Abstract
Rhabdomyoma (RHM) is a benign cardiac tumour usually associated with tuberous sclerosis complex (TSC). Most RHMs are asymptomatic and regress spontaneously during the first years of life. Haemodynamically significant RHMs are classically treated with surgical excision. We present a case of a premature infant, born to a mother having TSC, with a prenatal diagnosis of pulmonary valve atresia and a large ventricular septal defect. Multiple cardiac RHMs were also present, including a large tumour affecting the right ventricular filling. Owing to the prematurity and low birth weight, the infant was inoperable. In this report, we describe our approach to pharmacologically reduce the RHM size using oral everolimus in preparation for a two-ventricle surgical repair of the structural cardiac defect. We also specifically describe the dose of everolimus that was used in this case to achieve therapeutic serum levels, which was seven times lower than the conventional dose applicable for older infants.
Background
Tuberous sclerosis complex (TSC) is a multisystem disease associated with abnormal cellular proliferation and differentiation leading to the growth of hamartomas in various organs including the brain, skin, kidney, liver, lung and heart.1 This abnormal cellular differentiation and proliferation is a result of anomalies in TSC1 and TSC2 genes that lead to upregulation of the mammalian target of rapamycin (mTOR) pathway.2 3 Recent studies confirmed the efficacy and safety of mTOR inhibitor treatment for subependymal giant-cell astrocytoma and renal angiomyolipomas and proposed a disease-modifying effect on other aspects of TSC.4–7
Cardiac rhabdomyoma (RHM) is the most common congenital cardiac tumour and is often the first prenatal sign of TSC.8 RHM natural history is usually favourable with spontaneous regression in about 63% of cases, while the remaining cases remain stable with rare reported cases of them increasing in size.9 10 Most RHMs are asymptomatic but cases with haemodynamic instability leading to hydrops and fetal demise or the need of postnatal surgery have been reported.8 11–14 We report the effect of medical treatment using an oral mTOR inhibitor on a case of haemodynamically significant and inoperable RHM in a preterm infant with TSC.
Case presentation
A preterm female infant was born at 302/7 weeks of gestational age. She was the fourth child of a 34-year-old mother who has a diagnosis of TSC. The previous three siblings had TSC, with the first two infants being asymptomatic while the youngest sibling had antenatal diagnosis of RHM and postnatal refractory epilepsy. Fetal echocardiography of our case at 233/7 weeks of gestation revealed a double outlet right ventricle with pulmonary valve atresia, hypoplastic pulmonary artery and a large ventricular septal defect. No intracardiac tumour was detected on this echocardiography. A follow-up echocardiography at 273/7 weeks indicated the presence of two RHMs, one attached to the interventricular septum (IVS) of the right ventricle, measuring 9.8×4.4 mm, and the other originating from the lateral wall of the left ventricle and measuring 12×8.4 mm. There was no evidence of ventricular inlet or outflow obstruction at this time. Normal heart rate was noted. Cerebral MRI was performed the same day and showed no evidence of cerebral lesions. At 303/7 weeks, the follow-up echocardiography showed an increase in the size of the right ventricle RHM to 14.2×12 mm and an unchanged left ventricle RHM.
The infant was delivered at 305/7 postmenstrual age (PMA) by caesarean section due to worsening intrauterine growth restriction below 3rd centile. Her birth weight was 980 g. The transition to extrauterine life was smooth; only non-invasive ventilation was required with FiO2 of 21%. Transcutaneous oxygen saturation was above 90%. The infant's physical examination was normal except for a grade II/VI heart murmur. Owing to her pulmonary valve atresia, intravenous prostaglandin (PGE1) at 0.025 µg/kg/min was started on admission to the neonatal intensive care unit. Following several episodes of desaturation, PGE1 was gradually increased up to 0.15 µg/kg/min. This increase in the dose led to intubation and mechanical ventilation due to important episodes of apnoea. The baby was subsequently extubated and was successfully ventilated with non-invasive ventilation with few episodes of desaturation and apnoea.
Investigations
Postnatal echocardiography confirmed the double outlet right ventricle and pulmonary artery hypoplasia (2.7 mm main pulmonary artery, 3.2 mm right pulmonary artery and 3.4 mm left pulmonary artery). A total of six RHMs were identified. The largest (16×11 mm) was in the right ventricle occupying most of its cavity. The other tumours were one in the left ventricle (8×15 mm), attached to its lateral wall, the other was attached to the mitral papillary muscle (7×5 mm), and three smaller ones were attached to the interventricular septum and to the apex of the left ventricle (figure 1). The structural cardiac malformation being duct dependent, PGE1 could not be weaned prior to establishing supply to the pulmonary artery by surgical shunt. On the other hand, single ventricle-type palliation was considered due to the severe reduction of the right ventricular filling capacity by the massive right ventricular RHM, unless a surgical tumour resection was performed. Owing to the prematurity and low birth weight, the infant was inoperable.
Figure 1.
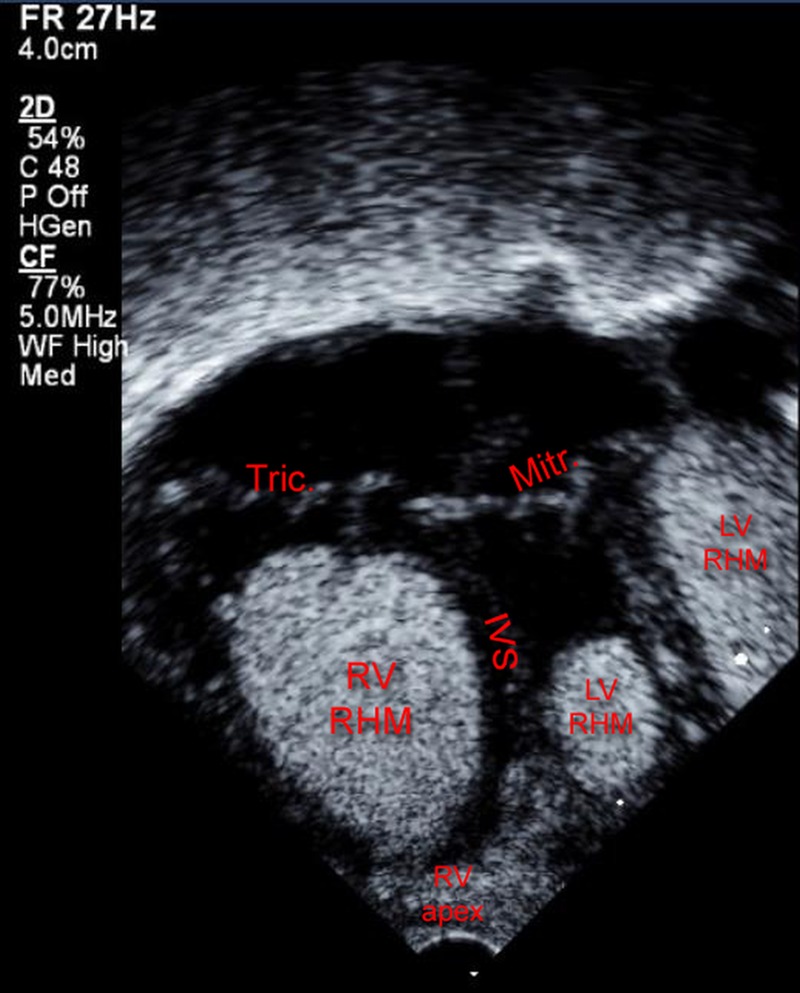
The left panel depicts a large RHM occupying most of the RV cavity, with other RHMs shown in the LV (IVS, interventricular septum; RHM, rhabdomyoma; RV, right ventricle; LV, left ventricle; Tric, tricuspid valve; Mitr, mitral valve).
Differential diagnosis
Systemic evaluation of the infant for other possible TSC lesions was performed. Dermatological examination and abdominal and head ultrasounds were normal. EEG was normal on day 17 of life (331/7 weeks PMA). MRI was performed at 371/7 weeks PMA showing two small cortical tubers at the left frontal and left parietal lobes, and an anomaly of the white matter in the left parietal region without any significant clinical neurological symptoms.
Treatment
Considering these circumstances, pharmacological size reduction of the RHM was attempted using an mTOR inhibitor (everolimus) after interdisciplinary discussion and parents’ informed consent. Oral everolimus was started on day 20 of life—333/7 weeks of PMA—at a daily dose of 0.1 mg. Everolimus level was drawn after four completed days of treatment and the trough was 13.7 ng/mL, which is within the target therapeutic range for children and adults (5–15 ng/mL). This therapeutic regimen did not require adjustments based on subsequent serum level that was 11 ng/mL 1 week later. Safety monitoring involved twice weekly electrolytes, complete blood count, blood urea nitrogen, creatinine and urinary proteins as well as full hepatic work up and triglyceride levels. A transient hypokalaemia was the only recorded biochemical imbalance, and was palliated with oral supplements. Owing to the immunosuppressive effect of everolimus, the patient was put in protective isolation. Everolimus therapy was discontinued after 34 days of treatment, at 38 2/7weeks PMA, because of suspected infection with high temperature and respiratory deterioration. Antibiotic therapy was empirically initiated and then discontinued based on a negative septic work up. Meanwhile, serial echocardiography studies demonstrated significant reduction of the right ventricular RHM and a near disappearance of the left ventricular RHM (figure 2). The size of the RHM remained insignificant on subsequent echocardiograms.
Figure 2.
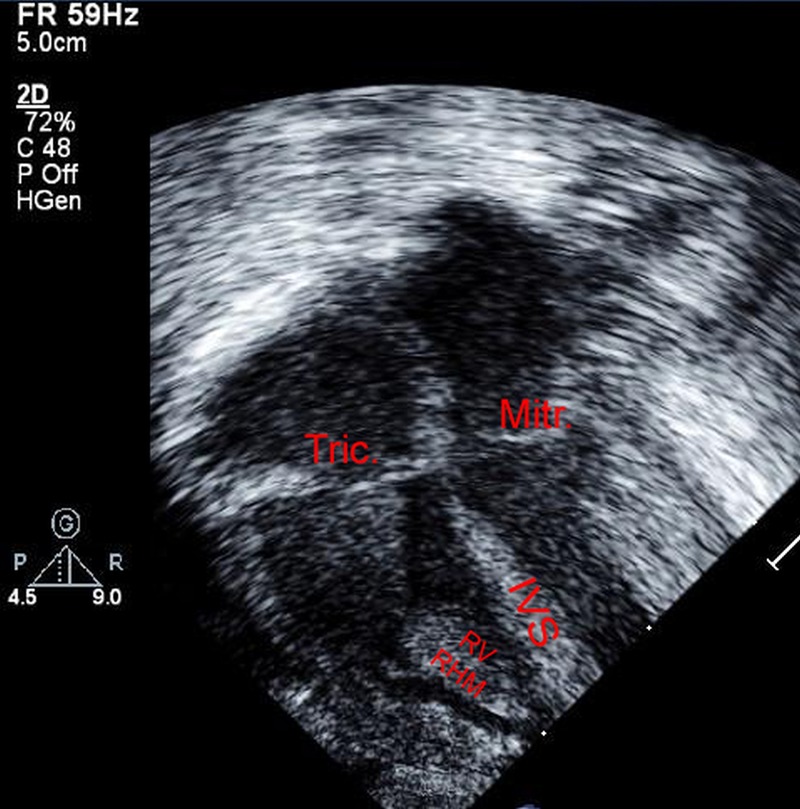
After 34 days of treatment with an mTOR inhibitor, the RHM had significantly decreased in size, as shown on the right panel (IVS, interventricular septum; RHM, rhabdomyoma; RV, right ventricle; LV, left ventricle; Tric, tricuspid valve; Mitr, mitral valve).
Outcome and follow-up
At 88 days of life (3 weeks post term), the infant was deemed operable because she had a good general condition and sufficient body weight (3.1 kg). A Goretex shunt was surgically implanted between the right subclavian artery and the pulmonary artery allowing the withdrawal of PGE1. The adequate blood flow through the right ventricle as well as its adequate growth made two ventricles repair possible in the near future without the need for surgical resection of the cardiac tumour. Subsequently, the patient underwent a successful two-ventricle surgical repair 4 months after the shunt at age 7 months. The latter operation consisted of patch closure of the ventricular septal defect with a right ventricle to pulmonary artery conduit and did not require surgical resection of the remaining RHM. Last follow-up 6 months later showed normal cardiac function, no residual shunt and a haemodynamically non-significant RHM in the right ventricle without outflow tract obstruction.
Discussion
This is the first report of the use of an mTOR inhibitor drug in a preterm infant for inoperable haemodynamically significant RHM. It illustrates a rapid regression of the RHM with everolimus treatment and minimal short-term side effects.
The traditional management of TSC has been challenged by the recent discovery that mTOR inhibitors can potentially compensate the lack of mTOR inhibition resulting from mutations in TSC1 or TSC2. Since the first report of the efficacy of the mTOR inhibitor rapamycine for the treatment of subependymal giant cell astrocytomas in 2006,15 multiple studies have confirmed the efficacy and safety of mTOR inhibitors for the treatment of different clinical manifestations of TSC. Many countries, including the USA and Canada, have approved the use of everolimus for the treatment of subependymal giant cell astrocytomas and of renal angiomyolipomas in association with TSC. In addition, animal models of TSC have suggested that mTOR inhibitors could have beneficial effects on cognitive deficits16 and on epileptogenesis.17 To the best of our knowledge, there is no study evaluating the efficacy of this agent in the specific treatment of RHM. Only two case reports described the effect of the use of everolimus on RHM in a paediatric population (a 7-year-old child and a full-term infant).18 19 Clinical studies that have shown an efficacy of everolimus in TSC have targeted a blood trough level of 5–15 ng/mL.4 7 In the paediatric population, the dose needed to achieve this trough target seems to be around 4.7–5.6 mg/m2/day.7 Data regarding pharmacokinetics and pharmacodynamics of mTOR inhibitors in the neonatal population are not available.
Demir et al18 reported the use of everolimus in a term newborn for large RHM with a twice weekly regimen to achieve therapeutic levels (0.25 mg twice a day administered 2 days/week). Our therapeutic dose schedule was calculated based on the serum levels provided in that report. In essence, very high trough levels (83.5 ng/mL) were reported with their initial 0.25 mg every 6 h 2 days/week regimen. Actually, a serum level of 83.5 ng/mL is roughly 10-fold the recommended therapeutic target. We estimated the dose needed to achieve therapeutic levels in Demir's case report to be 4.5 mg/m2/week, which is seven times lower than the reported paediatric dose. Therefore, we estimated that based on our patient's birth weight, a daily dose of 0.1 mg would be equivalent to the reported 4.5 mg/m2/week (divided in 7 daily doses). This estimation was further consolidated with follow-up serum trough levels. Therefore, it is plausible that the everolimus hepatic metabolism pathway (CYP 450 3A4)20 is immature in the newborn and requires a dosing adjustment in the neonatal period.
Based on this case, and on the previously reported neonatal case, everolimus could be an alternative to surgical excision in inoperable cases of RHM even in preterm infants. Minimal short-term side effects were noted in this case, but additional studies are needed to confirm efficacy and long-term safety of everolimus as a treatment of RHM. In addition, our report uncovers a pharmacokinetic profile of everolimus that seems to be specific to the newborn.
Learning points.
This is, to the best of our knowledge, the second neonate and the first prematurely born baby to be reported portraying beneficial therapy with a mammalian target of rapamycin inhibitor. Three particular points could be drawn from this case:
Everolimus is an alternative therapy in newborns with haemodynamically significant rhabdomyomas.
Dose titration in the neonate, particularly in the premature baby, is significantly lower than the recommended dose for children.
No side effects were encountered in the setting of this case.
Footnotes
Contributors: ND was involved in concept of the novel therapy, echocardiography and cardiac data acquisition, primary writing and editing the manuscript; IM was involved in writing and editing of the manuscript; GE was involved in clinical data gathering, drafting the manuscript and revision; IG was involved in dosage and safety data gathering, writing and editing the manuscript; and PM was involved in concept of the novel therapy; writing and editing the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Borkowska J, Schwartz RA, Kotulska K et al. Tuberous sclerosis complex: tumors and tumorigenesis. Int J Dermatol 2011;50:13–20. [DOI] [PubMed] [Google Scholar]
- 2.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 2008;412:179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JA, Zhang H, Roberts PS et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol 2004;63:1236–42. [DOI] [PubMed] [Google Scholar]
- 4.Franz DN, Belousova E, Sparagana S et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013;381:125–32. [DOI] [PubMed] [Google Scholar]
- 5.Franz DN. Everolimus in the treatment of subependymal giant cell astrocytomas, angiomyolipomas, and pulmonary and skin lesions associated with tuberous sclerosis complex. Biologics 2013;7:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bissler JJ, Kingswood JC, Radzikowska E et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013;381:817–24. [DOI] [PubMed] [Google Scholar]
- 7.Krueger DA, Care MM, Holland K et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med 2010;363:1801–11. [DOI] [PubMed] [Google Scholar]
- 8.Lacey SR, Donofrio MT. Fetal cardiac tumors: prenatal diagnosis and outcome. Pediatr Cardiol 2007;28:61–7. [DOI] [PubMed] [Google Scholar]
- 9.Bader RS, Chitayat D, Kelly E et al. Fetal rhabdomyoma: prenatal diagnosis, clinical outcome, and incidence of associated tuberous sclerosis complex. J Pediatr 2003;143:620–4. [DOI] [PubMed] [Google Scholar]
- 10.Holley DG, Martin GR, Brenner JI et al. Diagnosis and management of fetal cardiac tumors: a multicenter experience and review of published reports. J Am Coll Cardiol 1995;26:516–20. [DOI] [PubMed] [Google Scholar]
- 11.Schlaegel F, Takacs Z, Solomayer EF et al. Prenatal diagnosis of giant cardiac rhabdomyoma with fetal hydrops in tuberous sclerosis. J Prenat Med 2013;7:39–41. [PMC free article] [PubMed] [Google Scholar]
- 12.Pruksanusak N, Suntharasaj T, Suwanrath C et al. Fetal cardiac rhabdomyoma with hydrops fetalis: report of 2 cases and literature review. J Ultrasound Med 2012;31:1821–4. [DOI] [PubMed] [Google Scholar]
- 13.Yinon Y, Chitayat D, Blaser S et al. Fetal cardiac tumors: a single-center experience of 40 cases. Prenat Diagn 2010;30:941–9. [DOI] [PubMed] [Google Scholar]
- 14.Chao AS, Chao A, Wang TH et al. Outcome of antenatally diagnosed cardiac rhabdomyoma: case series and a meta-analysis. Ultrasound Obstet Gynecol 2008;31:289–95. [DOI] [PubMed] [Google Scholar]
- 15.Franz DN, Leonard J, Tudor C et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol 2006;59:490–8. [DOI] [PubMed] [Google Scholar]
- 16.Ehninger D, Han S, Shilyansky C et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med 2008;14:843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia 2010;51:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demir HA, Ekici F, Yazal Erdem A et al. Everolimus: a challenging drug in the treatment of multifocal inoperable cardiac rhabdomyoma. Pediatrics 2012;130:e243–7. [DOI] [PubMed] [Google Scholar]
- 19.Tiberio D, Franz DN, Phillips JR. Regression of a cardiac rhabdomyoma in a patient receiving everolimus. Pediatrics 2011;127:e1335–7. [DOI] [PubMed] [Google Scholar]
- 20.Novartis. Afinitor® (everolimus) tablets for oral administration: Canada prescribing information, Novartis Pharmaceuticals Corporation. http://www.novartis.ca/asknovartispharma/download.htm?res=afinitor_scrip_e.pdf&resTitleId=705 (Cited on 20 February 2014 2013).


