elicited not only by direct contact with food in the mouth, but also by the sight of food. The term “psychic secretions” has been replaced by “cephalic phase reflexes” (e.g., Zafra, Molina, & Puerto, 2006) in the modern literature. In addition to salivation, cephalic phase reflexes include a variety of hormonal, metabolic, and enzymatic reactions that are evoked by gustatory, visual, and auditory cues associated with food and with thoughts of food.
Cephalic phase responses are now understood to promote energy homeostasis by preparing the digestive system for the reception, digestion, absorption and optimal processing of food (e.g., Power & Schulkin, 2008; Smeets, Erkner, & de Graaf, 2010; Woods & Ramsay, 2000). Woods & Ramsay (2000) proposed that energy regulation may be critically dependent on Pavlovian conditioning of cephalic phase reflexes. They reviewed the evidence on the learned controls of both food and drug intake and concluded that “when individuals are able to predict accurately that a regulated bodily parameter is going to be altered by an external event, they can learn to initiate a conditioned response in anticipation of the perturbation that minimizes its impact. We believe that this general learning mechanism is an integral component of any centrally regulated system” (p. 175). As a result, it is possible that even small, consistent perturbations in parameters involved with energy homeostasis could lead to significant weight gain over the long-term (Katan & Ludwig, 2010).
A basis for the elicitation of cephalic phase responses by taste, flavor and other stimuli has been provided by research demonstrating the ability of animals to associate such sensory cues with an appetitive, post-ingestive unconditioned stimulus (US). For the purposes of our present analysis, we define this US as a biologically significant, post-oral, interoceptive sensory event, that is produced by the arrival of calories in the gut. The intensity of this US depends, within limits, on factors such as the speed and degree to which its serves to produce recovery from energy deficit. Evidence for associations based on learning about post-ingestive USs has been obtained within flavor-nutrient conditioning preparations, in which an animal comes to prefer a flavor that has been repeatedly paired with the intake of foods or fluids of relatively high compared to lower energy density or with a caloric US that is infused directly into the stomach or upper intestine (Brunstrom & Mitchell, 2007; Sclafani, 2004; Yeomans, Leitch, Gould, & Mobini, 2008). Forming these associations enables animals to predict the post-ingestive consequences of consuming a particular flavors or tastes. We propose that the inability to make these kinds of predictions reliably may be a significant contributor to the global increase in obesity.
Based on longstanding principles of Pavlovian conditioning (e.g., Rescorla, 1968, 1988; Waldmann, Schmid, Wong, & Blaisdell, 2012), the stronger the contingency between the presentation of an orosensory CS and the occurrence of a post-ingestive US, the greater the ability of the orosensory CS to excite the memorial representation of that post-ingestive US, and the more strongly that CS will evoke conditioned cephalic phase and other responses (e.g., Bouton & Moody, 2004; Pickens & Holland, 2004). Conversely, weakening this contingency should reduce the evocation of those conditioned responses (Bills, Dopheide, Pineno, & Schachtman, 2006) by reducing the ability of the orosensory CS to excite the representation of its post-ingestive outcome. Therefore, if cephalic phase responses contribute to efficient energy regulation, weakening the contingency between orosensory CSs and post-ingestive USs should have a negative impact on the control of food intake, body weight, and other homeostatic responses. Research in our laboratories confirms that dietary factors that are now ubiquitous in the current environment can reduce the ability of sweet and fatty taste cues to serve as strong signals for post-ingestive caloric outcomes.
Sweet-taste→calorie predictive relations
Until relatively recently, when sweet tasting food and beverages were consumed they were almost always accompanied by the arrival of calories in the gastrointestinal tract, with the intensity of sweetness strongly and positively correlated with energy density. For most of us, our first experience with this relationship occurred very early in life. However, based on elementary principles of Pavlovian conditioning (see Wasserman & Miller, 1997) one way to weaken the validity of sweet taste as a signal for calories would be to consume sweet-tasting, but low- or no-calorie foods and beverages. Beginning in the 1960s, the rapid and widespread proliferation of non-caloric sweeteners like saccharin, aspartame and sucralose in foods and beverages has introduced precisely this type of relationship into the Western diet. In essence, this could be viewed as a situation in which our early experience with sweet taste is continuously reinforced followed by experience later in life in which sweet taste is followed only intermittently by caloric consequences. Previous research in runway studies showed that continuous reinforcement followed by partial reinforcement leads to a reduction in the strength of conditioned appetitive responding (e.g., Sutherland, Mackintosh, and Wolfe, 1965). Our recent research indicates that adverse effects of consuming artificial sweeteners on sweet taste cue validity may be one factor that has helped create the current obesogenic environment.
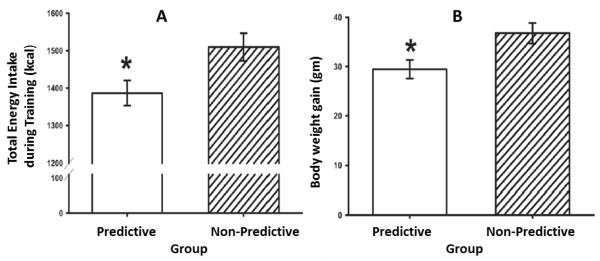
A series of experiments provided evidence for this hypothesis and shed light on the nature of both the physiological and associative mechanisms that may make intake of non-caloric sweeteners obseogenic. In one such experiment, Swithers & Davidson (2008) gave adult male rats maintained on a standard laboratory chow diet a daily supplement of 30 g of plain yogurt to which caloric or non-caloric sweeteners (0.3% saccharin) had been added. In rats, this concentration is at or near the peak of the preference function (Sclafani, Bahrani, Zukerman, & Ackroff, 2010). For one group, plain yogurt and yogurt sweetened with 20% glucose were provided so that animals had equal exposure to the unsweetened, lower calorie (0.6 kcal/gm) yogurt and the sweetened, higher calorie (1.2 kcal/gm) yogurt. We referred to this group as the Predictive group because sweet taste was a reliable signal for increased caloric density in the yogurt. In a second, Non-Predictive group, both the plain yogurt and the saccharin-sweetened yogurt were equal in calories (0.6 kcal/g). Thus, sweet taste did not predict increased calories for that group. For each group, exposure to the different sweet taste-calorie relationships took place daily over a 14-day training period with each type of yogurt (plain or sweetened) presented on an equal number of days, and animals in both the Predictive and Non-Predictive groups consumed identical quantities of the yogurt supplements. By the end of this training period, we found that rats in the Non-Predictive group had consumed significantly more total energy (their supplements + their chow maintenance diets; see Figure 1, Panel A) and had gained significantly more weight (Figure 1, Panel B) compared to the Predictive group. This result was obtained despite the fact that for the Predictive group the glucose sweetened yogurt supplement contained more calories than did the saccharin-sweetened yogurt that was given to the Non-Predictive group.
Figure 1.
(adapted from Swithers & Davidson, 2008). Panel A: Total energy intake during 14 days of consumption of sweet Predictive or sweet Non-Predictive yogurt diets. Panel B: Body weight gain during 14 days of consumption of sweet Predictive or sweet Non-Predictive yogurt diets. *p. < 0.05 compared to Sweet Non-Predictive.
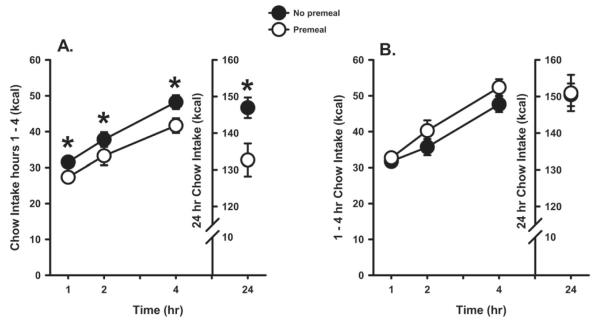
We then showed that one effect of this training for the Non-Predictive group was that they now had a reduced ability to compensate for calories contained in a novel sweet caloric food by reducing subsequent intake of chow after consuming this sweet “snack”. Rats in both groups were food deprived overnight before being tested on two occasions. For the test sessions, rats were given either 30 min access to a novel, sweet, high calorie premeal comprising 5g of chocolate Ensure Plus (with 2% guar added to approximate viscosity of yogurt diets) or 30 min with no premeal. The order of the premeal and no premeal tests was counterbalanced. Chow intake was assessed 1, 2, 4 and 24 hr following the completion of the premeal. All rats ate all of the Ensure that was given during the premeal. Figure 2 shows that total caloric intake (premeal + chow intake) was not significantly different between the premeal and no premeal tests for the rats in the Predictive group (Panel A). In other words, rats in the Predictive group compensated almost completely for the calories contained in a novel sweet-tasting premeal by reducing their lab chow intake during the subsequent test period. In contrast, total caloric intake for the Non-Predictive group (Panel B) was significantly greater on the days when they consumed the novel sweet-tasting premeal compared to the no premeal day, suggesting that animals in this group were unable to use the sweet taste of the Ensure to predict the energy delivered by the premeal. Consequently, compared to rats in the Predictive group, rats in the Non-Predictive group showed weaker compensation for the calories contained in a sweet premeal that they consumed for the first time. Similar results were obtained in a study examining the consequences of providing savory snack chips made with regular high-calorie fat compared to a non-caloric fat substitute; animals that experienced a predictive relationship between potato chips and calories showed more complete compensation for calories contained in a novel high-fat snack chip than animals given a non-predictive relationship between potato chips and calories (Swithers, Doerflinger, & Davidson, 2006).
Figure 2.
(adapted from Swithers & Davidson, 2008). Chow intake following a novel sweet premeal or no premeal in Sweet Predictive (A) or Sweet Non-Predictive (B) rats. * p.< 0.05 compared to premeal.
The findings of several additional studies lend credence to the idea that consumption of non-caloric sweeteners alters the ability of sweet taste to evoke cephalic phase responses. Previous research showed that ingestion of food evokes a reflexive thermogenic response (Jequier, 1983; Tappy, 1996) and this form of heat production can be evoked by pre-absorptive (e.g., orosensory) food cues. For example, when food is tasted but not swallowed, the magnitude of the thermic response is comparable and can even exceed that produced by normal food intake (Diamond, Brondel, & Leblanc, 1985; Leblanc & Cabanac, 1989). Conversely, when nutrients bypass the oropharyngeal cavity and are administered directly into the stomach via gavage or feeding tube (Diamond et al., 1985; Hashkes, Gartside, & Blondheim, 1997; LeBlanc, Cabanac, & Samson, 1984) cephalic phase thermogenic responses are either not observed or are much weaker than those produced by normal feeding. Moreover, previous reports showed that thermogenesis in response to oral intake of a palatable food is blunted in obese compared to non-obese humans (Hashkes et al., 1997). Swithers and Davidson (2008) reasoned that if sweet taste evokes thermic responses based, in part, on the degree to which they predict caloric post-ingestive consequences, one might expect that for rats that have had the validity of sweet taste→calorie relationship reduced by consuming non-caloric sweeteners, tasting a sweet, high-calorie food would evoke a weaker thermogenic response.
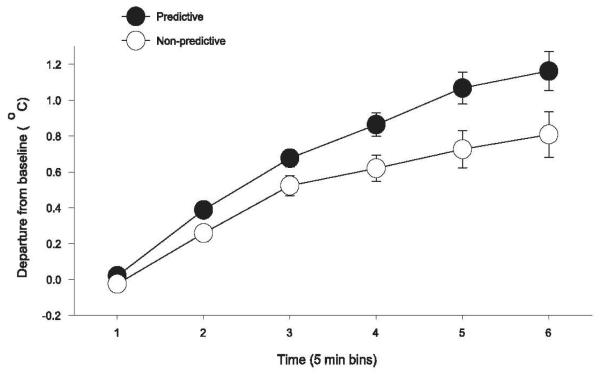
To test this possibility, we implanted small radio frequency transmitters in the abdominal cavities of two groups of rats prior to training them with caloric or non-caloric sweeteners as described above for Group Predictive and Group Non-Predictive. These transmitters supplied minute-to-minute body temperature readings during testing when each rat consumed a small (5g) premeal of a novel sweet, high calorie chocolate Ensure Plus. Figure 3 shows that the thermogenic response produced by consuming the premeal was attenuated for rats that had been trained with the non-predictive sweet taste→calorie relationship compared to controls for which sweet taste remained a remained a valid signal for calories. Given that total number of calories consumed in the premeal was the same for both groups, a weakened thermogenic response suggests that those calories were not being utilized as efficiently by the Non-Predictive compared to the Predictive group.
Figure 3.
(adapted from Swithers & Davidson, 2008). Changes in core body temperature over the first 60 min following yogurt presentation during Sweet Predictive (A) or Sweet Non-Predictive (B) training. * p. < 0.05 compared to unsweetened yogurt.
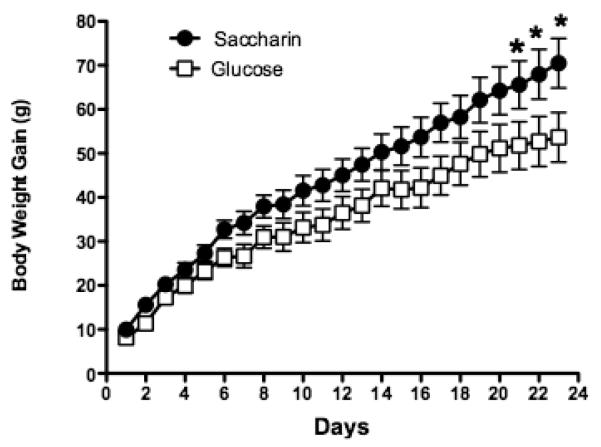
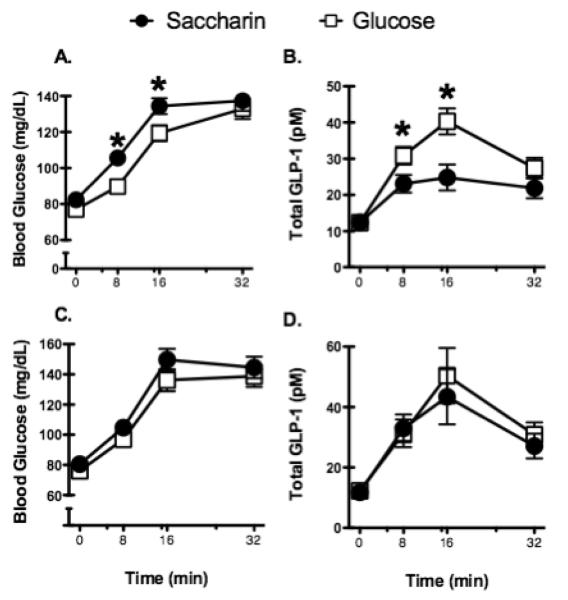
We also observed effects of consuming saccharin on glucoregulatory and hormonal responses to sweet tastes. Confirming earlier findings (e.g., Swithers, Baker, & Davidson, 2009; Swithers & Davidson, 2008; Swithers, Laboy, Clark, Cooper, & Davidson, 2012) showed that by the end of 24 days of exposure, rats given a daily supplement of saccharin-sweetened yogurt gained significantly more weight than rats that received yogurt sweetened with glucose during the same period (see Figure 4). Rats in both groups were then given fasting glucose tolerance tests under two conditions. In one condition, the rats consumed 5g of a 20% glucose solution by mouth, while in the other, the same glucose solution was delivered directly to the stomach via gavage. Blood samples were collected from each rat immediately prior to each of these two methods of glucose exposure and then again at 8, 16, and 32 minutes after the exposure. We found that the results of the glucose tolerance test depended on whether the rats (a) were trained with either saccharin- or glucose-sweetened yogurt and (b) consumed their glucose load orally or received it via gavage during glucose tolerance testing. Figure 5 (Panel A) shows that for rats that had been given saccharin-sweetened yogurt during training, blood glucose levels were elevated at 8 and 16 minutes following the oral glucose test, compared to rats that had received glucose-sweetened yogurt during training. That is, relative to experience with glucose-sweetened diets, experience with the saccharin-sweetened diet appeared to impair glycemic responses. This difference was not observed when glucose was delivered directly to the stomach (Figure 5C), supporting the hypothesis that it is learning to predict the outcome based on the sweet taste, and not in response to post-oral or post-absorptive stimuli, that is altering these physiological outcomes.
Figure 4.
(adapted from Swithers, Laboy, Clark, Cooper & Davidson, 2012). Body weight gain was significantly greater in animals given a saccharin-sweetened solution compared to animals given a glucose-sweetened solution when a high-fat, high sugar maintenance diet was provided. * p. < 0.05 compared to the glucose group.
Figure 5.
(adapted from Swithers, Laboy, Clark, Cooper & Davidson, 2012). Blood glucose levels were significantly higher in animals previously given access to a saccharin-sweetened solution when animals were allowed to consume 5 ml of a 20% glucose solution orally (A) but not when the same solution was delivered directly into the stomach by gavage (C). Levels of total GLP-1 were significantly lower 8 and 16 min following presentation of the glucose solution orally (B) but not following delivery of the glucose solution directly into the stomach by gavage (D). * p < 0.05 compared to glucose group.
Panel B of Figure 5 shows that circulating levels of the incretin hormone GLP-1 were significantly reduced for the saccharin-exposed group, relative to the glucose-trained group, at 8 and 16 minutes, the same time points that showed elevated glucose levels. Again, this did not occur when the oral stimulus was omitted by administering glucose directly to the stomach (Figure 5D). This finding may provide at least part of the explanation for the relative hyperglycemic response and excess weight gain shown by the saccharin-exposed group. Release of GLP-1 is known to inhibit emptying of the contents of the stomach into the intestine (i.e., gastric empyting). Thus, reduced secretion of GLP-1 in response to an oral glucose load could augment gastric emptying which would produce more rapid delivery of the oral glucose load to the intestines, and more rapid elevations of blood glucose levels. GLP-1 is also known to enhance glucose metabolism in skeletal muscle, liver and adipose tissue, and to suppress release of glucagon, all of which tie decreased GLP-1 to increased levels of glucose in the blood. With respect to body weight and intake regulation, both peripheral and central actions of GLP-1 during meals have been directly linked to satiety (e.g., Barrera et al., 2011; Dailey & Moran, 2013; Flint, Raben, Astrup, & Holst, 1998; Hayes, De Jonghe, & Kanoski, 2010). Decreased GLP-1 release in response to orosensory cues, such as sweet taste, due to weakened predictive ability (as in the saccharin-trained group) may lead to reduced satiety, increased intake and subsequent weight gain. Within our associative framework, experience with consuming a sweet taste that is not followed by the anticipated nutritive consequence would cause the sweet taste to become less effective at eliciting release of GLP-1 over time. Release of GLP-1 by sweet taste in the mouth would then become blunted even when caloric sweeteners are subsequently consumed.
Further support for this conceptualization is shown in the bottom portion of Figure 5. When the glucose load was delivered by gavage and thus bypassed taste receptors in the oral cavity, neither blood glucose levels (Panel C) nor GLP-1 release (Panel D) was influenced by prior exposure to saccharin. These findings suggest that the effects of prior exposure to saccharin on the ability of a glucose load to increase blood glucose levels and decrease GLP-1 release depended on a reduced ability of sweet taste in the mouth to evoke these responses.
These results clearly demonstrate that a prior history of consuming non-caloric sweeteners impaired glucoregulation and at least some hormonal responses typically evoked by orosensory contact with sweet caloric solutions and associated with regulation of ingestive behavior and metabolic processes. However, when those caloric solutions bypassed the mouth and were delivered directly to the stomach, prior experience with non-caloric sweeteners had little effect on those responses. These findings are consistent with our hypothesis that consuming non-caloric sweeteners can weaken the validity of sweet taste as a signal for caloric post-ingestive outcomes, and thereby reduce the strength of anticipatory cephalic phase responses that contribute to energy regulation.
The results of the studies reported in this section using an intact animal model, are consistent with findings from previous studies that employed a sham-feeding preparation in which the implantation of fistula in the esophagus or stomach prevented nutrients taken by mouth from being absorbed in gastrointestinal tract. For example, in an experiment with rats, Van Vort & Smith (1987) reported that as a result of prior experience with either flavor or location during sham feeding of milk, those flavors and locations produced a conditioned increase in meal size during subsequent real feeding with milk. The authors attributed this outcome to a decrease in the satiating potency of the milk. One reason for this decrease in satiating potency may have been because sham-feeding produced at least partial extinction of cephalic phase responses. Naim, Kare, & Merritt (1978) reported that cephalic phase responses extinguished rapidly in dogs that were allowed consume and swallow sweet tasting fluids that were drained from the GI tract prior to absorption via open gastric and intestinal fistulas.
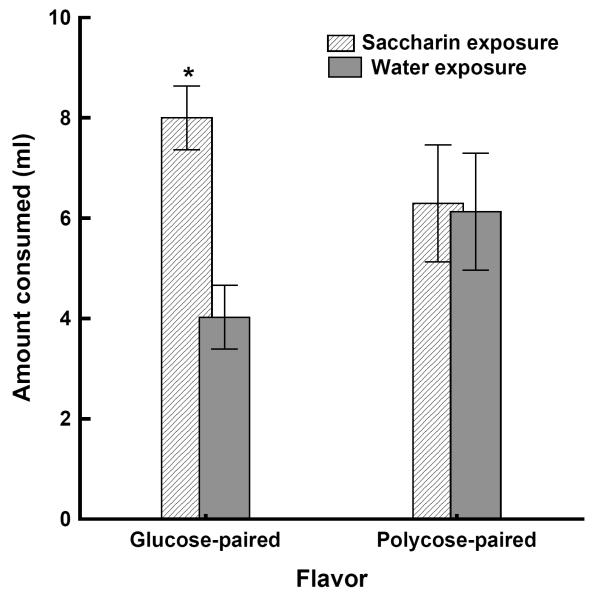
Another recent set of studies provided more direct evidence that oral consumption of non-caloric sweeteners weakens the ability of sweet taste to predict caloric consequences (Davidson, Martin, Clark, & Swithers, 2011). The first experiment was based on the Pavlovian concept that when multiple cues are presented, they can compete with one another for association with outcomes. One way to enhance the associability of a particular cue during such competition is to weaken the ability of the competing cues to predict that outcome. In this context, the idea was that if prior exposure to saccharin weakened the ability of sweet taste to signal calories, then one effect of that exposure would be to make it so that sweet taste was less able to compete with other stimuli for association with caloric consequences produced by consuming real sugars. To test this idea, we gave one group of rats 30 ml of a 0.3% saccharin solution for 14 days along with their regular chow diet. A control group received only water and chow during this period. We then gave both groups training in which a novel flavor A (e.g. cherry) was mixed in solution with sweet-tasting, caloric 20% glucose and novel flavor B (e.g. grape) was mixed with 20% polycose, a solution that was equicaloric and highly preferred by rats, but which does not appear to be perceived as sweet (e.g.,Treesukosol, Blonde, & Spector, 2009). These solutions were presented on alternating days for 10 days each. Our rationale was that prior experience consuming the saccharin solution had weakened the ability of sweet taste to signal calories; sweet taste should be less able to compete with the novel flavor A for association with calories contained in the sweet tasting glucose solution, compared to rats that had no experience with saccharin. At the same time, learning about novel flavor B that was presented in solution with polycose should not differ between the two groups because polycose does not taste sweet. To evaluate these predictions, we tested all rats by measuring intake of flavor A and flavor B when they were presented without glucose or polycose. Figure 6 shows that the rats that had been previously exposed to saccharin consumed significantly more of the flavor that had been mixed with glucose compared to rats that had been exposed to water. In contrast, intake of the flavor that had been mixed with polycose did not differ between the two groups. This pattern of results indicates that prior consumption of saccharin had selectively reduced the ability of sweet taste to signal calories, permitting enhanced learning about a novel flavor associated with a sweet-tasting caloric solution, but not learning about an equicaloric solution that did not taste sweet.
Figure 6.
(adapted from Davidson, Martin, & Swithers, 2011). Mean amount cons umed (±SEM) of the glucose-paired and polycose-paired flavor solution during a 4-hr test for rats that received 0.03% saccharin solution or water during pre-training. *denotes significant (p. < 0.05) difference between the saccharin and water pre-training conditions.
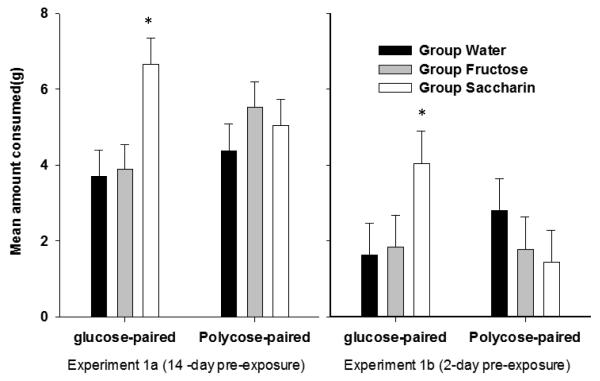
Three additional studies have confirmed the generality of this outcome. The first study used the same design and procedures that were employed by (Davidson et al., 2011), but added a group that was pre-exposed to a 20% sweet-tasting, caloric fructose solution when other groups were receiving saccharin and water to rule out the possibility that the results of our previous study were based on differential exposure to sweet taste per se for the saccharin group relative to the water group, rather than on a reduction in sweet taste cue validity (unpublished). If sweet taste exposure per se were the explanation, then the fructose pre-exposed group should exhibit responses to the novel flavors similar to the saccharin-exposed rather than to the water-exposed groups. On the other hand, because fructose is sweet and contains calories, prior consumption of fructose would not be expected to reduce the cue validity of sweet taste as a signal for calories and thus, would not weaken the ability of sweet taste to compete for associative strength with a novel flavor mixed with glucose. Thus, rats pre-exposed to fructose would be expected to show a pattern of intake less like the saccharin-pre-exposed rats and more like the rats that were pre-exposed to water. The results shown in the left panel of Figure 7 strongly supported this latter hypothesis while replicating the results previously reported by (Davidson et al., 2011). In addition, these findings were systematically replicated in a study of the same design that used the same procedures, except that the length of the pre-exposure to saccharin, fructose, and water groups was reduced from 14 days to only 2 days and the length of flavor conditioning phase with glucose and polycose was reduced from 10 days to 5 days with each solution (unpublished). The right side of Figure 7 shows that while intake of the flavors after this abbreviated pre-exposure and training regimen was reduced during testing for all groups, the results confirmed that (a) saccharin pre-exposure resulted in greater intake of the flavor paired with glucose relative to both water and fructose pre-exposed groups and; (b) intake of the flavor paired with polycose did not vary significantly among the three pre-exposure groups. This outcome indicates that the cue validity of sweet taste can be reduced following relatively short-term exposure to saccharin.
Figure 7.
(previously unpublished data). Comparison of the results following 14 - days (leftmost panels) and 2-days (rightmost panels) of pre-exposure for groups pre-exposed to saccharin, fructose, and, plain water respectively. * denotes saccharin group > both fructose and water groups, p. < 0.05.
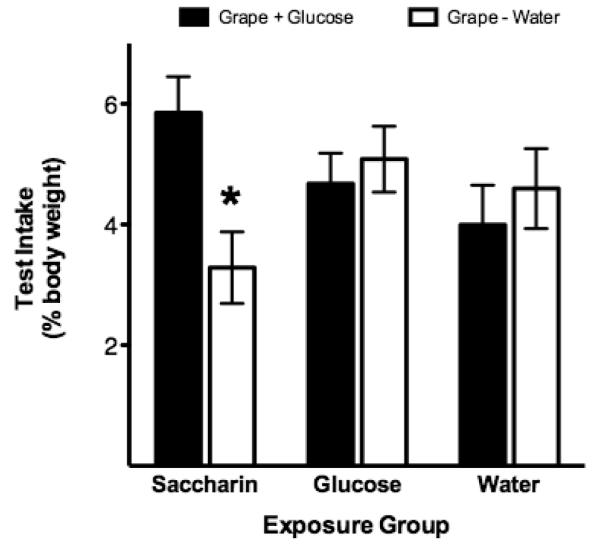
A third study examined the consequences of such cue pre-exposure during development in pups tested prior to weaning (Swithers, Ogden, Laboy, & Davidson, 2012). Using a similar strategy, rat pups were first exposed to oral infusions of water, saccharin or glucose on post-natal days (PND) 15 and 16. Then, on PND 17 and 18, separate animals in each pre-exposure group received oral infusions of a novel grape flavor presented either in water or mixed with 20% glucose. During testing on PND 19, intake of the grape flavor alone was assessed. The results were consistent with those observed in adult animals (see Figure 8). Namely, preweanling pups that were given pre-exposure to saccharin solutions showed enhanced responding to the grape flavor during testing if it was paired with sweet-tasting glucose during training. In contrast, animals pre-exposed to either water or glucose solutions showed similar intake of the grape flavor during testing whether it was trained with glucose or water. These results suggest that from early in life, exposure to artificial sweeteners can affect learning about the relationship between sweet tastes and caloric outcomes.
Figure 8.
(adapted from Swithers, Ogden, Laboy & Davidson, 2012). Intake of a grape flavor (expressed as percent body weight) during testing was significantly higher (p. < 0.05) in saccharin pre-exposed animals that had been trained with grape+glucose solutions (filled bars, left) compared to saccharin pre-exposed rats that had been trained with grape+water (open bars, left). For rats pre-exposed either to glucose (middle) or water (right), there were no differences in intake of the grape flavor during testing based on previous training.
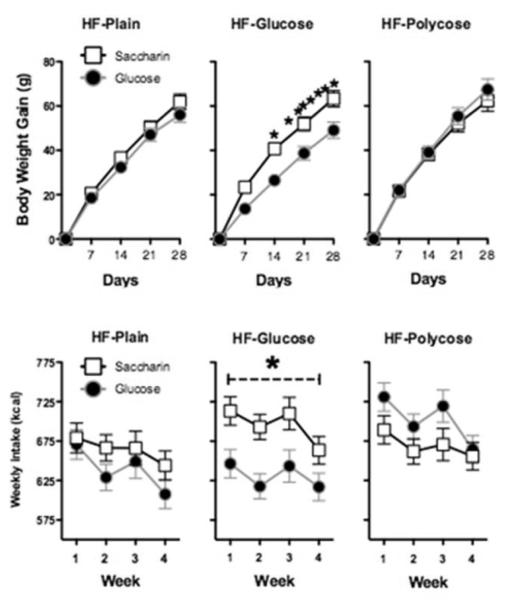
An obvious and important implication of these results is that if consumption of non-caloric sweeteners selectively reduces the validity of sweet taste as a signal for calories and this reduction interferes with the evocation of cephalic phase responses that promote efficient energy and body weight regulation, then the effects of non-caloric sweeteners on energy and body weight regulation should depend on the maintenance diet containing sweet taste cues. Davidson et al. (2011) confirmed this prediction. In that study, one group of rats was maintained on a high-fat diet with the added caloric sweetener glucose, (HF+glucose) another group was maintained on an equicaloric high-fat diet with non-sweet polycose (HF+polycose) added, and a third group was maintained on the HF diet without added glucose or polycose (HF plain). In addition to the assigned maintenance diets, rats in each of three were given daily supplements of yogurt (30 g) sweetened with saccharin (0.3%) or sweetened with glucose (20%) instead of saccharin. Figure 9 shows that total caloric intake (top panels) and weight gain (bottom panels) for rats given the HF+glucose diet, but not the HF-plain or HF+polycose diets, was significantly higher for rats that received saccharin compared to rats that received glucose in their daily supplements. Thus, the results of this study showed that consuming saccharin had an adverse effect compared to consuming glucose on the ability of rats to regulate their intake and body weight when they were maintained on a sweetened, but not on an unsweetened, high-fat maintenance diet.
Figure 9.
(adapted from Davidson, Martin, & Swithers, 2011). Rats that received yogurt sweetened with saccharin or glucose on some days and plain yogurt on other days gained significantly more weight per day (top panels) and consumed significantly more kcals per week (yogurt + maintenance diet; bottom panels) when they were maintained on a high fat (HF) + glucose diet (center panels) compared to rats maintained on a plain HF diet (right panels) or a HF diet + polycose (left panels). * denotes significant difference (p.< 0.05).
Similar effects were seen with regard to fat substitutes; animals given potato chip supplements containing a non-caloric fat substitute exhibited increased energy intake and body weight gain only when their maintenance diet was high in fat. Consistent with the idea that this exposure weakened the ability of fat to serve as a cue for high calories, animals who were given the fat-substituted chips while consuming a low-fat chow, then switched to a high-fat chow with no chips showed increased energy intake and weight gain relative to those previously given only regular high-fat, high calorie chips (Swithers, Ogden, & Davidson, 2011).
Our work has also generated evidence supporting the generality and the persistence of the adverse effects on energy regulation of disrupting sweet taste-calorie relations. Swithers et al. (2009) found that these effects could also be obtained with non-caloric sweeteners with chemical structures that are distinct from saccharin (acesulfame potassium (AceK)) and when non-caloric sweeteners were mixed in supplements other than yogurt (refried beans). In addition, this paper reported that weight gain induced by exposure to non-caloric sweeteners persisted for at least two weeks after the opportunity to consume those sweeteners was terminated and that weight gain was difficult to reverse when exposure to saccharin was followed by the opportunity to consume glucose sweetened supplements.
The findings that have been summarized in this section may have special significance when one considers that diets high in fat and sugars are currently so pervasive in western and westernized societies that they have been dubbed “western” diets. Our findings suggest that whatever obesogenic effects consuming a western diet may have on its own, its negative impact on the controls of intake and body weight are more likely to be exacerbated than ameliorated by consuming non-caloric sweeteners or other substitutes that mimic the sensory properties of high-calorie foods but without delivering the caloric consequences. Thus, one answer to the question of what makes the current environment obesogenic may be found in the widespread use of non-caloric sweeteners and their continued promotion as a means of managing caloric intake and body weight.
Furthermore, while the results reported here are from studies with rodents, there are many studies with adult humans, which indicate that consuming artificial sweeteners may have no positive effects on weight management and may even promote weight gain and co.morbidities that are associated with excess intake and body weight. However, not all studies agree (see Gardner, 2014; Shankar, Ahuja, & Sriram, 2013; Swithers, 2013 for recent reviews). For example, some evidence suggests that consuming artificially-sweetened beverages may reduce weight gain in normal weight children compared to consuming sugar-sweetened beverages (de Ruyter, Olthof, Seidell, & Katan, 2012). Whether children are protected somehow from the negative effects of artificial-sweeteners, whether they are more susceptible weight gain induced by sugars, whether the same results would be found if exposure to artificially-sweetened beverages was increased or whether artificially-sweetened beverages produce more weight gain by children when compared to other noncaloric drinks (e.g., water) is uncertain.
Integration of post-oral sensory information in the control of ingestive behavior
Associations between orosensory cues and post-ingestive consequences are likely to be early components in a larger cascade of predictive relationships that are formed as nutrients make their way through the gastrointestinal (GI) tract. Similar to cephalic phase responses that are evoked by orosensory cues, post-ingestive, pre-absorptive stimuli produced by the ingestion of foods and fluids also evoke numerous “gastric” or “intestinal phase” neurohormonal and metabolic responses that contribute to energy regulation (Guo, Singh, Gomez, Greeley, & Thompson, 1987).
Tracy, Phillips, Chi, Powley, & Davidson (2004) provided evidence that GI stimuli can influence ingestive behavior as a result of being associated with other GI events. Adapting a technique developed previously by Sclafani and his colleagues (e.g.,Elizalde & Sclafani, 1990), Tracy et al. (2004) yoked rats’ oral intake of flavored non-nutritive solutions (e.g., grape- or cherry-flavored Kool-Aid©) with the infusion directly into the GI tract of water or of equicaloric polycose (carbohydrate) or fat emulsions (each consistently paired with a specific flavor). This technique allowed for the assessment of learning about the post-oral stimulus properties of the different nutrients because the delivery of the nutrient solutions completely bypassed the oral cavity. Confirming previous results (e.g., Lucas and Sclafani, 1999), the rats quickly learned to prefer and increase consumption of a flavored solution that was paired with intragastric infusion of either fat or carbohydrate (the CS+) compared to the flavor that had been paired with intragastric infusion of water (the CS-).
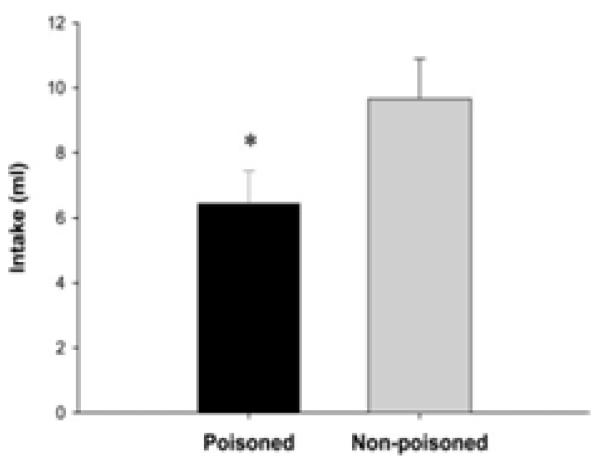
In a second experiment, Tracy et al. (2004) combined this technique with a conditioned taste aversion (CTA) procedure to create what was termed the “intestinal taste aversion” (ITA) paradigm. Here, oral intake of non-nutritive flavors was again yoked with infusion of polycose or corn oil, with each consistent flavor-nutrient pairing occurring on alternating days to allow for sufficient discrimination of the GI and post-absorbtive consequences of each nutrient. Then, in a second phase, the polycose infusion was followed by an intraperitonal (i.p.) injection of lithium chloride (LiCl) to induce gastric malaise for half the rats, whereas infusion of the fat emulsion was followed by i.p. injection of isotonic saline. The remaining rats received LiCl following GI infusion of the fat emulsion and saline following polycose infusions. As expected, after this training, the rats reduced their preference for the poisoned, LiCl-paired orally consumed flavor relative to the non-poisoned, saline-paired flavor (i.e., they showed a conventional CTA). However, the finding of most interest was obtained when the rats were given their first opportunity to orally consume the two nutrient solutions that had been infused intragastrically during training (Figure 10). Preference was significantly reduced for the LiCl-paired nutrient compared to the saline-paired nutrient, despite the fact that the neither nutrient had ever been consumed previously by mouth. Thus, Tracy et al. (2004) showed that (a) rats can associate the stimuli produced by the post-ingestive features of IG polycose and fat infusions with malaise and (b) such learning about nutrient cues detected solely in the gut modifies behavior when animals are given the opportunity to consume those same nutrients orally. These effects were replicated using intraduodenal infusions, indicating that nutrient stimuli were being detected in the intestine and the contribution of gastric receptors or responses are negligible in this process, hence, “intestinal taste aversion” (Tracy et al., 2004).
Figure 10.
(adapted from Tracy, Phillips, Chi, Powley, & Davidson, 2004). Intake of orally presented nutrients (polycose and peanut oil) following ITA training which paired GI infusion of one nutrient with injection of LiCl (Poisoned) and infusion of the other nutrient with saline injection (Non-poisoned). *denotes significance (p. < 0.05)
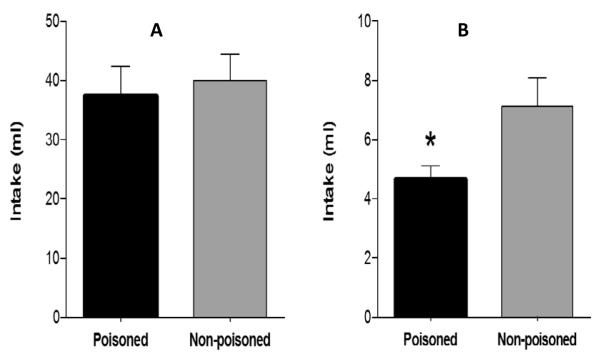
Although Tracy et al. (2004) showed that rats could learn to associate nutritive stimuli that were infused into the gut with malaise, they were unable to obtain evidence that ITA learning occurred following GI infusion of non-nutritive flavor cues. That is, when GI infusion of one non-nutritive flavor solution (e.g., orange Kool-Aid) was paired with LiCl poisoning and GI infusion of a different non-nutritive flavor solution (e.g., lime Kool-Aid) was not poisoned, there was no significant difference in amount consumed when the two flavors were subsequently offered in an oral intake test. Tracy & Davidson (2006) replicated this finding, but also modified the ITA technique to show that learning about non-nutritive stimuli does occur in the GI tract, but only under specific conditions. When rats were trained with GI infusions of non-nutritive flavor stimuli alone, there was little difference in a subsequent oral intake test between poisoned and non-poisoned flavor solutions (see Figure 11, panel A). In contrast, when the flavor solutions were co-infused into the gut with a nutrient stimulus (either polycose or corn oil) during ITA training, a significant difference in subsequent oral intake of the poisoned and non-poisoned flavor solutions was observed when those solution were presented without nutrients (Figure 11, Panel B). This study also confirmed that ITA training reduced preference for poisoned compared to non-poisoned IG nutrient emulsions, when oral intake of those emulsions was tested in the absence of the flavor solutions. The results of this study suggested that ITA training of non-nutritive GI flavor stimuli in compound with nutritive GI cues potentiated learning about the GI flavor stimuli.
Figure 11.
(adap ted from Tracy and Davidson, 2006). Learning about non-nutritive flavor solutions in the GI tract depends on co-infusion of nutrients. Panel A: oral intake of flavors following ITA training with IG infusions of flavors alone. Panel B: oral intake of flavors following ITA training with IG infusions of flavors combined with IG infusions of nutritive emulsions. * denote significance (p. < 0.05) difference between poisoned and non-poisoned conditions.
These results were confirmed and extended by Schier, Davidson, & Powley (2011) who modified the ITA procedure in ways that allowed continuous assessment of learning about GI stimuli based on rapid, moment-to-moment, changes in ingestive behavior. This study took advantage of the fact that rats have great difficulty orally discriminating two salts—NaCl and LiCl—that produce similar orosensory and post-oral taste stimuli, but produce very different unconditioned GI effects. Thirsty rats were trained to lick at a sipper spout for a safe hypotonic NaCl salt solution. A brief intestinal infusion of either the same salt or an equimolar, but toxic LiCl solution was yoked to their licking behavior for the first 6 min of each 30 min session. Because previous work (Tracy et al., 2004) had suggested the site of action to be post-gastric, intraduodenal infusions were selected for these studies as this allows for greater temporal control over the application of the taste stimuli to intestinal receptors by eliminating delays that could be associated with stomach emptying.
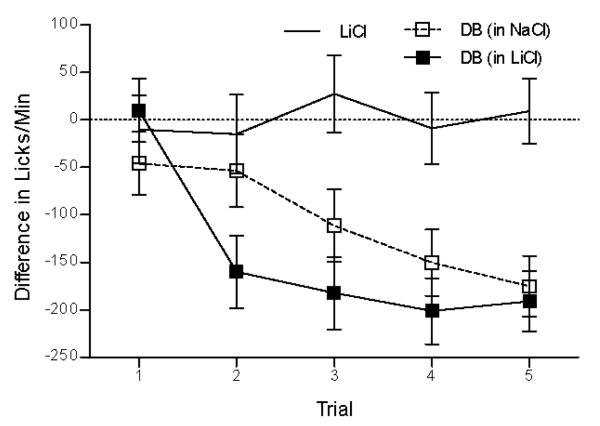
Training with saline and LiCl infusions was conducted on different days. Because rats were licking for the same NaCl solution at the sipper spout on both of these session types, oral taste cues were of no predictive value in discerning when the rat would be made ill by LiCl; instead, rats were required to use the intestinal stimulus to solve this discrimination. As expected, rats were not able to discriminate intestinal infusion of LiCl from NaCl during that early infusion period to predict the subsequent malaise. However, when a distinct chemical cue (bitter, denatonium benzoate, DB) was laced into the intestinal infusion of LiCl, rats learned to rapidly curb intake within minutes of the DB arriving in the intestine, ultimately reducing the further accumulation of the malaise-inducing LiCl. In this case, intestinal taste aversion was acquired after just a single trial (see Figure 12). This study extended previous findings that taste stimuli sensed at GI receptors are associatively integrated with subsequent gastric events (e.g., malaise), and also showed that these signals and associations not only control food selection and intake at distal time points, but are also rather rapidly integrated to control ongoing ingestive behavior. This latter phenomenon powerfully underscores the capacity for post-oral stimuli to associatively access and amend oral taste-evoked responses. In particular, the results provided the first evidence that an early intestinal CS can predict the occurrence of a subsequent intestinal US.
Figure 12.
(adapted from Schier, Davidson, & Powley, 2011). Mean ± SE difference in licks per minute of 0.12 M NaCl at the sipper spout during the “intestinal taste window” (minutes 3–8) across trials as a function of training group. For one group of rats (DB in LiCl, conditioned), licking for 0.12 M NaCl at the sipper spout was suppressed in response to ID 10 mM DB in 0.12 M LiCl infusions relative to plain ID 0.12 M NaCl. This early lick suppression in response to ID DB in LiCl was not evident on Trial 1, but emerged by Trial 2. By comparison, a second group of rats for which ID DB was laced into NaCl (ID DB in NaCl, unconditioned), licking for 0.12 M NaCl at the sipper spout was suppressed in response to ID DB in 0.12 M NaCl infusions relative to plain ID 0.12 M LiCl, but this suppression was slower to emerge across training. A third group of rats (ID LiCl) showed no lick suppression in response to ID 0.12 M LiCl alone relative to ID 0.12 M NaCl alone.
This ITA approach was also used by Schier, Davidson, & Powley (2012) to examine whether the sweet taste properties of an intestinal stimulus, like bitter taste, can also act as an early discriminative stimulus in the intestine. Under the concentrations and conditions tested in these studies, we found no evidence that naïve rats learned to respond to intestinal sweet taste alone (in the form of the non-caloric artificial sweeteners sodium saccharin or sucralose), despite repeated pairing with LiCl. However, the rats did learn to use an intestinal cue produced by infusion of sweet tasting caloric sugar (sucrose) mixed with LiCl to rapidly suppress licking to NaCl at the sipper spout. Furthermore, learning that an intraduodenal (ID) sucrose cue signaled LiCl later generalized to sweet taste alone in the form of ID sucralose. Consistent with this, rats trained with ID sucralose plus polycose (a carbohydrate as described above that does not taste sweet) mixed with LiCl also responded to ID sucralose alone during a subsequent test. These initial observations suggest that artificial sweeteners in the GI tract do not provide a salient CS in their own right. However, intestinal sweet taste cues can exert significant learned control over ingestive behavior as a result of being trained in compound with a nutritive cue. Thus, in addition to direct associations between oral taste and post-ingestive USs, these findings suggest that ‘taste’ features of a post-oral stimulus are also encoded in these types of associations.
The results of both Schier et al. (2012) and Tracy & Davidson (2006) are consistent with the idea that presenting the intestine with a less salient taste stimulus in compound with a more salient nutrient cue potentiates the capacity of gut taste signals to enter into the associative control of behavior. This type of potentiation is known to occur when an odor cue is trained in compound with an oral taste stimulus as a cue for illness (e.g., Rusiniak, Hankins, Garcia, & Brett, 1979; Rusiniak, Palmerino, & Garcia, 1982). When trained separately, the taste cue, but not the odor cue, becomes strongly associated with malaise. However, following compound training, the capacity of the odor alone to signal illness is significantly enhanced. It is conceivable that at the level of GI tract, nutrient cues are more salient than taste stimuli and when these types of cues are trained together the capacity for associative control by gut taste stimuli is enhanced.
Within the present analysis, a question of interest is: what are the effects of this type of exposure (i.e., sweet gut taste without nutrients) on the ability of sweet gut taste + nutrient compound stimuli to evoke conditioned responses? Although these questions have not been directly tested using gut taste and nutrient cues, findings from studies of potentiation with conventional oral taste and odor stimuli may be instructive with respect to this question. After demonstrating potentiation of CTA learning about an orange odor cue based on compound conditioning with a more salient bitter taste CS, Trost & Batsell (2004) assessed the effects of subsequent extinction of the odor cue alone, the taste cue alone, both the odor and taste cues presented to the same animals separately, and the originally trained odor + taste compound, on the ability of the odor + taste compound cue to evoke conditioned responding. The results showed that extinction of the odor cue alone produced a reduction in responding to the originally trained odor + taste compound cue. This reduction was significantly greater compared to that produced by extinction of the taste cue alone, and was equal to the reduction observed following extinction of both odor and taste either in compound or when each cue was presented separately. It should be noted that this pattern of results was not obtained when almond was the odor cue. However, almond odor was also more weakly potentiated compared to orange odor, following compound training with the bitter taste stimulus.
With respect to the current analysis, these findings raise the possibility that when sweet tastes (the less salient component of the taste-nutrient compound cue) are detected in the gut without nutrients (the more salient component of the compound this experience could weaken the capacity of gut nutrient cues to evoke conditioned responses that are normally under their control. From this perspective, consuming artificial sweeteners would not only disrupt the capacity of orally detected sweet tastes to signal nutritive post-ingestive outcomes, but could also impair the ability of nutrient cues detected in the gut to control a variety of downstream neural, hormonal, and metabolic responses that may be involved with the regulation of intake.
While the preceding analysis suggests how taste and nutrient cues may interact within the gut, how oral and intestinal taste cues might be integrated within an associative framework is another question of interest. Several, admittedly speculative, options seem possible:
(1). Intestinal sweet taste cues could improve the ability of animals to associate oral tastes with the absorption of specific nutrients in the gut by serving as a “gap filler” (e.g., Bolles, Collier, Bouton, & Marlin, 1978) during the temporal interval between oral taste in the mouth and nutrient absorption in the intestine. Gap fillers increase conditioned responding based on a CS →US association when the interval between the CS and US is relatively long. Weakening the relationship between a potentially gap filling intestinal sweet taste and a caloric US an energetic US might also reduce the strength of association between an oral taste cues and that US.
(2) Oral and gut sweet tastes may be associated with different post-ingestive USs. It is well-known that meal termination occurs well before most of the energy contained in ingested foods and fluids is absorbed. Receptors in the GI tract are sensitive to various qualities (e.g., macronutrient composition, energy density) of what has been consumed. These signals provide a source of negative feedback that contributes to the cessation of intake in anticipation of achieving energy, and perhaps metabolic, homeostasis (Meyer, Hlinka, Tabrizi, DiMaso, & Raybould, 1998). It may be that oral sweet taste is associated with a more immediate appetitive, post-oral, but largely pre-absorptive, US, that is sufficient to condition appetitive and consummatory responses. In contrast, sweet taste cues in the gut would be more temporally contiguous, and presumably more strongly conditioned, to any temporally-delayed US arising from the absorption of nutrients in the intestine. Thus, instead of conditioning appetitive and eating behaviors, this latter association might control the evocation of metabolic and hormonal responses that give rise to satiety signals. For example, release of GLP-1 from intestinal L-cells is glucose-dependent; in animal models, non-nutritive sweeteners alone fail to elicit GLP-1 release but do potentiate the glucose-stimulated release of GLP-1. It would make sense if these gut taste signals were too weak to evoke these responses unless they were accompanied by strong nutritive cues. Furthermore, interference with this type of GI signaling could make it more difficult for animals to terminate meals in anticipation of homeostasis.
(3) Oral taste and gut taste may produce a CS that has a common central representation. Tracy et al. (Tracy & Davidson, 2006; Tracy et al., 2004) showed that associating nutrient cues detected only in the gut with malaise altered subsequent intake of the same nutrient when it was first consumed by mouth. One implication of this finding is that oral and GI taste may be integrated within a common central representation. Thus, if this central representation of sweet taste is associated with a post-ingestive US, then a sweet taste detected without calories in the mouth might also reduce the ability of sweet taste detected in the gut to signal calories. This could be one way in which oral tastes consumed without calories, or with fewer calories than expected, might reduce the capacity of gut taste cues to generate satiety signals. From this perspective, consuming artificial sweeteners would not only disrupt the capacity of orally detected sweet tastes to signal nutritive post-ingestive outcomes, but could also impair the ability of nutrient cues detected in the gut to control a variety of downstream neural, hormonal, and metabolic responses that may be involved with the regulation of intake.
At present, there is little empirical basis for evaluating or selecting among these alternative hypotheses.
Learning and the modulatory control of intake
One way that intake could be inhibited in anticipation of achieving energy homeostasis is through the production of satiety signals. In fact, Woods (2004) proposed that humans and other animals will continue to eat in response to conditioned food-related cues until the intake is terminated by interoceptive satiety cues. From this perspective, overeating and excess weight gain is a consequence of the failure of these physiological satiety signals to exert sufficient inhibitory control. However, a complete account of energy and body weight regulation must do more than acknowledge that appetitive and consummatory behavior is suppressed by satiety. An important theoretical challenge is to describe the mechanisms that underlie the power of satiety cues to inhibit appetitive and consummatory responses and to identify factors in the “obesogenic environment” that may weaken those mechanisms.
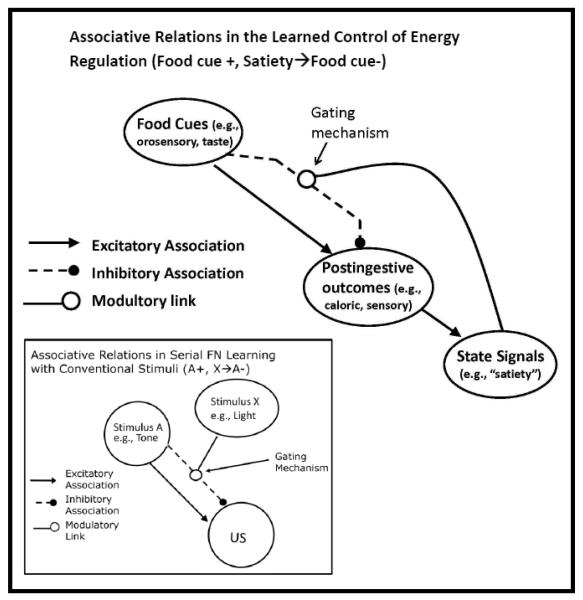
Refining earlier ideas about the learned modulatory role of energy state signals (e.g., Davidson, 1993, 2000; Davidson & Benoit, 1996; Harris, Gorissen, Bailey, & Westbrook, 2000), we recently proposed that the ability of satiety cues to inhibit eating behavior depends on the operation of a learning and memory mechanism analogous to the one underlying the solution to a Pavlovian serial feature negative (FN) problem (e.g., Davidson, Kanoski, Schier, Clegg, & Benoit, 2007; Davidson, Kanoski, Walls, & Jarrard, 2005; Davidson et al., 2014). Serial FN problems take the general form of A+, X→A-, where the presentation of Stimulus A (e.g., a tone) signals the delivery of a US (+) on trials when it is presented alone, but not on trials when it is preceded by the presentation a different cue, Stimulus X (e.g., a light). Animals show that they have solved the serial FN problem by exhibiting more conditioned responses on A+ trials than on X→A- trials. A variety of evidence indicates that the ability of the X cue (a.k.a., a negative feature stimulus) to suppress conditioned responding is not based on the formation of a direct inhibitory association with the US, but on its ability to modulate or gate the activation of the inhibitory association that is formed between Stimulus A and the US (e.g., see Swartzentruber 1995 for review). This function is often referred to as negative occasion setting (e.g., Morell & Holland, 1993).
Figure 13 depicts how a mechanism derived from accounts of serial FN learning (e.g., Bouton & Nelson, 1994; Swartzentruber & Rescorla, 1994) may also be applied to explain how satiety cues exert inhibitory control over eating behavior (see Davidson et al., 2014). This model adopts the simplifying assumption that all of the pathways shown in the figure are learned. In addition, we assume that the degree to which the memorial representation of the post-ingestive US is excited or retrieved is directly related to response strength. The main part of Figure 13 shows the set of hypothetical associative relations in which environmental food-related conditioned stimuli, unconditioned stimuli produced by the post-ingestive consequences (e.g., gastrointestinal, nutritive, caloric) and interoceptive satiety signals are embedded. The inset in Figure 13 shows the correspondence between these events and associative relations and the connections between the events that comprise the serial FN problem that was described above. Specifically, food-related cues correspond to Stimulus A in a serial FN problem and satiety cues correspond to Stimulus X. Like Stimulus A in the serial FN task, food-related cues signal the occurrence of an appetitive post-ingestive US in the absence, but not in the presence, of interoceptive satiety cues. This arrangement embeds food cues in two associations, an excitatory association that activates or retrieves the memorial representation of the US, and an inhibitory association that opposes the activation of that US. Satiety cues, like X cues in a serial FN problem, suppress the evocation of eating behavior by modulating or gating the inhibitory association between food cues and the representation of the appetitive post-ingestive US. The inhibitory association is formed when food-related CSs are encountered and the post-ingestive US is diminished or does not occur. For example, at the beginning of a meal the post-ingestive sensory consequences of intake might be highly rewarding, whereas at the end of a meal continued eating could make those consequences non-rewarding or even aversive. Thus, the same CSs are associated with an appetitive consequence on some occasions, which promotes the formation of an excitatory association and are associated with the absence of this appetitive post-ingestive stimulation at other times, thereby promoting the formation of an inhibitory association. The inset shows that Stimulus X gates the activation of the inhibitory association between Stimulus A and the US. Similarly, it is the strength of the inhibitory association that is modulated by interoceptive satiety cues. Like stimulus A, X, and the US in the inset, food-related, post-ingestive and satiety cues are assigned no special associative or motivational properties. However, the model depicting the learned control of energy intake includes the possibility that post-ingestive US may come to predict the occurrence of satiety signals. A similar link between the US and Stimulus X is typically not included in accounts of serial FN discrimination learning with conventional stimuli.
Figure 13.
(adapted from Davidson, Sample, & Swithers, 2014): This figure depicts a model of the assoc iative relations that underlie energy and body weight regulation. Environmental cues related to food become embedded in concurrent excitatory and inhibitory associations with the memorial representation of the post-ingestive US produced by intake. Satiety signals gate the activation of the inhibitory association thereby modulating the retrieval or activation of that post-ingestive US (see text for details). The inset shows the correspondence between this model and the mechanisms hypothesized to underlie serial feature negative discrimination learning.
The conceptualization diagrammed in Figure 13 suggests that excess food intake and body weight gain could be the result of impairing the ability of satiety cues to serve as feature negative stimuli. One way this could happen is by disrupting the function of brain substrates that underlie serial feature negative learning in general. Holland, Lamoureux, Han, & Gallagher (1999) reported that serial feature negative learning using conventional auditory and visual stimuli is impaired in rats following neurotoxic damage to the hippocampus. Kanoski, Zhang, Zheng, & Davidson (2010) found that a pattern of serial FN impairment quite similar to that reported by Holland et al. (1999), was exhibited by rats that had been maintained on a diet that was high in saturated fat and sugar. This diet was similar in composition to the “westernized diet” that is a staple of the current obesogenic environment and known to promote excess caloric intake and weight gain in both humans and rodents.
Furthermore, Kanoski et al. (2010) reported that rats fed westernized diet that were impaired on the nonspatial serial feature negative task also exhibited defects in the protection afforded to the brain by the blood-brain barrier (BBB) and these defects were associated with increased concentrations of exogenous substances in the hippocampus, but not in other brain areas (striatum, prefrontal cortex) that are known to play a role in learning and memory. In addition, more recent studies have shown that sensitivity to the adverse effects of western diet on serial FN learning and on BBB and hippocampal pathology is directly linked to the sensitivity of rats to the obesity-promoting effects of that diet (Davidson et al., 2013; Davidson et al., 2012). Thus, one way the consumption of westernized diet may make the current environment obesogenic is by interfering with the function of brain substrates that underlie the ability to solve the feature negative problem, which enables satiety cues to serve as effective inhibitory stimuli.
Performance on tasks that require animals to learn and remember spatial relationships of among objects or locations in their environment are often considered to provide benchmark assessments of hippocampal dependency. A variety of studies with nonhuman animals have reported that consuming a diet high in saturated fat and carbohydrate is accompanied by impaired spatial memory and by potentially pathological changes in the hippocampus, such as reduced hippocampal synaptic plasticity (Stranahan et al., 2008), reduced expression in the hippocampus of genes implicated in memory consolidation (Heyward et al., 2012), the emergence of hippocampal insulin resistance (McNay et al., 2010), reduced hippocampal neurogenesis (Grayson et al., 2013) and long lasting changes in hippocampal morphology (Valladolid-Acebes et al., 2013). Recent human studies with both adults (Francis & Stevenson, 2011) and children (Baym et al., 2014) also indicate that consumption of such diets is associated with poor performance on tests of cognitive processes (e.g., relational memory; verbal paired associate learning) that are thought to depend on the hippocampus.
Figure 13 also points to another way that dietary factors in the obesogenic environment could reduce inhibitory control of eating by satiety cues. As noted by others, animals terminate meals well before most of the energy in the meal has been absorbed (e.g., Booth, 1977). This indicates that the production of satiety signals occurs in anticipation of energy homeostasis. Figure 13 suggests that the anticipatory release of satiety signals may be the endpoint of a series of associations in which tastes and other food-related cues activate the representation of the post-ingestive caloric or nutritive consequences of intake and those consequences and their memorial representations are associated with the activation of satiety signals. The findings of Schier et al. (2012) indicate that post-ingestive stimuli detected in the GI tract can suppress appetitive and consummatory behavior in anticipation of interoceptive state produced by intake of LiCl (i.e., malaise). It seems plausible that these types of post-ingestive stimuli might also suppress appetitive and consummatory behavior in anticipation of the interoceptive state produced by intake of foods and fluids (e.g., satiety). While it is conceivable that oral taste stimuli could exert some direct conditioned effect on satiety, this possibility is not emphasized in the current model. We think that more proximal stimuli arising within the internal milieu (e.g., cues produced gastric distention, GI taste or nutritive cues) would be more reliable predictors of impending satiety signals compared to oral stimuli.
Earlier in this paper we described how consumption of non-caloric sweeteners could reduce the validity of sweet tastes as predictors of appetitive, post-ingestive outcomes. If animals are less able to use sweet taste to predict the post-ingestive consequences of intake and if the production of satiety signals themselves is dependent on the accurate prediction of those consequences, then weakening the validity of taste cues might also weaken the strength of satiety signals. Results reviewed above (Swithers, Laboy, et al., 2012) which showed that prior experience consuming non-caloric sweeteners reduced the ability of a sweet glucose solution to elicit release of GLP-1 are consistent with this hypothesis. The results of several earlier studies suggest that GLP-1 functions as a physiological satiety signal when its release from the intestine is stimulated by nutrient intake (e.g., Sam, Troke, Tan, & Bewick, 2012; Williams, Baskin, & Schwartz, 2009). Thus, as suggested by Swithers, Laboy, et al. (2012), it may be that consuming non-caloric sweeteners promotes increased intake and body weight gain, in part, by suppressing the production of interoceptive satiety signals. This reduction in the physiological presence of these signals would thereby reduce the strength of the inhibitory gating action of satiety signals within the serial feature negative conceptualization of ingestive control.
Provisos and qualifications
This paper describes a model of how Pavlovian conditioning mechanisms may be involved with both energy and body weight regulation and dysregulation. This model is not antagonistic toward views that emphasize other, non-Pavlovian processes as important contributors to regulatory control. For example, we have no doubt that hormonal, metabolic, and neural mechanisms other than those that underlie learning and memory are involved with the control of food and fluid intake and that these types of mechanisms may be disordered in overweight and obese people (see Begg & Woods, 2013 for recent review). The model we propose describes how physiological signals of satiety, post-ingestive sensory stimuli and gastrointestinal events) may be embedded in sets of predictive relations that, along with environmental cues, can participate in the associative control of eating and appetitive behavior. Furthermore, it is conceivable that disorders of metabolic and neurohormonal control mechanisms are due, in part, to disruption of associative processes. This integration of Pavlovian and physiological perspectives has the potential to provide an account that is more complete than could be provided by either perspective independently.
Neither does our model deny that social factors (e.g., Cunningham, Vaquera, Maturo, & Narayan, 2012), stress (e.g., Bartoli, Carra, Crocamo, Carretta, & Clerici, 2013), gene-environment interactions (e.g., Lee, 2013), epigenetics (e.g., Drummond & Gibney, 2013), palatability (e.g., Finlayson, King, & Blundell, 2007), or other variables may impact energy regulation and dysregulation. Rather than deny a role for such factors, our model has the potential to identify new ways that learning and memory processes could underlie their effects on intake and body weight.
For example, Schachter (Schachter, 1968) proposed the hypothesis that increased sensitivity to external food-related cues relative to internal satiety signals (i.e., “externality”) contributes to overeating and obesity and recent studies have reported findings that are consistent with this hypothesis (e.g., Ferriday & Brunstrom, 2011; Wansink, Payne, & Chandon, 2007). It has been suggested that differences in externality, like obesity, may reflect genetic predispositions (e.g., Carnell & Wardle, 2008) or could result from parental practices that promote increased attention to food and food-related cues during childhood (e.g., Jansen et al., 2003). Our model suggests that externality may have different origins. We’ve proposed that consuming a diet impairs the ability of animals to use their interoceptive satiety cues to inhibit activation or retrieval of the appetitive post-ingestive consequences of intake by external food-related stimuli. Within this framework, consuming a westernized diet could promote externality because a reduction in inhibitory stimulus control by satiety signals would enable excitatory stimulus control by external food cues to be expressed more strongly. This is the hallmark of the externality hypothesis.
There are other phenomena that may be addressed for within our framework. Higgs (2002, 2008) provided persuasive evidence that merely reminding humans of a recent meal can inhibit subsequent intake and that the ability of the memory of recent meal to suppress subsequent eating depends on how well that meal was encoded. In addition, data provided Brunstrom et al. (2012) suggests that under some circumstances, memory of the perceived energy content of a meal may be more effective in suppressing subsequent intake than are interoceptive signals produced by the actual energetic content of the meal. As we have suggested elsewhere (Davidson et al., 2014), these findings might be accounted for by assuming that the memory of a recent meal, like a physiological satiety cue, functions as a negative feature stimulus that is informative about the likely post-ingestive sensory consequences of intake. Within our perspective, memories, physiological satiety cues, and conventional external stimuli have no special associative properties and thus they will operate in the same way when they are embedded in the set of associative relationships that define a serial feature negative discrimination problem.
Finally, the approach we have outlined is Pavlovian rather than instrumental in the sense that it relies on associations among different stimuli, rather than among stimuli and responses, to describe the mechanisms that underlie the elicitation of appetitive and consummatory behavior. This choice has historical precedents, in that, as noted previously, Pavlov characterized cephalic phase reflexes as classically conditioned responses. Furthermore, most of the research on which our model is based has relied on food approach responses evoked by a signal for sucrose pellets to index the strength of appetitive behavior. A recent study concluded that appetitive food approach responses trained under these conditions are controlled by a Pavlovian, as opposed to an instrumental, contingency because the food magazine approach performance was largely insensitive to an omission training procedure in which responses resulted in the withholding of the food US (Harris, Andrew, & Kwok, 2013). While such findings encourage an emphasis on Pavlovian principles, we accept that further refinements of our model will likely need to describe the role of instrumental learning in energy and body weight regulation.
Summary and conclusions
Highly palatable, energy dense foods and beverages are widely available in our current environment. In addition, our environment is rich with food-related cues that are strongly associated with the rewarding consequences of eating and drinking. This environment has often been termed “obesogenic” in large part because so many people who reside in it are overweight and obese. This review set out to describe how the capacity of our obesogenic environment to promote excess food intake and weight gain may be based on interference with associative mechanisms that underlie the learned control of energy regulation. We outlined a system for the associative control of energy regulation in which taste cues, nutritive post-ingestive USs, gastrointestinal stimuli, and interoceptive satiety signals exert control over intake based on the operation of fundamental principles of Pavlovian conditioning.
This theoretical framework was used to show how dietary factors which are prevalent in the current obesogenic environment can produce overeating, weight gain, and metabolic disease by interfering with these learned controls of energy intake. For many years, the consumption of non-caloric sweeteners has been promoted by the food industry and by various health advocacy groups as an effective way to reduce caloric intake and manage body weight. In contrast to this view, our analysis shows how intake of caloric sweeteners may actually interfere with the learned control of energy and body regulation by degrading the ability of sweet taste to signal the calories contained in sweet, energy-rich foods and beverages. This conclusion is supported by findings which show that intake of non-caloric sweeteners promotes excess intake and body weight gain in rats by reducing the validity of sweet taste as a signal for energetic and nutritive post-ingestive outcomes. Consistent with this analysis, many, though not all, studies with humans provide evidence that energy dysregulation is associated with consuming non-caloric sweeteners (see Swithers, 2013), for review of recent human findings). Interference of this type may involve a cascade of associations beginning with taste stimuli detected in the mouth as well as in the GI tract.
We also discussed evidence that consumption of a “western” diet that is high in sugar and saturated fat may promote overeating and weight gain by interfering with the ability of interoceptive satiety cues to act as ingestive behavior inhibitors within a Pavlovian feature negative framework. We reviewed evidence which suggests that interference with hippocampal-dependent learning and memory processes, such as feature negative learning, could originate with western diet-induced pathologies that impair the function of the BBB and the hippocampus itself.
Applying an expanded learning and memory framework, one that recognizes the diversity of events and predictive relationships about which animals can learn, has the potential to generate novel and testable hypotheses that may yield much new information about the causes of overeating and obesity. Within the approach we have described in the present paper, a complete understanding of how the current environment promotes excess food intake and body weight gain will require us to think not only about the way food and food-related stimuli can excite appetitive and consummatory behaviors, but also about how dietary and presumably other factors can weaken the associative mechanisms that underlie the inhibition of those responses. This will require an appreciation of all the events, exteroceptive, and those arising within the internal milieu, which an animal may use to determine when to eat and when to refrain from eating and greater knowledge about the types of associative relations in which those cues are embedded.
Acknowledgments
The preparation of this manuscript and much of the research that is described herein was supported by Grants P01 HD052112 and R01 HD29792 from the National Institutes of Child Health and Development and Grant R01 DK076078 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
“If we could give every individual the right amount of nourishment and exercise, not too little and not too much, we would have found the safest way to health”. From Hippocrates. Hippocratic Writings. Chicago: Encyclopedia Britannica, 1955.
References
- Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011;31(10):3904–3913. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli F, Carra G, Crocamo C, Carretta D, Clerici M. Metabolic Syndrome in People Suffering from Posttraumatic Stress Disorder: A Systematic Review and Meta-Analysis. Metabolic Syndrome and Related Disorders. 2013;11(5):301–308. doi: 10.1089/met.2013.0010. [DOI] [PubMed] [Google Scholar]
- Baym CL, Khan NA, Monti KM, Raine LB, Drollette ES, Moore RD, Cohen NJ. Differential impact of dietary lipids on hippocampal-dependent relational memory in prepubescent children. American Journal of Clinical Nutrition. 2014 doi: 10.3945/ajcn.113.079624. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg DP, Woods SC. The endocrinology of food intake. Nature Reviews Endocrinology. 2013;9(10):584–597. doi: 10.1038/nrendo.2013.136. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Current Opinion in Neurobiology. 2011;21(6):888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71(4):478–487. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bills CH, Dopheide M, Pineno O, Schachtman TRS. Effects of an extinguished CS on competition with another CS. Behavioural Processes. 2006;72(1):14–22. doi: 10.1016/j.beproc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Birch LL, Anzman SL. Learning to eat in an obesogenic environment: A developmental systems perspective on childhood obesity. Child Development Perspectives. 2010;4(2):138–143. [Google Scholar]
- Boggiano MM, Dorsey JR, Thomas JM, Murdaugh DL. The Pavlovian power of palatable food: lessons for weight-loss adherence from a new rodent model of cue-induced overeating. Int J Obes (Lond) 2009;33(6):693–701. doi: 10.1038/ijo.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC, Collier AC, Bouton ME, Marlin NA. Some tricks for ameliorating trace-conditioning deficit. Bulletin of the Psychonomic Society. 1978;11(6):403–406. [Google Scholar]
- Booth DA. Satiety and appetite are conditioned reactions. Psychosom Med. 1977;39(2):76–81. doi: 10.1097/00006842-197703000-00002. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Moody EW. Memory processes in classical conditioning. Neurosci Biobehav Rev. 2004;28(7):663–674. doi: 10.1016/j.neubiorev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Nelson JB. Context-specificity of target versus feature inhibition in a feature-negative discrimination. J Exp Psychol Anim Behav Process. 1994;20(1):51–65. [PubMed] [Google Scholar]
- Brunstrom JM, Burn JF, Sell NR, Collingwood JM, Rogers PJ, Wilkinson LL, Ferriday D. Episodic memory and appetite regulation in humans. PLoS One. 2012;7(12):e50707. doi: 10.1371/journal.pone.0050707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstrom JM, Mitchell GL. Flavor-nutrient learning in restrained and unrestrained eaters. Physiol Behav. 2007;90(1):133–141. doi: 10.1016/j.physbeh.2006.09.016. doi: 10.1016/j.physbeh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Capaldi ED, Davidson TL, Myers DE. Resistance to satiation-reinforcing effectd of food and eating under satiation. Learning and Motivation. 1981;12(2):171–195. [Google Scholar]
- Capaldi ED, Myers DE. Resistance to satiation as a function of 3 satiation procedures. Bulletin of the Psychonomic Society. 1979;14(1):53–56. [Google Scholar]
- Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. American Journal of Clinical Nutrition. 2008;88(1):22–29. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Klingenberg L, Astrup A, Sjodin AM. Modern sedentary activities promote overconsumption of food in our current obesogenic environment. Obesity Reviews. 2011;12(501):e12–e20. doi: 10.1111/j.1467-789X.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Babey SH. Contextual influences on eating behaviours: heuristic processing and dietary choices. Obesity Reviews. 2012;13(9):766–779. doi: 10.1111/j.1467-789X.2012.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsica JA, Hood MM. Eating disorders in an obesogenic environment. J Am Diet Assoc. 2011;111(7):996–1000. doi: 10.1016/j.jada.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Cunningham SA, Vaquera E, Maturo CC, Narayan KMV. Is there evidence that friends influence body weight? A systematic review of empirical research. Social Science & Medicine. 2012;75(7):1175–1183. doi: 10.1016/j.socscimed.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends in Endocrinology and Metabolism. 2013;24(2):85–91. doi: 10.1016/j.tem.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL. The nature and function of interoceptive signals to feed: toward integration of physiological and learning perspectives. Psychol Rev. 1993;100(4):640–657. doi: 10.1037/0033-295x.100.4.640. [DOI] [PubMed] [Google Scholar]
- Davidson TL. Pavlovian occasion setting: a link between physiological change and appetitive behavior. Appetite. 2000;35(3):271–272. doi: 10.1006/appe.2000.0355. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Benoit SC. The learned function of food deprivation cues: A role for conditioned modulation. Animal Learning & Behavior. 1996;24:46–56. [Google Scholar]
- Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, Zheng W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253C:110–122. doi: 10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7(6):613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiology & Behavior. 2005;86(5):731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Martin AA, Clark K, Swithers SE. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q J Exp Psychol (Hove) 2011;64(7):1430–1441. doi: 10.1080/17470218.2011.552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol Behav. 2012;107(1):26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Sample CH, Swithers SE. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiol Learn Mem. 2014;108C:172–184. doi: 10.1016/j.nlm.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A Trial of Sugar-free or Sugar-Sweetened Beverages and Body Weight in Children. New England Journal of Medicine. 2012;367(15):1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- Diamond P, Brondel L, Leblanc J. Palatability and postprandial thermogenesis in dogs. American Journal of Physiology. 1985;248(1):E75–E79. doi: 10.1152/ajpendo.1985.248.1.E75. [DOI] [PubMed] [Google Scholar]
- Drummond EM, Gibney ER. Epigenetic regulation in obesity. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16(4):392–397. doi: 10.1097/MCO.0b013e3283620f45. [DOI] [PubMed] [Google Scholar]
- Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric polycose infusions: a detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47(1):63–77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Lin H, Carr KA, Fletcher KD. Food reinforcement and obesity. Psychological moderators. Appetite. 2012;58(1):157–162. doi: 10.1016/j.appet.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM. ’I just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. International Journal of Obesity. 2011;35(1):142–149. doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- Finlayson G, King N, Blundell JE. Liking vs. wanting food: Importance for human appetite control and weight regulation. Neurosci Biobehav Rev. 2007;31(7):987–1002. doi: 10.1016/j.neubiorev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Global Burden Metab Risk Factors, C. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav Neurosci. 2011;125(6):943–955. doi: 10.1037/a0025998. [DOI] [PubMed] [Google Scholar]
- Gardner C. Non-nutritive sweeteners: evidence for benefit vs. risk. Current Opinion in Lipidology. 2014;25(1):80–84. doi: 10.1097/MOL.0000000000000034. [DOI] [PubMed] [Google Scholar]
- Giskes K, van Lenthe F, Avendano-Pabon M, Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: are we getting closer to understanding obesogenic environments? Obesity Reviews. 2011;12(501):e95–e106. doi: 10.1111/j.1467-789X.2010.00769.x. [DOI] [PubMed] [Google Scholar]
- Grayson BE, Fitzgerald MF, Hakala-Finch AP, Ferris VM, Begg DP, Tong J, Benoit SC. Improvements in hippocampal-dependent memory and microglial infiltration with calorie restriction and gastric bypass surgery, but not with vertical sleeve gastrectomy. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.100. In press. [DOI] [PubMed] [Google Scholar]
- Guo YS, Singh P, Gomez G, Greeley GH, Thompson JC. Effect of peptide-YY on cephalic, gastric, and intestinal phases of gastric-acid secretion and on the release of gastrointestinal hormones. Gastroenterology. 1987;92(5):1202–1208. doi: 10.1016/s0016-5085(87)91078-x. [DOI] [PubMed] [Google Scholar]
- Harris JA, Andrew BJ, Kwok DW. Magazine approach during a signal for food depends on Pavlovian, not instrumental, conditioning. J Exp Psychol Anim Behav Process. 2013;39(2):107–116. doi: 10.1037/a0031315. [DOI] [PubMed] [Google Scholar]
- Harris JA, Gorissen MC, Bailey GK, Westbrook RF. Motivational state regulates the content of learned flavor preferences. J Exp Psychol Anim Behav Process. 2000;26(1):15–30. doi: 10.1037//0097-7403.26.1.15. [DOI] [PubMed] [Google Scholar]
- Hashkes PJ, Gartside PS, Blondheim SH. Effect of food palatability on early (cephalic) phase of diet-induced thermogenesis in nonobese and obese man. International Journal of Obesity. 1997;21(7):608–613. doi: 10.1038/sj.ijo.0800449. [DOI] [PubMed] [Google Scholar]
- Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 2010;100(5):503–510. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyward FD, Walton RG, Carle MS, Coleman MA, Garvey WT, Sweatt JD. Adult mice maintained on a high-fat diet exhibit object location memory deficits and reduced hippocampal SIRT1 gene expression. Neurobiol Learn Mem. 2012;98(1):25–32. doi: 10.1016/j.nlm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs S. Memory for recent eating and its influence on subsequent food intake. Appetite. 2002;39(2):159–166. doi: 10.1006/appe.2002.0500. [DOI] [PubMed] [Google Scholar]
- Higgs S. Cognitive influences on food intake: the effects of manipulating memory for recent eating. Physiol Behav. 2008;94(5):734–739. doi: 10.1016/j.physbeh.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9(2):143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jansen A, Theunissen N, Slechten K, Nederkoorn C, Boon B, Mulkens S, Roefs A. Overweight children overeat after exposure to food cues. Eat Behav. 2003;4(2):197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Jequier E. Does a thermogenic defect play a role in the pathogenesis of human obesity? Clinical Physiology. 1983;3(1):1–7. doi: 10.1111/j.1475-097x.1983.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21(1):207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanakis V, Brown M, McPherson K, Webber L, Rtveladze K, Marsh T. By-state comparison of obesity trends in the adult population of the the United States of America. Journal of Epidemiology and Community Health. 2012;66:A10–A11. [Google Scholar]
- Katan MB, Ludwig DS. Extra Calories Cause Weight Gain-But How Much? Jama-Journal of the American Medical Association. 2010;303(1):65–66. doi: 10.1001/jama.2009.1912. [DOI] [PubMed] [Google Scholar]
- Kimokoti RW, Millen BE. Diet, the Global Obesity Epidemic, and Prevention. J Am Diet Assoc. 2011;111(8):1137–1140. doi: 10.1016/j.jada.2011.05.016. [DOI] [PubMed] [Google Scholar]
- King BM. The modern obesity epidemic, ancestral hunter-gatherers, and the sensory/reward control of food intake. Am Psychol. 2013;68(2):88–96. doi: 10.1037/a0030684. [DOI] [PubMed] [Google Scholar]
- Leblanc J, Cabanac M. Cephalic postprandial thermogenesis in human subjects. Physiology & Behavior. 1989;46(3):479–482. doi: 10.1016/0031-9384(89)90024-3. [DOI] [PubMed] [Google Scholar]
- LeBlanc J, Cabanac M, Samson P. Reduced postprandial heat production with gavage as compared with meal feeding in human subjects. Am J Physiol. 1984;246(1 Pt 1):E95–101. doi: 10.1152/ajpendo.1984.246.1.E95. [DOI] [PubMed] [Google Scholar]
- Lee YS. Genetics of nonsyndromic obesity. Current Opinion in Pediatrics. 2013;25(6):666–673. doi: 10.1097/MOP.0b013e3283658fba. [DOI] [PubMed] [Google Scholar]
- Majer IM, Mackenbach JP, van Baal PHM. Time trends and forecasts of body mass index from repeated cross-sectional data: a different approach. Statistics in Medicine. 2013;32(9):1561–1571. doi: 10.1002/sim.5558. [DOI] [PubMed] [Google Scholar]
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nature Reviews Endocrinology. 2013;9(1):13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- Martin AA, Davidson TL. Human Cognitive Function and the Obesogenic Environment. Physiology & Behavior. 2014 doi: 10.1016/j.physbeh.2014.02.062. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93(4):546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Hlinka M, Tabrizi Y, DiMaso N, Raybould HE. Chemical specificities and intestinal distributions of nutrient-driven satiety. Am J Physiol. 1998;275(4 Pt 2):R1293–1307. doi: 10.1152/ajpregu.1998.275.4.R1293. [DOI] [PubMed] [Google Scholar]
- Morell JR, Holland PC. Summation and transfer of negative occasion setting. Animal Learning & Behavior. 1993;21(2):145–153. [Google Scholar]
- Morgan MJ. Resistance to satiation. Animal Behaviour. 1974;22:449–466. [Google Scholar]
- Naim M, Kare MR, Merritt AM. Effects of oral stimulation on the cephalic phase of pancreatic exocrine secretion in dogs. Physiology & Behavior. 1978;20:563–570. doi: 10.1016/0031-9384(78)90248-2. [DOI] [PubMed] [Google Scholar]
- Petrovich GD. Learning and the motivation to eat: Forebrain circuitry. Physiology & Behavior. 2011;104(4):582–589. doi: 10.1016/j.physbeh.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Holland PC. Conditioning and cognition. Neurosci Biobehav Rev. 2004;28(7):651–661. doi: 10.1016/j.neubiorev.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Power ML, Schulkin J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite. 2008;50(2-3):194–206. doi: 10.1016/j.appet.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD. Learned food-cue stimulates persistent feeding in sated rats. Appetite. 2012;59(2):437–447. doi: 10.1016/j.appet.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. J Comp Physiol Psychol. 1968;66(1):1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning: Its not what you think it is. American Psychologist. 1988;43(3):151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rusiniak KW, Hankins WG, Garcia J, Brett LP. Flavor-illness aversions: potentiation of odor by taste in rats. Behav Neural Biol. 1979;25(1):1–17. doi: 10.1016/s0163-1047(79)90688-5. [DOI] [PubMed] [Google Scholar]
- Rusiniak KW, Palmerino CC, Garcia J. Potentiation of odor by taste in rats: tests of some nonassociative factors. J Comp Physiol Psychol. 1982;96(5):775–780. doi: 10.1037/h0077915. [DOI] [PubMed] [Google Scholar]
- Sam AH, Troke RC, Tan TM, Bewick GA. The role of the gut/brain axis in modulating food intake. Neuropharmacology. 2012;63(1):46–56. doi: 10.1016/j.neuropharm.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Schachter S. Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science. 1968;161(3843):751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- Schier LA, Davidson TL, Powley TL. Ongoing ingestive behavior is rapidly suppressed by a preabsorptive, intestinal “bitter taste” cue. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1557–1568. doi: 10.1152/ajpregu.00344.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier LA, Davidson TL, Powley TL. Rapid stimulus-bound suppression of intake in response to an intraduodenal nonnutritive sweetener after training with nutritive sugars predicting malaise. Am J Physiol Regul Integr Comp Physiol. 2012;302(11):R1351–1363. doi: 10.1152/ajpregu.00702.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81(5):773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Bahrani M, Zukerman S, Ackroff K. Stevia and saccharin preferences in rats and mice. Chem Senses. 2010;35(5):433–443. doi: 10.1093/chemse/bjq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar P, Ahuja S, Sriram K. Non-nutritive sweeteners: Review and update. Nutrition. 2013;29(11-12):1293–1299. doi: 10.1016/j.nut.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Smeets PA, Erkner A, de Graaf C. Cephalic phase responses and appetite. Nutr Rev. 2010;68(11):643–655. doi: 10.1111/j.1753-4887.2010.00334.x. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18(11):1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. International Journal of Obesity. 2013;37(6):889–891. doi: 10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland N, Mackintosh N, Wolfe J. Extinction as a function of the order of partial and consistent reinforcement. Journal of Experimental Psychology. 1965;69(1):56–59. doi: 10.1037/h0021631. [DOI] [PubMed] [Google Scholar]
- Swartzentruber D. Modulatory mechanisms in Pavlovian conditioning. Animal Learning & Behavior. 1995;23(2):123–143. [Google Scholar]
- Swartzentruber D, Rescorla RA. Modulation of trained and extinguished stimuli by facilitators and inhibitors. Animal Learning & Behavior. 1994;22(3):309–316. [Google Scholar]
- Swinburn B, Egger G. Preventive strategies against weight gain and obesity. Obes Rev. 2002;3(4):289–301. doi: 10.1046/j.1467-789x.2002.00082.x. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. Obesity 1 The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- Swithers SE. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab. 2013;24(9):431–441. doi: 10.1016/j.tem.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Baker CR, Davidson TL. General and Persistent Effects of High-Intensity Sweeteners on Body Weight Gain and Caloric Compensation in Rats. Behav Neurosci. 2009;123(4):772–780. doi: 10.1037/a0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122(1):161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Doerflinger A, Davidson TL. Consistent relationships between sensory properties of savory snack foods and calories influence food intake in rats. Int J Obes (Lond) 2006;30(11):1685–1692. doi: 10.1038/sj.ijo.0803329. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Laboy AF, Clark K, Cooper S, Davidson TL. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav Brain Res. 2012;233(1):1–14. doi: 10.1016/j.bbr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Ogden SB, Davidson TL. Fat substitutes promote weight gain in rats consuming high-fat diets. Behav Neurosci. 2011;125(4):512–518. doi: 10.1037/a0024404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Ogden SB, Laboy AF, Davidson TL. Saccharin pre-exposure enhances appetitive flavor learning in pre-weanling rats. Dev Psychobiol. 2012;54(8):818–824. doi: 10.1002/dev.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappy L. Thermic effect of food and sympathetic nervous system activity in humans. Reproduction Nutrition Development. 1996;36(4):391–397. doi: 10.1051/rnd:19960405. [DOI] [PubMed] [Google Scholar]
- Todes DP. From the machine to the ghost within - Pavlov’s transition from digestive physiology to conditional reflexes. American Psychologist. 1997;52(9):947–955. [Google Scholar]
- Tracy AL, Davidson TL. Comparison of nutritive and nonnutritive stimuli in intestinal and oral conditioned taste aversion paradigms. Behav Neurosci. 2006;120(6):1268–1278. doi: 10.1037/0735-7044.120.6.1268. [DOI] [PubMed] [Google Scholar]
- Tracy AL, Phillips RJ, Chi MM, Powley TL, Davidson TL. The gastrointestinal tract “tastes” nutrients: evidence from the intestinal taste aversion paradigm. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1086–1100. doi: 10.1152/ajpregu.00047.2004. [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R855–865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost CA, Batsell WR., Jr. Taste + odor interactions in compound aversion conditioning. Learn Behav. 2004;32(4):440–453. doi: 10.3758/bf03196040. [DOI] [PubMed] [Google Scholar]
- Valladolid-Acebes I, Fole A, Martin M, Morales L, Cano MV, Ruiz-Gayo M, Del Olmo N. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol Learn Mem. 2013;106:18–25. doi: 10.1016/j.nlm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Van Vort W, Smith GP. Sham feeding experience produces a conditioned increase of meal size. Appetite. 1987;9(1):21–29. doi: 10.1016/0195-6663(87)90050-x. [DOI] [PubMed] [Google Scholar]
- Waldmann MR, Schmid M, Wong J, Blaisdell AP. Rats distinguish between absence of events and lack of evidence in contingency learning. Animal Cognition. 2012;15(5):979–990. doi: 10.1007/s10071-012-0524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansink B, Payne CR, Chandon P. Internal and external cues of meal cessation: The French paradox redux? Obesity. 2007;15(12):2920–2924. doi: 10.1038/oby.2007.348. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Miller RR. What’s elementary about associative learning? Annu Rev Psychol. 1997;48:573–607. doi: 10.1146/annurev.psych.48.1.573. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220(4595):431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150(4):1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol. 2004;286(1):G7–13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000;110(1-2):175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Leitch M, Gould NJ, Mobini S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiol Behav. 2008;93(4-5):798–806. doi: 10.1016/j.physbeh.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Zafra MA, Molina F, Puerto A. The neural/cephalic phase reflexes in the physiology of nutrition. Neurosci Biobehav Rev. 2006;30(7):1032–1044. doi: 10.1016/j.neubiorev.2006.03.005. [DOI] [PubMed] [Google Scholar]