Abstract
Traditionally, urologists recommend an interval of at least 4 weeks after prostate biopsy before radical prostatectomy. The aim of our study was to evaluate whether the interval from prostate biopsy to radical prostatectomy affects immediate operative outcomes, with a focus on differences in surgical approach. The study population of 1,848 radical prostatectomy patients was divided into two groups according to the surgical approach: open or minimally invasive. Open group included perineal and retropubic approach, and minimally invasive group included laparoscopic and robotic approach. The cut-off of the biopsy-to-surgery interval was 4 weeks. Positive surgical margin status, operative time and estimated blood loss were evaluated as endpoint parameters. In the open group, there were significant differences in operative time and estimated blood loss between the <4-week and ≥4-week interval subgroups, but there was no difference in positive margin rate. In the minimally invasive group, there were no differences in the three outcome parameters between the two subgroups. Multivariate analysis revealed that the biopsy-to-surgery interval was not a significant factor affecting immediate operative outcomes in both open and minimally invasive groups, with the exception of the interval ≥4 weeks as a significant factor decreasing operative time in the minimally invasive group. In conclusion, performing open or minimally invasive radical prostatectomy within 4 weeks of prostate biopsy is feasible for both approaches, and is even beneficial for minimally invasive radical prostatectomy to reduce operative time.
Graphical Abstract
Keywords: Biopsy, Prostate, Prostatectomy, Prostatic Neoplasms
INTRODUCTION
Prostate cancer is the most common cancer and the second leading cause of cancer death in North American men. In Korea, it is the fifth most common cancer and eighth leading cause of cancer death in the male population (1). At the current time, most cases of prostate cancer are diagnosed by transrectal ultrasound-guided prostate biopsy. For treatment, radical prostatectomy is the gold standard for localized prostate cancer. The number of radical prostatectomies performed annually has increased over the past decade, and in particular over the past 5 yr (2). Traditionally, urologists tend to recommend an interval of at least 4 weeks after prostate biopsy before radical prostatectomy to allow biopsy-induced inflammation or hematoma to subside (3). However, studies have shown that early radical prostatectomy does not affect immediate operative outcomes such as operative time, estimated blood loss, and positive surgical margin status for either conventional radical retropubic prostatectomy (4, 5, 6) or robot-assisted laparoscopic radical prostatectomy (7, 8, 9). Nonetheless, to our knowledge there are no studies evaluating this subject with respect to the surgical approach.
Therefore, we aimed to evaluate the influence of biopsy-to-surgery interval on immediate operative outcome with a focus on differences according to the surgical approach, for open radical prostatectomy (perineal and retropubic approach) and minimally invasive radical prostatectomy (laparoscopic and robotic approach).
MATERIALS AND METHODS
Data collection and study design
We retrospectively reviewed and analyzed the medical records of 2,062 patients with prostate cancer who underwent radical prostatectomy, including 573 (31.0%) radical perineal prostatectomies, 308 (16.7%) radical retropubic prostatectomies, 158 (8.5%) laparoscopic radical prostatectomies, and 809 (43.8%) robot-assisted laparoscopic radical prostatectomies. All radical prostatectomies were performed by five surgeons in our institution between September 1995 and November 2011. The cut-off value of prostate-specific antigen for prostate biopsy was mostly 2.5 ng/mL, but it was 4 ng/mL in earlier periods. The majority of prostate biopsies were 12-core biopsies, but 10-core biopsy was performed in earlier periods. If abnormal lesions suspicious for malignancy were detected, additional targeted biopsies were performed. For cancer grading, the conventional Gleason grading system was used before January 2006, and the modified Gleason grading system proposed at the 2005 International Society of Urological Pathology Consensus Conference (10) has been used since January 2006. Clinical T staging was conducted by digital rectal examination and/or prostatic magnetic resonance imaging, and the metastatic status was assessed by chest radiography and whole-body bone scans.
The exclusion criteria were: 1) patients who underwent neoadjuvant treatment such as androgen deprivation therapy and/or radiotherapy before radical prostatectomy; and 2) diagnosis at the time of surgery of benign prostatic hyperplasia such as transurethral resection of the prostate or holmium laser enucleation of the prostate. A cohort of 1,848 men was finally included in this study. Patients were divided into two groups according to the surgical approach (open radical prostatectomy and minimally invasive radical prostatectomy). Radical perineal prostatectomy and radical retropubic prostatectomy were subgroups of open radical prostatectomy, and laparoscopic radical prostatectomy and robot-assisted laparoscopic radical prostatectomy were subgroups of minimally invasive radical prostatectomy. The cut-off for biopsy-to-surgery interval was 4 weeks because it has been the traditional recommendation as mentioned above (3). We recorded demographic and clinicopathologic variables and compared these variables between subgroups of biopsy-to-surgery interval <4 weeks and ≥4 weeks for each group. To assess the immediate operative outcome, we set the primary endpoint as positive surgical margin status and the secondary endpoints as operative time and estimated blood loss, as in most of the previous studies (4, 5, 6, 7, 8, 9).
Statistical analyses
To compare the distribution of important clinical and pathologic variables across the biopsy-to-surgery interval, we used the independent t-test, chi-square test, and Fisher's exact test. For multivariate analysis to determine independent predictors for positive surgical margin status, the binary logistic regression test was used. For multivariate analysis to predict factors for operative time and estimated blood loss, the multiple linear regression test was used. All analyses were performed using SPSS v.19.0 (SPSS Inc., Chicago, IL, USA), and a P<0.05 was considered statistically significant.
Ethics statement
The study protocol was approved by the institutional review board of the Samsung Medical Center (IRB File No. 2013-09-013). Informed consent was waived by the board.
RESULTS
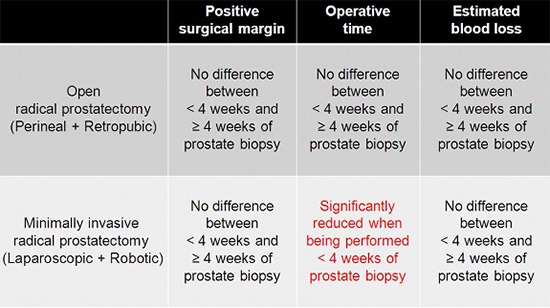
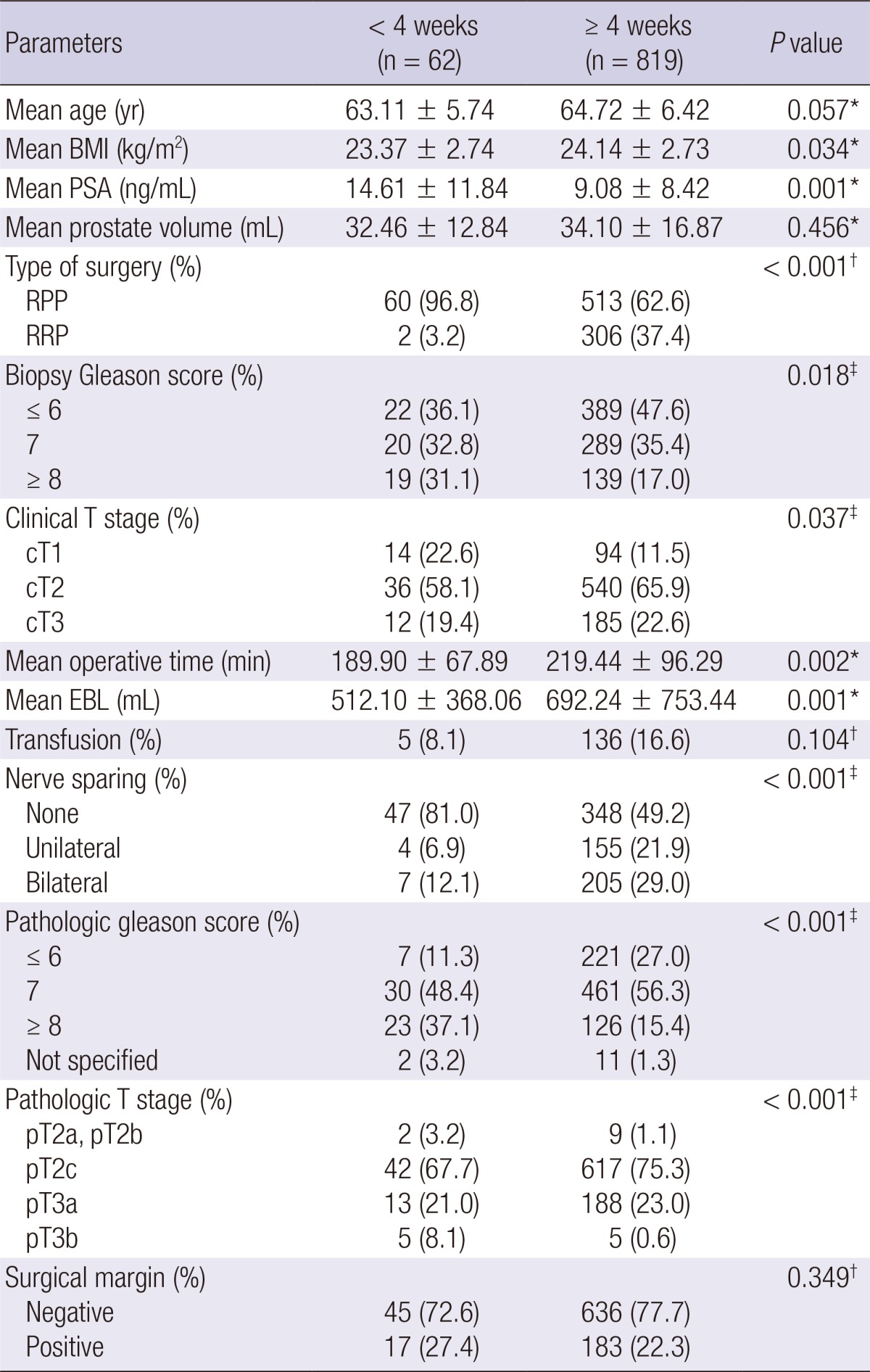
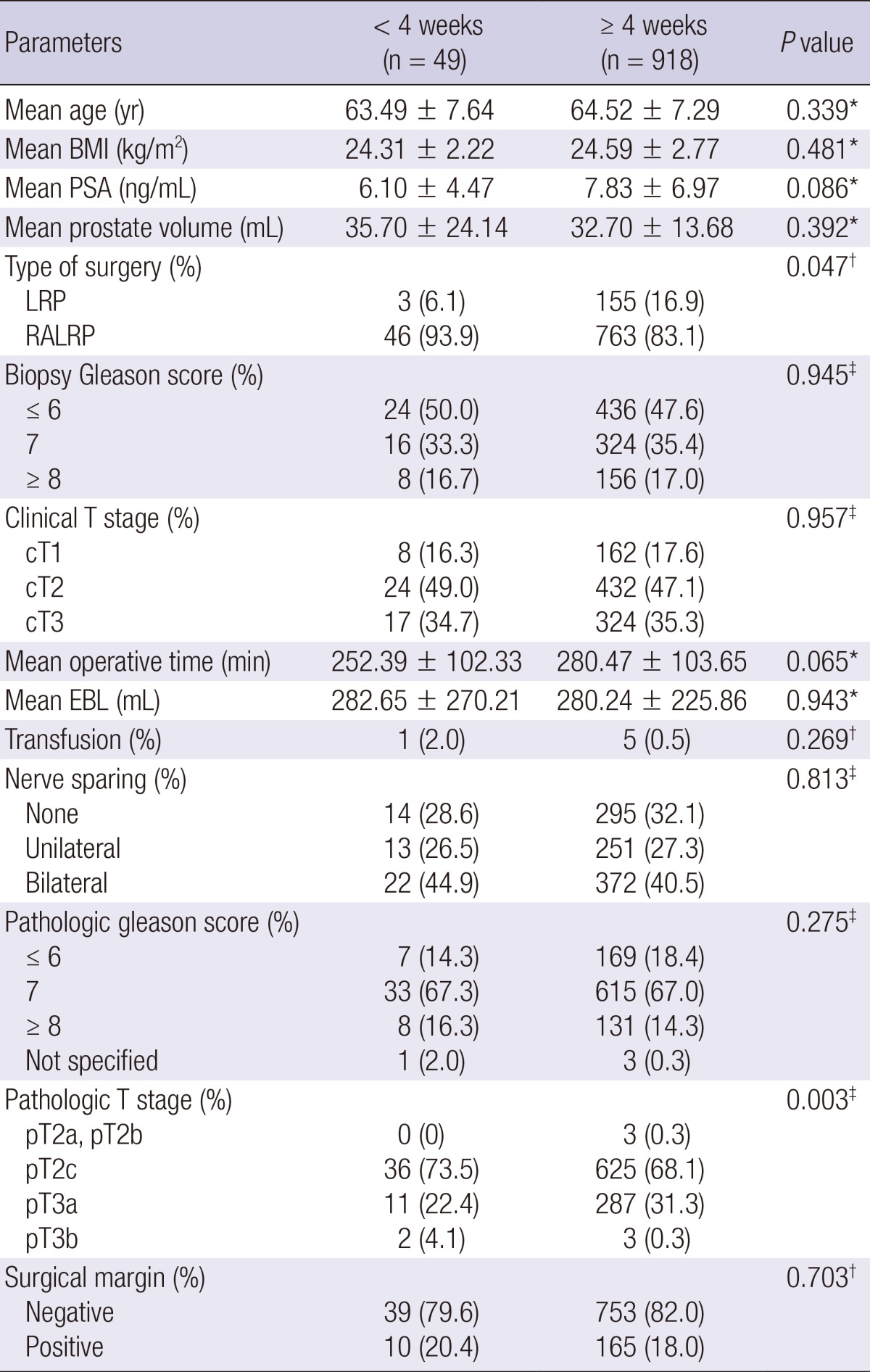
The mean biopsy-to-surgery interval in the overall radical prostatectomy cohort was 8.40±6.16 weeks. In the open radical prostatectomy group, there were significant differences in body mass index, prostate-specific antigen level, type of surgery (radical perineal prostatectomy or radical retropubic prostatectomy), biopsy Gleason score, clinical T stage, nerve sparing procedure, pathologic Gleason score, and pathologic T stage between the <4 and ≥4 weeks subgroups (Table 1). However, in the minimally invasive radical prostatectomy group, only type of surgery and pathologic T stage showed significant differences between the two subgroups. Laparoscopic radical prostatectomy was performed more often after 4 weeks of biopsy and robot-assisted laparoscopic radical prostatectomy was performed more often within 4 weeks of biopsy (P=0.047) (Table 2).
Table 1.
Comparison of clinicopathologic features among open radical prostatectomy groups classified by the interval from prostate biopsy to surgery
*Independent t-test; †Fisher's exact test; ‡Chi-square test. BMI, body mass index; PSA, prostate-specific antigen; RPP, radical perineal prostatectomy; RRP, radical retropubic prostatectomy; EBL, estimated blood loss.
Table 2.
Comparison of clinicopathologic features among minimally invasive radical prostatectomy groups classified by the interval from prostate biopsy to surgery
*Independent t-test; †Fisher's exact test; ‡Chi-square test. BMI, body mass index; PSA, prostate-specific antigen; LRP, laparoscopic radical prostatectomy; RALRP, robot-assisted laparoscopic radical prostatectomy; EBL, estimated blood loss.
Primary endpoint: positive surgical margin status
Overall positive surgical margin rate in the entire cohort was 20.3% (375/1,848). There were no significant differences in positive surgical margin rate between the <4 and ≥4 weeks subgroups for both the open radical prostatectomy group (Table 1) and minimally invasive radical prostatectomy group (Table 2).
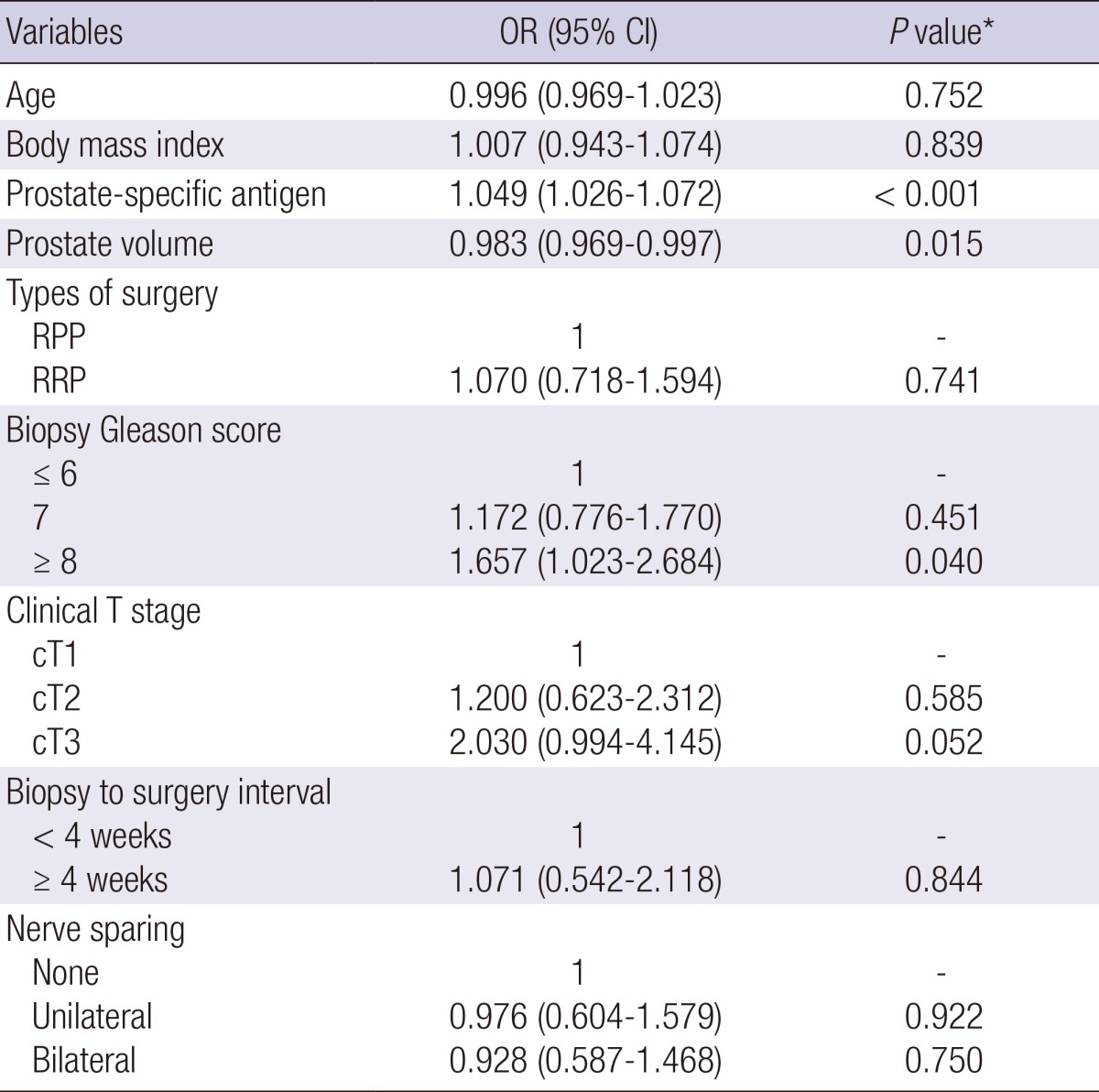
On binary logistic regression analysis, prostate-specific antigen level, prostate volume, and biopsy Gleason score ≥8 were independent factors for positive surgical margin status in open radical prostatectomy group (Table 3). With regard to minimally invasive prostatectomy group, independent factors for positive surgical margin status were prostate-specific antigen level, clinical T stage 3, and unilateral and bilateral nerve sparing (Table 4). However, biopsy-to-surgery interval was not a significant factor for positive surgical margin status for both groups.
Table 3.
Multivariate analysis of biopsy-to-surgery interval to evaluate factors affecting positive surgical margins in patients who underwent open radical prostatectomy
*Binary logistic regression test. OR, odds ratio; CI, confidence interval; RPP, radical perineal prostatectomy; RRP, radical retropubic prostatectomy.
Table 4.
Multivariate analysis of biopsy-to-surgery interval to evaluate factors affecting positive surgical margins in patients who underwent minimally invasive radical prostatectomy
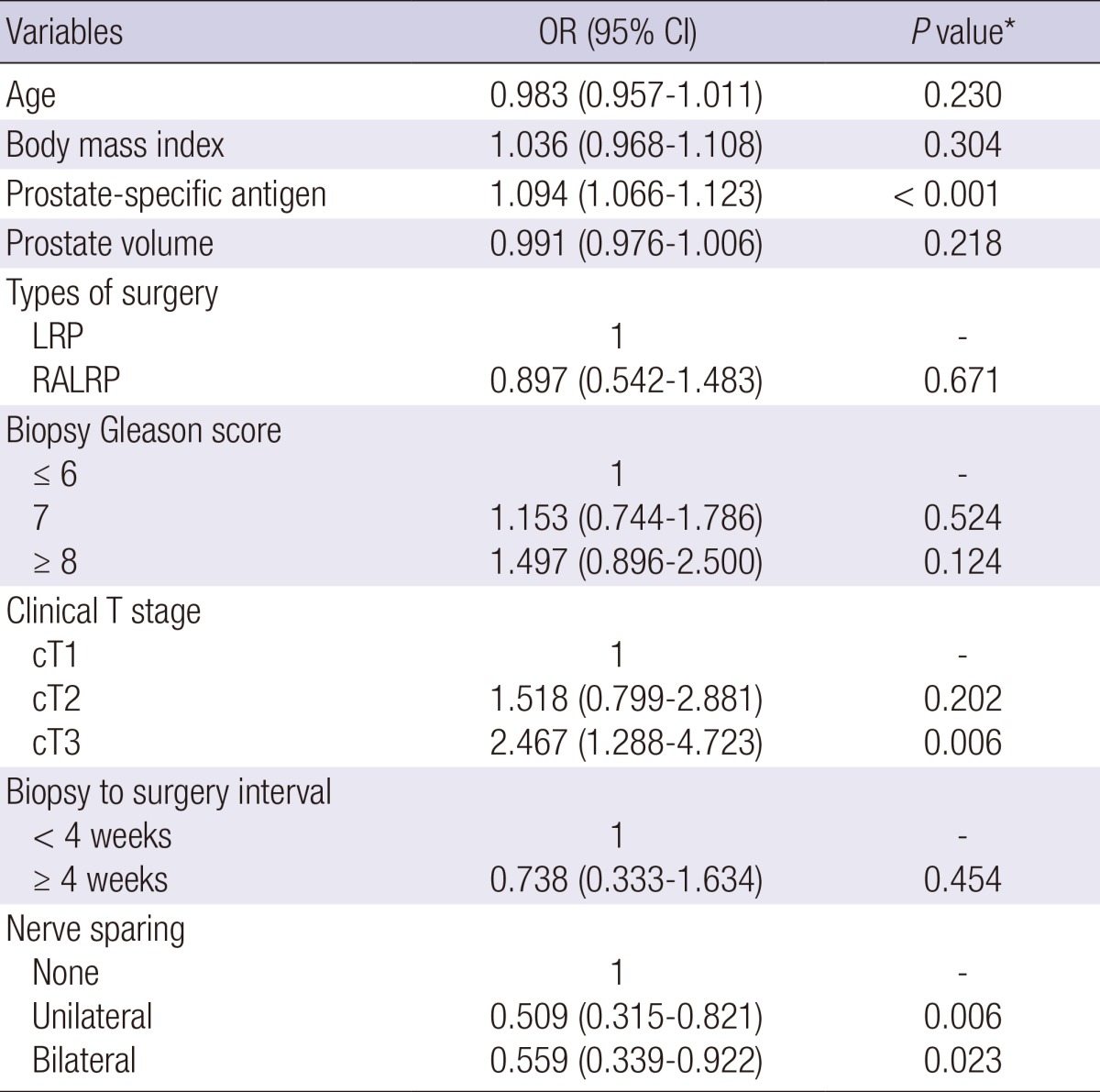
*Binary logistic regression test. OR, odds ratio; CI, confidence interval; LRP, laparoscopic radical prostatectomy; RALRP, robot-assisted laparoscopic radical prostatectomy.
Secondary endpoint: operative time
For the entire cohort, the mean operative time was 249.64 min. In the open radical prostatectomy group, operative time was significantly shorter when radical prostatectomy was performed within 4 weeks of prostate biopsy (P=0.002) (Table 1). However, in the minimally invasive radical prostatectomy group, there was no significant difference in operative time between the <4 and ≥4 weeks subgroups (Table 2).
On multiple linear regression analysis, factors that increased operative time were body mass index (t=5.361, P<0.001), prostate-specific antigen level (t=4.602, P<0.001), prostate volume (t=3.605, P<0.001), and clinical T stage (t=2.399, P=0.017 for cT1; t=6.193, P<0.001 for cT2; compared to cT3), whereas factors to decrease operative time was radical perineal prostatectomy (t=-20.534, P<0.001) compared to robot-assisted laparoscopic radical prostatectomy (data not shown). The biopsy-to-surgery interval was not a significant factor affecting operative time. However, on the multivariate analysis assessed for surgical approach, biopsy-to-surgery interval <4 weeks was a significant factor to decrease operative time with regard to minimally invasive radical prostatectomy (t=-2.064, P=0.039) (Table 5).
Table 5.
Multivariate analysis* of biopsy-to-surgery interval to evaluate factors affecting operative time and estimated blood loss overall and according to surgical approach
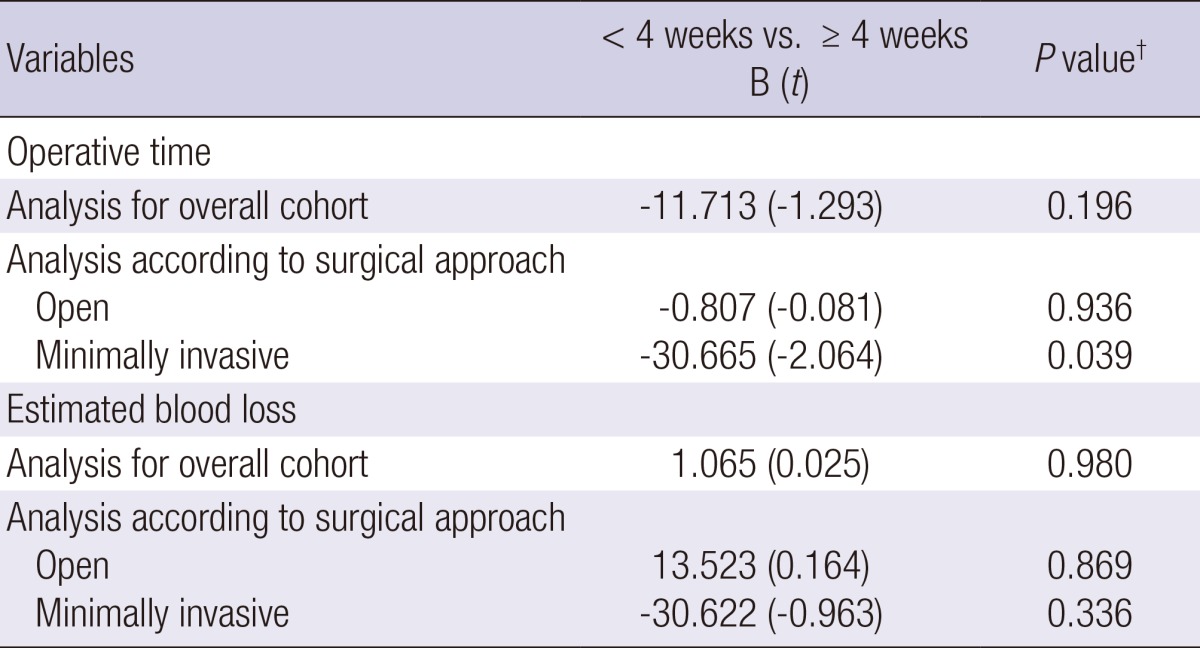
*Other covariates not shown in this table included age, body mass index, prostate-specific antigen, prostate volume, biopsy Gleason score, clinical T stage, and nerve sparing; †Multiple linear regression test.
Secondary endpoint: estimated blood loss
Mean estimated blood loss was 470.55 mL in the entire cohort. In the open radical prostatectomy group, estimated blood loss was significantly lower when the radical prostatectomy was performed within 4 weeks of prostate biopsy (P=0.001) (Table 1). However, in minimally invasive radical prostatectomy group, there was no significant difference in estimated blood loss between the <4 weeks and ≥4 weeks subgroups (Table 2).
Multiple linear regression analysis revealed that factors that increased the estimated blood loss were body mass index (t=2.682, P=0.007), prostate volume (t=5.012, P<0.001), radical perineal prostatectomy (t=7.105, P<0.001), radical retropubic prostatectomy (t=21.442, P<0.001) compared to robot-assisted laparoscopic radical prostatectomy, biopsy Gleason score of 7 (t=2.035, P=0.042) compared to a score of ≥ 8, cT2 stage (t=2.993, P=0.003) compared to cT3 stage, and not performing nerve sparing procedure (t=2.049, P=0.041) compared to bilateral nerve sparing (data not shown). However, biopsy-to-surgery interval was not a significant factor affecting estimated blood loss. On multivariate analysis assessed with respect to surgical approach, biopsy-to-surgery interval remained not significant for estimated blood loss (Table 5).
DISCUSSION
The objective of our study was to investigate the impact of the biopsy-to-surgery interval on immediate operative outcome with a focus on differences according to the surgical approach. Our data showed that the biopsy-to-surgery interval had no impact on the positive surgical margin status in both open radical prostatectomy and minimally invasive radical prostatectomy groups. In addition, it did not affect estimated blood loss in both groups or operative time in the open radical prostatectomy group, although biopsy-to-surgery interval <4 weeks was a significant factor for decreased operative time in the minimally invasive radical prostatectomy group.
The results of the present study are partly consistent with those of earlier studies. Lee and associates reported that the biopsy-to-surgery interval was not an independent factor of positive surgical margin status, operative time, and estimated blood loss in a group of men who underwent a total of 169 radical retropubic prostatectomies (4). A larger study with a cohort of 2,996 radical retropubic prostatectomies also showed no significant association between early surgery and positive surgical margin status, operative time, and estimated blood loss using an interval of 4 weeks or less (5). For robot-assisted laparoscopic radical prostatectomy, a study of 559 patients who underwent robot-assisted laparoscopic radical prostatectomy reported no association between biopsy-to-surgery interval and the three outcome parameters using an interval of 4 weeks or less (7). Another study used intervals of ≤2 weeks, >2 to ≤4 weeks, >4 to ≤6 weeks, >6 to ≤8 weeks, and >8 weeks with a cohort of 237 robot-assisted laparoscopic radical prostatectomies, and revealed that biopsy-to-surgery interval was not significantly associated with positive surgical margin status, operative time, and estimated blood loss (9). However, our findings are in disagreement with the results of Choi et al., who found a strong trend that robot-assisted laparoscopic radical prostatectomy performed within 4 weeks of biopsy was associates with a longer operative time (232.6 vs. 208.8 min, P=0.07) (8).
An interesting finding in our study, which is not in accord with earlier studies, is that performing minimally invasive radical prostatectomy within 4 weeks of prostate biopsy significantly shortened the operative time. The explanation for this finding is not clear. Inflammatory reaction will occur to some extent in the post-biopsy prostate because the biopsy results in local tissue injury. It is known that the presence of histologic inflammation within the prostate correlates significantly with serum prostate-specific antigen levels (11, 12). Oesterling et al. (13) found that the median time required for the serum prostate-specific antigen value to return to a stable level after prostate biopsy was 15-17 days. Thus, it is inferred that the most of the post-biopsy inflammation within the prostate would resolve 15-17 days after biopsy. The resolution of acute inflammation is followed by tissue proliferation and remodeling and it is known that tissue remodeling with collagen scar formation begins 3-4 weeks after tissue injury (14, 15, 16). Thus, local tissue remodeling and scar formation within the prostate may start 3-4 weeks after biopsy and might lead to intraprostatic tissue adhesions.
A tissue adhesion is known to be one of the factors that make the laparoscopic procedure difficult to perform (17). Studies have shown that the most common cause of a conversion to laparotomy during laparoscopic adhesiolysis for small bowel obstruction was matted, dense adhesions (18, 19, 20). Regarding prostatectomy, Jaffe et al. reported that significantly more operative time was needed when performing laparoscopic radical prostatectomy for patients who underwent previous transurethral resection of the prostate than those who did not, and this finding was explained by the more difficult dissection resulting from periprostatic inflammation and fibrosis (21). For robot-assisted laparoscopic radical prostatectomy, a previous study reported a trend that robot-assisted laparoscopic radical prostatectomy with previous transurethral resection of the prostate required more operative time than without previous transurethral resection of the prostate (200 min vs. 186 min, P=0.112), although this was not statistically significant (22). Therefore, the longer operative time when performing minimally invasive radical prostatectomy after 4 weeks of biopsy might be explained by the development of intraprostatic or periprostatic adhesions more than 4 weeks after prostate biopsy.
Our study has limitations, including its retrospective design and performance at a single institution. There is a possibility of selection bias associated with the referral patterns to a tertiary medical center. Another limitation is the heterogeneous nature of the cohort with four different prostatectomies performed by five different surgeons in a long time period. Therefore, certain confounding factors such as the difference of surgeon experience and learning curve might have affected the outcomes. The most critical limitation is that many of the baseline demographic data showed significant differences in the open radical prostatectomy group (Table 1). This might stem from the heterogeneity of the radical prostatectomy cohort, which included two different types of open radical prostatectomies (radical perineal prostatectomy and radical retropubic prostatectomy) performed by three different surgeons. As shown in Table 1, significantly more radical perineal prostatectomies were performed within 4 weeks of biopsy, and more radical retropubic prostatectomies were performed after 4 weeks of biopsy. This difference most likely accounts for many of the differences in baseline parameters. However, because multivariate analyses with adjustment for possible confounders showed that the biopsy-to-surgery interval was not a significant factor for positive surgical margin status, operative time, and estimated blood loss (except for operative time in the minimally invasive radical prostatectomy group analysis), the baseline differences would not have much impact on the conclusions of our study.
In conclusion, our study showed that biopsy-to-surgery interval did not affect positive surgical margin status, operative time, or estimated blood loss in both open radical prostatectomy and minimally invasive radical prostatectomy groups with the exception of a significantly shorter operative time when minimally invasive radical prostatectomy was performed within 4 weeks of biopsy. Therefore, it is feasible to perform open or minimally invasive radical prostatectomies within 4 weeks of prostate biopsy, and this might even be beneficial when performing minimally invasive radical prostatectomy to reduce the operative time.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stitzenberg KB, Wong YN, Nielsen ME, Egleston BL, Uzzo RG. Trends in radical prostatectomy: centralization, robotics, and access to urologic cancer care. Cancer. 2012;118:54–62. doi: 10.1002/cncr.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh PC, Partin AW. Anatomic radical retropubic prostatectomy. In: Wein AJ, Kavoussi LR, Novick AC, Peters CA, Partin AW, editors. Campbell-Walsh Urology. 9th ed. Philadelphia: W. B. Saunders; 2007. pp. 2956–1978. [Google Scholar]
- 4.Lee DK, Allareddy V, O'Donnell MA, Williams RD, Konety BR. Does the interval between prostate biopsy and radical prostatectomy affect the immediate postoperative outcome? BJU Int. 2006;97:48–50. doi: 10.1111/j.1464-410X.2006.05861.x. [DOI] [PubMed] [Google Scholar]
- 5.Eggener SE, Yossepowitch O, Serio AM, Vickers AJ, Scardino PT, Eastham JA. Radical prostatectomy shortly after prostate biopsy does not affect operative difficulty or efficacy. Urology. 2007;69:1128–1133. doi: 10.1016/j.urology.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 6.Adiyat KT, Murugesan M, Katkoori D, Eldefrawy A, Soloway MS. Total prostatectomy within 6 weeks of a prostate biopsy: is it safe? Int Braz J Urol. 2010;36:177–181. doi: 10.1590/s1677-55382010000200007. discussion 82. [DOI] [PubMed] [Google Scholar]
- 7.Martin GL, Nunez RN, Humphreys MD, Martin AD, Ferrigni RG, Andrews PE, Castle EP. Interval from prostate biopsy to robot-assisted radical prostatectomy: effects on perioperative outcomes. BJU Int. 2009;104:1734–1737. doi: 10.1111/j.1464-410X.2009.08685.x. [DOI] [PubMed] [Google Scholar]
- 8.Choi H, Ko YH, Kang SG, Kang SH, Park HS, Cheon J, Patel VR. Biopsy related prostate status does not affect on the clinicopathological outcome of robotic assisted laparoscopic radical prostatectomy. Cancer Res Treat. 2009;41:205–210. doi: 10.4143/crt.2009.41.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim IS, Na W, Nam JS, Oh JJ, Jeong CW, Hong SK, Byun SS, Lee SE. Interval from prostate biopsy to robot-assisted laparoscopic radical prostatectomy (RALP): effects on surgical difficulties. Korean J Urol. 2011;52:664–668. doi: 10.4111/kju.2011.52.10.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 11.Okada K, Kojima M, Naya Y, Kamoi K, Yokoyama K, Takamatsu T, Miki T. Correlation of histological inflammation in needle biopsy specimens with serum prostate-specific antigen levels in men with negative biopsy for prostate cancer. Urology. 2000;55:892–898. doi: 10.1016/s0090-4295(00)00519-7. [DOI] [PubMed] [Google Scholar]
- 12.Ozden C, Ozdal OL, Guzel O, Han O, Seckin S, Memis A. The correlation between serum prostate specific antigen levels and asymptomatic inflammatory prostatitis. Int Urol Nephrol. 2007;39:859–863. doi: 10.1007/s11255-006-9125-2. [DOI] [PubMed] [Google Scholar]
- 13.Oesterling JE, Rice DC, Glenski WJ, Bergstralh EJ. Effect of cystoscopy, prostate biopsy, and transurethral resection of prostate on serum prostate-specific antigen concentration. Urology. 1993;42:276–282. doi: 10.1016/0090-4295(93)90616-i. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Krafts KP. Tissue repair: The hidden drama. Organogenesis. 2010;6:225–233. doi: 10.4161/org.6.4.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 17.Davey AK, Maher PJ. Surgical adhesions: a timely update, a great challenge for the future. J Minim Invasive Gynecol. 2007;14:15–22. doi: 10.1016/j.jmig.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Nagle A, Ujiki M, Denham W, Murayama K. Laparoscopic adhesiolysis for small bowel obstruction. Am J Surg. 2004;187:464–470. doi: 10.1016/j.amjsurg.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Farinella E, Cirocchi R, La Mura F, Morelli U, Cattorini L, Delmonaco P, Migliaccio C, De Sol AA, Cozzaglio L, Sciannameo F. Feasibility of laparoscopy for small bowel obstruction. World J Emerg Surg. 2009;4:3. doi: 10.1186/1749-7922-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dindo D, Schafer M, Muller MK, Clavien PA, Hahnloser D. Laparoscopy for small bowel obstruction: the reason for conversion matters. Surg Endosc. 2010;24:792–797. doi: 10.1007/s00464-009-0658-1. [DOI] [PubMed] [Google Scholar]
- 21.Jaffe J, Stakhovsky O, Cathelineau X, Barret E, Vallancien G, Rozet F. Surgical outcomes for men undergoing laparoscopic radical prostatectomy after transurethral resection of the prostate. J Urol. 2007;178:483–487. doi: 10.1016/j.juro.2007.03.114. discussion 7. [DOI] [PubMed] [Google Scholar]
- 22.Martin AD, Desai PJ, Nunez RN, Martin GL, Andrews PE, Ferrigni RG, Swanson SK, Pacelli A, Castle EP. Does a history of previous surgery or radiation to the prostate affect outcomes of robot-assisted radical prostatectomy? BJU Int. 2009;103:1696–1698. doi: 10.1111/j.1464-410X.2008.08276.x. [DOI] [PubMed] [Google Scholar]


