Abstract
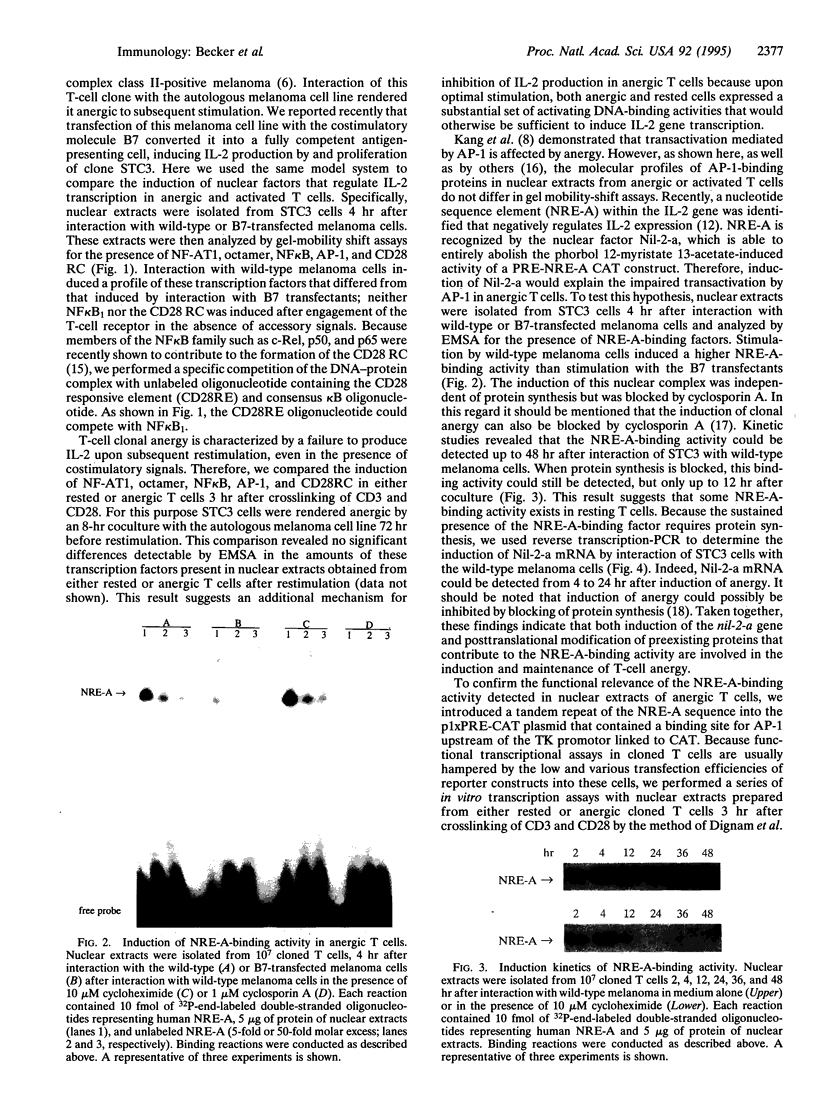
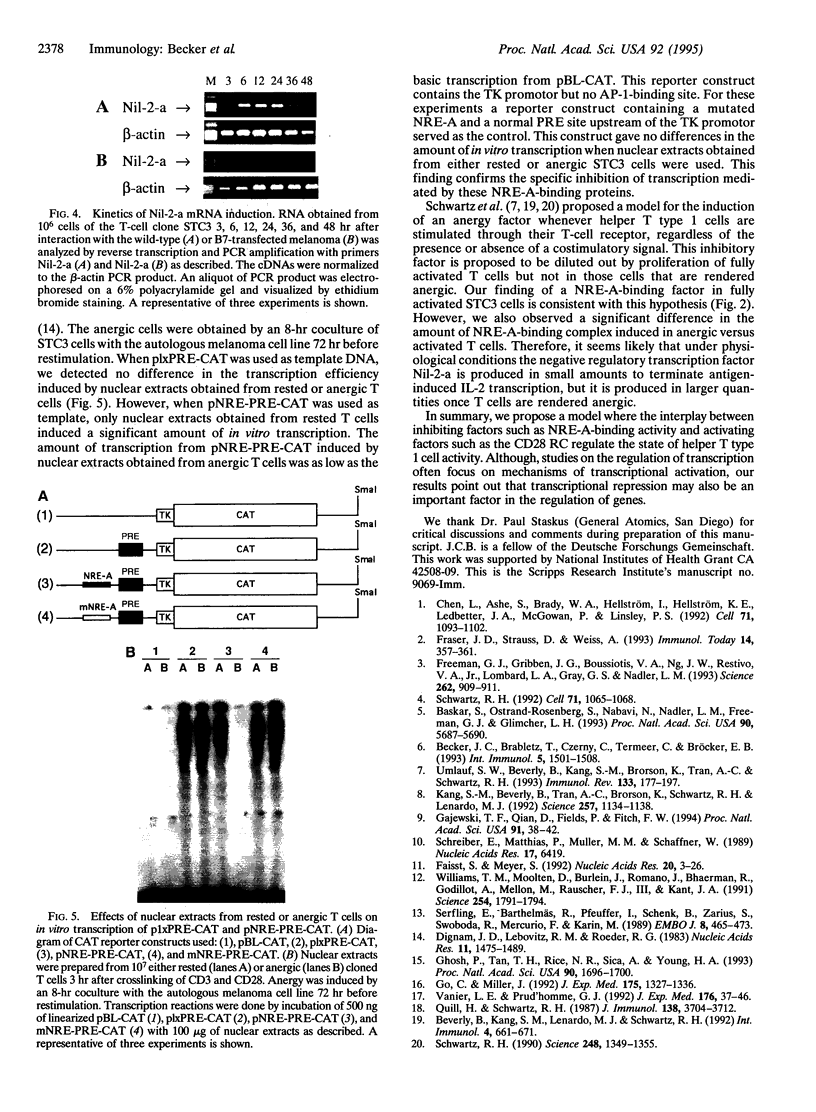
Anergy is a mechanism of T-lymphocyte tolerance induced by antigen-receptor stimulation in the absence of costimulation, whereby T cells exhibit a defect in antigen-induced transcription of the interleukin 2 (IL-2) gene. Here we present evidence for a mechanism of negative IL-2 gene regulation in anergic T cells. High amounts of binding activity to the negative regulatory element A (NRE-A) of the IL-2 promotor were detected in nuclear extracts from human T cells shortly after induction of anergy. Rapid induction of this nuclear complex is blocked by cyclosporin A and is found to be independent of protein synthesis. Plasmid DNAs, containing either the human phorbol 12-myristate 13-acetate-responsive element (PRE) or both NRE-A and PRE, were used as template for in vitro transcription assays in the presence of T-cell nuclear extracts. Under these conditions nuclear extracts from both anergic and rested T-cell clones, after crosslinking of CD3 and CD28, induced transcription of plasmids containing only PRE. However, when plasmids containing NRE-A and PRE were used, transcription was only induced by nuclear extracts from rested but not anergic T cells. These findings suggest the functional relevance of transcriptional repression of the IL-2 gene in anergic T cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskar S., Ostrand-Rosenberg S., Nabavi N., Nadler L. M., Freeman G. J., Glimcher L. H. Constitutive expression of B7 restores immunogenicity of tumor cells expressing truncated major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5687–5690. doi: 10.1073/pnas.90.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. C., Brabletz T., Czerny C., Termeer C., Bröcker E. B. Tumor escape mechanisms from immunosurveillance: induction of unresponsiveness in a specific MHC-restricted CD4+ human T cell clone by the autologous MHC class II+ melanoma. Int Immunol. 1993 Dec;5(12):1501–1508. doi: 10.1093/intimm/5.12.1501. [DOI] [PubMed] [Google Scholar]
- Beverly B., Kang S. M., Lenardo M. J., Schwartz R. H. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992 Jun;4(6):661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- Chen L., Ashe S., Brady W. A., Hellström I., Hellström K. E., Ledbetter J. A., McGowan P., Linsley P. S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992 Dec 24;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D., Straus D., Weiss A. Signal transduction events leading to T-cell lymphokine gene expression. Immunol Today. 1993 Jul;14(7):357–362. doi: 10.1016/0167-5699(93)90236-E. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Gribben J. G., Boussiotis V. A., Ng J. W., Restivo V. A., Jr, Lombard L. A., Gray G. S., Nadler L. M. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993 Nov 5;262(5135):909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Qian D., Fields P., Fitch F. W. Anergic T-lymphocyte clones have altered inositol phosphate, calcium, and tyrosine kinase signaling pathways. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):38–42. doi: 10.1073/pnas.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Tan T. H., Rice N. R., Sica A., Young H. A. The interleukin 2 CD28-responsive complex contains at least three members of the NF kappa B family: c-Rel, p50, and p65. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1696–1700. doi: 10.1073/pnas.90.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go C., Miller J. Differential induction of transcription factors that regulate the interleukin 2 gene during anergy induction and restimulation. J Exp Med. 1992 May 1;175(5):1327–1336. doi: 10.1084/jem.175.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. M., Beverly B., Tran A. C., Brorson K., Schwartz R. H., Lenardo M. J. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992 Aug 21;257(5073):1134–1138. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- Quill H., Schwartz R. H. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987 Jun 1;138(11):3704–3712. [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992 Dec 24;71(7):1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umlauf S. W., Beverly B., Kang S. M., Brorson K., Tran A. C., Schwartz R. H. Molecular regulation of the IL-2 gene: rheostatic control of the immune system. Immunol Rev. 1993 Jun;133:177–197. doi: 10.1111/j.1600-065x.1993.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Vanier L. E., Prud'homme G. J. Cyclosporin A markedly enhances superantigen-induced peripheral T cell deletion and inhibits anergy induction. J Exp Med. 1992 Jul 1;176(1):37–46. doi: 10.1084/jem.176.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. M., Moolten D., Burlein J., Romano J., Bhaerman R., Godillot A., Mellon M., Rauscher F. J., 3rd, Kant J. A. Identification of a zinc finger protein that inhibits IL-2 gene expression. Science. 1991 Dec 20;254(5039):1791–1794. doi: 10.1126/science.1840704. [DOI] [PubMed] [Google Scholar]