Abstract
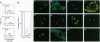
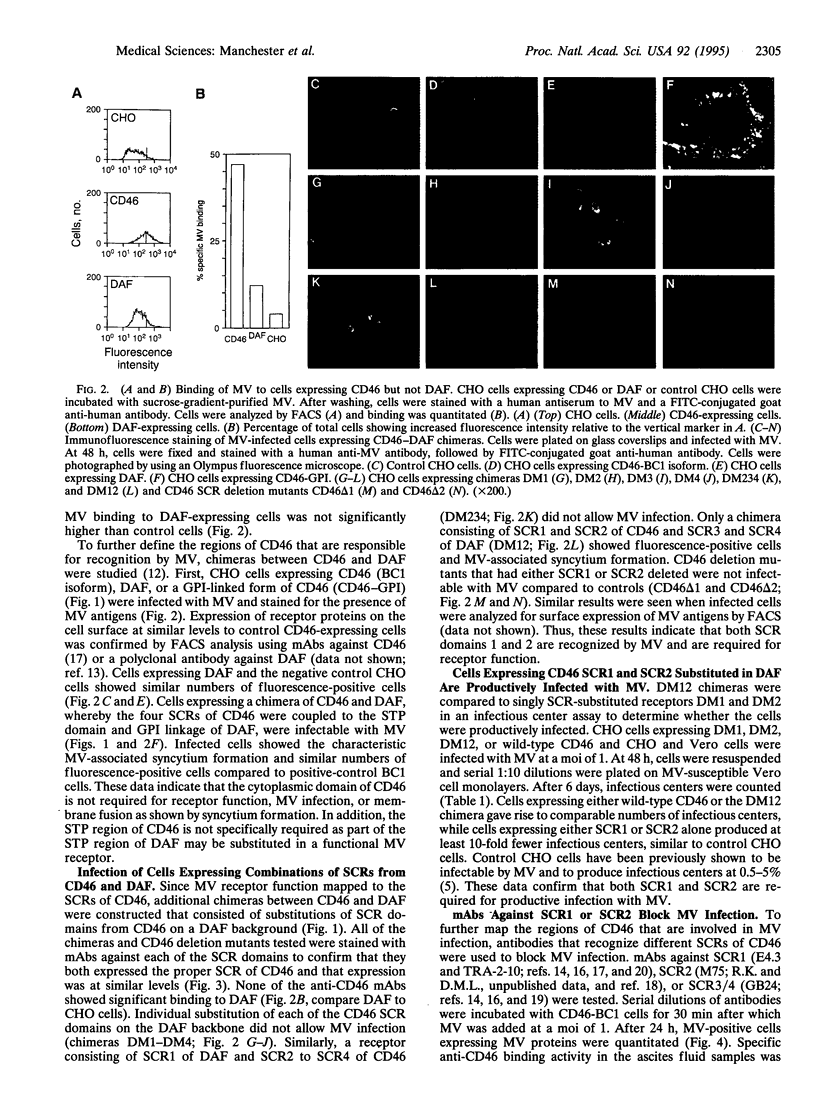
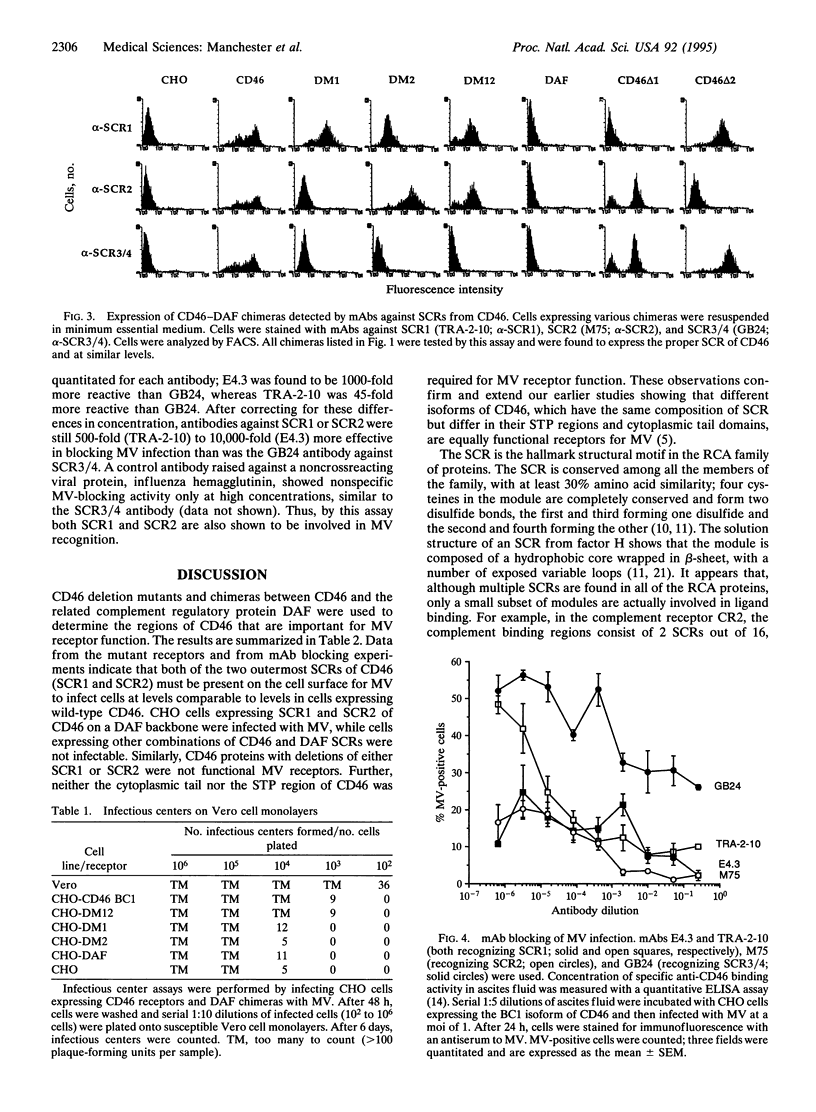
The human complement regulatory protein membrane cofactor protein (CD46) is the cellular receptor for measles virus (MV), whereas decay accelerating factor (DAF; CD55), a structurally similar complement regulatory protein, does not bind MV. To characterize the interaction between MV and CD46, mutants of the CD46 protein and hybrid molecules between CD46 and DAF were tested for their ability to act as MV receptors. The transmembrane domain and cytoplasmic tail of CD46 were not required for receptor function as cells expressing the CD46 extracellular domain linked to the glycosyl-phosphatidylinositol tail of DAF were rendered susceptible to MV infection. Chimeric proteins exchanging the four extracellular short consensus repeat (SCR) domains between CD46 and DAF indicated that only molecules with both SCR1 and SCR2 from CD46 allowed a productive MV infection. Further, monoclonal antibodies (mAbs) against SCR1 or SCR2 of CD46 blocked MV infection, whereas a mAb against SCR3 and SCR4 did not. The latter mAb blocks C3b/C4b binding (which maps to SCR3 and SCR4) whereas the former mAbs do not. Thus, our data indicate that both SCR1 and SCR2 make up the MV receptor determinant in CD46. These results also suggest avenues for development of therapeutic agents to inhibit MV binding and thus infection and disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaby P., Samb B., Simondon F., Knudsen K., Seck A. M., Bennett J., Whittle H. Divergent mortality for male and female recipients of low-titer and high-titer measles vaccines in rural Senegal. Am J Epidemiol. 1993 Nov 1;138(9):746–755. doi: 10.1093/oxfordjournals.aje.a116912. [DOI] [PubMed] [Google Scholar]
- Adams E. M., Brown M. C., Nunge M., Krych M., Atkinson J. P. Contribution of the repeating domains of membrane cofactor protein (CD46) of the complement system to ligand binding and cofactor activity. J Immunol. 1991 Nov 1;147(9):3005–3011. [PubMed] [Google Scholar]
- Andrews P. W., Knowles B. B., Parkar M., Pym B., Stanley K., Goodfellow P. N. A human cell-surface antigen defined by a monoclonal antibody and controlled by a gene on human chromosome 1. Ann Hum Genet. 1985 Jan;49(Pt 1):31–39. doi: 10.1111/j.1469-1809.1985.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Barlow P. N., Baron M., Norman D. G., Day A. J., Willis A. C., Sim R. B., Campbell I. D. Secondary structure of a complement control protein module by two-dimensional 1H NMR. Biochemistry. 1991 Jan 29;30(4):997–1004. doi: 10.1021/bi00218a016. [DOI] [PubMed] [Google Scholar]
- Barlow P. N., Norman D. G., Steinkasserer A., Horne T. J., Pearce J., Driscoll P. C., Sim R. B., Campbell I. D. Solution structure of the fifth repeat of factor H: a second example of the complement control protein module. Biochemistry. 1992 Apr 14;31(14):3626–3634. doi: 10.1021/bi00129a011. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. Vaccines for the Third World. Nature. 1989 Nov 9;342(6246):115–120. doi: 10.1038/342115a0. [DOI] [PubMed] [Google Scholar]
- Cho S. W., Oglesby T. J., Hsi B. L., Adams E. M., Atkinson J. P. Characterization of three monoclonal antibodies to membrane co-factor protein (MCP) of the complement system and quantification of MCP by radioassay. Clin Exp Immunol. 1991 Feb;83(2):257–261. doi: 10.1111/j.1365-2249.1991.tb05624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne K. E., Hall S. E., Thompson S., Arce M. A., Kinoshita T., Fujita T., Anstee D. J., Rosse W., Lublin D. M. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol. 1992 Nov 1;149(9):2906–2913. [PubMed] [Google Scholar]
- Dörig R. E., Marcil A., Chopra A., Richardson C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993 Oct 22;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Fine S. M., Unanue E. R., Chaplin D. D. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5649–5653. doi: 10.1073/pnas.85.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garenne M., Leroy O., Beau J. P., Sene I. Child mortality after high-titre measles vaccines: prospective study in Senegal. Lancet. 1991 Oct 12;338(8772):903–907. doi: 10.1016/0140-6736(91)91771-l. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Holers V. M., Atkinson J. P. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- Hsi B. L., Yeh C. J., Fénichel P., Samson M., Grivaux C. Monoclonal antibody GB24 recognizes a trophoblast-lymphocyte cross-reactive antigen. Am J Reprod Immunol Microbiol. 1988 Sep;18(1):21–27. doi: 10.1111/j.1600-0897.1988.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Janatova J., Reid K. B., Willis A. C. Disulfide bonds are localized within the short consensus repeat units of complement regulatory proteins: C4b-binding protein. Biochemistry. 1989 May 30;28(11):4754–4761. doi: 10.1021/bi00437a036. [DOI] [PubMed] [Google Scholar]
- Kalli K. R., Hsu P. H., Bartow T. J., Ahearn J. M., Matsumoto A. K., Klickstein L. B., Fearon D. T. Mapping of the C3b-binding site of CR1 and construction of a (CR1)2-F(ab')2 chimeric complement inhibitor. J Exp Med. 1991 Dec 1;174(6):1451–1460. doi: 10.1084/jem.174.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krych M., Clemenza L., Howdeshell D., Hauhart R., Hourcade D., Atkinson J. P. Analysis of the functional domains of complement receptor type 1 (C3b/C4b receptor; CD35) by substitution mutagenesis. J Biol Chem. 1994 May 6;269(18):13273–13278. [PubMed] [Google Scholar]
- Liszewski M. K., Post T. W., Atkinson J. P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Coyne K. E. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J Exp Med. 1991 Jul 1;174(1):35–44. doi: 10.1084/jem.174.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisner A., Schneider-Schaulies J., Liszewski M. K., Atkinson J. P., Herrler G. Binding of measles virus to membrane cofactor protein (CD46): importance of disulfide bonds and N-glycans for the receptor function. J Virol. 1994 Oct;68(10):6299–6304. doi: 10.1128/jvi.68.10.6299-6304.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester M., Liszewski M. K., Atkinson J. P., Oldstone M. B. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. D., Cannon M. J., Sewall A., Finlayson M., Okimoto M., Nemerow G. R. Inhibition of Epstein-Barr virus infection in vitro and in vivo by soluble CR2 (CD21) containing two short consensus repeats. J Virol. 1991 Jul;65(7):3559–3565. doi: 10.1128/jvi.65.7.3559-3565.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., Wolfert R., McNaughton M. E., Cooper N. R. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2). J Virol. 1985 Aug;55(2):347–351. doi: 10.1128/jvi.55.2.347-351.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J Immunol. 1990 Jul 1;145(1):238–245. [PubMed] [Google Scholar]
- Sparrow R. L., McKenzie I. F. Hu Ly-m5: a unique antigen physically associated with HLA molecules. Hum Immunol. 1983 May;7(1):1–15. doi: 10.1016/0198-8859(83)90002-2. [DOI] [PubMed] [Google Scholar]