Abstract
Malaria kills nearly 1 million people each year, and the protozoan parasite Plasmodium falciparum has become increasingly resistant to current therapies. Isoprenoid synthesis via the methylerythritol phosphate (MEP) pathway represents an attractive target for the development of new antimalarials. The phosphonic acid antibiotic fosmidomycin is a specific inhibitor of isoprenoid synthesis and has been a helpful tool to outline the essential functions of isoprenoid biosynthesis in P. falciparum. Isoprenoids are a large, diverse class of hydrocarbons that function in a variety of essential cellular processes in eukaryotes. In P. falciparum, isoprenoids are used for tRNA isopentenylation and protein prenylation, as well as the synthesis of vitamin E, carotenoids, ubiquinone, and dolichols. Recently, isoprenoid synthesis in P. falciparum has been shown to be regulated by a sugar phosphatase. We outline what is known about isoprenoid function and the regulation of isoprenoid synthesis in P. falciparum, in order to identify valuable directions for future research.
INTRODUCTION
Severe malaria remains a threat to human health worldwide, with over 250 million cases per year. Malaria is a leading cause of death in children, with almost one million deaths each year (1, 2). Despite ongoing and intensive control efforts, malaria remains endemic on five continents. Widespread resistance to former first-line agents, most notably chloroquine, has severely limited malaria control efforts (2). Currently, the recommended standard of care for malaria infection is combination therapy using artemisinin-based therapeutics. However, decreased sensitivity to artemisinin has been recognized in the field, particularly in Southeast Asia. The spread of artemisinin resistance threatens the progress that has been made in control of malaria, particularly in sub-Saharan Africa (3–5). New antimalarial agents, particularly agents with novel mechanisms of action, are urgently needed.
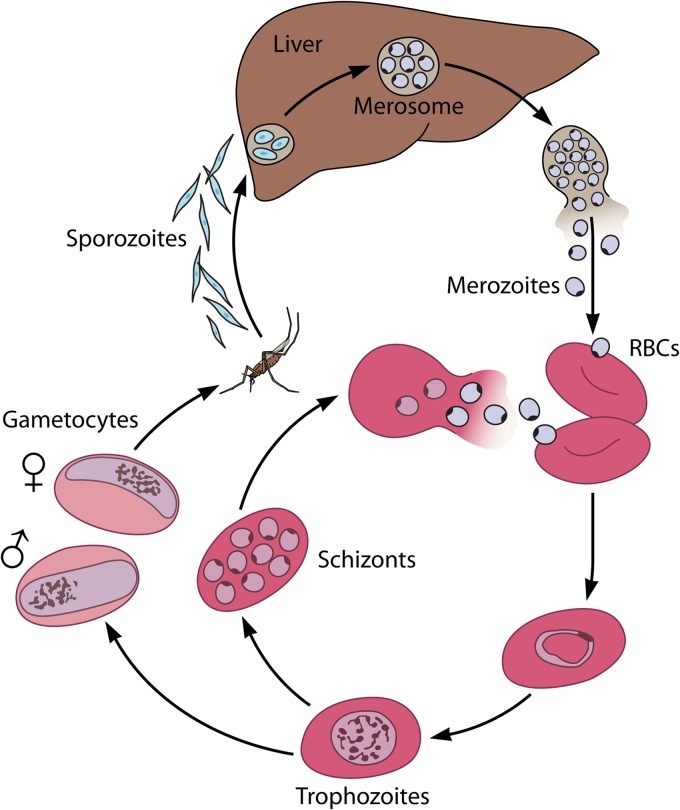
Malaria is caused by infection with protozoan parasites in the genus Plasmodium. Most cases of life-threatening malaria are attributable to infection with a single species, Plasmodium falciparum, although P. vivax and P. knowlesi have also been associated with severe disease (6–9). Plasmodium infection is transmitted through the bite of anopheline mosquitoes (Fig. 1 depicts their life cycle). Expelled from mosquito salivary glands, malaria sporozoites first traffic to the liver, where 10 to 100,000 daughter parasites are generated from a single invading cell. Upon egress from the liver, the parasite enters the host bloodstream. There, the malaria parasite begins an asexual cycle of growth and development within erythrocytes. This intraerythrocytic cycle leads to the signs and symptoms associated with malaria infection, including fever, anemia, and multiorgan dysfunction due to vascular adherence of parasitized red blood cells. New antimalarials must therefore target this pathogenic stage of parasite development. A small proportion of asexual-stage parasites leave the asexual cycle and commit to the production of sexual forms, known as gametocytes. Upon a new blood meal, gametocytes return to the mosquito midgut, where they complete sexual development and begin the life cycle anew.
FIG 1.
Life cycle of Plasmodium falciparum. Infection begins with the injection of sporozoites into the host bloodstream by the bite of an Anopheles mosquito. Parasites multiply in the liver and are released back into the host bloodstream as merozoites, where they begin the intraerythrocytic developmental cycle (RBCs, red blood cells). Inside the erythrocyte, parasites grow into large trophozoites. They eventually divide to become multinucleate schizonts, which erupt from the host cell and reenter the blood as merozoites. A proportion of these blood-stage parasites become gametocytes and are taken up by the mosquito vector, where they complete sexual replication.
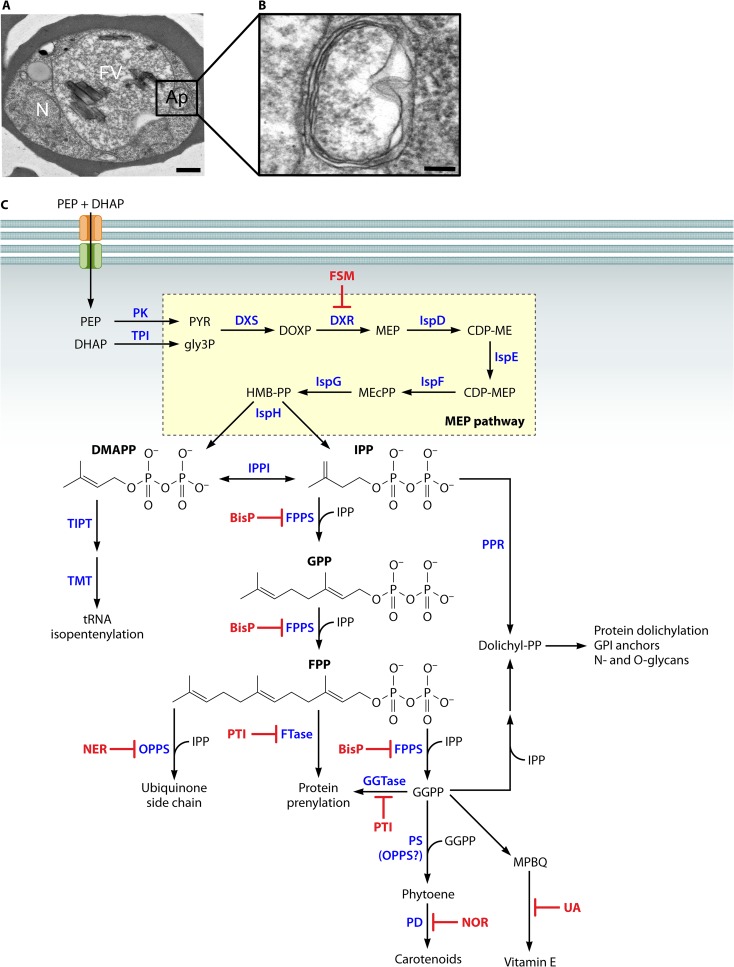
One cellular peculiarity of Plasmodium species, as well as other apicomplexan parasites, such as Toxoplasma and Babesia species, is the presence of an unusual plastid organelle, the apicoplast (Fig. 2A and B). The apicoplast is surrounded by four membranes, suggesting an ancient secondary endosymbiotic event between a protozoan parasite ancestor and red algae, similar to that of the chloroplast (10–12). While the apicoplast was previously believed to be of green algal origin, the recent discovery and genome sequencing of the alveolate Chromera velia has revealed C. velia as an evolutionary link between apicomplexans and their red algal ancestors (11, 12). C. velia can potentially serve as a useful tool to study the evolution of plastid pathways in apicomplexan parasites. While photosynthetic capabilities have been lost over time, the malaria parasite has retained some plantlike metabolic pathways that hold particular value as targets for antimalarial drug development, since these pathogen-specific processes are not present in humans.
FIG 2.
Synthesis of isoprenoid products in P. falciparum. (A) Electron micrograph of P. falciparum cell, with labels showing the red blood cell (RBC), nucleus (N), food vacuole (FV), and apicoplast (Ap). Scale bar represents 500 nm. (B) The P. falciparum apicoplast is the site of isoprenoid synthesis by the MEP pathway. It is surrounded by four membranes, indicative of secondary endosymbiotic origins. Scale bar represents 100 nm. (C) Isoprenoid products produced by P. falciparum. Abbreviations used: phosphoenol pyruvate (PEP), dihydroxyacetone phosophate (DHAP), pyruvate kinase (PK), triose phosphate isomerase (TPI), 1-deoxy-d-xylulose 5-phosphate synthase (DXS), 1-deoxy-d-xylulose 5-phosphate (DOXP), DOXP reductoisomerase (DXR), fosmidomycin (FSM), 2-C-methyl-d-erythriol 4-phosphate (MEP), MEP cytidyltransferase (IspD), 4-diphosphocytidyl-2-C-methylerythritol (CDP-ME), CDP-ME kinase (IspE), 4-diphosphocytidyl-2-C-methylerythritol 2-phosphate (CDP-MEP), 2-C-methyl-d-erythritol 2,4-cyclopyrophosphate (MEcPP), MEcPP synthase (IspF), (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP), HMB-PP synthase (IspG), HMB-PP reductase (IspH), dimethylallyl pyrophosphate (DMAPP), isopentenyl pyrophosphate (IPP), isopentenyl pyrophosphate isomerase (IPPI), tRNA isopentenyltransferase (TIPT), tRNA methylthiolase (TMT), farnesyl pyrophosphate synthase (FPPS), bisphosphate (BisP), geranyl pyrophosphate (GPP), polyprenol reductase (PPR), farnesyl pyrophosphate (FPP), octaprenyl pyrophosphate synthase (OPPS), nerolidol (NER), farnesyl transferase (FTase), prenyltransferase inhibitors (PTI), geranylgeranyl transferase (GGTase), geranylgeranyl pyrophosphate (GGPP), phytoene synthase (PS), phytoene desaturase (PD), norflurazon (NOR), dolichyl pyrophosphate (dolichyl-PP), 2-methyl-6-phytyl-1,4-benzoquinol (MPBQ), and usnic acid (UA).
Key among apicoplast metabolic pathways is that of isoprenoid biosynthesis. Isoprenoids comprise a very large and diverse group of biomolecules derived from the sequential assembly of two 5-carbon isomers, isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). Chains of isoprene units are subsequently modified through cyclizations, oxidations, reductions, and additions to generate the array of over 25,000 isoprenoids found in nature (13). In humans (as well as fungi, archeabacteria, cytoplasm of plants, and other metazoans), the isoprenoid building blocks IPP and DMAPP are produced through a mevalonate-dependent pathway from acetyl-coenzyme A (CoA). The rate-limiting step in the mevalonate pathway is the conversion of 3-hydroxy-3-methyl-glutaryl (HMG)-CoA to mevalonic acid by the enzyme HMG-CoA reductase; this enzyme is the target for the widely used statin class of cholesterol-lowering drugs (14).
In Plasmodium species, IPP and DMAPP are produced via an alternative biosynthetic route that does not utilize mevalonate (15, 16). This pathway, also called the MEP (2-C-methyl-d-erythritol 4-phosphate) pathway or DOXP (1-deoxy-d-xylulose 5-phosphate) pathway, converts glyceraldehyde 3-phosphate and pyruvate to IPP and DMAPP through seven enzymatic steps (Fig. 2C). At least two enzymes of this pathway catalyze rate-limiting steps in IPP production: DOXP synthase (DXS) (EC 2.2.1.7, PlasmoDB identifier [ID] PF3D7_1337200) converts glyceraldehyde 3-phosphate and pyruvate to DOXP, and DOXP reductoisomerase (DXR) (EC 1.1.1.267, PF3D7_1467300) converts DOXP to MEP (17, 18). The mechanism by which IPP and DMAPP are exported from the apicoplast for use in the cytoplasm remains unknown.
Given the structural diversity of isoprenoids, it is not surprising that these molecules serve diverse cellular functions. Plants in particular elaborate an incredible range of specialized isoprenoid end products, including pharmacologically active compounds like paclitaxel (originally named taxol) (19) and artemisinin (20), as well as terpenes, volatile isoprenoids that confer the characteristic odors, flavors, and colors of plants. Other roles of isoprenoids include regulation of cell growth and energy production, intracellular signaling, and membrane structural support (15, 21, 22). Recent reviews discuss apicoplast metabolism and, specifically, isoprenoid synthesis, as drug targets in P. falciparum (23, 24). Here, we address the key questions in the field: what isoprenoids does the malaria parasite make, and why?
FOSMIDOMYCIN
An important reagent in the study of the MEP pathway has been the selective MEP pathway inhibitor, fosmidomycin. Fosmidomycin is a small, three-carbon phosphonate compound that was first identified from Streptomyces lavendulae by its antibacterial properties (25). Subsequent in vitro studies revealed that fosmidomycin competitively inhibits DXR, the first dedicated enzyme of the MEP pathway (26–28). The charged nature of fosmidomycin means that this compound is typically excluded from cells unless actively imported, which has limited its utility against many organisms, including the apicomplexan Toxoplasma gondii (29) and the agent of tuberculosis, Mycobacterium tuberculosis (30). Intraerythrocytic malaria parasites elaborately remodel the host red blood cell, significantly increasing the cellular uptake of many nutrients (31–33). These so-called new permeability pathways likely facilitate the uptake of fosmidomycin, as fosmidomycin is excluded from uninfected red blood cells but inhibits the growth of Plasmodium and a related, tick-borne intraerythrocytic apicomplexan pathogen, Babesia divergens (34). It remains unclear what cellular machinery is required for fosmidomycin uptake into P. falciparum cells.
Fosmidomycin is well validated as a specific inhibitor of DXR. Analysis of MEP pathway intermediates in bacteria and P. falciparum has established that fosmidomycin reduces the intracellular levels of downstream MEP pathway metabolites and isoprenoid products (35–37). In addition, the growth inhibitory effects of fosmidomycin are chemically rescued in bacteria and malaria parasites through supplementation of the medium with IPP or unphosphorylated isoprenols (farnesol and geranylgeraniol). The 50% inhibitory concentration (IC50) for fosmidomycin increases 10-fold when the medium is supplemented with farnesol or geranylgeraniol (35, 38). Supplementation of the medium with geranylgeraniol also rescues protein mislocalization and the organelle disruption effects of fosmidomycin treatment (39). Treatment with high concentrations of fosmidomycin is not completely rescued by prenyl alcohol supplementation, perhaps due to the toxicity of these compounds at high concentrations (40).
In asexual parasites, the MEP pathway may be the only essential function of the apicoplast organelle in which it resides. Treating parasites with inhibitors of apicoplast replication forces P. falciparum to lose its apicoplast genome and structure. These parasites nonetheless survive when supplemented with exogenous IPP (38).
Small-molecule inhibitors that target apicoplast replication often result in a delayed-death phenotype in P. falciparum, in which drug-treated parasites complete the first cell cycle after treatment and arrest in the second (41). In contrast, fosmidomycin treatment inhibits intraerythrocytic growth of P. falciparum during the first cell cycle. Interestingly, fosmidomycin-treated parasites develop within the red blood cell, begin hemoglobin digestion, and initiate DNA replication prior to cell cycle arrest as multinucleate schizonts (39). The requirement of the new permeability pathways mentioned above for fosmidomycin import into P. falciparum cells may explain this delayed action, as these pathways are not fully developed until the trophozoite stage (31, 32). Liver-stage parasites are also sensitive to fosmidomycin. Treatment of liver-stage Plasmodium berghei inhibits the development of the apicoplast and reduces the number of merosomes, the result of liver-stage replication. Thus, the MEP pathway appears to be required for optimal growth in hepatocytes (29). Little is known about isoprenoid synthesis in the gametocyte and mosquito stages of the parasite life cycle, although proteomics studies have identified MEP pathway enzymes expressed in late gametocytes (42).
Below, we detail the isoprenoid products downstream from IPP (Fig. 2C; Table 1) and what is known about their production and/or function in P. falciparum.
TABLE 1.
Classes of isoprenoid products in P. falciparum
| Isoprenoid class (no. of carbons) | Present in parasite? | Role(s) in asexual stage | Enzyme(s) (PlasmoDB ID[s]) | Notable reference(s) |
|---|---|---|---|---|
| IPP, DMAPP (5) | Yes | tRNA isopentenylation | tRNA isopentenyltransferase (PF3D7_1207600), isopentenyl-adenosine tRNA methylthiolase (PF3D7_0622200) | |
| Monoterpenes (10) | No | |||
| Sesquiterpenes, diterpenes (15 and 20) | Yes | Protein prenylation, vitamin E synthesis | FPP-GGPP synthase (PF3D7_1128400), farnesyl transferase (PF3D7_1242600 [α subunit] and PF3D7_1147500 [β subunit]), geranylgeranyl transferase type I (PF3D7_1242600 [α subunit] and PF3D7_0602500 [β subunit]), geranylgeranyl transferase type II (PF3D7_1442500 [α subunit] and PF3D7_1214300 [β subunit]), REP/GDI superfamily members (PF3D7_1242800 and PF3D7_1038100) | 35, 49, 63, 64, 68, 78 |
| Sterols (30) | Yes, but taken from host | Membrane stability | 86, 87, 89, 90 | |
| Carotenoids (40) | Yes | Unknown, possibly response to oxidative stress | Phytoene synthase (PF3D7_0202700) | 104 |
| Ubiquinone (40 and 45) | Yes | Electron acceptor in pyrimidine synthesis | Dihydroorotate dehydrogenase (PF3D7_0603300), octaprenyl pyrophosphate synthase (PF3D7_0202700), 4-hydroxybenzoate octaprenyltransferase (PF3D7_0607500) | 104, 113, 117, 120 |
| Dolichols (55 and 60) | Yes | Protein modifications: dolichylation, GPI anchors, O-and N-linked glycosylation | Polyprenol reductase (PF3D7_1455900), dolichyldiphosphatase (PF3D7_0805600), GPI1 (PF3D7_0618900), dolichol phosphate mannose synthase (PF3D7_1141600) | 123, 124, 125, 130, 131, 134, 135 |
5-CARBON ISOPRENOIDS: DMAPP AND IPP
The most proximally produced compounds of the MEP pathway are the end products and isoprenoid building blocks, isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMAPP). In bacteria, DMAPP is used as a substrate for tRNA isopentenylation (43). In this process, an isopentenyl group is added to an adenosine in the tRNA, targeting the tRNA to the ribosome and improving translation fidelity. Evidence suggests that P. falciparum produces isopentenylated tRNAs, as its apicoplast genome encodes four tRNAs that represent probable candidates for isopentenylation (44). P. falciparum encodes a homologue of the Escherichia coli tRNA isopentenyltransferase MiaA (EC 2.5.1.75, PlasmoDB ID PF3D7_1207600) (44). P. falciparum also possesses a homolog of the isopentenyl-adenosine tRNA methylthiolase MiaB (EC 2.8.4.-, PlasmoDB ID PF3D7_0622200), an enzyme whose bacterial homologs participate in downstream tRNA isopentenylation steps (44, 45).
As previously described, supplementation of medium with farnesol or geranylgeraniol rescues fosmidomycin treatment of malaria parasites. These isoprenols are presumed to be phosphorylated intracellularly by nonspecific kinases to generate their cognate diphosphates, farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). Fosmidomycin-treated parasites supplemented with farnesol (15-carbon) or geranylgeraniol (20-carbon) therefore do not have a known source of 5- or 10-carbon isoprenoids but are still capable of intraerythrocytic growth (35). These studies cannot rule out a role for tRNA isopentenylation in maintaining apicoplast function, but they do suggest there may not be additional roles for tRNA isopentenylation outside the maintenance of isoprenoid biosynthesis within the apicoplast.
10-, 15-, AND 20-CARBON ISOPRENOIDS
MONOTERPENES
Condensation of two isoprene units produces the 10-carbon isoprenoid geranyl pyrophosphate (GPP). GPP is utilized by monoterpene synthases and monoterpene cyclases to produce 10-carbon monoterpenes. Monoterpenes are the most abundant compounds found in plant essential oils. Terpene mixtures such as citronellal (citronella) and citral (lemon) are produced by plants to deter herbivores (46). Some monoterpenes have been found to have antimicrobial properties; thymol, a monoterpene component of the essential oil in thyme, has been shown to decrease counts of Salmonella enterica serovar Typhimurium and Staphylococcus aureus (47). To date, no studies have identified evidence of monoterpene synthesis in P. falciparum. Homology-based searches do not identify potential monoterpene synthases or monoterpene cyclases in the P. falciparum genome, although this class of enzymes is remarkably diverse (48).
SESQUITERPENES AND DITERPENES
FPP and GGPP are also used to produce sesquiterpenes and diterpenes. In P. falciparum, the condensation of GPP and IPP (to produce FPP) and of FPP and IPP (to produce geranylgeranyl diphosphate [GGPP]) appears to be catalyzed by a bifunctional FPP-GGPP synthase (PlasmoDB ID PF3D7_1128400) (49). The crystal structure of the P. vivax enzyme has been solved (50, 51). This bifunctional enzyme is sensitive to bisphosphonates, such as zoledronate and risedronate (49). These compounds bind to bone minerals and are traditionally used to inhibit bone resorption in the treatment of diseases like osteoporosis (52). Metabolic-labeling studies using [14C]mevalonate, [14C]IPP, and [14C]DMAPP validate FPP synthesis as the target of bisphosphonate inhibition (53). Bisphosphonates, as well as their analogs, have been shown to bind FPP-GGPP synthase in the active site of both the human and parasite enzymes (51). Bisphosphonates compete with GPP for binding, and their efficacy is enhanced by IPP stabilization of the enzyme inhibitor complex (54, 55). Treatment with bisphosphonates inhibits parasite growth and decreases protein prenylation (50, 51, 56–58). Partial rescue of parasite growth can be achieved by the addition of FPP or GGPP to the culture medium, indicating that bisphosphonates target FPP and GGPP synthesis in P. falciparum (56).
P. falciparum utilizes sesquiterpenes and diterpenes for protein prenylation. In protein prenylation, lipophilic farnesyl (15-carbon) and geranylgeranyl groups (20-carbon) are attached to C-terminal cysteines, which results in protein association with membranes. Prenylation is crucial for the function of a variety of membrane-bound enzymes, such as the Ras, Rho, and Rab families of small GTPases. Farnesyl transferase (EC 2.5.1.58, PlasmoDB IDs PF3D7_1242600 [α subunit] and PF3D7_1147500 [β subunit]) and geranylgeranyl transferase type I (EC 2.5.1.59, PlasmoDB IDs PF3D7_1242600 [α subunit] and PF3D7_0602500 [β subunit]) transfer FPP and GGPP moieties to the target protein via recognition of a C-terminal CaaX motif (59). This motif is composed of a cysteine (C), two aliphatic amino acids (aa), and the C-terminal amino acid (X). These two prenyltransferases share an α subunit but have distinct β subunits (60). Geranylgeranyl transferase type II (EC 2.5.1.60, Rab geranylgeranyltransferase, PlasmoDB IDs PF3D7_1442500 [α subunit] and PF3D7_1214300 [β subunit]) utilizes a different mechanism of substrate recognition, which requires a Rab escort protein (REP) (61). The REP binds Rab proteins, facilitates their prenylation, and delivers them to their target membrane. REPs are part of the REP/GDI superfamily, which also includes GDP dissociation inhibitor (GDI) proteins. GDI proteins are involved in cycling the Rab between membranes and the cytosol (61). Two members of the REP/GDI superfamily are found in the P. falciparum genome (PlasmoDB IDs PF3D7_1242800 and PF3D7_1038100) (62). Further studies are required to determine if these proteins function as REPs or GDIs.
Protein prenylation appears to be an essential use of isoprenoids in P. falciparum, as the parasite is sensitive to chemical inhibition of protein prenylation. Prenyltransferase inhibitors, major candidates for anticancer therapy, have shown potent antimalarial activity (63–65). These include a number of peptidomimetics of the CaaX motif (63, 66, 67). Additionally, certain monoterpenes have been shown to inhibit the growth of P. falciparum via inhibition of prenylation (40, 68). Limonene has been shown to inhibit the prenylation of 21- to 26-kDa proteins in mammalian cell culture and in vitro (69–71). P. falciparum parasites treated with limonene are unable to progress from the ring to the trophozoite stage (68).
Feeding P. falciparum with labeled [3H]FPP and [3H]GPP identifies 21- to 24-kDa and 50-kDa prenylation target proteins that are differentially labeled by FPP and GPP (68). Overall, it appears that the majority of prenylated proteins are small and preferentially geranylgeranylated in vivo, with the exception of a single 50-kDa protein (63). Bioinformatic methods have been used to compile a list of predicted prenylation targets in P. falciparum (60). These include a number of GTP-binding proteins, such as Rab2 and Rab11a.
A number of studies have identified specific prenylation targets in P. falciparum. The localization of the small GTPase Rab7 to endosomal vesicles was shown to be prenylation dependent. These vesicles are predicted to participate in endosomal trafficking (72). The SNARE protein Ykt6.1 of P. falciparum (PfYkt6.1) has been shown to be a farnesyltransferase substrate in vitro, and its localization is disrupted when lacking a CaaX motif, suggesting it is also a prenylation target in vivo (73). Similarly, the P. falciparum tyrosine phosphatase PfPRL is a substrate for farnesylation in vitro (74).
Inhibition of isoprenoid synthesis by fosmidomycin produces prenylation phenotypes in P. falciparum. Similar to metabolic labeling, probing for prenylation using a prenylation-specific antibody identifies proteins of 21 to 24 kDa and 50 kDa (39, 68). Prenylation of these targets is reduced in fosmidomycin-treated parasites, confirming that prenyl groups are indeed products of the MEP pathway. The P. falciparum geranylgeranyltransferase substrates PfRab5a and PfRab5c mislocalize from hemoglobin-containing vesicles to the host cell membrane upon treatment with fosmidomycin, and this mislocalization correlates with changes to food vacuole morphology and integrity. Proper localization is restored by geranylgeraniol supplementation (39). Geranylgeraniol supplementation also substantially rescues growth inhibition by fosmidomycin, suggesting that geranylgeranylation may be the only essential form of protein prenylation in P. falciparum (35, 38).
The group of compounds collectively known as vitamin E (tocopherols and tocotrienols) function as antioxidants and membrane stabilizers (75–77). Recently, a metabolic labeling study using [3H]FPP and [3H]GPP identified de novo vitamin E synthesis by P. falciparum. Parasite growth is sensitive to usnic acid, an inhibitor of vitamin E biosynthesis. Growth is partially rescued by α-tocopherol, indicating that vitamin E synthesis is essential in malaria parasites (78).
In plants, the homogentisic acid head group of vitamin E is synthesized via the shikimate pathway. This head group is then prenylated with phytyl diphosphate (20-carbon) or geranylgeranyl diphosphate to generate 2-methyl-6-phytyl-1,4-benzoquinol, the first committed intermediate for the synthesis of tocopherols and tocotrienols. This prenylation is catalyzed by homogentisate prenyltransferases (EC 2.5.1.115 and EC 2.5.1.116) (79). As no obvious homogentisate prenyltransferase homologs exist in the P. falciparum genome, further work will be required to understand the mechanism by which the parasite synthesizes vitamin E.
STEROLS
Sterols are 30-carbon isoprenoids that are ubiquitous among eukaryotes and are utilized for a variety of cellular functions. In particular, cholesterol is essential for membrane architecture in eukaryotes, and its production is tightly regulated (80, 81). Cholesterol is also a precursor for signaling molecules, such as sex steroids and mineralocorticoids in mammals and brassinosteroids in plants. Squalene synthase (EC 2.5.1.21) commits the isoprenoid pathway to sterol biosynthesis by converting two molecules of FPP to squalene; squalene then serves as the backbone for subsequent modifications (82).
The animal host synthesizes cholesterol de novo and is also able to import it from dietary sources (83). Radioactive labeling experiments show no evidence for cholesterol biosynthesis in P. falciparum (84, 85). Homology searches do not identify a squalene synthase in the P. falciparum genome. Instead, Plasmodium spp. appear to obtain cholesterol from the host cell. P. knowlesi was shown to import host-derived 14C-labeled cholesterol, and cellular uptake by the host cell itself was also increased upon infection with malaria parasites (86–88). P. falciparum import of cholesterol has been studied within hepatocytes, a site of high cholesterol synthesis and parasite replication early in infection. Inhibition of host cell isoprenoid synthesis decreases sterol levels in the liver-stage parasite (89). While cholesterol is essential for the maintenance of parasite membrane stability (90), these studies suggest that cholesterol synthesis does not occur in P. falciparum and is therefore not an essential function of de novo isoprenoid synthesis by the parasite.
CAROTENOIDS
Carotenoids are 40-carbon isoprenoids derived from the condensation of two GGPP molecules by phytoene synthase (EC 2.5.1.32, PlasmoDB ID PF3D7_0202700). Carotenoids are synthesized by plants and algae, as well as some bacteria and fungi. In plants, algae, and photosynthetic bacteria, carotenoids like carotene, lycopene, xanthophyll, and lutein function in photosynthesis and protect against free radical damage (91–95). In plants, carotenoid synthesis occurs in the chloroplast (96, 97). Fungi also utilize carotenoid pigments for protection against free radicals (98, 99). In animals, which cannot synthesize carotenoids, dietary carotenoids are used for the synthesis of vitamin A (91). An exception is found in insects that have acquired carotenoid synthesis from fungi through lateral gene transfer (100, 101). Commercial synthesis of carotenoids is of interest for their use as nutraceuticals, dietary supplements, and pigments (102, 103).
Carotenoids have recently been detected in the intraerythrocytic stages of P. falciparum; schizonts contain the highest concentrations, indicating that carotenoid synthesis begins in the ring stage and builds during the schizont stage (104). Geranyl pyrophosphate serves as a substrate for carotenoid synthesis by phytoene synthase (EC 2.5.1.32, PlasmoDB ID PF3D7_0202700). Phytoene is then converted to carotenoid products by phytoene desaturase (EC 1.3.99.30, locus unknown). P. falciparum is sensitive to the small molecular herbicide norflurazon, which inhibits phytoene desaturase. Norflurazon treatment causes an accumulation of phytoene and a decrease in carotenoid content. Inhibition by norflurazon can be partially rescued with lycopene (104). While carotenoids serve important functions in plants, algae, bacteria, and fungi, it is not yet known what physiological role they play in Plasmodium. As in other organisms, they may play a role in the cellular response to oxidative stress.
In plants, the phytohormone abscisic acid is also produced from carotenoid intermediates (105). The Plasmodium relative and apicomplexan parasite, Toxoplasma gondii, has been shown to produce abscisic acid to control calcium signaling for processes like protein secretion and parasite egress. The abscisic acid response genes identified in T. gondii are conserved in P. falciparum, but it is not known whether P. falciparum also synthesizes this isoprenoid product. The route for abscisic acid synthesis from isoprenoid precursors in apicomplexan parasites remains unknown, as no clear biosynthetic route is readily identified bioinformatically (106).
COENZYME Q (UBIQUINONE)
In most eukaryotes, mitochondria are the site of energy generation through oxidative phosphorylation. Within the mitochrondrial matrix, the tricarboxylic acid cycle uses 2-carbon metabolites generated from the breakdown of glucose, amino acids, and fatty acids to produce high-energy electron carriers. In the inner mitochondrial membrane, the electron transport chain uses high-energy electrons to harness energy in the form of ATP. In the mitochondria of asexual Plasmodium parasites, however, the electron transport chain is not a primary source of ATP and the parasite instead relies on glycolysis for most of its ATP production (107). Indeed, little of the parasitic glucose supply is completely oxidized, and glucose is instead excreted as lactic acid (108, 109). Additionally, the parasites show relatively little oxygen consumption, consistent with minimal respiration (110). However, ATP generation by the electron transport chain may be essential for parasite stages within the mosquito host, where extracellular glucose levels are lower and the parasite cannot rely solely on glycolysis for ATP production (111, 112).
In asexual-stage parasites, the electron transport chain operates to provide a continuous supply of reduced coenzyme Q. Coenzyme Q, or ubiquinone, typically functions as an electron acceptor in the electron transport chain. It is maintained in asexual Plasmodium parasites as an electron acceptor for dihydroorotate dehydrogenase (DHODH) (EC 1.3.98.1, PlasmoDB ID PF3D7_0603300), an enzyme required for pyrimidine synthesis. DHODH is essential for survival, as the parasite is incapable of pyrimidine salvage, and small molecules targeting DHODH have potent antimalarial activity (39, 113–116). Ubiquinone levels have been shown to peak at the beginning of schizogony and are sensitive to fosmidomycin treatment (36).
Synthesis of coenzyme Q requires the addition of an isoprenyl side chain to a benzoquinone ring. The parasite possesses an octaprenyl pyrophosphate synthase (EC 2.5.1.90, PlasmoDB ID PF3D7_0202700) that is capable of synthesizing these side chains. This multifunctional enzyme produces 40-carbon, 45-carbon, and 55-carbon isoprenoid products and has been shown to also have phytoene synthase activity (104, 117). The addition of these isoprenoids to 4-hydroxybenzoate is performed by 4-hydroxybenzoate octaprenyltransferase (EC 2.5.1.39, PlasmoDB ID PF3D7_0607500) (118, 119). Labeling studies in P. falciparum identify coenzyme Q isoforms coenzyme Q8 and Q9, which have 8 and 9 isoprene units (40-carbon and 45-carbon), respectively, in their side chains (120, 121). Incorporation of labeled FPP results in the detection of coenzyme Q8, and incorporation of labeled GGPP detects coenzyme Q9 (120). In another study using labeled p-hydrobenzoic acid, coenzyme Q8 was found to be the dominant form of coenzyme Q (121).
Nerolidol, a sesquiterpene alcohol, was found to inhibit the synthesis of the isoprenyl side chain destined for coenzyme Q, likely because of its structural similarity to FPP. Treatment with nerolidol inhibits the intraerythrocytic development of P. falciparum (117, 120).
DOLICHOLS
Dolichols are long-chain hydrocarbon compounds made of various numbers of isoprene units. In the form of dolichyl phosphate or pyrophosphate, dolichols are essential for the transfer of sugars onto proteins, i.e., dolichylation, O-linked glycosylation, N-linked glycosylation, and the production of glycophosphatidylinositol (GPI) anchors, which are essential for successful infection (122).
Multiple studies have demonstrated the presence of dolichols and their intermediates in P. falciparum, specifically, those composed of 11 and 12 isoprene units (55 and 60 carbons). Labeling experiments demonstrate that these dolichols are formed from FPP and GPP, respectively (123, 124). As expected, dolichol synthesis is also sensitive to fosmidomycin treatment (37).
Dolichyl pyrophosphate is produced from IPP by a polyprenol reductase (EC 1.3.1.94) and from GGPP via a dehydrodolichol pyrophosphate intermediate. Dolichyl pyrophosphate is then converted to dolichyl phosphate by dolichyldiphosphatase (EC 3.6.1.43). Dolichyl phosphate is utilized for glycosylation and synthesis of GPI anchors. P. falciparum possesses homologs of both polyprenol reductase (PlasmoDB ID PF3D7_1455900) and dolichyldiphosphatase (PlasmoDB ID PF3D7_0805600).
Posttranslational addition of dolichols to proteins has been demonstrated in P. falciparum. Labeling using [3H]FPP and [3H]GGPP identified a dolichol with 11 isoprene units attached to 21- to 28-kDa protein(s). The target proteins and enzyme(s) responsible for dolichylation of proteins in P. falciparum remain unknown (124).
P. falciparum synthesizes GPI anchors for protein modification (125). The parasite is sensitive to known inhibitors of GPI synthesis, such as the mannose analogue 2-deoxyglucose (126, 127). Studies have identified a number of parasite proteins as targets for GPI addition, including merozoite surface antigens, a serine protease, and a heat shock 70 protein (128, 129). Merozoite surface antigens appear to be the primary targets of GPI anchor addition and are of great interest as antigens for vaccine development (130). The enzyme GPI1 (EC 2.4.1.198, PlasmoDB ID PF3D7_0618900) transfers N-acetylglucosamine to phosphatidylinositol in GPI biosynthesis. The P. falciparum GPI1 homolog was shown to complement a yeast gpi1 mutant, confirming its function (131). Dolichol phosphate mannose synthase (EC 2.4.1.83, PlasmoDB ID PF3D7_1141600) catalyzes the addition of sugar moieties in both GPI anchor glycosylations and N-linked glycosylations. The P. falciparum enzyme has been shown to be of a novel clade distinct from animal or yeast synthases of the same type (132).
While GPI anchors appear to constitute most of the glycosylation in P. falciparum, there is also evidence for O- and N-linked glycosylation of proteins (133–135). The presence of N-linked glycosylation has long been debated (136, 137). However, the parasite is sensitive to tunicamycin, an inhibitor of N-linked glycosylation (135), and parasite proteins have been shown to be capable substrates of N-linked glycosylation when expressed in heterologous systems (138).
REGULATION OF ISOPRENOID SYNTHESIS
MEP pathway regulation has been thoroughly studied in plants, which utilize a large variety of isoprenoids for signaling and environmental interactions (139–141). However, little is known about regulation of the MEP pathway in P. falciparum. Given the variety and essentiality of isoprenoid products in P. falciparum, it is likely that isoprenoid synthesis by the MEP pathway is regulated to control the product availability of IPP and DMAPP. Two ATPs and 3 NADPHs are consumed for the production of each IPP molecule from glucose (142). The cell likely regulates this pathway to optimize energy consumption. A better understanding of MEP pathway regulation in the parasite will facilitate new strategies to inhibit the MEP pathway and may contribute an improved understanding of MEP pathway regulation in other plastid-possessing eukaryotic pathogens.
Regulation of the MEP pathway may operate at the level of gene expression, protein activity, or metabolite availability. Studies in Arabidopsis thaliana have identified an RNA processing protein, Rif10, which posttranscriptionally regulates the levels of MEP pathway enzymes (143). However, studies in P. falciparum have indicated that the transcript levels of MEP pathway enzymes do not change significantly upon chemical inhibition of the MEP pathway, suggesting that modulating transcript levels is likely not a primary mechanism of MEP pathway regulation in the parasite (37). In the asexual intraerythrocytic cycle, the transcript levels of MEP pathway genes peak in the late trophozoite stage (144). It is possible that the expression levels of MEP pathway genes may respond to other environmental and cellular cues, but other perturbation studies have yet to uncover such regulation (145).
There is evidence for regulation of the MEP pathway by MEP pathway metabolites. A recent study identified a feed-forward mechanism of MEP pathway regulation in E. coli. The MEP pathway enzyme 2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase (EC 4.6.1.12, PlasmoDB ID PF3D7_0209300) is activated by the upstream intermediate MEP (146). Additionally, IPP and DMAPP have been shown to cause feedback inhibition of the rate-limiting enzyme DXS in Populus trichocarpa (147). Further study is required to determine whether similar regulatory mechanisms exist in P. falciparum.
A recent study has identified a novel regulator of the MEP pathway in P. falciparum. Its HAD1 (PfHAD1) (PlasmoDB ID PF3D7_1033400), a sugar phosphatase member of the haloacid dehalogenase-like hydrolase (HAD) superfamily, was shown to be a negative regulator that acts upstream from the MEP pathway. Loss of PfHAD1 results in resistance to fosmidomycin and increased levels of MEP pathway metabolites, primarily DOXP. PfHAD1 appears to utilize cellular sugar phosphates upstream from the MEP pathway. PfHAD1 is predicted to restrict the availability of precursors to the apicoplast-localized MEP pathway (148).
CONCLUSION
While gaps remain in our understanding of isoprenoid biology in P. falciparum, it is clear that isoprenoids are essential and diverse in the malaria parasite. The efficacy of fosmidomycin and the recent screening for apicoplast inhibitors demonstrates that apicoplast biology and, specifically, isoprenoid synthesis are promising, druggable targets for the development of new antimalarials (149). Therefore, developing a complete understanding of the biology and regulation of the MEP pathway should be a priority for the field. This may identify additional drug targets and will inform future antimalarial development. For example, targeting MEP pathway regulation may prove synergistic in combination therapies with direct MEP pathway enzyme inhibitors, such as fosmidomycin.
The discovery of PfHAD1 as a negative regulator of isoprenoid precursor synthesis has begun to expand our understanding of the regulation of this essential pathway. Whether PfHAD1 itself will be a useful antimalarial target will depend on whether PfHAD1 is required for parasite growth during human infection, which remains unknown. Parasite strains selected for fosmidomycin resistance lose PfHAD1 function, and this loss is necessary for resistance. However, the loss of PfHAD1 is not the only genetic change found in these strains, and other changes may be required for parasites to tolerate the loss of PfHAD1 (148). Alternatively, PfHAD1 function may be dispensable under laboratory culture conditions but necessary for development in vivo. The strong sequence conservation of PfHAD1 in P. falciparum field isolates suggests that it likely plays an important role in the cell, but further studies are required to demonstrate whether this is the case. Inactivating alleles of PfHAD1 were readily obtained during in vitro culture with fosmidomycin. If PfHAD1 is not essential during natural infection, this locus may represent an important biomarker for clinical resistance to fosmidomycin, its analogs, or other MEP pathway inhibitors as they are developed.
PfHAD1 belongs to the Cof-like hydrolase subfamily (InterPro accession number IPR000150) of the haloacid dehalogenase-like hydrolase (HAD) superfamily (150). Two additional members of this subfamily exist in the P. falciparum genome, PfHAD2 (PlasmoDB ID PF3D7_1226300) and PfHAD3 (PlasmoDB ID PF3D7_1226100), whose functions are unknown. PfHAD2 and PfHAD3 protein sequences possess 25 to 30% identity and approximately 50% similarity with PfHAD1, which is typical for enzymes within this subfamily. Sequence homology predicts that, like PfHAD1, they also utilize sugar phosphates as substrates. Ongoing studies are needed to evaluate whether these PfHAD1 homologs also function as regulators of isoprenoid synthesis or regulators of other essential biosynthetic processes in P. falciparum.
The malaria parasite, Plasmodium falciparum, is relatively slow growing and difficult to genetically manipulate. Classical genetics in this organism have been hampered by the regulatory and ethical challenges raised by the need for primate infection. The rise in modern next-generation sequencing technologies has been an enormous technical advance in genetic studies of nonmodel organisms, including P. falciparum. The presence of PfHAD1 homologs in other organisms that utilize the MEP pathway, such as bacteria and plants, demonstrates that P. falciparum can be a useful model eukaryote for study of the isoprenoid metabolism. Since PfHAD1 homologs are closely conserved in organisms containing the MEP pathway (eubacteria, algae, and plants) but are absent in organisms that do not utilize the MEP pathway (archaea, fungi, and animals), HAD homologs may be regulators of the MEP pathway in multiple systems (148). Perhaps sugar phosphatase members of the HAD superfamily coevolved alongside the energetically expensive MEP pathway, in order to regulate substrate availability and control energy consumption when downstream products are not required.
Other MEP pathway-utilizing and HAD-containing organisms are used to produce commercially important isoprenoids. These products include pharmaceuticals (such as paclitaxel and artemisinin), food additives (such as lycopene), fragrances, and biofuel precursors (151–155). Because complex isoprenoids are challenging to synthesize chemically, there is great interest in increasing and optimizing bioproduction of these compounds. Antimalarial drug development is naturally of critical importance in the malaria field. However, as we advance our understanding of isoprenoid biology and regulation in malaria parasites, we may also gain insight into fundamental aspects of MEP pathway biology that will have impacts on the diverse disciplines touched by this ancient cellular process.
ACKNOWLEDGMENTS
We thank Rachel L. Edwards and Wandy Beatty (Molecular Microbiology Imaging Facility, Washington University) for providing electron micrographs. We thank Aakash Y. Gandhi and Chad Schaber for critical reading of the manuscript.
This work was supported by grant MD-LI-2011-171 from the Children's Discovery Institute of Washington University and St. Louis Children's Hospital, grant R01AI103280 from the NIH/NIAID, a Basil O'Connor Starter Scholar Research Award from the March of Dimes, and a Clinical Scientist Development award from the Doris Duke Charitable Foundation. A.M.G. is supported by training grant 5T32GM007067 from the NIGMS and a Monsanto Excellence Fund Graduate Fellowship.
The funders had no role in the decision to publish or preparation of the manuscript.
Footnotes
Published ahead of print 12 September 2014
REFERENCES
- 1.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. 2005. WHO estimates of the causes of death in children. Lancet 365:1147–1152. 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 2.Sachs J, Malaney P. 2002. The economic and social burden of malaria. Nature 415:680–685. 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 3.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat DWN. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samarasekera U. 2009. Countries race to contain resistance to key antimalarial. Lancet 374:277–280. 10.1016/S0140-6736(09)61349-0. [DOI] [PubMed] [Google Scholar]
- 5.Hyde JE. 2005. Drug-resistant malaria. Trends Parasitol. 21:494–498. 10.1016/j.pt.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genton B, D'Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Müller I. 2008. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 5:e127. 10.1371/journal.pmed.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN. 2008. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 5:e128. 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.William T, Menon J, Rajahram G, Chan L, Ma G, Donaldson S, Khoo S, Fredrick C, Jelip J, Anstey N, Yeo TW. 2011. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg. Infect. Dis. 17:1248–1255. 10.3201/eid1707.101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox-Singh J, Hiu J, Lucas SB, Divis PC, Zulkarnaen M, Chandran P, Wong KT, Adem P, Zaki SR, Singh B, Krishna S. 2010. Severe malaria–a case of fatal Plasmodium knowlesi infection with post-mortem findings: a case report. Malar. J. 9:10. 10.1186/1475-2875-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Köhler S, Delwiche CF, Denny PW, Tilney LG, Webster P, Wilson RJ, Palmer JD, Roos DS. 1997. A plastid of probable green algal origin in apicomplexan parasites. Science 275:1485–1489. 10.1126/science.275.5305.1485. [DOI] [PubMed] [Google Scholar]
- 11.Janouskovec J, Horák A, Oborník M, Lukes J, Keeling PJ. 2010. A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc. Natl. Acad. Sci. U. S. A. 107:10949–10954. 10.1073/pnas.1003335107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore RB, Oborník M, Janouskovec J, Chrudimský T, Vancová M, Green DH, Wright SW, Davies NW, Bolch CJS, Heimann K, Slapeta J, Hoegh-Guldberg O, Logsdon JM, Carter DA. 2008. A photosynthetic alveolate closely related to apicomplexan parasites. Nature 451:959–963. 10.1038/nature06635. [DOI] [PubMed] [Google Scholar]
- 13.Gershenzon J, Dudareva N. 2007. The function of terpene natural products in the natural world. Nat. Chem. Biol. 3:408–414. 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 14.Tobert JA. 2003. Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2:517–526. 10.1038/nrd1112. [DOI] [PubMed] [Google Scholar]
- 15.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. 1993. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem. J. 295(Pt 2):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuzuyama T. 2002. Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci. Biotechnol. Biochem. 66:1619–1627. 10.1271/bbb.66.1619. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi S, Kuzuyama T, Watanabe H, Seto H. 1998. A 1-deoxy-d-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-d-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 95:9879–9884. 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichtenthaler HK. 1999. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50:47–65. 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- 19.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. 1971. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 93:2325–2327. 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 20.Tu Y. 2011. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 17:1217–1220. 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- 21.Holstein SA, Hohl RJ. 2004. Isoprenoids: remarkable diversity of form and function. Lipids 39:293–309. 10.1007/s11745-004-1233-3. [DOI] [PubMed] [Google Scholar]
- 22.Eisenreich W, Bacher A, Arigoni D, Rohdich F. 2004. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell. Mol. Life Sci. 61:1401–1426. 10.1007/s00018-004-3381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botté CY, Dubar F, McFadden GI, Maréchal E, Biot C. 2012. Plasmodium falciparum apicoplast drugs: targets or off-targets? Chem. Rev. 112:1269–1283. 10.1021/cr200258w. [DOI] [PubMed] [Google Scholar]
- 24.Qidwai T, Jamal F, Khan MY, Sharma B. 2014. Exploring drug targets in isoprenoid biosynthetic pathway for Plasmodium falciparum. Biochem. Res. Int. 2014:657189. 10.1155/2014/657189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Okuhara M, Kuroda Y, Goto T, Okamoto M, Terano H, Kohsaka M, Aoki H, Imanaka H. 1980. Studies on new phosphonic acid antibiotics: isolation and characterization of FR-31564, FR-32863 and FR-33289. J. Antibiot. (Tokyo) 33:24–28. 10.7164/antibiotics.33.24. [DOI] [PubMed] [Google Scholar]
- 26.Kuzuyama T, Shimizu T, Takahashi S, Seto H. 1998. Fosmidomycin, a specific inhibitor of 1-deoxy-d-xylulose 5-phosphate reductoisomerase in the nonmevalonate pathway for terpenoid biosynthesis. Tetrahedron Lett. 39:7913–7916. 10.1016/S0040-4039(98)01755-9. [DOI] [Google Scholar]
- 27.Koppisch AT, Fox DT, Blagg BSJ, Poulter CD. 2002. E. coli MEP synthase: steady-state kinetic analysis and substrate binding. Biochemistry 41:236–243. 10.1021/bi0118207. [DOI] [PubMed] [Google Scholar]
- 28.Steinbacher S, Kaiser J, Eisenreich W, Huber R, Bacher A, Rohdich F. 2003. Structural basis of fosmidomycin action revealed by the complex with 2-C-methyl-d-erythritol 4-phosphate synthase (IspC). Implications for the catalytic mechanism and anti-malaria drug development. J. Biol. Chem. 278:18401–18407. 10.1074/jbc.M300993200. [DOI] [PubMed] [Google Scholar]
- 29.Nair SC, Brooks CF, Goodman CD, Sturm A, McFadden GI, Sundriyal S, Anglin JL, Song Y, Moreno SNJ, Striepen B. 2011. Apicoplast isoprenoid precursor synthesis and the molecular basis of fosmidomycin resistance in Toxoplasma gondii. J. Exp. Med. 208:1547–1559. 10.1084/jem.20110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AC, Parish T. 2008. Dxr is essential in Mycobacterium tuberculosis and fosmidomycin resistance is due to a lack of uptake. BMC Microbiol. 8:78. 10.1186/1471-2180-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elford BC, Haynes JD, Chulay JD, Wilson RJM. 1985. Selective stage-specific changes in the permeability to small hydrophilic solutes of human erythrocytes infected with Plasmodium falciparum. Mol. Biochem. Parasitol. 16:43–60. 10.1016/0166-6851(85)90048-9. [DOI] [PubMed] [Google Scholar]
- 32.Ginsburg H, Krugliak M, Eidelman O, Ioav Cabantchik Z. 1983. New permeability pathways induced in membranes of Plasmodium falciparum infected erythrocytes. Mol. Biochem. Parasitol. 8:177–190. 10.1016/0166-6851(83)90008-7. [DOI] [PubMed] [Google Scholar]
- 33.Desai SA, Bezrukov SM, Zimmerberg J. 2000. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 406:1001–1005. 10.1038/35023000. [DOI] [PubMed] [Google Scholar]
- 34.Baumeister S, Wiesner J, Reichenberg A, Hintz M, Bietz S, Harb OS, Roos DS, Kordes M, Friesen J, Matuschewski K, Lingelbach K, Jomaa H, Seeber F. 2011. Fosmidomycin uptake into Plasmodium and Babesia-infected erythrocytes is facilitated by parasite-induced new permeability pathways. PLoS One 6:e19334. 10.1371/journal.pone.0019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang B, Watts KM, Hodge D, Kemp LM, Hunstad DA, Hicks LM, Odom AR. 2011. A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry 50:3570–3577. 10.1021/bi200113y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassera MB, Gozzo FC, D'Alexandri FL, Merino EF, del Portillo HA, Peres VJ, Almeida IC, Eberlin MN, Wunderlich G, Wiesner J, Jomaa H, Kimura EA, Katzin AM. 2004. The methylerythritol phosphate pathway is functionally active in all intraerythrocytic stages of Plasmodium falciparum. J. Biol. Chem. 279:51749–51759. 10.1074/jbc.M408360200. [DOI] [PubMed] [Google Scholar]
- 37.Cassera MB, Merino EF, Peres VJ, Kimura EA, Wunderlich G, Katzin AM. 2007. Effect of fosmidomycin on metabolic and transcript profiles of the methylerythritol phosphate pathway in Plasmodium falciparum. Mem. Inst. Oswaldo Cruz 102:377–384. 10.1590/S0074-02762007000300019. [DOI] [PubMed] [Google Scholar]
- 38.Yeh E, DeRisi JL. 2011. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 9:e1001138. 10.1371/journal.pbio.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howe R, Kelly M, Jimah J, Hodge D, Odom AR. 2013. Isoprenoid biosynthesis inhibition disrupts Rab5 localization and food vacuolar integrity in Plasmodium falciparum. Eukaryot. Cell 12:215–223. 10.1128/EC.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodrigues Goulart H, Kimura EA, Peres VJ, Couto AS, Aquino Duarte FA, Katzin AM. 2004. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob. Agents Chemother. 48:2502–2509. 10.1128/AAC.48.7.2502-2509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ. 2006. Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrob. Agents Chemother. 50:3124–3131. 10.1128/AAC.00394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, Younis Younis S, Sauerwein R, Alano P. 2010. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol. Cell. Proteomics 9:1437–1448. 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung H-CE, Chen Y, Winkler ME. 1997. Regulation of substrate recognition by the MiaA tRNA prenyltransferase modification enzyme of Escherichia coli K-12. J. Biol. Chem. 272:13073–13083. 10.1074/jbc.272.20.13073. [DOI] [PubMed] [Google Scholar]
- 44.Ralph SA, van Dooren GG, Waller RF, Crawford MJ, Fraunholz MJ, Foth BJ, Tonkin CJ, Roos DS, McFadden GI. 2004. Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Microbiol. 2:203–216. 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 45.Esberg B, Leung HC, Tsui HC, Björk GR, Winkler ME. 1999. Identification of the miaB gene, involved in methylthiolation of isopentenylated A37 derivatives in the tRNA of Salmonella typhimurium and Escherichia coli. J. Bacteriol. 181:7256–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loza-Tavera H. 1999. Monoterpenes in essential oils. Biosynthesis and properties. Adv. Exp. Med. Biol. 464:49–62. 10.1007/978-1-4615-4729-7_5. [DOI] [PubMed] [Google Scholar]
- 47.Juven BJ, Kanner J, Schved F, Weisslowicz H. 1994. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 76:626–631. 10.1111/j.1365-2672.1994.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 48.Degenhardt J, Köllner TG, Gershenzon J. 2009. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70:1621–1637. 10.1016/j.phytochem.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 49.Jordão FM, Gabriel HB, Alves JM, Angeli CB, Bifano TD, Breda A, de Azevedo MF, Basso LA, Wunderlich G, Kimura EA, Katzin AM. 2013. Cloning and characterization of bifunctional enzyme farnesyl diphosphate/geranylgeranyl diphosphate synthase from Plasmodium falciparum. Malar. J. 12:184. 10.1186/1475-2875-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Artz JD, Wernimont AK, Dunford JE, Schapira M, Dong A, Zhao Y, Lew J, Russell RGG, Ebetino FH, Oppermann U, Hui R. 2011. Molecular characterization of a novel geranylgeranyl pyrophosphate synthase from Plasmodium parasites. J. Biol. Chem. 286:3315–3322. 10.1074/jbc.M109.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.No JH, de Macedo Dossin F, Zhang Y, Liu Y-L, Zhu W, Feng X, Yoo JA, Lee E, Wang K, Hui R, Freitas-Junior LH, Oldfield E. 2012. Lipophilic analogs of zoledronate and risedronate inhibit Plasmodium geranylgeranyl diphosphate synthase (GGPPS) and exhibit potent antimalarial activity. Proc. Natl. Acad. Sci. U. S. A. 109:4058–4063. 10.1073/pnas.1118215109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodan GA, Fleisch HA. 1996. Bisphosphonates: mechanisms of action. J. Clin. Invest. 97:2692–2696. 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Beek E, Pieterman E, Cohen L, Löwik C, Papapoulos S. 1999. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem. Biophys. Res. Commun. 264:108–111. 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- 54.Rondeau J-M, Bitsch F, Bourgier E, Geiser M, Hemmig R, Kroemer M, Lehmann S, Ramage P, Rieffel S, Strauss A, Green JR, Jahnke W. 2006. Structural basis for the exceptional in vivo efficacy of bisphosphonate drugs. ChemMedChem 1:267–273. 10.1002/cmdc.200500059. [DOI] [PubMed] [Google Scholar]
- 55.Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, Rogers MJ, Russell RGG, Oppermann U. 2006. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc. Natl. Acad. Sci. U. S. A. 103:7829–7834. 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordão FM, Saito AY, Miguel DC, de Jesus Peres V, Kimura EA, Katzin AM. 2011. In vitro and in vivo antiplasmodial activities of risedronate and its interference with protein prenylation in Plasmodium falciparum. Antimicrob. Agents Chemother. 55:2026–2031. 10.1128/AAC.01820-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh S, Chan JMW, Lea CR, Meints GA, Lewis JC, Tovian ZS, Flessner RM, Loftus TC, Bruchhaus I, Kendrick H, Croft SL, Kemp RG, Kobayashi S, Nozaki T, Oldfield E. 2004. Effects of bisphosphonates on the growth of Entamoeba histolytica and Plasmodium species in vitro and in vivo. J. Med. Chem. 47:175–187. 10.1021/jm030084x. [DOI] [PubMed] [Google Scholar]
- 58.Martin MB, Grimley JS, Lewis JC, Heath HT, Bailey BN, Kendrick H, Yardley V, Caldera A, Lira R, Urbina JA, Moreno SNJ, Docampo R, Croft SL, Oldfield E. 2001. Bisphosphonates inhibit the growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii, and Plasmodium falciparum: a potential route to chemotherapy. J. Med. Chem. 44:909–916. 10.1021/jm0002578. [DOI] [PubMed] [Google Scholar]
- 59.Zhang FL, Casey PJ. 1996. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65:241–269. 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 60.Buckner FS, Eastman RT, Yokoyama K, Gelb MH, Van Voorhis WC. 2005. Protein farnesyl transferase inhibitors for the treatment of malaria and African trypanosomiasis. Curr. Opin. Investig. Drugs 6:791–797. [PubMed] [Google Scholar]
- 61.Leung KF, Baron R, Seabra MC. 2006. Thematic review series: lipid posttranslational modifications. Geranylgeranylation of Rab GTPases. J. Lipid Res. 47:467–475. 10.1194/jlr.R500017-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Attal G, Langsley G. 1996. A Plasmodium falciparum homologue of a rab specific GDP dissociation inhibitor. Mol. Biochem. Parasitol. 79:91–95. 10.1016/0166-6851(96)02606-0. [DOI] [PubMed] [Google Scholar]
- 63.Chakrabarti D, Da Silva T, Barger J, Paquette S, Patel H, Patterson S, Allen CM. 2002. Protein farnesyltransferase and protein prenylation in Plasmodium falciparum. J. Biol. Chem. 277:42066–42073. 10.1074/jbc.M202860200. [DOI] [PubMed] [Google Scholar]
- 64.Eastman RT, White J, Hucke O, Bauer K, Yokoyama K, Nallan L, Chakrabarti D, Verlinde CLMJ, Gelb MH, Rathod PK, Van Voorhis WC. 2005. Resistance to a protein farnesyltransferase inhibitor in Plasmodium falciparum. J. Biol. Chem. 280:13554–13559. 10.1074/jbc.M413556200. [DOI] [PubMed] [Google Scholar]
- 65.Carrico D, Ohkanda J, Kendrick H, Yokoyama K, Blaskovich MA, Bucher CJ, Buckner FS, Van Voorhis WC, Chakrabarti D, Croft SL, Gelb MH, Sebti SM, Hamilton AD. 2004. In vitro and in vivo antimalarial activity of peptidomimetic protein farnesyltransferase inhibitors with improved membrane permeability. Bioorg. Med. Chem. 12:6517–6526. 10.1016/j.bmc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 66.Qian Y, Marugan JJ, Fossum RD, Vogt A, Sebti SM, Hamilton AD. 1999. Probing the hydrophobic pocket of farnesyltransferase: aromatic substitution of CAAX peptidomimetics leads to highly potent inhibitors. Bioorg. Med. Chem. 7:3011–3024. 10.1016/S0968-0896(99)00252-7. [DOI] [PubMed] [Google Scholar]
- 67.Ohkanda J, Lockman JW, Yokoyama K, Gelb MH, Croft SL, Kendrick H, Harrell MI, Feagin JE, Blaskovich MA, Sebti SM, Hamilton AD. 2001. Peptidomimetic inhibitors of protein farnesyltransferase show potent antimalarial activity. Bioorg. Med. Chem. Lett. 11:761–764. 10.1016/S0960-894X(01)00055-5. [DOI] [PubMed] [Google Scholar]
- 68.Moura IC, Wunderlich G, Uhrig ML, Couto AS, Peres VJ, Katzin AM, Kimura EA. 2001. Limonene arrests parasite development and inhibits isoprenylation of proteins in Plasmodium falciparum. Antimicrob. Agents Chemother. 45:2553–2558. 10.1128/AAC.45.9.2553-2558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gelb MH, Tamanoi F, Yokoyama K, Ghomashchi F, Esson K, Gould MN. 1995. The inhibition of protein prenyltransferases by oxygenated metabolites of limonene and perillyl alcohol. Cancer Lett. 91:169–175. 10.1016/0304-3835(95)03747-K. [DOI] [PubMed] [Google Scholar]
- 70.Hohl RJ, Lewis K. 1995. Differential effects of monoterpenes and lovastatin on RAS processing. J. Biol. Chem. 270:17508–17512. 10.1074/jbc.270.29.17508. [DOI] [PubMed] [Google Scholar]
- 71.Crowell PL, Chang RR, Ren ZB, Elson CE, Gould MN. 1991. Selective inhibition of isoprenylation of 21–26-kDa proteins by the anticarcinogen d-limonene and its metabolites. J. Biol. Chem. 266:17679–17685. [PubMed] [Google Scholar]
- 72.Krai P, Dalal S, Klemba M. 2014. Evidence for a Golgi-to-endosome protein sorting pathway in Plasmodium falciparum. PLoS One 9:e89771. 10.1371/journal.pone.0089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ayong L, DaSilva T, Mauser J, Allen CM, Chakrabarti D. 2011. Evidence for prenylation-dependent targeting of a Ykt6 SNARE in Plasmodium falciparum. Mol. Biochem. Parasitol. 175:162–168. 10.1016/j.molbiopara.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pendyala PR, Ayong L, Eatrides J, Schreiber M, Pham C, Chakrabarti R, Fidock DA, Allen CM, Chakrabarti D. 2008. Characterization of a PRL protein tyrosine phosphatase from Plasmodium falciparum. Mol. Biochem. Parasitol. 158:1–10. 10.1016/j.molbiopara.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 75.Asensi-Fabado MA, Munné-Bosch S. 2010. Vitamins in plants: occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 15:582–592. 10.1016/j.tplants.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 76.Falk J, Munné-Bosch S. 2010. Tocochromanol functions in plants: antioxidation and beyond. J. Exp. Bot. 61:1549–1566. 10.1093/jxb/erq030. [DOI] [PubMed] [Google Scholar]
- 77.Gruszka J, Pawlak A, Kruk J. 2008. Tocochromanols, plastoquinol, and other biological prenyllipids as singlet oxygen quenchers—;determination of singlet oxygen quenching rate constants and oxidation products. Free Radic. Biol. Med. 45:920–928. 10.1016/j.freeradbiomed.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 78.Sussmann RAC, Angeli CB, Peres VJ, Kimura EA, Katzin AM. 2011. Intraerythrocytic stages of Plasmodium falciparum biosynthesize vitamin E. FEBS Lett. 585:3985–3991. 10.1016/j.febslet.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 79.DellaPenna D. 2005. A decade of progress in understanding vitamin E synthesis in plants. J. Plant Physiol. 162:729–737. 10.1016/j.jplph.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Simons K. 2000. How cells handle cholesterol. Science 290:1721–1726. 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 81.Goldstein JL, Brown MS. 1990. Regulation of the mevalonate pathway. Nature 343:425–430. 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 82.Rilling HC. 1985. The mechanism of the condensation reactions of cholesterol biosynthesis. Fourth Morton lecture. Biochem. Soc. Trans. 13:997–1003. [DOI] [PubMed] [Google Scholar]
- 83.Dietschy JM. 1984. Regulation of cholesterol metabolism in man and in other species. Klin. Wochenschr. 62:338–345. 10.1007/BF01716251. [DOI] [PubMed] [Google Scholar]
- 84.Vial HJ, Philippot JR, Wallach DFH. 1984. A reevaluation of the status of cholesterol in erythrocytes infected by Plasmodium knowlesi and P. falciparum. Mol. Biochem. Parasitol. 13:53–65. 10.1016/0166-6851(84)90101-4. [DOI] [PubMed] [Google Scholar]
- 85.Mbaya B, Rigomier D, Edorh GG, Karst F, Schrevel J. 1990. Isoprenoid metabolism in Plasmodium falciparum during the intraerythrocytic phase of malaria. Biochem. Biophys. Res. Commun. 173:849–854. 10.1016/S0006-291X(05)80864-2. [DOI] [PubMed] [Google Scholar]
- 86.Rock RC. 1971. Incorporation of 14 C-labelled fatty acids into lipids of rhesus erythrocytes and Plasmodium knowlesi in vitro. Comp. Biochem. Physiol. B. 40:893–906. 10.1016/0300-9629(71)90278-7. [DOI] [PubMed] [Google Scholar]
- 87.Trigg PI. 1968. Sterol metabolism of Plasmodium knowlesi in vitro. Ann. Trop. Med. Parasitol. 62:481–487. [DOI] [PubMed] [Google Scholar]
- 88.Holz GG. 1977. Lipids and the malarial parasite. Bull. World Health Organ. 55:237–248. [PMC free article] [PubMed] [Google Scholar]
- 89.Labaied M, Jayabalasingham B, Bano N, Cha S-J, Sandoval J, Guan G, Coppens I. 2011. Plasmodium salvages cholesterol internalized by LDL and synthesized de novo in the liver. Cell. Microbiol. 13:569–586. 10.1111/j.1462-5822.2010.01555.x. [DOI] [PubMed] [Google Scholar]
- 90.Lauer S, VanWye J, Harrison T, McManus H, Samuel BU, Hiller NL, Mohandas N, Haldar K. 2000. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 19:3556–3564. 10.1093/emboj/19.14.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cazzonelli CI. 2011. Goldacre review: carotenoids in nature: insights from plants and beyond. Funct. Plant Biol. 38:833–847. 10.1071/FP11192. [DOI] [PubMed] [Google Scholar]
- 92.Krinsky NI. 1979. Carotenoid protection against oxidation. Pure Appl. Chem. 51:649–660. [Google Scholar]
- 93.Griffiths M, Sistrom WR, Cohen-Bazire G, Stanier RY. 1955. Function of carotenoids in photosynthesis. Nature 176:1211–1214. 10.1038/1761211a0. [DOI] [PubMed] [Google Scholar]
- 94.Cogdell RJ, Howard TD, Bittl R, Schlodder E, Geisenheimer I, Lubitz W. 2000. How carotenoids protect bacterial photosynthesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:1345–1349. 10.1098/rstb.2000.0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gruszecki WI, Strzałka K. 2005. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta 1740:108–115. 10.1016/j.bbadis.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 96.Joyard J, Ferro M, Masselon C, Seigneurin-Berny D, Salvi D, Garin J, Rolland N. 2009. Chloroplast proteomics and the compartmentation of plastidial isoprenoid biosynthetic pathways. Mol. Plant. 2:1154–1180. 10.1093/mp/ssp088. [DOI] [PubMed] [Google Scholar]
- 97.Shumskaya M, Bradbury LMT, Monaco RR, Wurtzel ET. 2012. Plastid localization of the key carotenoid enzyme phytoene synthase is altered by isozyme, allelic variation, and activity. Plant Cell 24:3725–3741. 10.1105/tpc.112.104174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Daub ME, Payne GA. 1989. The role of carotenoids in resistance of fungi to cercosporin. Phytopathology 79:180. 10.1094/Phyto-79-180. [DOI] [Google Scholar]
- 99.Jensen SL. 1965. On fungal carotenoids and the natural distribution of spirilloxanthin. Phytochemistry 4:925–931. 10.1016/S0031-9422(00)86270-6. [DOI] [Google Scholar]
- 100.Moran NA, Jarvik T. 2010. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328:624–627. 10.1126/science.1187113. [DOI] [PubMed] [Google Scholar]
- 101.Altincicek B, Kovacs JL, Gerardo NM. 2012. Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae. Biol. Lett. 8:253–257. 10.1098/rsbl.2011.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sandmann G. 2001. Genetic manipulation of carotenoid biosynthesis: strategies, problems and achievements. Trends Plant Sci. 6:14–17. 10.1016/S1360-1385(00)01817-3. [DOI] [PubMed] [Google Scholar]
- 103.Spolaore P, Joannis-Cassan C, Duran E, Isambert A. 2006. Commercial applications of microalgae. J. Biosci. Bioeng. 101:87–96. 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- 104.Tonhosolo R, D'Alexandri FL, de Rosso VV, Gazarini ML, Matsumura MY, Peres VJ, Merino EF, Carlton JM, Wunderlich G, Mercadante AZ, Kimura EA, Katzin AM. 2009. Carotenoid biosynthesis in intraerythrocytic stages of Plasmodium falciparum. J. Biol. Chem. 284:9974–9985. 10.1074/jbc.M807464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nambara E, Marion-Poll A. 2005. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 56:165–185. 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 106.Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. 2008. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature 451:207–210. 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olszewski KL, Llinás M. 2011. Central carbon metabolism of Plasmodium parasites. Mol. Biochem. Parasitol. 175:95–103. 10.1016/j.molbiopara.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scheibel LW, JM 1969. Glycolytic and cytochrome oxidase activity in Plasmodia. Mil. Med. 134:1074–1080. [PubMed] [Google Scholar]
- 109.Bowman IB, Grant PT, Kermack WO, Ogston D. 1961. The metabolism of Plasmodium berghei, the malaria parasite of rodents. 2. An effect of mepacrine on the metabolism of glucose by the parasite separated from its host cell. Biochem. J. 78:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krungkrai J, Burat D, Kudan S, Krungkrai S, Prapunwattana P. 1999. Mitochondrial oxygen consumption in asexual and sexual blood stages of the human malarial parasite, Plasmodium falciparum. Southeast Asian J. Trop. Med. Public Health 30:636–642. [PubMed] [Google Scholar]
- 111.MacRae JI, Dixon MW, Dearnley MK, Chua HH, Chambers JM, Kenny S, Bottova I, Tilley L, McConville MJ. 2013. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 11:67. 10.1186/1741-7007-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boysen KE, Matuschewski K. 2011. Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase. J. Biol. Chem. 286:32661–32671. 10.1074/jbc.M111.269399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. 2007. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature 446:88–91. 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 114.Phillips MA, Gujjar R, Malmquist NA, White J, El Mazouni F, Baldwin J, Rathod PK. 2008. Triazolopyrimidine-based dihydroorotate dehydrogenase inhibitors with potent and selective activity against the malaria parasite Plasmodium falciparum. J. Med. Chem. 51:3649–3653. 10.1021/jm8001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gujjar R, Marwaha A, El Mazouni F, White J, White KL, Creason S, Shackleford DM, Baldwin J, Charman WN, Buckner FS, Charman S, Rathod PK, Phillips MA. 2009. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J. Med. Chem. 52:1864–1872. 10.1021/jm801343r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heikkilä T, Ramsey C, Davies M, Galtier C, Stead AMW, Johnson AP, Fishwick CWG, Boa AN, McConkey GA. 2007. Design and synthesis of potent inhibitors of the malaria parasite dihydroorotate dehydrogenase. J. Med. Chem. 50:186–191. 10.1021/jm060687j. [DOI] [PubMed] [Google Scholar]
- 117.Tonhosolo R, D'Alexandri FL, Genta FA, Wunderlich G, Gozzo FC, Eberlin MN, Peres VJ, Kimura EA, Katzin AM. 2005. Identification, molecular cloning and functional characterization of an octaprenyl pyrophosphate synthase in intra-erythrocytic stages of Plasmodium falciparum. Biochem. J. 392:117–126. 10.1042/BJ20050441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Winrow MJ, Rudney H. 1969. The incorporation of p-hydroxybenzoic acid and isopentenyl pyrophosphate into ubiquinone precursors by cell-free preparations of rat tissues. Biochem. Biophys. Res. Commun. 37:833–840. 10.1016/0006-291X(69)90967-X. [DOI] [PubMed] [Google Scholar]
- 119.Suzuki K, Ueda M, Yuasa M, Nakagawa T, Kawamukai M, Matsuda H. 1994. Evidence that Escherichia coli ubiA product is a functional homolog of yeast COQ2, and the regulation of ubiA gene expression. Biosci. Biotechnol. Biochem. 58:1814–1819. 10.1271/bbb.58.1814. [DOI] [PubMed] [Google Scholar]
- 120.de Macedo CS, Uhrig ML, Kimura EA, Katzin AM. 2002. Characterization of the isoprenoid chain of coenzyme Q in Plasmodium falciparum. FEMS Microbiol. Lett. 207:13–20. 10.1111/j.1574-6968.2002.tb11021.x. [DOI] [PubMed] [Google Scholar]
- 121.Schnell JV, Siddiqui WA, Geiman QM, Skelton FS, Lunan KD, Folkers K. 1971. Coenzyme Q. 114. Biosynthesis of coenzymes Q by malarial parasites. 2. Coenzyme Q synthesis in blood cultures of monkeys infected with malarial parasites (Plasmodium falciparum and P. knowlesi). J. Med. Chem. 14:1026–1029. 10.1021/jm00293a002. [DOI] [PubMed] [Google Scholar]
- 122.Cowman AF, Crabb BS. 2006. Invasion of red blood cells by malaria parasites. Cell 124:755–766. 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 123.Couto AS, Kimura EA, Peres VJ, Uhrig ML, Katzin AM. 1999. Active isoprenoid pathway in the intra-erythrocytic stages of Plasmodium falciparum: presence of dolichols of 11 and 12 isoprene units. Biochem. J. 341(Pt 3):629–637. 10.1042/0264-6021:3410629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.D'Alexandri FL, Kimura EA, Peres VJ, Katzin AM. 2006. Protein dolichylation in Plasmodium falciparum. FEBS Lett. 580:6343–6348. 10.1016/j.febslet.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 125.Gowda DC, Gupta P, Davidson EA. 1997. Glycosylphosphatidylinositol anchors represent the major carbohydrate modification in proteins of intraerythrocytic stage Plasmodium falciparum. J. Biol. Chem. 272:6428–6439. 10.1074/jbc.272.10.6428. [DOI] [PubMed] [Google Scholar]
- 126.Udeinya IJ, VanDyke K. 1981. 2-Deoxyglucose: inhibition of parasitemia and of glucosamine incorporation into glycosylated macromolecules, in malarial parasites (Plasmodium falciparum). Pharmacology 23:171–175. 10.1159/000137546. [DOI] [PubMed] [Google Scholar]
- 127.de Macedo CS, Shams-Eldin H, Smith TK, Schwarz RT, Azzouz N. 2003. Inhibitors of glycosyl-phosphatidylinositol anchor biosynthesis. Biochimie 85:465–472. 10.1016/S0300-9084(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 128.Braun-Breton C, Rosenberry TL, da Silva LP. 1988. Induction of the proteolytic activity of a membrane protein in Plasmodium falciparum by phosphatidyl inositol-specific phospholipase C. Nature 332:457–459. 10.1038/332457a0. [DOI] [PubMed] [Google Scholar]
- 129.Braun Breton C, Rosenberry TL, Pereira da Silva LH. 1990. Glycolipid anchorage of Plasmodium falciparum surface antigens. Res. Immunol. 141:743–755. 10.1016/0923-2494(90)90005-J. [DOI] [PubMed] [Google Scholar]
- 130.Gilson PR, Nebl T, Vukcevic D, Moritz RL, Sargeant T, Speed TP, Schofield L, Crabb BS. 2006. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol. Cell. Proteomics 5:1286–1299. 10.1074/mcp.M600035-MCP200. [DOI] [PubMed] [Google Scholar]
- 131.Shams-Eldin H, Azzouz N, Kedees MH, Orlean P, Kinoshita T, Schwarz RT. 2002. The GPI1 homologue from Plasmodium falciparum complements a Saccharomyces cerevisiae GPI1 anchoring mutant. Mol. Biochem. Parasitol. 120:73–81. 10.1016/S0166-6851(01)00434-0. [DOI] [PubMed] [Google Scholar]
- 132.Shams-Eldin H, de Macedo CS, Niehus S, Dorn C, Kimmel J, Azzouz N, Schwarz RT. 2008. Plasmodium falciparum dolichol phosphate mannose synthase represents a novel clade. Biochem. Biophys. Res. Commun. 370:388–393. 10.1016/j.bbrc.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 133.Nasir-ud-Din Drager-Dayal R, Decrind C, Hu BH, Del Giudice G, Hoessli D. 1992. Plasmodium falciparum synthesizes O-glycosylated glycoproteins containing O-linked N-acetylglucosamine. Biochem. Int. 27:55–64. [PubMed] [Google Scholar]
- 134.Dieckmann-Schuppert A, Bause E, Schwarz RT. 1993. Studies on O-glycans of Plasmodium-falciparum-infected human erythrocytes. Evidence for O-GlcNAc and O-GlcNAc-transferase in malaria parasites. Eur. J. Biochem. 216:779–788. 10.1111/j.1432-1033.1993.tb18198.x. [DOI] [PubMed] [Google Scholar]
- 135.Kimura EA, Couto AS, Peres VJ, Casal OL, Katzin AM. 1996. N-linked glycoproteins are related to schizogony of the intraerythrocytic stage in Plasmodium falciparum. J. Biol. Chem. 271:14452–14461. 10.1074/jbc.271.24.14452. [DOI] [PubMed] [Google Scholar]
- 136.Dieckmann-Schuppert A, Bender S, Odenthal-Schnittler M, Bause E, Schwarz RT. 1992. Apparent lack of N-glycosylation in the asexual intraerythrocytic stage of Plasmodium falciparum. Eur. J. Biochem. 205:815–825. 10.1111/j.1432-1033.1992.tb16846.x. [DOI] [PubMed] [Google Scholar]
- 137.von Itzstein M, Plebanski M, Cooke BM, Coppel RL. 2008. Hot, sweet and sticky: the glycobiology of Plasmodium falciparum. Trends Parasitol. 24:210–218. 10.1016/j.pt.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 138.Kedees MH, Azzouz N, Gerold P, Shams-Eldin H, Iqbal J, Eckert V, Schwarz RT. 2002. Glycosylation status of Plasmodium falciparum circumsporozoite protein expressed in the baculovirus system. Exp. Parasitol. 101:64–68. 10.1016/S0014-4894(02)00030-9. [DOI] [PubMed] [Google Scholar]
- 139.Cazzonelli CI, Pogson BJ. 2010. Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 15:266–274. 10.1016/j.tplants.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 140.Rodríguez-Concepción M. 2006. Early steps in isoprenoid biosynthesis: multilevel regulation of the supply of common precursors in plant cells. Phytochem. Rev. 5:1–15. 10.1007/s11101-005-3130-4. [DOI] [Google Scholar]
- 141.Cordoba E, Salmi M, León P. 2009. Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. J. Exp. Bot. 60:2933–2943. 10.1093/jxb/erp190. [DOI] [PubMed] [Google Scholar]
- 142.Partow S, Siewers V, Daviet L, Schalk M, Nielsen J. 2012. Reconstruction and evaluation of the synthetic bacterial MEP pathway in Saccharomyces cerevisiae. PLoS One 7:e52498. 10.1371/journal.pone.0052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sauret-Güeto S, Botella-Pavía P, Flores-Pérez U, Martínez-García JF, San Román C, León P, Boronat A, Rodríguez-Concepción M. 2006. Plastid cues posttranscriptionally regulate the accumulation of key enzymes of the methylerythritol phosphate pathway in Arabidopsis. Plant Physiol. 141:75–84. 10.1104/pp.106.079855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.López-Barragán MJ, Lemieux J, Quiñones M, Williamson KC, Molina-Cruz A, Cui K, Barillas-Mury C, Zhao K, Su X. 2011. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genomics 12:587. 10.1186/1471-2164-12-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu G, Cabrera A, Kono M, Mok S, Chaal BK, Haase S, Engelberg K, Cheemadan S, Spielmann T, Preiser PR, Gilberger T-W, Bozdech Z. 2010. Transcriptional profiling of growth perturbations of the human malaria parasite Plasmodium falciparum. Nat. Biotechnol. 28:91–98. 10.1038/nbt.1597. [DOI] [PubMed] [Google Scholar]
- 146.Bitok JK, Meyers CF. 2012. 2C-Methyl-d-erythritol 4-phosphate enhances and sustains cyclodiphosphate synthase IspF activity. ACS Chem. Biol. 7:1702–1710. 10.1021/cb300243w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Banerjee A, Wu Y, Banerjee R, Li Y, Yan H, Sharkey TD. 2013. Feedback inhibition of deoxy-d-xylulose-5-phosphate synthase regulates the methylerythritol 4-phosphate pathway. J. Biol. Chem. 288:16926–16936. 10.1074/jbc.M113.464636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guggisberg AM, Park J, Edwards RL, Kelly ML, Hodge DM, Tolia NH, Odom AR. 2014. A sugar phosphatase regulates the methylerythritol phosphate (MEP) pathway in malaria parasites. Nat. Commun. 5:4467. 10.1038/ncomms5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bowman JD, Merino EF, Brooks CF, Striepen B, Carlier PR, Cassera MB. 2014. Antiapicoplast and gametocytocidal screening to identify the mechanisms of action of compounds within the malaria box. Antimicrob. Agents Chemother. 58:811–819. 10.1128/AAC.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hunter S, Jones P, Mitchell A, Apweiler R, Attwood TK, Bateman A, Bernard T, Binns D, Bork P, Burge S, de Castro E, Coggill P, Corbett M, Das U, Daugherty L, Duquenne L, Finn RD, Fraser M, Gough J, Haft D, Hulo N, Kahn D, Kelly E, Letunic I, Lonsdale D, Lopez R, Madera M, Maslen J, McAnulla C, McDowall J, McMenamin C, Mi H, Mutowo-Muellenet P, Mulder N, Natale D, Orengo C, Pesseat S, Punta M, Quinn AF, Rivoire C, Sangrador-Vegas A, Selengut JD, Sigrist CJA, Scheremetjew M, Tate J, Thimmajanarthanan M, Thomas PD, Wu CH, Yeats C, Yong S-Y. 2012. InterPro in 2011: new developments in the family and domain prediction database. Nucleic Acids Res. 40:D306–D312. 10.1093/nar/gkr948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ajikumar PK, Xiao W-H, Tyo KEJ, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G. 2010. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science 330:70–74. 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao Y, Yang J, Qin B, Li Y, Sun Y, Su S, Xian M. 2011. Biosynthesis of isoprene in Escherichia coli via methylerythritol phosphate (MEP) pathway. Appl. Microbiol. Biotechnol. 90:1915–1922. 10.1007/s00253-011-3199-1. [DOI] [PubMed] [Google Scholar]
- 153.Mahmoud SS, Croteau RB. 2001. Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc. Natl. Acad. Sci. U. S. A. 98:8915–8920. 10.1073/pnas.141237298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zeng Q, Qiu F, Yuan L. 2008. Production of artemisinin by genetically-modified microbes. Biotechnol. Lett. 30:581–592. 10.1007/s10529-007-9596-y. [DOI] [PubMed] [Google Scholar]
- 155.Yuan LZ, Rouvière PE, Larossa RA, Suh W. 2006. Chromosomal promoter replacement of the isoprenoid pathway for enhancing carotenoid production in E. coli. Metab. Eng. 8:79–90. 10.1016/j.ymben.2005.08.005. [DOI] [PubMed] [Google Scholar]