Abstract
We developed a simple system that regulates CO2 and O2 levels within a microfluidic chip. This system enables long-term cell culture under hypoxic conditions without the need of a CO2 incubator or a multi-gas incubator. Hypoxic conditions were generated using a miniature water jacket containing dissolved ascorbate as an oxygen scavenger. Formulations of the water jacket were determined that enables both 5% pCO2 and desired pO2 levels ranging from 5 to 15%. We also cultured PC-12 cells and primary neuronal cells from chick embryos under hypoxia and observed hypoxia-induced cell death and inhibition of neurite outgrowth.
Physiological oxygen concentration in vivo ranges from 1 to 11%1 and is lower than the atmospheric level (20.9%). Hypoxic conditions can also result in some cellular responses such as angiogenesis,2 cell death,3 and differentiation.4 To study these cellular responses, an experimental cell culture system is needed in which the oxygen concentration can be maintained at a low level. Conventional systems that create a hypoxic environment, such as multi-gas incubators and modular incubation chambers, are expensive and bulky. The cost and size of these devices are particularly problematic when one is interested in examining the degree of cellular responses under different oxygen levels. Moreover, the conventional systems do not allow researchers to mimic short-term hypoxia because they require >3 h to equilibrate the dissolved oxygen in the medium with the surrounding gas-phase O2.5
To facilitate the preparation of various oxygen tensions, microfluidic oxygen gradient generators have proven to be effective in observing cellular responses under varying oxygen levels at low spatial cost.6,7 However, to temporally and spatially stabilize the oxygen concentration gradient through gas exchange, these systems still require precise control of flow rates. As a result, most microfluidic gradient generators require cumbersome components such as syringe pumps. It is also time consuming and troublesome to calibrate the concentration gradient before each experiment. These complexities can limit research which requires specialized oxygen tensions for cell cultures.
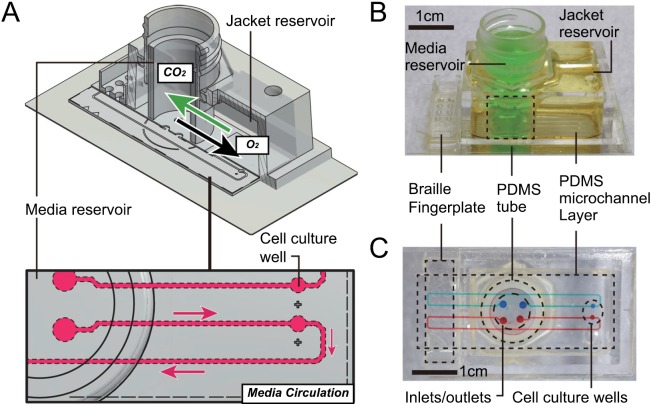
Here, we report on a simple system for accurately maintaining the CO2 and O2 levels of the cell culture medium that is introduced to microfluidic channels. We designed a flat glass-bottom microfluidic chip that enables long-term maintenance of CO2/O2 levels in cell culture media without any incubator, recirculates media using a Braille device, and allows for observation by confocal laser microscope (Fig. 1). As shown in Figure 1(a), the microfluidic chip has two nested reservoirs partitioned with a tube made of PDMS, as previously described.8 When the jacket (outer) reservoir has low O2 and high CO2 levels, the O2/CO2 levels of the media (inner) change likewise due to diffusion of O2/CO2 through the PDMS tube. The microchannel feature layer was fabricated by typical softlithographic processes with backside lithography.9 The thin glass layer placed on top of the microfluidic channel layer can stop gas exchange at the interface of the microfluidic channels. By limiting the diffusion of gas through the channel surface, one can accurately estimate O2/CO2 levels of the medium flowing through the entire length of the channels by measuring the O2/CO2 levels of the media reservoir.
FIG. 1.
Microfluidic cell culture chip with on-chip multi-gas incubation. (a) Diagram showing the maintenance of cell culture media in the microfluidic chip. Gas exchange (O2 and CO2) and media flow in the microfluidic channel are shown. (b) Exterior appearance of the entire cell culture chip. (c) Upper view of the microfluidic channel layer.
We tested a buffer system that contains sodium ascorbate (NaHAsc) as an oxygen scavenger. Ascorbic acid (AscH2) is a weak diprotic acid: it can dissociate into ascorbate monoanion (AscH−) and ascorbate dianion (Asc2−)
Although both ascorbate anions autoxidize into ascorbyl radical (AscH−), deprotonation from AscH− to AscH− is unlikely to occur at near neutral pH because the midpoint reduction potential is high. Asc2− easily donates electrons and forms AscH− (Ref. 10) and then forms dehydroascorbic acid (DHA). Therefore, a simplified description of the reaction is
Since the second pKa of AscH2 is large, the autoxidation of ascorbate is slow at near neutral pH. However, it increases by tenfold for one pH rise.11 This slow but regulated dissociation shows how stabilization is possible in parallel with O2 reduction and stabilization of other buffers such as bicarbonate buffer.
Temporal stability of partial O2 pressure (pO2) in the media reservoir was evaluated using a fiber optic oxygen sensor. Figure 2 shows the pO2 values in the media reservoir measured for 72 h. As shown in Figure 2(a), incubating the media reservoir with NaHAsc-containing jacket solution immediately decreased the pO2. The pO2 then plateaued approximately after 6 h incubation. The variations of pO2 at their plateau were within ±0.3% atm for all cases. The result showed that NaHAsc in the jacket reservoir effectively stabilized the pO2 to hypoxic values for at least 3 days.
FIG. 2.
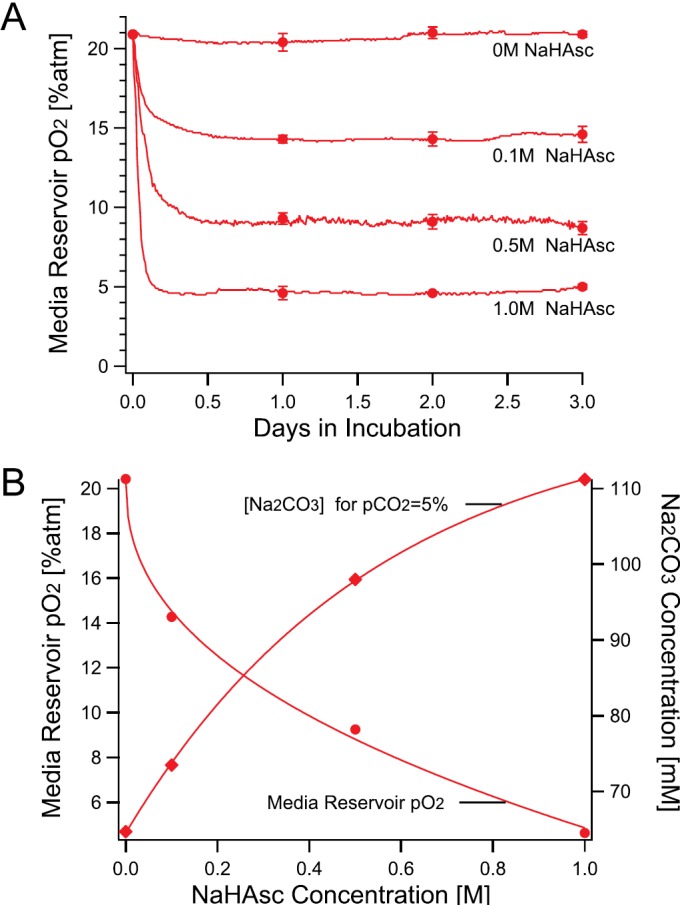
Hypoxic conditioning of the microfluidic chip with NaHAsc. (a) Time evolution of partial O2 pressure (pO2) measured in the media reservoir in contact with jacket solutions containing 0.8 M NaHCO3, 65 mM Na2CO3, and different concentration of NaHAsc. Observed typical pO2 values at 10-min intervals are plotted as lines; the average values at 24, 48, and 72 h are plotted as representative values (N = 3, ±SD). (b) The relationship among the NaHAsc concentration, the media reservoir pO2, and the Na2CO3 concentration when the media reservoir pCO2 is 5% on Day 1. Interpolated Na2CO3 concentrations values at pCO2 = 5% atm shown in (a) with corresponding NaHAsc concentrations were plotted.
Figure 2(b) shows the relationship among the NaHAsc concentration in the jacket reservoir and the pO2 in the media reservoir after 24 h incubation with NaHAsc-containing jacket reservoir, and the Na2CO3 concentration to obtain 5% atm pCO2. As expected, we observed a monotonic decrease of pO2 with increasing NaHAsc concentration. In contrast, the pCO2 was decreased by addition of NaHAsc and could be compensated by addition of Na2CO3. To determine the formulation of a jacket solution from Figure 2(b), one needs to: (1) fix NaHCO3 concentration to 0.8 M, (2) find a NaHAsc concentration against the desired pO2, and (3) find the Na2CO3 concentration against the NaHAsc concentration obtained in (2). We can infer that the addition of ascorbate in the jacket solution changes it to the ascorbic acid molecule (AscH2) and the reaction suppresses excess production of carbonic acid. As the bicarbonate buffer system reaches equilibrium and CO32− accumulates, ascorbate donates protons to pull its equilibrium to Asc2− generation, resulting in pCO2 increase.
We examined a neuronal cell culture under hypoxia using the microfluidic and on-chip multi-gas incubation system. Figures 3(a) and 3(b) show rat adrenal pheochromocytoma (PC-12) cells cultured on-chip for 24 h under normoxia and hypoxia conditions (pO2 approximately 5%; see Fig. 2(b)). Whereas PC-12 cells under normoxia condition (a) were healthy at 24 h, however, cells under hypoxia died (b), as shown by EthD-1 red fluorescence. These results are consistent with a report that showed hypoxia induced death of PC-12 cells,12 in which the survival rate of PC-12 cells under 2% O2 was 80% at 24 h and 20% at 72 h. The reason for the PC-12 cells dying more rapidly at 24 h in the microfluidic device than that of a previous report12 may be explained by the fast diffusion velocity of gas-phase oxygen from a liquid of high surface-to-volume ratio through PDMS, a highly oxygen-permeable material compared with the velocity of conventional cell culture vessels.5
FIG. 3.
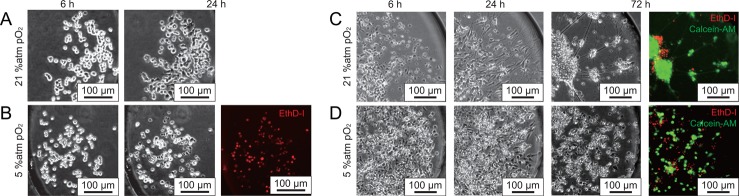
Microfluidic neuronal cell cultures under hypoxia using the on-chip multi-gas incubation system. Used jacket solutions are without NaHAsc and with 1.0 M NaHAsc. The pCO2 was adjusted to 5% atm based on data from Figure 2(b). Hours denotes the time after seeding. (a and b) Microfluidic cell culture of PC-12 cells. (c and d) On-chip culture of dorsal root ganglion neurons isolated from chick embryo.
Figures 3(c) and 3(d) show dorsal root ganglion cells from chick embryos cultured on-chip for 72 h under normoxia and hypoxia (pO2 approximately 5%). Based on the fluorescent cell viability assay, the overall survival rate, including all cell species, was approximately 50% at 72 h. The surviving cells, however, aggregated and significantly extended their neurites only under normoxia. Neurons under hypoxia separated and extended neurites only slightly. These results reasonably agree with a previous report that showed inhibition of neurite growth and a survival rate of 75% at 48 h under 1% O2.13
In conclusion, we developed a simple, standalone microfluidic chip that is suitable for on-chip cell culturing under hypoxia. Taking advantage of the slow dissociation of AscH−, we successfully maintained hypoxic pO2 levels (5% or higher) for at least 3 days. We also demonstrated successful on-chip hypoxic cell culturing of PC-12 cells and neural cells from chick embryos and found that hypoxia-induced cellular changes were similar to those observed using conventional multi-gas CO2 incubators. The simplicity of this system is advantageous in evaluating effects of hypoxia under multiple conditions of pO2 levels.
Acknowledgments
This research was supported by KAKENHI (25350576) and a Grant-in-Aid for JSPS fellows 24-11210.
References
- 1.Carreau A., Hafny-Rahbi B. E., Matejuk A., Grillon C., and Kieda C., J. Cell. Mol. Med. 15(6), 1239–1253 (2011). 10.1111/j.1582-4934.2011.01258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pugh C. W. and Ratcliffe P. J., Nat. Med. 9(6), 677–684 (2003). 10.1038/nm0603-677 [DOI] [PubMed] [Google Scholar]
- 3.Bunn H. F. and Poyton R. O., Physiol. Rev. 76(3), 839–885 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Simon M. C. and Keith B., Nat. Rev. Mol. Cell Biol. 9(4), 285–296 (2008). 10.1038/nrm2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen C. B., Schneider B., Kelly W., and Carl W., Am. J. Physiol. 281(4), L1021–L1027 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Wang L., Liu W., Wang Y., Wang J.-c., Tu Q., Liu R., and Wang J., Lab Chip 13(4), 695–705 (2013). 10.1039/c2lc40661f [DOI] [PubMed] [Google Scholar]
- 7.Funamoto K., Zervantonakis I. K., Liu Y. C., Ochs C. J., Kim C., and Kamm R. D., Lab Chip 12(22), 4855–4863 (2012). 10.1039/c2lc40306d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takano A., Tanaka M., and Futai N., Microfluid. Nanofluid. 12(6), 907–915 (2012). 10.1007/s10404-011-0925-z [DOI] [Google Scholar]
- 9.Futai N., Gu W., and Takayama S., Adv. Mater. 16(15), 1320–1323 (2004). 10.1002/adma.200400595 [DOI] [Google Scholar]
- 10.Njus D., Jalukar V., Zu J., and Kelley P. M., Am. J. Clin. Nutr. 54(6), 1179S–1183S (1991). [DOI] [PubMed] [Google Scholar]
- 11.Buettner G. R., J Biochem. Bioph. Meth. 16(1), 27–40 (1988). 10.1016/0165-022X(88)90100-5 [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura S., Banno Y., Nakashima S., Takenaka K., Sakai H., Nishimura Y., Sakai N., Shimizu S., Eguchi Y., and Tsujimoto Y., J. Biol. Chem. 273(12), 6921–6927 (1998). 10.1074/jbc.273.12.6921 [DOI] [PubMed] [Google Scholar]
- 13.Honma H., Gross L., and Windebank A. J., Neurosci. Lett. 354(2), 95–98 (2004). 10.1016/j.neulet.2003.08.084 [DOI] [PubMed] [Google Scholar]