Abstract
Background
Cardiac surgery has been shown to result in a significant decrease of the antioxidant selenium, which is associated with the development of multiorgan dysfunction and increased mortality. Thus, a large-scale study is needed to investigate the effect of perioperative selenium supplementation on the occurrence of postoperative organ dysfunction.
Methods/Design
We plan a prospective, randomized double-blind, multicenter controlled trial, which will be conducted in North and South America and in Europe. In this trial we will include 1,400 high-risk patients, who are most likely to benefit from selenium supplementation. This includes patients scheduled for non-emergent combined and/or complex procedures, or with a predicted operative mortality of ≥5% according to the EuroSCORE II. Eligible patients will be randomly assigned to either the treatment group (bolus infusion of 2,000 μg sodium selenite immediately prior to surgery, followed by an additional dosage of 2,000 μg at ICU admission, and a further daily supplementation of 1,000 μg up to 10 days or ICU discharge) or to the control group (placebo administration at the same time points).
The primary endpoint of this study is a composite of 'persistent organ dysfunction’ (POD) and/or death within 30 days from surgery (POD + death). POD is defined as any need for life-sustaining therapies (mechanical ventilation, vasopressor therapy, mechanical circulatory support, continuous renal replacement therapy, or new intermittent hemodialysis) at any time within 30 days from surgery.
Discussion
The SUSTAIN-CSX™ study is a multicenter trial to investigate the effect of a perioperative high dosage sodium selenite supplementation in high-risk cardiac surgical patients.
Trial registration
This trial was registered at Clinicaltrials.gov (identifier: NCT02002247) on 28 November 2013.
Electronic supplementary material
The online version of this article (doi:10.1186/1745-6215-15-339) contains supplementary material, which is available to authorized users.
Keywords: Selenium, Inflammatory response, Oxidative stress, Antioxidant capacity, Myocardial ischemia/reperfusion, Postoperative organ failure
Background
Cardiac surgery is performed annually in approximately one million patients worldwide. If current healthcare use and service delivery patterns continue the demand for cardiac surgery is expected to increase on the basis of population growth and ageing [1].
Patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) are exposed to various ischaemic stimuli, resulting from global ischaemic cardioplegic arrest of the heart and/or from embolic events. Cessation of CPB and reperfusion of ischemic vascular beds evokes oxidative stress and triggers an intense inflammatory response, which is associated with endothelial dysfunction, microvascular thrombosis, immune dysfunction, and eventually the potential for injury of virtually all vital organs, including heart, lungs, brain, kidneys and intestines (Figure 1) [2, 3]. In mammals, a sophisticated endogenous defense system protects tissues from oxidative stress. Several enzymes such as catalase, superoxide dismutase and glutathione peroxidase (GPx) are specifically designed to neutralize reactive oxygen species [4]. For these antioxidant (AOX) enzymes, interest in the essential trace elements selenium has arisen as it is involved in multiple steps of intracellular AOX defense [5, 6] and thus can neutralize both reactive oxygen and nitrogen species [4]. Selenium is considered as the cornerstone of the antioxidant defense mechanism and may be one of the most important antioxidants [7]. When incorporated into the various selenoenzymes, selenium increases its antioxidant capacity and influences the inflammatory signaling pathways that modulate reactive oxygen species (ROS) by inhibiting the nuclear factor-kappa b (NF-kB) cascade, resulting in a suppressed production of interleukins and tumor necrosis factor alpha (TNFα) [8].
Figure 1.
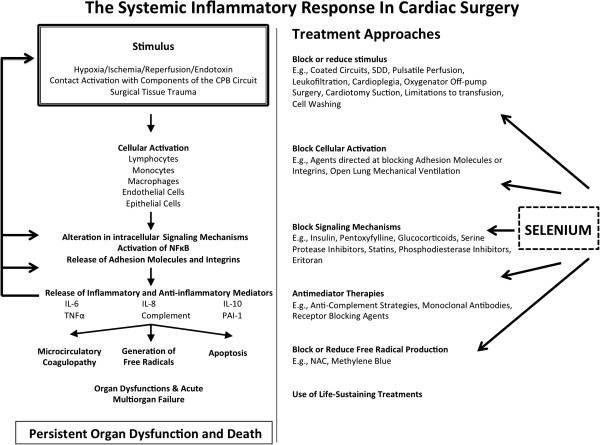
Multiple stimulus and treatment approaches in cardiac surgery. The present figure was modified and reproduced with permission from Hall [2]. CPB = cardiopulmonary bypass; IL = interleukin; TNF = tumor necrosis factor; PAI = Plasminogen activator inhibitor; NAC = N-acetylcysteine.
In addition to critically ill patients, a significant selenium deficiency and hence an insufficient endogenous antioxidant capacity was recently demonstrated among patients after cardiac surgery. Selenium levels fell with increasing CPB time and continued to decrease postoperatively, when compared to selenium levels from a healthy population not undergoing surgery. Importantly, low selenium levels were associated with postoperative complications and the intraoperative selenium decrease showed a predictive accuracy for subsequent development of multi-organ failure [9, 10]. Given those findings and recent results from a non-randomized open-label study which indicated a beneficial effect of perioperative sodium selenite supplementation [11], we aim to study a perioperative sodium selenite supplementation in a large-scale randomized trial, targeting patients who have a high risk of pronounced selenium deficiency. We hypothesize that the therapeutic strategy in this randomized trial may contribute to a lower rate of organ dysfunction postoperatively, and thus, improved health outcomes.
Methods/Design
Design
This is a randomized, double-blind (patients and physician), placebo controlled multicenter study, which will be conducted in North America (Canada and United States) and Europe (Belgium, Switzerland and Germany), with an aim to enroll approximately 1,400 patients to evaluate the overall study hypothesis.
Ethics
The present study (study protocol, patient information and informed consent) is approved by the ethics committee of RWTH Aachen University (Ethik-Kommission an der Medizinischen Fakultät der RWTH Aachen, ethic vote EK 249/13) and Queens University (Kingston, Canada). Furthermore it has been approved by Health Canada and the Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) in Germany. Written informed consent from the patient and treating anesthesiologist or surgeon will be obtained before enrolment in the study.
Inclusion criteria
Participants in the trial must be adult patients (>18 years of age) scheduled to undergo elective cardiac surgery with the use of cardiopulmonary bypass (CPB) and cardioplegic arrest that exhibit a high perioperative risk profile as defined by either: 1.) planned valve surgery combined with coronary artery bypass grafting or 2.) multiple valve replacement and/or repair surgeries or 3.) combined cardiac surgical procedures involving the thoracic aorta or 4.) scheduled cardiac surgery with a high perioperative risk profile, defined as a predicted operative mortality of ≥5% (EuroSCORE II).
These high-risk patients have recently been shown to experience an excessive systemic inflammatory response, with the most pronounced decrease of selenium during surgery [10, 12]. Furthermore it has been demonstrated that postoperative selenium blood levels were inversely correlated with duration of CPB. More precisely, the longer the surgical procedure, the more pronounced the postoperative decrease in circulating selenium levels (r = -0.121, P <0.05) [9]. In addition, the results of our collaborators and those from a recently published study revealed that the preoperatively assessed EuroSCORE I inversely correlated to the postoperatively measured selenium levels (r = -0.312, P <0.01), indicating that this group would be most likely to benefit from perioperative selenium supplementation [9]. These same criteria enabled the identification of patients who were likely to experience a prolonged ICU course [13], which will offer some statistical efficiencies given the higher rate of organ dysfunction and need for prolonged administration of life-sustaining therapies.
Since previous randomized trials in cardiac surgery often lacked generalizability, particularly in the case of elderly patients, we decided to enroll patients in accordance to the recommendations of the American Heart Association Council on Clinical Cardiology without any age restriction [14].
Exclusion criteria
We will exclude patients who meet any of the following criteria: 1) known hypersensitivity to sodium selenite or to any of the constituents of the solution, 2) severe renal dysfunction as evidenced by preoperative creatinine clearance <50 ml/min and/or preoperative value of serum creatinine level >200 μmol/L, 3) chronic liver disease as evidenced by a preoperative total bilirubin >2 mg/dl or 34 umol/L, 4) disabling neuropsychiatric disorders (severe dementia, severe Alzheimer’s disease, advanced Parkinson’s disease), 5) pregnancy or lactation period, 6.) simultaneous participation in another clinical trial of an experimental therapy (co-enrolment acceptable in observational studies or randomized trials of existing therapies if permitted by both steering committees and local ethics boards), 7) patients undergoing heart transplantation or preoperative planned LVAD insertion or complex congenital heart surgery.
These exclusion criteria will enable us to exclude patients who have the lowest likelihood of deriving benefit from the study intervention, and those at high risk of having an atypical postoperative course (patients undergoing heart transplantation), and thus potentially interfering with the valid determination of our primary endpoint.
Random allocation of patients
At each participating centre the local coordinating investigator will screen daily all cardiac surgical patients scheduled to undergo cardiac surgery in the near future or on the next day. A screening log will be kept at each site to determine the number of patients meeting the inclusion criteria, those truly eligible patients, those who consent and are randomized, and reasons why potentially eligible patients were not enrolled. Following a full explanation of the nature and purpose of the study, written informed consent will be sought from the patients participating in the study. At the time of enrolment into the study, patients will be randomized to receive either sodium selenite or a matching placebo similar in appearance, consistency, volume, and smell so as to blind patients, investigators and healthcare practitioners as to the nature of the study medication. Patients will be consecutively randomized by a web-based randomization system (concealed and blinded) developed by the Clinical Evaluation Research Unit at the Kingston General Hospital and will be based on the method of permutated blocks of undisclosed random size and stratified by centre.
Intervention
Patients will receive daily perioperative treatment of either high-dose sodium selenite or placebo. As with our previously referenced preliminary studies, all patients will receive an intravenous bolus of 2,000 μg sodium selenite (equal to 40 ml prepared solution) or the same volume of normal saline (placebo) within 30 minutes after induction of anesthesia via the central venous catheter. This bolus dose will be completed prior to commencing cardiopulmonary bypass. After termination of surgery, immediately after admission to the ICU, all patients will receive a second bolus of 2,000 μg sodium selenite or placebo, accordingly. This additional postoperative bolus is in response to the postoperative drop in selenium levels observed on day 1 in a recently completed open label trial (Figure 2) [11]. This postoperative drop may be due to sustained oxidative stress, bleeding or transfusions that frequently occur in the first 24 hours of surgery in patients with prolonged CPB. Then, on every subsequent morning (8:00 am) during ICU stay, patients will receive a continuous infusion of 1,000 μg sodium selenite or placebo over 30 minutes via central or peripheral venous access. The daily administration of study solution will continue until death, discharge from ICU (or a step down/intermediate care unit), or for a maximum of 10 days. This dosing regimen was chosen according to efficacy, tolerability and safety that was confirmed in previous supplementation trials in patients with systemic inflammation and cardiac surgery [11, 15, 16]. The intervention drug and placebo solution will be supplied by biosyn Arzneimittel GmBH (Fellbach, Germany), an industry partner that manufactures intravenous sodium selenite in accordance to good manufacture practice (GMP) guidelines and will be provided in such a way as to maintain blinding. Since there exist numerous treatments which may influence the study outcome, we will attempt to guide and standardize at least the management of anaesthesia, intensive and nutritional support (please see Additional file 1).
Figure 2.
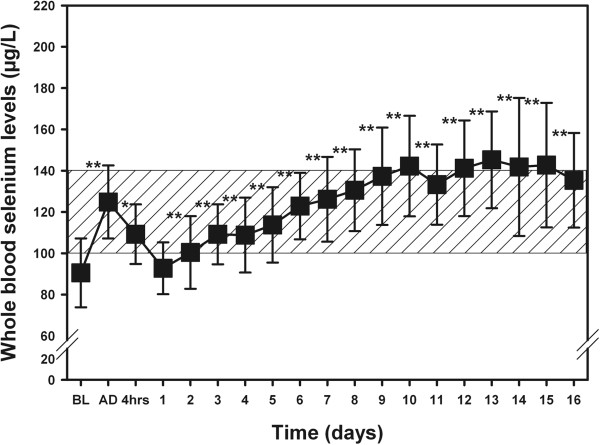
Perioperative time course of whole blood concentrations of selenium. The shaded area indicates the reference range for whole blood selenium concentration in Germany. BL: Baseline before induction of anesthesia; AD: admission to the ICU; 4 hrs: 4 hours after admission to ICU. *P <0.05 versus baseline. **P <0.01 versus baseline. This picture was taken from [11]. The shaded area indicates the reference range. Data are given as means ± SD.
Blinding and protection against bias
At the time of enrolment into the study, researchers will be blinded to the treatment assignment (guarding against selection bias). Patients will be randomized to receive either sodium selenite or a matching placebo similar in appearance (consistency, volume and smell) so as to blind patients, investigators and healthcare practitioners as to the nature of the study medication (guarding against performance and detection bias). Recording of relevant clinical data and neurocognitive assessments, will be performed by study personnel blinded to group allocation during the ICU stay (well established CAM-ICU questionnaire, which evaluates the acute onset or fluctuating course, inattention, altered level of consciousness, and disorganized thinking [17]).
Consistent with the intention-to-treat principle, all randomized patients will be included in the analysis. Large numbers of patients lost to follow-up would threaten the validity of this trial. Given that the study occurs in hospital and the majority of the outcomes, including the primary outcome, will be assessed in hospital, we anticipate no loss to follow up in hospital and less than 10% for post-discharge follow-up assessments. With respect to the planned mid-term follow-up portion of the study (secondary endpoint), to guard against attrition bias, we will take the following proactive strategies shown to enhance retention: 1) we will obtain the contact information of a family member in case we cannot contact the patient and/or if the patient is not able to provide information, to obtain the most important patient-centered physical function on SF-36 from the family member [18]; 2) the study coordinator will contact the patient and family member at the time of ICU discharge to build a relationship and reinforce the need for subsequent contact (after three and six months); 3) respondents will be notified of upcoming interviews by means of mail-out reminders; and 4) we will obtain survival status of all patients lost to follow-up from public registries.
The monitoring of sites will be performed by the CERU at the Kingston General Hospital, Ontario, Canada (for the Canadian sites) and by the Clinical Trial Center (CTC) Aachen at RWTH Aachen University, Germany.
Endpoints
Primary outcome
The primary objective of the definitive trial is to investigate the effect of a high-dose sodium selenite perioperative supplementation on clinical outcomes in cardiac surgical patients at high risk for perioperative complications. The selection of a primary clinical outcome for large-scale trials in cardiac surgery is associated with a number of challenges. Options include mortality, length of stay in the ICU and various composite endpoints. Mortality rates are too low in this population to be a responsive primary outcome for this intervention. At the Ottawa Heart Institute, over the past five years in-hospital mortality rate for all patients(elective and emergent) was 3.1%. A total sample size of over 22,000 patients would be required to achieve 80% power to detect a 20% relative risk reduction (RRR) to 2.5% at a two-sided alpha = 0.05. Furthermore, focusing on mortality alone misses the beneficial (or adverse) effect of treatments on morbidity and quality of life. Length of stay in an ICU may be a potential outcome but discharge practices are tremendously variable across units, and are dependent on non-clinical factors such as availability of beds, rendering this outcome less sensitive to detect a treatment effect. A standard composite endpoint of all-cause mortality, myocardial infarction, stroke, renal failure, and prolonged mechanical ventilation could be considered but the inclusion of stroke, particularly due to embolic origins, would not be expected to be modified by our proposed treatment strategy. Moreover, combining myocardial infarction defined by a biochemical change (troponin rise) with an event such as death challenges the validity of the composite endpoint.
A fourth option could be to use persistent organ dysfunction (POD) + death as a composite endpoint. POD is defined as the need for life-sustaining therapies at any time postoperatively (life-sustaining therapy, mechanical ventilation (includes non-invasive ventilation), any vasopressor therapy, mechanical circulatory support, continuous renal replacement therapy or intermittent hemodialysis). The primary endpoint will be the number of days alive and POD-free, which will be evaluated by the composite outcome POD + death within the first 30 days after surgery.
We have validated this endpoint in critically ill patients before and have shown that patients who develop POD are at higher risk for subsequent death and long-term disability or lower quality of life compared to those who do not have POD [19]. In a similar study of cardiac surgery patients, Williams et al. showed that the persistence of multiple organ failure identifies a subpopulation of surgical patients that have a high likelihood for death or poor physical function over the subsequent two years [20]. Moreover, given our biological model illustrated in Figure 1, POD would be the most direct outcome and the most sensitive to detecting a treatment effect of sodium selenite (Figure 1).
The 30-day time frame is commonly used in the cardiac surgery literature because there is virtually no loss to follow-up during this period and there are only about 5% of outlying patients who remain alive and dependent on life-sustaining therapy after (sometimes months after) 30 days. Patients who die within 30 days will be given a value of 0 free days. This outcome will have properties similar to 'ventilator free days’ which has been widely accepted in critical care medicine and used in several recent major RCTs [21–23]. Free days will only be counted if they persist for at least 48 hours prior to re-application of life sustaining therapy and are not followed by death within 30 days. We propose to compare the primary outcome between arms using the Wilcoxon rank sum test. The daily proportion of patients alive and free of life-sustaining therapy by arm over the first 30 days will be depicted graphically. The primary analysis will follow the intent-to-treat principle, including all patients in the arm they were randomized to regardless of treatment compliance. The analysis and study reporting will be in accordance with the CONSORT statement [24].
Secondary outcomes
Secondary outcomes for the larger scale trial will include perioperative hemodynamic profile (for example mean arterial blood pressure, cardiac output, systemic vascular resistance and so on), cardiovascular complications (such as arrhythmias, cardiac arrest and infarction), ventilator free days, the occurrence of postoperative delirium (assessed by CAM-ICU score) over time, length of stay in the ICU and hospital, re-admission rates, hospital-acquired infections (proven bacteremia) and 30-day mortality. We plan to follow study patients prospectively during the ICU stay, documenting the compliance with the study procedure, reasons for interruptions of study medications and primary and secondary outcomes. Additionally, we will contact patients by telephone at three and six months to assess survival and health-related quality of life (using SF-36 scores) as in our previous antioxidant trial of critically ill patients [25].
Besides these clinical data, we plan to obtain an extensive pharmacokinetic and pharmacodynamic evaluation of our sodium selenite supplementation regimen. Blood samples will be drawn prior to surgery (pre-treatment), after surgery (ICU admission), at the first postoperative day (before start of selenium application) and then daily until ICU discharge or postoperative day 10 to determine whole blood selenium levels (measured by atomic absorption spectroscopy to ensure determination of selenium independent from the compartment). In addition, incorporation of selenium into selenoproteins will be measured through monitoring of GPx activity and selenoprotein P (Sel-P) levels, both of which have been repeatedly shown to correlate with the circulating selenium levels [26, 27]. In addition, we aim to perform genotyping analysis to evaluate the effects of genotype on metabolism, selenium status and health outcomes. The relative ratio between two isoforms of Sel-P has been reported to be influenced by genotype with respect to two single nucleotide polymorphisms (SNPs) in the Sel-P gene, the effect of which was abrogated under conditions of selenium supplementation [27]. Since Sel-P is crucially involved in the systemic selenium transport (blood and tissue), we thus aim to evaluate the significance of the underlying genotype on patients’ response to supplementation and patients’ outcome after surgery. Furthermore, we hope to reveal new insights into the underlying genotype with respect to further selenoproteins.
We shall also aim to assess the extent of oxidative stress and inflammation by measuring MDA-LDL IgM (antibodies against oxidized LDL), asymmetric dimethyl-arginine (ADMA), endogenous peroxidase activity (EPA) and the well-established markers of inflammation: interleukin (IL)-6, IL-10 and TNFα in a substudy. All collected blood samples will be stored at -80°C until final analysis in a central lab at Aachen University. Standard lab tests to detect myocardial ischemia (to detect troponin, for example), inflammation (C-reactive protein) and organ function (creatinine, urea, arterial blood gases, bilirubin and hemoglobin) will be performed according to clinical routine and as clinically indicated.
Statistical analysis
Justification of sample size for the definitive trial
From a database of all patients that underwent cardiac surgery between 1 January 2011 and 31 December 2011 at the University Hospital of Aachen, Germany (n = 1127) and met present inclusion criteria (n = 170), we estimated the control arm distribution of POD free days. In this dataset the mean (SD) POD free days was 23.2 (9.2), factoring in a mortality rate of 4%. The distribution and cumulative distribution of POD free days shows that 4% of the patients died, 6% survived but had 0 POD free days and 58% had 26 to 29 free days. Only 15% of patients had between 1 and 20 free days. These numbers are consistent with the 4% 30-day mortality rate and the 6% not yet free from life-sustaining therapy by 30 days that we observed from the 83 patients seen at the University of Ottawa Heart Institute between July 2012 and June 2013 who met the eligibility criteria of the current calculation. Similar to ventilator free days, the distribution of free days is clearly not Gaussian, with most of its distribution near the minimum and maximum possible values of 0 and 30 respectively.
We performed simulation in order to accurately estimate the actual power of applying the Wilcoxon rank-sum test to scenarios with various effect sizes where the control arm had the same distribution as observed from our German data. The simulation generated data in the control arm where the mortality rate was 4% and the daily rate (hazard) of being liberated from life-sustaining therapy was the same as observed within our German data. The intervention arm was then generated by multiplying the control arm daily rate of liberation by a fixed factor (hazard ratio). The mean days on life-sustaining therapy was then subtracted from 30 to obtain the free days. All estimates are based on simulating 10,000 samples of the required sample size, so power estimates will have more than a 95% chance of being accurate to within 1%. It derived from a virtual calculation, which showed that the distribution of the simulated control arm was nearly identical to the distribution of the actual observed German data. The calculation also provided the distribution of the intervention arm when the intervention causes a 20% relative increase in the daily rate of liberation from life-sustaining therapy compared to the control arm.
We expect that the sample size of the definitive trial will be approximately 700 per arm. This would provide about 90% power to detect a 20% relative increase in the daily rate of liberation from life-sustaining therapy. Such an effect size would result in a mean decrease of 1.5 days in the days of life-sustaining therapy from 6.8 to 5.3 days, or equivalently, a 1.5 day increase from 23.2 to 24.7 free days. We believe such an effect size is plausible and is in line with minimally clinically important differences accepted in other recent major trials in the ICU setting.
Pilot study
Before advancing to a large-scale trial, a randomized, double-blind, placebo controlled pilot study of 80 patients will be carried out to determine the feasibility of a multicenter RCT, uncover problems regarding recruitment of patients, adherence with the study protocol and any contaminations (such as the co-application of selenium) and to develop study procedures for a large-scale RCT. The primary focus of the pilot study thus is to evaluate: 1) Recruitment of trial patients. Successful recruitment will be defined as >2 patients per month per site on average. A recruitment of 2 to 3 patients per month is considered as reasonable given the high numbers of competing studies in the field of cardiac surgery, missed patients and consent failure rates, all of which might limit our ability to enroll all of these potentially eligible patients. We expect to monitor success with recruitment throughout the study and make revisions to the recruitment procedures and eligibility criteria as necessary. 2.) Adherence to protocol. Successful adherence will be defined as ≥90% of prescribed intervention being administered across all patients. Preliminary estimates of non-administration of the trial intervention are needed, along with experience with behavioral strategies that maximize exposure to the intervention. 3.) Contamination. Success will be defined if <5% of patients have any non-study open-label intravenous selenium, in either group, during their hospital stay. Intravenous selenium is not currently the standard of care in any participating center but this outcome is important because contamination introduces risk of bias. A careful evaluation of the threat of contamination is key and mitigating strategies need to be identified.
After completion of this pilot trial, we will initiate the definitive randomized, double-blind, placebo controlled multicenter trial to evaluate our overall study hypothesis. Sites participating in the Canadian component of the multinational, multicenter pilot study include: University of Ottawa Heart Institute, Montreal Heart Institute and Sunnybrook Health Sciences Center. In addition, three European centers (RWTH Aachen University, Germany, University Hospital Frankfurt, Germany and Kiel University, Germany) will also be implementing the pilot study at their respective institutions. An additional objective of the pilot study is to finalize the primary outcome for the large-scale trial and develop estimates that can be used for a more precise calculation of the sample size for a large-scale trial. While a data safety monitoring committee has not yet been established for the pilot trial, it will be implemented for the definitive trial.
After completion of this pilot phase, descriptive and inferential statistics (mostly rates with 95% confidence intervals) will be presented to describe the pilot study feasibility outcomes. Safety and pharmacokinetic variables will be described by arm. However, due to the limited sample size and the potential of rolling the pilot study into the definitive study, clinical efficacy outcomes will be reported overall but not by arm. There are no planned interim or subgroup analyses for this pilot trial.
Discussion
A significant number of patients require cardiac surgery for the management of their underlying heart disease. In addition to death, major morbidity from organ failure remains frequent following cardiac surgery, particularly in patients at high risk for complications and poor clinical outcomes. The perioperative inflammatory response as it occurs during the process in cardiac surgery is considered a major contributor to surgery-associated complications [3].
Various studies have previously demonstrated the crucial role of the essential trace element selenium within the multiples steps of antioxidant defense [8], and circulating selenium levels have been shown to correlate with the activity of GPx and other selenoenzymes in different clinical settings. In addition, selenium affects both the cell-mediated and the humoral immune defense mechanisms, and depressed selenium levels are associated with the reduction of natural killer cells [28, 29]. Interestingly the administration of sodium selenite is postulated to have a biphasic action, initially as a pro-oxidant and then as an antioxidant [30]. The early transient pro-oxidant effect of selenite might be a useful therapeutic strategy. In fact, an intravenous loading dose given as a bolus could have the following effects: 1) direct reversible inhibition of NF-κB binding to DNA controlling gene expression and thus downregulation of the synthesis of proinflammatory cytokines [31], 2) induction of apoptosis and cytotoxicity at the microcirculation level [32], and 3) a direct virucidal and bactericidal effect [30]. Wang et al. [33] demonstrated that a selenite loading bolus causes a transient peak plasma concentration of selenium and beneficial improvements in hemodynamics status, proinflammatory cytokines profile and survival. However, these findings have never been proven in ICU patients where plasma selenium significantly decreases [34].
In view of an impressive body of evidence that indicates a beneficial role of sodium selenite during inflammation, various preliminary clinical trials attempted to enhance the antioxidant and immunological defense mechanisms in critically ill patients by a sodium selenite supplementation strategy [15, 16, 35–38]. Accordingly, results from a recent meta-analysis indicated a significantly reduced incidence of infections and mortality in critically ill patients who had received a high dose (>500 μg) selenium supplementation during their ICU stay [28]. In the same way, Alhazzani et al. [39] and Huang et al. [40] very recently demonstrated reduced mortality in critically ill patients after selenium supplementation. While there is good evidence of benefit from selenium supplementation in critical illness, the optimal timing and dosage of selenium supplementation remains unclear.
A cardiac surgical setting with myocardial ischemia and reperfusion seems to be an ideal model to investigate the effect of perioperative sodium selenite supplementation. Indeed, two recently published studies investigated the effect of a perioperative sodium selenite supplementation in cardiac surgical patients. In a randomized trial, 117 patients received preoperative metabolic therapy, which consisted of several antioxidants (coenzyme Q10, magnesium, lipoic acid, omega-3 fatty acids and sodium selenite) and was compared to a placebo. In this study, the application of a metabolic cocktail resulted in reduced oxidative stress, less myocardial injury and reduced length of hospital stay [41]. In addition, results from a recently published open label trial, investigating the safety and pharmacokinetics of high-dose sodium selenite supplementation in cardiac surgical patients, demonstrated that the chosen doses were effective in preventing the intraoperative decrease of circulating selenium levels. When comparing sodium selenite-treated patients with a historical control group of cardiac surgical patients, the treatment group had less organ dysfunction, as assessed by the Sequential Organ Failure Assessment (SOFA) score on the first postoperative day. However, the loading dose was apparently not high enough to prevent the fall in circulating selenium levels to baseline levels on the first postoperative day. For this reason, the supplementation strategy in the present trial has been modified and a second loading dose of sodium selenite was added on admission to the ICU to compensate for this.
Pharmacokinetic studies suggest that optimal incorporation of supplemental selenium into selenoproteins requires approximately 72 hours [29], and there is some evidence to suggest that post-injury normalization of selenium levels is unable to significantly affect the incurred oxidative damage [42]. In this regard, we did consider including a preoperative oral sodium selenite supplementation in addition to our intravenous perioperative supplementation as part of a 2 × 2 factorial design. However, most participating cardiac centers report a high volatility in surgical booking and subsequent cancellation and re-scheduling (for example at University of Ottawa Heart Institute over a recent six month period 80% of the original elective and/or in-hospital urgent bookings were rescheduled from their original booked date (BM, personal communication)). Thus, it appeared highly likely that there would be major issues surrounding compliance and variability of any preoperative supplementation intervention, which would impact greatly on such a trial. In the current proposed pilot trial we will track timing and occurrences of screening, booking, cancellation and surgical dates to determine if and how a preoperative supplementation strategy might be feasible in a definitive trial.
In the present trial only high-risk cardiac surgical patients will be included. Previous data indicate that these patients are exposed to the most pronounced decrease of selenium during surgery [10], leading to the development of various organ dysfunctions. In addition, previous studies indicated that postoperative selenium blood levels were inversely correlated with duration of CPB, that is, the longer the surgical procedure, the more pronounced the postoperative decrease in circulating selenium levels [9, 10]. In addition, it was demonstrated that the preoperatively assessed EuroSCORE I inversely correlated to the postoperatively measured selenium levels (r = -0.312, P <0.01), indicating that this is the group of people who are most likely to benefit from perioperative selenium supplementation [9].
In conclusion, the SUSTAIN-CSX™-study is a prospective, randomized, double-blind, multicenter controlled multinational trial to investigate the effect of perioperative high-dose sodium selenite supplementation on the persistence of organ dysfunction in approximately 1,400 high-risk cardiac surgical patients.
Trial status
Recruitment and enrollment of the first patients (pilot study) in both countries will start end of August 2014.
Electronic supplementary material
Additional file 1: Management of anesthesia and cardiopulmonary bypass: recommendations for general anesthesia and management of cardiopulmonary bypass in enrolled patients. Intensive care unit and nutritional support: recommendations and rules for postoperative management of enrolled patients during the ICU stay with respect to extubation, discharge from ICU and nutrition. (DOCX 83 KB)
Acknowledgements
This study is supported by the Canadian Institute of Health Research (Canada) and biosyn Arzneimittel GmBH (Fellbach, Germany), which provides the study medication and financial support for the study initiation. This study has been presented in part in the 34th International Symposium on Intensive Care and Emergency Medicine (ISICEM), Brussels.
Abbreviations
- ADMA
Asymmetric Dimethyl-Arginin
- AOX
Antioxidant
- CPB
Cardiopulmonary bypass
- EPA
Endogenous peroxidase activity
- GMP
Good manufacture practice
- GPX
Glutathione peroxidase
- ICU
Intensive care unit
- IL
Interleukin
- LVAD
Left ventricular assist device
- NF-kB
Nuclear factor-kappa b
- POD
Persistent organ dysfunction
- ROS
Reactive oxygen species
- Sel-P
Selenoprotein P
- SNP
Single nucleotide polymorphisms
- TNFα
Tumor necrosis factor alpha.
Footnotes
Competing interests
CS, SR and WM have previously received a speaker’s fee from biosyn Arzneimittel GmbH (Fellbach, Germany).
Authors’ contributions
CS, BM, SR, WM, RW, SF, RF, YL, PM, CH, RR, AG, GE, AD and DH have substantially contributed to the interpretation of current scientific knowledge, which resulted in the conception and design of the present study. CS, BM, SR, WM, RW, SF, RF, YL, PM, CH, RR, AG, GE, and DH are investigators of the present trial and participated in the acquisition of funding and contribute to the collection of data. All authors have been involved in drafting the manuscript or revising it critically for important intellectual content. AD performed the statistical analysis. CS, BM and DH conceived of the study, coordinated and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Christian Stoppe, Email: christian.stoppe@gmail.com.
Bernard McDonald, Email: bmcdonald@ottawaheart.ca.
Steffen Rex, Email: steffen.rex@gmail.com.
William Manzanares, Email: wmanzanares@adinet.com.uy.
Richard Whitlock, Email: Richard.Whitlock@phri.ca.
Stephen Fremes, Email: Stephen.Fremes@sunnybrook.ca.
Robert Fowler, Email: Rob.Fowler@sunnybrook.ca.
Yoan Lamarche, Email: yoanlamarche@gmail.com.
Patrick Meybohm, Email: patrick.Meybohm@kgu.de.
Christoph Haberthür, Email: christoph.haberthuer@hirslanden.ch.
Rolf Rossaint, Email: rrossaint@ukaachen.de.
Andreas Goetzenich, Email: agoetzenich@ukaachen.de.
Gunnar Elke, Email: Gunnar.Elke@uksh.de.
Andrew Day, Email: daya@KGH.KARI.NET.
Daren K Heyland, Email: dkh2@queensu.ca.
References
- 1.Grover A, Gorman K, Dall TM, Jonas R, Lytle B, Shemin R, Wood D, Kron I. Shortage of cardiothoracic surgeons is likely by 2020. Circulation. 2009;120:488–494. doi: 10.1161/CIRCULATIONAHA.108.776278. [DOI] [PubMed] [Google Scholar]
- 2.Hall R. Identification of inflammatory mediators and their modulation by strategies for the management of the systemic inflammatory response during cardiac surgery. J Cardiothorac Vasc Anesth. 2013;27:983–1033. doi: 10.1053/j.jvca.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Laffey JG, Boylan JF, Cheng DCH. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Klotz L-O, Kröncke K-D, Buchczyk DP, Sies H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J Nutr. 2003;133:1448S–1451S. doi: 10.1093/jn/133.5.1448S. [DOI] [PubMed] [Google Scholar]
- 5.Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta. 2009;1790:1478–1485. doi: 10.1016/j.bbagen.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Venardos KM, Perkins A, Headrick J, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Curr Med Chem. 2007;14:1539–1549. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- 7.Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med. 2004;31:327–337. doi: 10.1007/s00134-004-2522-z. [DOI] [PubMed] [Google Scholar]
- 8.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 9.Koszta G, Kacska Z, Szatmári K, Szerafin T, Fülesdi B. Lower whole blood selenium level is associated with higher operative risk and mortality following cardiac surgery. J Anesth. 2012;26:812–821. doi: 10.1007/s00540-012-1454-y. [DOI] [PubMed] [Google Scholar]
- 10.Stoppe C, Schälte G, Rossaint R, Coburn M, Graf B, Spillner J, Marx G, Rex S. The intraoperative decrease of selenium is associated with the postoperative development of multiorgan dysfunction in cardiac surgical patients. Crit Care Med. 2011;39:1879–1885. doi: 10.1097/CCM.0b013e3182190d48. [DOI] [PubMed] [Google Scholar]
- 11.Stoppe C, Spillner J, Rossaint R, Coburn M, Schälte G, Wildenhues A, Marx G, Rex S. Selenium blood concentrations in patients undergoing elective cardiac surgery and receiving perioperative sodium selenite administration. Nutrition. 2013;29:158–165. doi: 10.1016/j.nut.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Motoyama T, Okamoto K, Kukita I, Hamaguchi M, Kinoshita Y, Ogawa H. Possible role of increased oxidant stress in multiple organ failure after systemic inflammatory response syndrome. Crit Care Med. 2003;31:1048–1052. doi: 10.1097/01.CCM.0000055371.27268.36. [DOI] [PubMed] [Google Scholar]
- 13.Silberman S, Bitran D, Fink D, Tauber R, Merin O. Very prolonged stay in the intensive care unit after cardiac operations: early results and late survival. Ann Thorac Surg. 2013;96:15–21. doi: 10.1016/j.athoracsur.2013.01.103. [DOI] [PubMed] [Google Scholar]
- 14.Alexander KP, Newby LK, Cannon CP, Armstrong PW, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, Gore JM, Krumholz HM, Ohman EM, American Heart Association Council on Clinical Cardiology, Society of Geriatric Cardiology Acute coronary care in the elderly, part I: non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 15.Manzanares W, Biestro A, Galusso F, Torre MH, Mañáy N, Facchin G, Hardy G. High-dose selenium for critically ill patients with systemic inflammation: Pharmacokinetics and pharmacodynamics of selenious acid: a pilot study. Nutrition. 2010;26:634–640. doi: 10.1016/j.nut.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Angstwurm MWA, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, Strau R, Meier-Hellmann A, Insel R, Radke J, Schüttler J, Gärtner R, Selenium in Intensive Care (SIC) Results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock*. Crit Care Med. 2007;35:118–126. doi: 10.1097/01.CCM.0000251124.83436.0E. [DOI] [PubMed] [Google Scholar]
- 17.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 18.Kosinski M, Keller SD, Hatoum HT, Kong SX, Ware JE. The SF-36 Health Survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: tests of data quality, scaling assumptions and score reliability. Med Care. 1999;37:MS10–MS22. doi: 10.1097/00005650-199905001-00002. [DOI] [PubMed] [Google Scholar]
- 19.Heyland DK, Muscedere J, Drover J, Jiang X, Day AG, Canadian Critical Care Trials Group Persistent organ dysfunction plus death: a novel, composite outcome measure for critical care trials. Crit Care. 2011;15:R98. doi: 10.1186/cc10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams MR, Wellner RB, Hartnett EA, Thornton B, Kavarana MN, Mahapatra R, Oz MC, Sladen R. Long-term survival and quality of life in cardiac surgical patients with prolonged intensive care unit length of stay. Ann Thorac Surg. 2002;73:1472–1478. doi: 10.1016/S0003-4975(02)03464-1. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfeld DA, Bernard GR, ARDS Network Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Heart N, Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice TW, Wheeler AP, Thompson BT, de Boisblanc BP, Steingrub J, Rock P, NIH NHLBI Acute Respiratory Distress Syndrome Network of Investigators, NHLBI ARDS Clinical Trials Network Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Schulz KF, Altman D, CONSORT Group (Consolidated Standards of Reporting Trials) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 25.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG, Canadian Critical Care Trials Group A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489–1497. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Reyes R, Mathieu F, Boelaert M, Begaux F, Suetens C, Rivera MT, Neve J, Perlmutter N, Vanderpas J. Selenium and iodine supplementation of rural Tibetan children affected by Kashin-Beck osteoarthropathy. Am J Clin Nutr. 2003;78:137–144. doi: 10.1093/ajcn/78.1.137. [DOI] [PubMed] [Google Scholar]
- 27.Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R. Selenium in human health and disease. Antioxida Redox Signal. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 28.Holzer R, Bockenkamp B, Booker P, Newland P, Ciotti G, Pozzi M. The impact of cardiopulmonary bypass on selenium status, thyroid function, and oxidative defense in children. Pediatr Cardiol. 2004;25:522–528. doi: 10.1007/s00246-004-0659-8. [DOI] [PubMed] [Google Scholar]
- 29.Manzanares W, Dhaliwal R, Jiang X, Murch L, Heyland DK. Antioxidant micronutrients in the critically ill: a systematic review and meta-analysis. Crit Care. 2012;16:R66. doi: 10.1186/cc11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent J-L, Forceville X. Critically elucidating the role of selenium. Curr Opin Anaesthesiol. 2008;21:148–154. doi: 10.1097/ACO.0b013e3282f49afe. [DOI] [PubMed] [Google Scholar]
- 31.Maehira F, Miyagi I, Eguchi Y. Selenium regulates transcription factor NF-kappaB activation during the acute phase reaction. Clin Chim Acta. 2003;334:163–171. doi: 10.1016/S0009-8981(03)00223-7. [DOI] [PubMed] [Google Scholar]
- 32.Chung YW, Kim TS, Lee SY, Lee SH, Choi Y, Kim N, Min B-M, Jeong D-W, Kim IY. Selenite-induced apoptosis of osteoclasts mediated by the mitochondrial pathway. Toxicol Lett. 2006;160:143–150. doi: 10.1016/j.toxlet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Forceville X, Antwerpen PV, Piagnerelli M, Ahishakiye D, Macours P, Backer DD, Neve J, Vincent J-L. A large-bolus injection, but not continous infusion of sodium selenite improves outcome in peritonitis. Shock. 2009;32:140–146. doi: 10.1097/SHK.0b013e318193c35d. [DOI] [PubMed] [Google Scholar]
- 34.Hardy G, Hardy I, Manzanares W. Selenium supplementation in the critically ill. Nutr Clin Pract. 2012;27:21–33. doi: 10.1177/0884533611434116. [DOI] [PubMed] [Google Scholar]
- 35.Manzanares W, Biestro A, Torre MH, Galusso F, Facchin G, Hardy G. High-dose selenium reduces ventilator-associated pneumonia and illness severity in critically ill patients with systemic inflammation. Intensive Care Med. 2011;37:1120–1127. doi: 10.1007/s00134-011-2212-6. [DOI] [PubMed] [Google Scholar]
- 36.Valenta J, Brodska H, Drabek T, Hendl J, Kazda A. High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med. 2011;37:808–815. doi: 10.1007/s00134-011-2153-0. [DOI] [PubMed] [Google Scholar]
- 37.Mishra V, Baines M, Perry SE, McLaughlin PJ, Carson J, Wenstone R, Shenkin A. Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clin Nutr. 2007;26:41–50. doi: 10.1016/j.clnu.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Andrews PJD, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, Vale LD, Battison CG, Jenkinson DJ, Cook JA, and the SIGNET (Scottish Intensive care Glutamine or seleNium Evaluative Trial) Trials Group Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ. 2011;342:d1542. doi: 10.1136/bmj.d1542. [DOI] [PubMed] [Google Scholar]
- 39.Alhazzani W, Jacobi J, Sindi A, Hartog C, Reinhart K, Kokkoris S, Gerlach H, Andrews P, Drabek T, Manzanares W, Cook DJ, Jaeschke RZ. The effect of selenium therapy on mortality in patients with sepsis syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2013;41:1555–1564. doi: 10.1097/CCM.0b013e31828a24c6. [DOI] [PubMed] [Google Scholar]
- 40.Huang T-S, Shyu Y-C, Chen H-Y, Lin L-M, Lo C-Y, Yuan S-S, Chen P-J. Effect of parenteral selenium supplementation in critically ill patients: a systematic review and meta-analysis. PLoS One. 2013;8:e54431. doi: 10.1371/journal.pone.0054431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leong J-Y, van der Merwe J, Pepe S, Bailey M, Perkins A, Lymbury R, Esmore D, Marasco S, Rosenfeldt F. Perioperative metabolic therapy improves redox status and outcomes in cardiac surgery patients: a randomised trial. Heart Lung Circ. 2010;19:584–591. doi: 10.1016/j.hlc.2010.06.659. [DOI] [PubMed] [Google Scholar]
- 42.Sandre C, Agay D, Ducros VR, Faure H, Cruz C, Alonso A, Chancerelle Y, Roussel A-M. Kinetic changes of oxidative stress and selenium status in plasma and tissues following burn injury in selenium-deficient and selenium-supplemented rats. J Trauma. 2006;60:627–634. doi: 10.1097/01.ta.0000205640.82459.d6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Management of anesthesia and cardiopulmonary bypass: recommendations for general anesthesia and management of cardiopulmonary bypass in enrolled patients. Intensive care unit and nutritional support: recommendations and rules for postoperative management of enrolled patients during the ICU stay with respect to extubation, discharge from ICU and nutrition. (DOCX 83 KB)


