Abstract
The pathophysiological role of the adenosine A3 receptor in the central nervous system is largely unknown. We have investigated the effects of the selective A3 receptor agonist 2-chloro-N6-(3-iodobenzyl)-adenosine, Cl-IB-MECA, in cells of the astroglial lineage (human astrocytoma ADF cells). A marked reorganization of the cytoskeleton, with appearance of stress fibers and numerous cell protrusions, was found following exposure of cells to low (nM) concentrations of Cl-IB-MECA. These “trophic” effects were accompanied by induction of the expression of Rho, a small GTP-binding protein, which was virtually absent in control cells, and by changes of the intracellular distribution of the antiapoptotic protein Bcl-xL, that, in agonist-exposed cells, became specifically associated to cell protrusions. This is the first demonstration that the intracellular organization of Bcl-xL can be modulated by the activation of a G-protein-coupled membrane receptor, such as the A3 adenosine receptor. Moreover, modulation of the astrocytic cytoskeleton by adenosine may have intriguing implications in both nervous system development and in the response of the brain to trauma and ischemia.
Adenosine regulates various physiological functions through four distinct G-protein-coupled receptors (the A1, A2A, A2B and A3 subtypes) (1) and participates in physiological neurotransmission (2). Activation of the A1 receptor results in potent neuroprotection (2), white the A2 receptor (particularly the A2A subtype) has been implicated in regulation of motor functions (1, 2). On the other hand, very little is known about the A3 receptor, which has a unique structure-activity relationship profile, tissue distribution and effector coupling (3).
Insights into the possible pathophysiological role of the A3 receptor have recently come from the use of the first really selective A3 receptor agonists, such as N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (IB-MECA, which is 50-fold selective for A3 versus A1 or A2A receptor) (4) and its derivative 2-chloro-IB-MECA (Cl-IB-MECA), which shows a selectivity for the A3 versus A1 and A2A receptors of 2500- and 1400-fold, respectively (5). Thanks to these selective compounds, we now know that the A3 receptor may play a role in inflammation (6), hypotension (7), mast cell degranulation (8), and, most strikingly, in regulation of cell survival, by promoting both cell protection and cell death, depending upon the cell type and the agonist concentration (9–12). Protective effects against ischemia/hypoxia are induced by low (nM) concentrations of A3 receptor agonists in both cardiac myocytes and in brain (13, 14). The molecular mechanisms at the basis of these effects are totally unknown. In this study, we have investigated the effects of nM Cl-IB-MECA concentrations on human cells of the astroglial lineage, a brain cell type which plays a key role in both brain development and in brain repair and remodelling following trauma and ischemia (15). In particular, based on the hypothesized role of this receptor in cell survival, we have evaluated possible effects of Cl-IB-MECA on the Bcl-2-related antiapoptotic protein Bcl-xL. Finally, since the Bcl-2 family of proteins has recently been demonstrated to regulate growth and regeneration of cellular processes in neural cells (16), we have investigated the effects of the A3 receptor agonist on the actin cytoskeleton and on Rho, a small GTP-binding protein that has recently been associated with actin assembly and cell spreading (17).
MATERIALS AND METHODS
Cell cultures and treatments
Human astrocytoma cells (ADF cells) (18) were grown at 37°C in a humidified atmosphere in RPMI 1640 (HyClone, Celhio. Milan, Italy) supplemented with 10% FCS, 1% non-essential amino acids (BioWhittaker, Verves, Belgium), penicillin (100 IU/ml) and streptomycin (100 µg/ml) (GIBCO, Life Technologies European Division). Cells were seeded on 35 mm petri dishes containing 6 glass coverslips (3.5 × 105 viable cells/ml) and were left 24 h in culture to allow attachment to the substratum. Cultures were then exposed to the drug by direct addition to the culture medium for 24–72 h. The A3 selective agonist Cl-IB-MECA was synthesized as previously described (4, 5); 10 mM stock solutions were prepared in DMSO, and serial dilutions were prepared in HBSS without calcium and magnesium.
Scanning electron microscopy
ADF cells were grown as described above and fixed as previously described (19) with 2.5% glutaraldeyde in 0.1% cacodylate buffer (pH 7.4) at room temperature for 20 min. Following post fixation in 1% OsO4 for 30 min, cells were dehydrated through exposure to increasingly concentrated ethanol solutions, critical point dried in CO2 and gold-coated by sputtering. Samples were then examined with a Cambridge 360 scanning electron microscope.
Analytical cytology
Cells grown on glass coversiips were fixed with 3.7% formaldehyde in phosphate buffered saline (pH 7.4) for 10 min at room temperature and permeabilized with 0.5% Triton X-100 (Sigma Chemicals Co., USA) in phosphate buffered saline for 5 min at room temperature. Samples were then incubated at 37°C for 30 min with: i) the fluorescein-phalloidin (Sigma Chemicals Co.) for detection of F-actin filaments: ii) anti- Rho antibodies (anti Rho-GDI is a polyclonal antibody interacting with several ras-like GTP-binding proteins including RhoA, RhoB, rac and CDC42; Santa Cruz Biotechnology, CA, USA; final dilution 1:50 in phosphate buffered saline) and iii) anti Bcl-xL polyclonal antibody (20) (Santa Cruz Biotechnology, CA, USA; final dilution 1:100 in phosphate buffered saline). For detection of Rho and Bcl-xL proteins, cells were then incubated with anti-rabbit IgG-fluorescein-linked whole antibody (Sigma Chemicals Co., USA: final dilution 1:100) at 37°C for 30 min. Finally, all samples were mounted with glycerol:phosphate buffered saline (1:1) and analyzed with a Nikon Microphot fluorescence microscope.
Western blot analysis
Cultures were incubated at room temperature for 10 min with lysis buffer (20 mM Tris-HCl: 10 mM EGTA; 2 mM EDTA; 1, mM DTT; 0.1 mM PMSF), scraped from the culture dish, transferred to a glass/glass potter and homogenized (10 strokes in ice). Twenty µg of total protein from each sample were run on 11% sodiumdodecylsulphatepolyacrylamide gels and blotted onto nitrocellulose filters which had been hydrated with water followed by blotting buffer containing 0.192 M glycine, 0.025 M Tris-HCl and 20% methanol (pH 8.3). Filters were first coated with TBS buffer (10 mM Tris-HCl, 150 mM NaCl, pH 8.0) containing 10% milk (w/v) at 4°C overnight, and then incubated at room temperature for 1 h with either 0.4 µg/10 ml of anti-Bcl-xL rabbit polyclonal IgG antibody (Santa Cruz Biotechnology, USA) or 5 µg/10 ml of anti-Bcl-2 mouse monoclonal antibody (Calbiochem. USA), After washing with TBST (TBS buffer containing 0.1% Tween 20), the blots were incubated with either goat anti-rabbit IgG antibody conjugated to horseradish peroxidase (1:4,000: Sigma Chemicals Co., USA) for Bcl-xL, or anti-mouse IgG antibody conjugated to horseradish peroxidase (1:10,000, Sigma Chemicals Co., USA) for Bcl-2. The respective proteins were visualized using ECL Western blotting kit (RPN 2106; Amersham, Buckinghamshire, UK). Semiquantitative evaluation of autoradiograms was performed by densitometric analysis on a computerized image analyser using a program (NIH-Image) developed by Dr. Wayne Rasband (NIH, Bethesda, MD, USA).
RESULTS
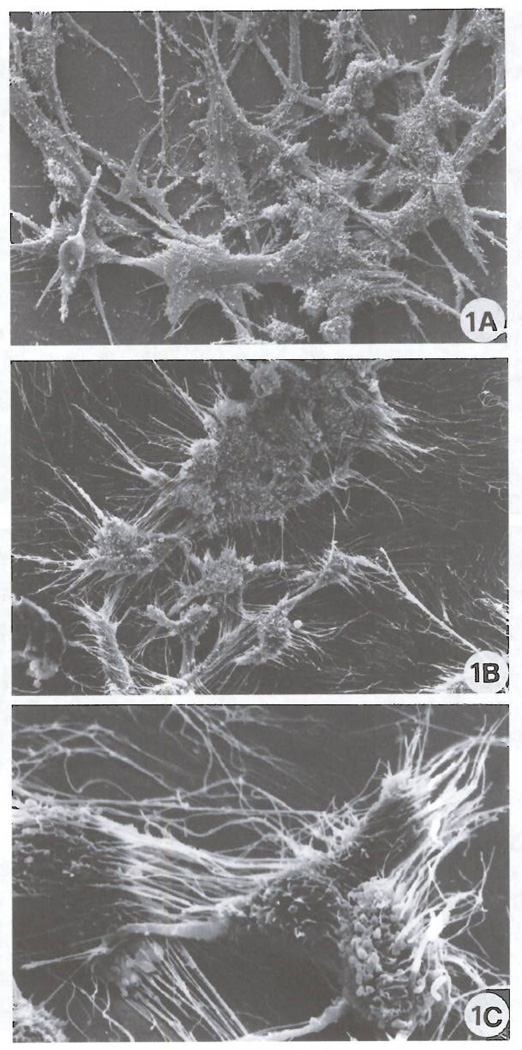
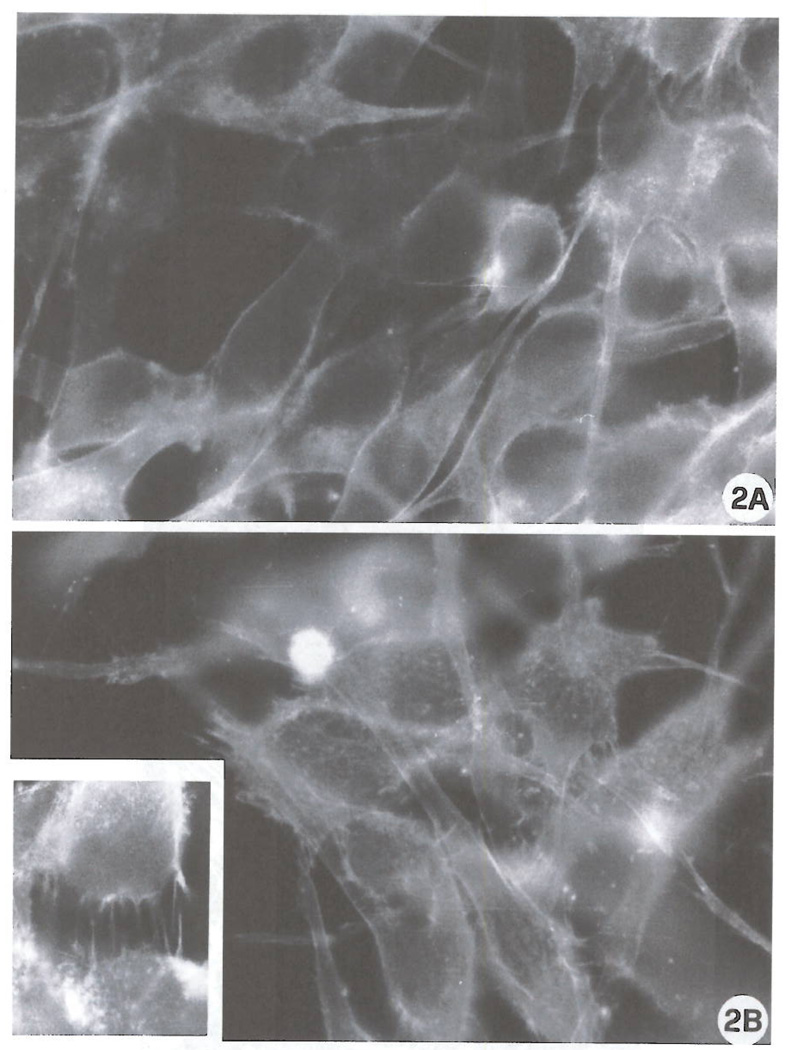
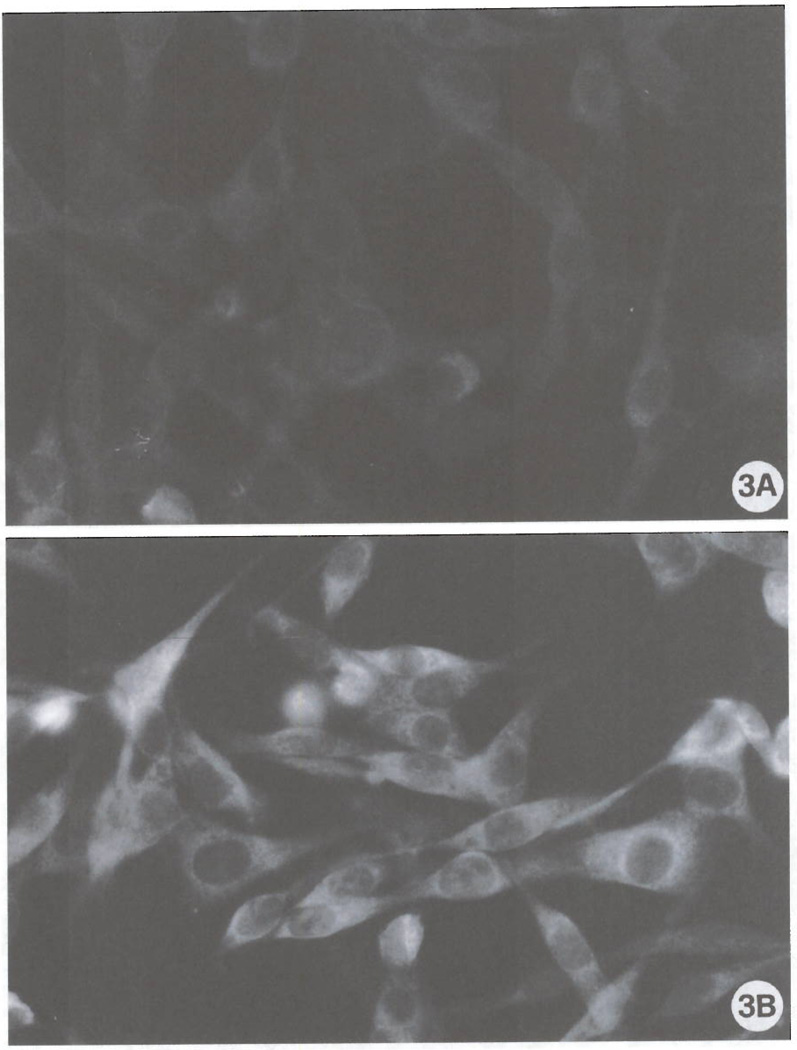
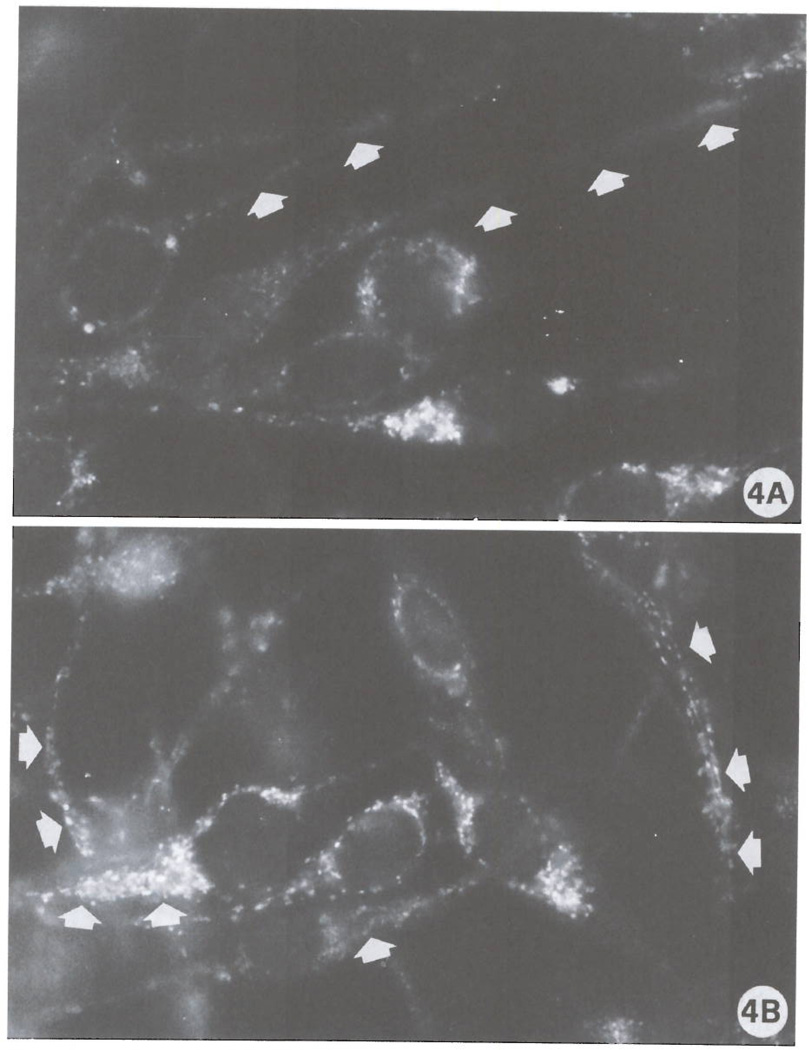
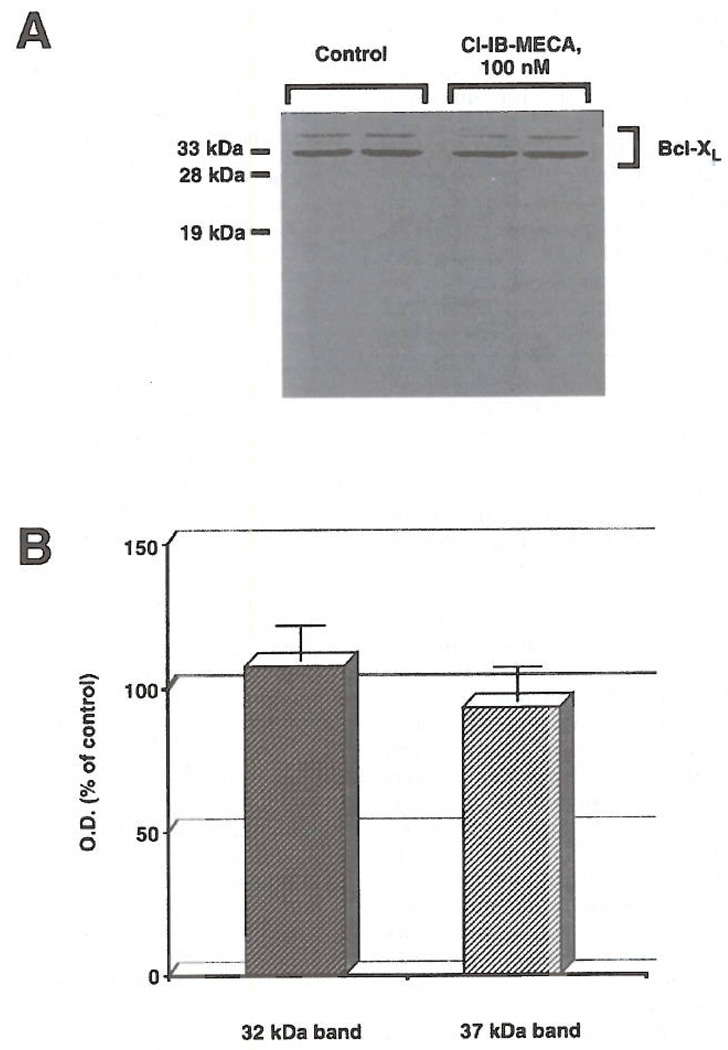
Ultrastructural evaluation of the effects of the A3 agonist clearly showed massive modifications of cell shape. As depicted in the scanning electron micrographs (Fig. 1) control cells showed a typical bipolar shape with a relatively low number of short cell protrusions (Fig. 1A). After a 72 h exposure to 100 nM Cl-IB-MECA (Fig. 1B), a striking alteration of cell shape and morphology with an increase of both the number and length of cellular processes was detected. These are mainly represented by long, thin cell protrusions leading to the formation of numerous cell-substrate contacts (Fig. 1C). In view of the importance of cytoskeletal components in the dendritic protrusions and growth cone formation (21), an analysis of F-actin microfilament distribution was also performed. Fig. 2 shows the fluorescence staining of actin microfilaments in control cultures (Fig. 2A) and in cultures treated for 72 h with 100 nM Cl-IB-MECA (Fig. 2B). Exposure to the A3 agonist was capable of inducing formation of stress fibers (Fig. 2B), elongation of cell protrusions and formation of F-actin positive spikes (Fig. 2B; inset), suggesting a reorganization of the cytoskeleton accompanying the induction of morphologic differentiation. Hence, in consideration of the importance recently ascribed to the Rho GTP-binding protein in F-actin cytoskeleton assembly and cell spreading (17), a specific immunocyto-chemical analysis of the expression of this molecule was conducted (Fig. 3). Results indicate that a remarkable expression of Rho protein, mainly localized in the perinuclear regions and in the dendritic protrusions (Fig. 3B), is detectable as a consequence of A3 agonist exposure. In consideration of the possible importance of A3 receptors in regulation of cell viability and function (9–12), expression of proteins related to the anti-apoptotic protein Bcl-2, which is involved in cell survival and has recently been associated to the cytoskeleton in neuronal cells (16), was also evaluated. ADF cells do not significantly express Bcl-2, as determined by Western blot analysis utilizing an anti-Bcl-2 monoclonal antibody (data not shown). They instead express significant amounts of the Bcl-2-related cell death inhibitory protein Bcl-xL. In control adhering cultures, immunoreactivity to anti Bcl-xL antibody was mainly distributed in the perinuclear region (Fig. 4A). Instead, in Cl-IB-MECA-treated cultures, immunoreactivity was evident as bright dot spots that were particularly localized to cellular protrusions (Figs. 4B). To establish whether A3 agonists can change the expression of Bcl-xL, we have also performed a semiquantitative Western blot analysis in control cells and in cultures that had been preincubated with Cl-IB-MECA for 72 h. As reported by other authors (22, 23), Bcl-xL was detected as a doublet of proteins with an apparent molecular mass of 29–33 kDa (32 and 37 kDa in our system, mean of 4 experiments. Fig. 5A). The explanation for the two forms of the proteins is unknown at present, but could be due to proteolytic processing, covalent modifications, or additional alternatively spliced forms that have not yet been identified. No statistically significant differences in overall Bcl-xL expression were found following exposure of cells to 100 nM Cl-IB-MECA for 72 h (Fig. 5B), suggesting that A3 agonists do not affect the total amount of protein expressed, but rather its intracellular distribution.
FIG 1.
Scanning election micrographs of ADF control cells (A) and ADF cells treated with 100 nM Cl-IB-MECA for 72 h (B, C). Control cells show a typical bipolar shape with a relatively low number of cell protrusions (A). Exposure to Cl-IB-MECA induces marked morphological changes represented by a dramatic increase of both the number and length of cellular processes (B, C). (Original magnification: A, B 1000X; C 3300X).
FIG 2.
F-actin analysis. Stress fibers, which were hardly detectable in control cultures (Fig. 2A), are markedly increased after exposure to 100 nM Cl-IBMECA for 72 h (Fig. 2B). Note elongated processes (Fig. 2B, inset), (Original magnification: A, B, inset 3000X).
FIG 3.
The expression of Rho in control cells (A) and in cultures exposed to 100 nM Cl-IB-MECA for 72 h (B). Note the marked positivity in the cell body and cell protrusions in agonist-exposed cells. (Original magnification: A, B 1500X).
FIG 4.
Intracellular distribution of Bcl-xL in ADF control cells (A) and in ADF cells exposed to Cl-IB-MECA for 72 h (B). Arrows in (A) indicate cell protrusions expressing low levels of Bcl-xL. By contrast, in treated cultures, positivity to the anti-Bcl-xL antibody is mainly found as very bright dots particularly abundant in cell protrusions (arrows). (Original magnifications: A, B, 3000X).
FIG 5.
Immunoblot analysis of Bcl-x in control ADF cells and in ceils exposed for 48 h to 100 nM Cl-IB-MECA. Bcl-xL is detected as a doublet of proteins with an apparent molecular mass of 32 and 37 kDa. In B, immunoblot analysis from 4 independent experiments run in triplicate and subjected to densitometric analysis of autoradiograms; data (optical density, O.D., in arbitrary units) are expressed as % of control.
DISCUSSION
The present study was undertaken to clarify the effects induced by trie activation of the A3 adenosine receptor in mammalian astroglial cells. Results show that low concentrations of selective A3 receptor agonists induce: (i) a dramatic redistribution of the cytoskeleton with elongation of cellular processes; (ii) increased expression and reorganization of Rho molecules and of F-actin stress fibers; and (iii) a marked intracellular redistribution of the Bcl-2-related protein Bcl-xL, that, in treated cells, is concentrated in cellular protrusions. Based on the role of F-actin microfilament assembly in stress fibers and on the activity of the Rho protein family in cell attachment and spreading (17), we suggest a functional relationship between these effects and the increased dendritic-like protrusions observed in A3 agonist-exposed cells. Our results also show for the first time that the anti-apoptotic protein Bcl-xL may be preferentially associated to cytoskeletal components. In fact, the presence of this protein is well detectable in cell samples specifically extracted and Triton-permeated for cytoskeleton analyses. More important, we show that the intracellular distribution of this molecule can be modulated by activation of a specific membrane neurotransmitter receptor, i.e. the A3 adenosine receptor. Exposure of cells to a selective adenosine A3 receptor agonist indeed resulted in the association of this protein with cytoskeletal elements in long dendritic-like cell protrusions. This last finding is consistent with the recent demonstration that proteins belonging to the Bcl-2 family may play key roles in crucial functions other than cell survival, or in functions that may only indirectly affect cell viability. For example, Bcl-2 was shown to promote growth and regeneration of retinal axons (16), whereas intracytoplasmic movements of Bcl-xL have been hypothesized to represent an important step in the regulation of apoptosis in human leukemic cells, again indirectly suggesting a regulatory role for cytoskeletal components (24). As previously demonstrated for other cell systems (25–26), these findings support, also for human astrocytes, the existence of a close relationship between integrity of the cytoskeleton, adhesion processes and molecules belonging to the Bcl-2 family: modifications of these relationships may eventually influence cell survival and death. Resistance of actin to cleavage has indeed been suggested to be important for apoptotic regulation (27), while cell spreading, more than cell attachment per se, seems to represent a pre-requisite for cell survival (26, 28). A3 agonist-induced astrocytic spreading, the first step of the dendritic-like protrusion formation and elongation, may play a role in nervous system development, when glial processes address migration of neuroblasts to their final destination. Moreover, reinforcement of the cytoskeleton by adenosine may also result in a secondary “protection” of cells from injury and death (M.P. Abbracchio et al., manuscript in preparation), which may be relevant to brain repair following trauma and ischemia.
ACKNOWLEDGMENTS
This work was partially supported by National Research Council (CNR) grants n° 96.04972.St74 to Daniela Monti and n° 96.04967.St74 to WM, by a grant of the Associazione Italians per la Ricerca sul Cancro (AIRC) to CF, and by The Italian Ministero dell’Universita’ e Ricerca Scientificafe Tecnologica (40% to MPA, FC and CF). M.P.A., S.C., R.B. and F.C, have been involved in the concerted action ADEURO (EU, BIOMED1, “Physiology and pharmacology of brain adenosine receptors-implications for the rational design of neuroactive drugs” supported by the European Union within the Biomedical and Health Research Programme). Cl-IB-MECA was kindly provided by Research Biochemical International as part of the Chemical Synthesis Program of the National Institute of Mental Health, Contract N°01MH30O03.
REFERENCES
- 1.Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolphi KA, Schubert P. In: Novel Therapies for CNS Injuries: Rationales and Results. Peterson PL, Phillis JW, editors. 1996. pp. 327–346. [Google Scholar]
- 3.Jacobson KA, Kim HO, Siddiqi SM, Olah ME, Stiles GL, von Lubitz DKJE. Drugs of the Future. 1995;20:689–699. doi: 10.1358/dof.1995.020.07.531583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallo-Rodriguez C, Ji X-D, Melman N, Siegman BD, Sanders LH, Orlina J, Pu Q, Olah ME, van Galen PJM, Stiles GL, Jacobson KA. J. Med. Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HO, Ji KD, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. J. Med. Chem. 1994;37:3614–3621. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linden J. Trends Pharmacol. Sci. 1994;15:298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 7.Hannon JP, Pfannkuche HJ, Fozard JR. Br. J. Pharmacol. 1995;115:945–952. doi: 10.1111/j.1476-5381.1995.tb15902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fozard JR, Pfannkuche HJ, Schuurman HJ. Eur. J. Pharmacol. 1996;298:293–297. doi: 10.1016/0014-2999(95)00822-5. [DOI] [PubMed] [Google Scholar]
- 9.Abbracchio MP, Ceruti S, Barbieri D, Franceschi C, Malorni W, Biondo L, Burnstock G, Cattabeni F. Biochem. Biophys. Res. Comm. 1995;213:908–915. doi: 10.1006/bbrc.1995.2215. [DOI] [PubMed] [Google Scholar]
- 10.Kohno Y, Sei Y, Koshiba M, Kim HO, Jacobson KA. Biochem. Biophys. Res. Comm. 1996;219:904–910. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Y, Sei Y, Abbracchio MP, Jiang JL, Kim YC, Jacobson KA. Biochem. Biophys. Res. Comnmn. 1997;232:317–322. doi: 10.1006/bbrc.1997.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceruti S, Barbieri D, Franceschi C, Giammarioli AM, Rainaldi G, Malorni W, Kim HO, von Lubitz DKJE, Jacobson KA, Cattabeni F, Abbracchio MP. Drug Dev. Res. 1996;37:177. [Google Scholar]
- 13.Stambaugh C, Jiang JL, Jacobson KA, Liang BT. Am. J. Physiol. 1997;273:H501–H505. doi: 10.1152/ajpheart.1997.273.1.H501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Lubitz DKJE, Lin RCS, Popik P, Carter MF, Jacobson KA. Eur. J. Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eddleston M, Mucke L. Neuroscience. 1993;64:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen DF, Schneider GE, Martinou JC, Tonegawa S. Nature. 1997;385:434–439. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- 17.Burridge K, Chrzanowska-Wodnicka M, Zhonz C. Trends Cell Biol. 1997;7:342–347. doi: 10.1016/S0962-8924(97)01127-6. [DOI] [PubMed] [Google Scholar]
- 18.Malorni W, Rainaldi G, Rivabene R, Santini MT. Cell Biol. Toxicol. 1994;10:207–218. doi: 10.1007/BF00756761. [DOI] [PubMed] [Google Scholar]
- 19.Malorni W, Rivabene R, Matarrese P. Chem. Biol. Interact. 1995;96:113–123. doi: 10.1016/0009-2797(94)03576-t. [DOI] [PubMed] [Google Scholar]
- 20.Akbar AN, Borthwich NJ, Wickremasinghe RG. Eur. J. Immunol. 1996;26:294–296. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 21.Ridley AJ. Bioessay. 1994;16:321–327. doi: 10.1002/bies.950160506. [DOI] [PubMed] [Google Scholar]
- 22.Ohta K, Iwai K, Kasahara Y, Taniguchi N, Krajewski S, Reed JC, Miyawaki T. Internat. Immunol. 1995;7:1817–1825. doi: 10.1093/intimm/7.11.1817. [DOI] [PubMed] [Google Scholar]
- 23.Krajewski S, Krajewska M, Shabaik A, Wang HG, Irie S, Fong L, Reed JC. Cancer Res. 1994;54:5501–5507. [PubMed] [Google Scholar]
- 24.Hsu YT, Wolter KG, Youle RJ. Proc. Natl. Acad. Sci. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brancolini C, Benedetti M, Schneider C. EMBO J. 1995;14:5179–5190. doi: 10.1002/j.1460-2075.1995.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frisch SM, Francis H. J. Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Q, Wei T, Lees-Miller S, Alnemri A, Watters D, Lavin MF. Proc. Natl. Acad. Sci. 1997;94:157–162. doi: 10.1073/pnas.94.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruoslahti E, Reed JC. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]