Abstract
Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei (the Bptm group) are close relatives with very different lifestyles: B. pseudomallei is an opportunistic pathogen, B. thailandensis is a nonpathogenic saprophyte, and B. mallei is a host-restricted pathogen. The acyl-homoserine lactone quorum-sensing (QS) systems of these three species show a high level of conservation. We used transcriptome sequencing (RNA-seq) to define the quorum-sensing regulon in each species, and we performed a cross-species analysis of the QS-controlled orthologs. Our analysis revealed a core set of QS-regulated genes in all three species, as well as QS-controlled factors shared by only two species or unique to a given species. This global survey of the QS regulons of B. pseudomallei, B. thailandensis, and B. mallei serves as a platform for predicting which QS-controlled processes might be important in different bacterial niches and contribute to the pathogenesis of B. pseudomallei and B. mallei.
INTRODUCTION
Our interest in Burkholderia thailandensis, Burkholderia pseudomallei, and Burkholderia mallei, which we call the Bptm group (1), stems from the fact that this triad shares a high degree of genetic similarity but the species have very divergent lifestyles. B. thailandensis is a soil saprophyte common to tropical and subtropical regions and is not a human pathogen (2, 3). B. pseudomallei is found in environments similar to those for B. thailandensis, but it is also an opportunistic pathogen that causes the emerging infectious disease melioidosis (4). B. mallei is the causative agent of a zoonotic disease that most commonly causes glanders in equines (5). Unlike B. pseudomallei and B. thailandensis, B. mallei is a host-restricted pathogen and does not have a saprophytic reservoir.
B. thailandensis and B. pseudomallei diverged from a common ancestor about 47 million years ago and have close 16S rRNA sequence similarities (6). More than 85% of their genes are conserved and their genomes are highly syntenic, with only four large-scale inversions (6). Genomic islands provide a major source of species-specific genes in B. thailandensis and B. pseudomallei (6, 7). The third member of the Bptm group, B. mallei, is believed to have evolved from an ancestral B. pseudomallei isolate following an animal infection. The B. mallei genome (5.8 Mb) is 20% smaller than the B. pseudomallei genome (7.2 Mb) yet retains high nucleotide sequence identity (99%) (8, 9). The expansion of genomic insertion sequences (ISs) facilitated numerous deletion events that resulted in reductive evolution of the B. mallei genome (8). Presumably, many genes needed for environmental survival were lost from B. mallei, while those important for host survival were maintained (8, 10). B. mallei has few species-specific genes; a multi-isolate query of B. mallei variable genes showed that all have B. pseudomallei orthologs (10). Despite this, the B. mallei genome is highly plastic due to the large number of IS elements and simple sequence repeats that facilitate homologous recombination (8–10).
Many Proteobacteria, including Burkholderia species, use acyl-homoserine lactone (AHL) quorum-sensing (QS) cell-to-cell signaling systems to differentiate between a low-population-density and a high-population-density state. AHL signals are made by members of the LuxI family of signal synthases and can diffuse in and out of cells. Once a critical AHL concentration is reached, the AHL binds to and influences the activity of a LuxR family receptor that is also a transcription factor. The active LuxR then initiates changes in transcription. In this way, bacteria can sense their population density (AHL concentration) and coordinate their behavior (see reference 11 for a review).
Our group is interested in AHL signaling and its role in different bacterial lifestyles. Frequently, AHL QS allows opportunistic pathogens and symbionts to sense and respond to lifestyle shifts that occur as a bacterium cycles between a free-living (low-population-density) state and a host-associated (high-population-density) state (12–15). In fact, QS is important for the virulence of many species (16). There is also mounting evidence that QS is important in environmental reservoirs where it can allow bacteria to mount interspecies attacks and compete for limited resources or facilitate other survival strategies (17, 18). Though many genes are controlled by QS, genes coding for secreted products such as virulence factors, toxins, biofilm components, and antimicrobials are frequently activated by AHL signaling.
Members of the Bptm group share homologous AHL QS systems (19–21). B. thailandensis and B. pseudomallei each contain three complete AHL QS circuits, QS-1 through QS-3 (which consist of the cognate pairs BtaI1-BtaR1, BtaI2-BtaR2, and BtaI3-BtaR3 for B. thailandensis and BpsI1-BpsR1, BpsI2-BpsR2, and BpsI3-BpsR3 for B. pseudomallei). During B. mallei's reductive evolution, it lost a large genomic region containing the QS-2 genes and thus only contains the QS-1 and QS-3 systems (BmaI1-BmaR1 and BmaI3-BmaR3). Additionally, each member of the Bptm group contains two orphan or solo LuxRs (receptors that do not have a cognate LuxI signal synthase and may or may not respond to an AHL). These are designated for each species (B. thailandensis, B. pseudomallei, or B. mallei) as R4 and R5. The QS systems are highly conserved across the Bptm group; orthologous LuxI and LuxR proteins show between 95 and 100% amino acid identity. Furthermore, the AHLs that each homologous circuit produces and responds to are identical. The QS-1 signal is N-octanoyl homoserine lactone (C8-HSL) (22–26), the QS-2 signal is N-3-hydroxy-decanoyl homoserine lactone (3OHC10-HSL) (24, 27), and the QS-3 signal is N-3-hydroxy-octanoyl homoserine lactone (3OHC8-HSL) (22, 24, 28).
There is limited information on the global roles of the QS circuits in the Bptm group. Of particular interest, we do not know if the QS-controlled factors among these species are conserved, as their LuxR and LuxI homologs are, or if they are divergent. Here, we describe a global analysis and comparison of the QS regulons of each member of the Bptm group, identify a core group of QS-controlled genes shared by all members of the group, and highlight similarities and differences between the three species. We believe this information will provide a foundation on which to generate ideas about how QS is used in different bacterial lifestyles and how signaling systems might change with niche adaptation. Additionally, QS has been associated with the virulence of both B. pseudomallei and B. mallei. B. pseudomallei mutants are attenuated in multiple infection models and show aberrant intracellular replication (21, 26, 29, 30). These studies imply a requirement for QS in melioidosis, yet it is not known which QS-controlled factor or factors are used in the host. A current hypothesis is that QS regulates the acute-to-chronic disease shift in B. mallei (31). As is the case for B. pseudomallei, the QS-controlled factors utilized by B. mallei in the host are unknown. Characterization of the QS-controlled factors in these pathogens should lead to a deeper understanding of meliodosis and glanders.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used are listed in Table S1 in the supplemental material. Bacteria were grown in Luria-Bertani (LB) broth (10 g tryptone, 5 g yeast extract, 5 g NaCl per liter) supplemented with 50 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 7.0) where indicated. For B. pseudomallei, 1.6 mM adenine sulfate and 0.005% thiamine-HCl were added to the growth medium. Antibiotics were used at the following concentrations: for Escherichia coli, 35 μg/ml kanamycin (Kan) and 25 μg/ml zeocin (Zeo); for B. pseudomallei, 2 mg/ml Zeo and 1 mg/ml Kan. Where stated, synthetic 3OHC10-HSL (4 μM; University of Nottingham [http://www.nottingham.ac.uk/quorum/compounds.htm]), 3OHC8-HSL (2 μM; synthesized as previously described [31]) and C8-HSL (2 μM; Sigma Chemical Co.) were added. Except where indicated, bacteria were grown at 37°C with shaking. All experiments with B. mallei were performed in a class II biological safety cabinet housed in a biological safety level 3 (BSL-3) enhanced laboratory. All experiments with B. pseudomallei employed strain Bp82, which is exempt from the select agent list, and were performed in a BSL-2 laboratory.
For RNA isolation from B. mallei and B. pseudomallei cells, inocula were from 5-ml overnight cultures. Fresh medium with or without AHLs (20 ml in 125-ml flasks) were inoculated to a starting optical density at 600 nm (OD600) of 0.05. All RNA samples were from cells at the transition from exponential growth to stationary phase (T phase; OD600, 2.0).
Measurements of C8-HSL, 3OHC8-HSL, and 3OHC10-HSL in B. pseudomallei cultures.
To measure AHLs in B. pseudomallei cultures, we twice extracted 5 ml of a culture grown to an OD600 of 4.0 with acidified ethyl acetate. The extract was then dried under N2 gas, dissolved in 50 μl 50% methanol, and subjected to C18 reverse-phase high-performance liquid chromatography, and fractions containing C8-HSL, 3OHC8-HSL, and 3OHC10-HSL were individually collected. We used previously described bioassays to measure the AHLs: C8-HSL was measured using the bioreporter E. coli(pBD4, pBD5) (23), and 3OHC8-HSL and 3OHC10-HSL were measured using the bioreporter E. coli(pJNR2, pI2P50) (27). Standard curves were generated by using synthetic C8-l-HSL, 3OHC10-l-HSL, and 3OHC8-l-HSL.
Mutant construction.
The bpsI2 (BP1026B_II1251) and bpsI3 (BP1026B_II1673) deletions were constructed by using the dual-plasmid method of Lopez et al. (32) and standard molecular biology procedures with E. coli DH10B as a cloning vehicle. To create gene deletion vectors, we used overlap extension PCR to generate approximately 1,000 bp of DNA flanking each gene with genomic DNA from B. pseudomallei Bp82 as a template. The flanking sequences were joined together and PCR amplified to generate each deletion construct. Primers used in cloning are listed in Table S1 in the supplemental material and included two sets of four primers each: OCM53-OCM56 for bpsI2 and OCM64-65 and OCM70-71 for bpsI3. The bpsI2 deletion construct and pEXKm5 were digested with SmaI and ligated to yield pCM139. The bpsI3 deletion construct and pEXKm5 were digested with XhoI and ExoRI and ligated to yield pCM134.
To create the B. pseudomallei ΔbpsI1 ΔbpsI2 ΔbpsI3 triple mutant, the unmarked successive deletions were generated in strain CM135 (B. pseudomallei Bp82 ΔbpsI1) with pCM139, pCM134, and pBADSce, as previously described (33), to yield strain CM153 (B. pseudomallei Bp82 ΔbpsI1 ΔbpsI2 ΔbpsI3).
RNA isolation, RNA-seq library construction, and RNA-seq analysis.
RNA isolation, library construction, and transcriptome sequencing (RNA-seq) analysis were done as described previously using a primer set we developed to limit rRNA amplification in the Bptm group (34). Sequencing reads were aligned to the B. pseudomallei 1026b genome or to the B. mallei ATCC 23344 genome and analyzed by using Avadis NGS software. Differentially regulated genes were determined for biological replicates by using differential expression sequence analysis (DESeq; with a false discovery rate [FDR] cutoff of 0.05) and showed 2-fold or more regulation relative to the reference condition. The data for biological replicates were deposited in the NCBI sequence read archive (SRA) database.
Ortholog and pseudogene analysis.
We used the Burkholderia Prokaryotic Genome Analysis tool (35) to identify orthologs and pseudogenes among the QS-controlled genes for each B. pseudomallei 1026b, B. mallei ATCC 23344, and B. thailandensis E264 list. We compared these lists with each other to identify areas of overlap and divergence. For orthologs that had more than one paralog in another species, each paralog was also considered.
Microarray data accession number.
The data from the RNA-seq analysis have been deposited in the NCBI SRA database under BioProject ID PRJNA241448. The tables are organized by locus tag.
RESULTS
Approach to identify QS-controlled genes in the Bptm group.
We sought to identify and compare QS-controlled factors in B. pseudomallei, B. thailandensis, and B. mallei by using an RNA-seq method we recently employed for B. thailandensis (34). For each species, we compared transcripts from the wild-type (QS-proficient parent) strain, B. thailandensis E264, B. pseudomallei Bp82, or B. mallei GB8 and the corresponding AHL-negative mutant, B. thailandensis JBT112 (22), B. pseudomallei CM153, or B. mallei CM38 (31). Next, we compared transcripts from each AHL-negative mutant grown with and without added QS signals, either individually or added together. The AHL-negative mutants contained wild-type copies of the LuxR family regulators and as such could respond to added AHL. Each RNA sample was isolated from cells transitioning from exponential growth to stationary phase (T phase; OD600 of 2). The B. thailandensis data have been published previously (34). The comparison of wild types to quorum-sensing signal synthesis mutants should reveal responses to all of the signals as they accumulate normally during the growth cycle, but this approach is limited in that we cannot derive any information about which signals might be responsible for any given response. A second issue with this approach is that, unavoidably, we are comparing two different strains with each other. Although the mutants are derived from the wild-type strains and one would expect them to be isogenic, the Bptm group is known to be genetically plastic, and there might be genomic changes other than those of which we are aware. The experiments in which a specific signal or all signals are added back to the signal synthesis mutant is not subject to either of the issues discussed above, but in these experiments signal concentrations are artificially high early in growth (see below).
We sought to add an excess and consistent amount of each AHL during RNA-seq sampling for all three species, to ensure analogous sampling conditions and facilitate cross-species comparisons. We previously reported the AHL abundances in stationary-phase cultures of B. thailandensis E264 (34) and B. mallei GB8 (31). For B. pseudomallei Bp82, we found that stationary-phase cultures contained 98 ± 10 nM (mean ± standard deviation) C8-HSL, 28 ± 2 nM 3OHC8-HSL, and 648 ± 144 nM 3OHC10-HSL. Although each species accumulated different concentrations of AHLs, we added equal amounts of synthetic AHLs in our experiments (2 μM C8-HSL, 2 μM 3OHC8-HSL, and 4 μM 3OHC10-HSL). We acknowledge that these concentrations exceed the physiological concentrations measured for each species, but we chose high concentrations in an effort to saturate the QS systems.
For B. thailandensis and B. pseudomallei, we identified genes activated and repressed by addition of C8-HSL, 3OHC10-HSL, 3OHC8-HSL, or all three AHLs together. For B. mallei, which only contains the QS-1 and QS-3 systems, we identified genes controlled by C8-HSL, 3OHC8-HSL, or both AHLs together. The single addition of an AHL to an AHL-negative strain allows us to evaluate the contribution of an individual AHL to gene regulation, which cannot be achieved by looking at single AHL synthesis mutants, as such strains would contain the other AHL synthases and data interpretation would be complex. A complete list of QS-controlled genes under all conditions can be found in the supplemental material. Figure 1 (and also Table S2 in the supplemental material) shows the QS-controlled genes of B. pseudomallei. Figure 1 (and also Table S3 in the supplemental material) shows those of B. mallei. The QS regulon of B. thailandensis has been described previously (34), but it is shown in Fig. 1 (and has been reformatted in Table S4 in the supplemental material) for the purposes of this report.
FIG 1.

Venn diagrams showing the relationship between QS-controlled genes in B. pseudomallei (Bp), using strain Bp82 as the wild type (WT) and strain CM153 as the AHL-negative mutant, B. mallei (Bm), using strains GB8 as WT and CM38 as the AHL-negative mutant, and B. thailandensis (Bt), using strains E264 as WT and JBT1122 as the AHL-negative mutant. The circles show overlapping regulons under different conditions (the numbers of genes are also given). For each species, the top diagrams show QS-controlled genes identified when the AHL-negative mutant was grown without any signals, compared to growth with the indicated AHL. The bottom diagrams show QS-controlled genes when the WT or the AHL-negative mutant grown with multiple AHLs (AHL− plus all signals or AHL− plus both) was compared to the AHL-negative mutant grown without added AHLs.
Table 1 summarizes the number of QS-activated and QS-repressed genes in each species under each condition tested. B. thailandensis showed the highest number of QS-controlled genes, followed by B. pseudomallei and then B. mallei. The majority of the QS-controlled genes under each condition were QS activated.
TABLE 1.
Summary of QS-controlled genes identified in the Bptm group
| Species | No. of QS-controlled genes induced (+) or repressed (−) in the presence of: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WTa |
C8-HSLb |
3OHC10-HSLb |
3OHC8-HSLb |
All AHLsc |
C8-HSL and 3OHC8-HSLc |
|||||||
| + | − | + | − | + | − | + | − | + | − | + | − | |
| B. pseudomallei | 40 | 1 | 2 | 0 | 53 | 4 | 65 | 7 | 120 | 55 | NDd | ND |
| B. thailandensis | 161 | 88 | 24 | 11 | 69 | 46 | 125 | 62 | 271 | 106 | ND | ND |
| B. mallei | 14 | 2 | 14 | 0 | ND | ND | 14 | 0 | ND | ND | 32 | 1 |
Numbers of QS-controlled genes that were induced or repressed in the WT compared to the corresponding AHL-negative mutant.
Numbers of QS-controlled genes that were induced or repressed when the indicated AHL was added to the medium for the corresponding AHL-negative mutant.
Numbers of QS-controlled genes that were induced or repressed when either all three AHLs or both C8-HSL and 3OHC8-HSL were added simultaneously to the medium for the corresponding AHL-negative mutant of the species.
ND, not done.
Cross-species comparison of QS-controlled orthologs in the Bptm group.
We predicted that the identification of conserved and divergent factors across the Bptm QS regulons could provide functional clues as to why certain bacterial processes are QS controlled in different niches. For example, QS-controlled factors common to only the saprophytic species (B. thailandensis and B. pseudomallei) may be most useful in saprophytic life. QS-controlled factors shared by the pathogenic species (B. pseudomallei and B. mallei) might be important in host association. Factors common to all members of the Bptm group may serve a conserved function important for Burkholderia physiology in several habitats.
To compare the QS-controlled orthologs in the Bptm group, we first determined if the genes in each regulon had orthologs in the other species of the group. A total of 517 genes showed QS control in B. thailandensis. Of these, 449 have B. pseudomallei orthologs and 310 have B. mallei orthologs. A total of 216 genes were QS controlled in B. pseudomallei. Of these, 143 have B. mallei orthologs and 175 have B. thailandensis orthologs. A total of 43 genes showed QS control in B. mallei. Of these, all have B. pseudomallei orthologs and 35 have B. thailandensis orthologs. We next compared the orthologs from the QS regulon of one species to the QS regulon of the other species. Systematically, we determined the number of shared orthologs among the QS regulons of the Bptm group.
Seven conserved orthologs are present in the QS regulons of B. pseudomallei, B. thailandensis, and B. mallei and represent a core group of QS-controlled factors. Seventy-one orthologs are shared by the QS regulons of B. thailandensis and B. pseudomallei. Two QS-controlled orthologs are shared by only B. mallei and B. pseudomallei, and nine orthologs are shared by the QS regulons of B. mallei and B. thailandensis. The Venn diagram in Fig. 2 shows a comparison of all QS-controlled orthologs across the species in the Bptm group.
FIG 2.

Comparison of QS-controlled orthologs in the Bptm group. A Venn diagram shows the quantity of shared and unique QS-controlled orthologs in B. thailandensis (Bt), B. pseudomallei (Bp), and B. mallei (Bm).
The core Bptm QS-controlled factors.
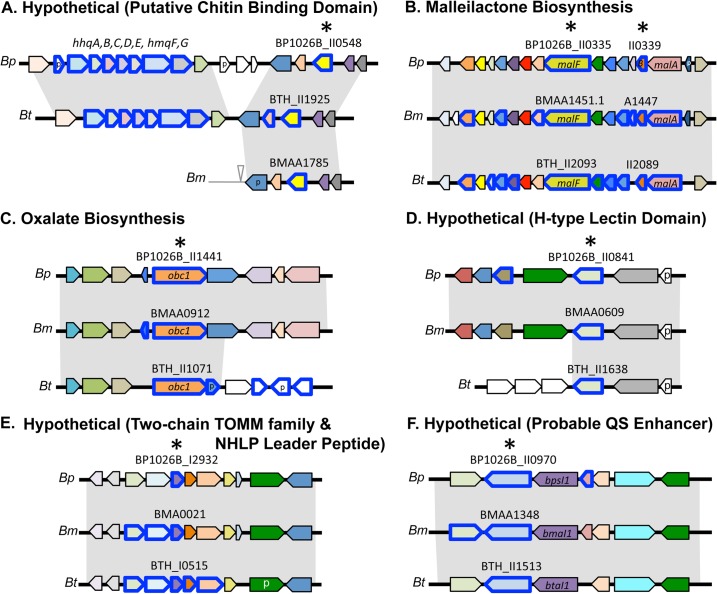
We identified seven orthologs that showed QS regulation in all three Burkholderia species. These orthologs code for a predicted chitin-binding protein (CBP), two products involved with malleilactone synthesis, the Obc1 enzyme for oxalate biosynthesis, and three hypothetical proteins of unknown function (Table 2). Figure 3 shows synteny maps of the orthologs in the core regulon. Nearly all of the core QS-controlled genes showed QS activation.
TABLE 2.
The Bptm core of QS-controlled genesa
| Locus tag number inb: |
Gene, description | Fold change |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
B. pseudomallei |
B. mallei |
B. thailandensis |
|||||||||||||||
| B. pseudomallei | B. mallei | B. thailandensis | WTc | QS-1d | QS-2d | QS-3d | All AHLsd | WTc | QS-1d | QS-3d | Bothd | WTc | QS-1d | QS-2d | QS-3d | All AHLsd | |
| I2932 | 0021 | I0515 | Hypothetical protein | 8.1+ | 3.2+ | 4.3+ | 8.8+ | 3.8+ | 12.8+ | ||||||||
| II0335 | A1451.1 | II2093 | malF, polyketide nonribosomal peptide synthase | 5.5+ | 5.2+ | 7.1+ | 7.0− | 7.0− | 6.2+ | ||||||||
| II0339 | A1447 | II2089 | malB, hypothetical protein | 7.4+ | 6.7+ | 6.6+ | 10.0+ | 16.6− | 18.4− | ||||||||
| II0548 | A1785 | II1925 | Putative chitin-binding protein | 46.2+ | 74.5+ | 185.9+ | 20.3+ | 24.5+ | 218.9+ | 23.4+ | 54.6+ | 71.5+ | 242.7+ | ||||
| II0841 | A0609 | II1638 | Hypothetical protein | 19.5+ | 17.1+ | 20.7+ | 3.7+ | 18.1+ | 33.1+ | 6.4+ | |||||||
| II0970 | A1348 | II1513 | Hypothetical protein | 7.4+ | 7.7+ | 7.2+ | 9.5+ | 7.9+ | |||||||||
| II1441 | A0912 | II1071 | obc1, oxalate biosynthesis | 23.3+ | 2.2+ | 3.3+ | 11.7+ | 8.7+ | 10.0+ | 15.4+ | 31.7+ | ||||||
Orthologs present in the QS regulons of B. pseudomallei, B. mallei, and B. thailandensis.
Locus tags correspond to the B. pseudomallei 1026b genome (BP1026B_[locus tag number]), the B. mallei ATCC 23344 genome (BMA_[locus tag number]), or the B. thailandensis E264 genome (BTH_[locus tag number]).
The fold change due to induction (+) or repression (−) in the B. pseudomallei Bp82, B. mallei GB8, or B. thailandensis E264 WT parent compared to the AHL-negative mutant CM153, CM38, or JRC112, without added AHLs.
The fold change due to induction (+) or repression (−) by AHLs (QS-1 for C8-HSL, QS-2 for 3OHC10-HSL, QS-3 for 3OHC8-HSL, both for C8-HSL and 3OHC8-HSL, or for all three AHLs) when added to the AHL-negative mutant CM153, CM38, or JRC112.
FIG 3.
Synteny maps of chromosome regions in B. pseudomallei 1026b (Bp), B. mallei ATCC 23344 (Bm), and B. thailandensis E264 (Bt), showing orthologs of the core QS regulon. Relevant genes are indicated by the locus tag or gene name. Genes are shown as arrows, and orthologs are color coded across species. An asterisk above a gene indicates that it is part of the core. Orthologs outlined in blue show QS control. An open triangle indicates an insertion sequence, and pseudogenes are labeled with a p. (A) Genes surrounding a hypothetical protein with a predicted chitin-binding domain. (B) Mallielactone biosynthesis region. (C) Region containing the oxalate biosynthetic gene, obc1. (D) Region containing a hypothetical protein with an H-type lectin domain. (E) Region containing a hypothetical protein with a two-chain TOMM family domain and an NHLP leader peptide. (F) Region containing a hypothetical protein that has a reductase (HMGR) domain and likely acts as a QS-1 enhancer.
QS-controlled genes common to B. pseudomallei and B. thailandensis.
Comparison of the Bptm QS regulons showed that there are 71 orthologs uniquely QS controlled in B. pseudomallei and B. thailandensis (Fig. 2; see also Table S5 in the supplemental material). The majority of these, 39 (55%), do not have orthologs in the B. mallei ATCC 23344 genome. This majority exceeds the genome-wide distributions in B. pseudomallei and B. thailandensis; 20.7% of the genes in B. pseudomallei 1026b and 30.5% of the genes in B. thailandensis E264 do not have a B. mallei ATCC 23344 ortholog. Thus, the QS regulon uniquely shared by the saprophytic species is enriched for genes no longer present in B. mallei.
QS-controlled genes unique to B. pseudomallei and B. mallei.
We identified two orthologs unique to the B. pseudomallei and B. mallei QS regulons, BP1026B_I1678/BMA1121 (Fig. 4B) and BP1026B_I1564/BMA1011. Neither of these orthologs is found in the genome of B. thailandensis. The BP1026B_I1564/BMA1011 ortholog codes for a hypothetical protein with no conserved domains. Genes neighboring BP1026B_I1564 are QS controlled in B. pseudomallei (BP1026B_I1563 to -I1566), but the orthologous region is not in B. mallei. BP1026B_I1678/BMA1011 is discussed in greater detail below.
FIG 4.
Synteny maps of the chromosome regions in B. pseudomallei 1026b (Bp), B. mallei ATCC 23344 (Bm), and B. thailandensis E264 (Bt), showing QS-controlled genes. Relevant genes are indicated by locus tags that correspond to the Bp genome (BP1026B_[locus tag number]), Bm genome (BMA_[locus tag number]), or the Bt genome (BTH_[locus tag number]). Genes are shown as arrows, and orthologs are color coded across species. Species-specific genes are white. Orthologs outlined in blue show QS control. An open triangle indicates an insertion sequence, pseudogenes are indicated with a p, and genomic islands (GI) are indicated with a bulleted black horizontal line. (A) The CPS II genes. (B) Genes for a B. pseudomallei-specific secondary metabolite and orthologs unique to the B. mallei and B. pseudomallei QS regulons.
The B. pseudomallei QS regulon.
We identified a total of 216 QS-controlled genes in B. pseudomallei (Table 1). Two genes (BP1026B_II1267 and BP1026B_II1268) were activated by C8-HSL. 3OHC10-HSL regulated 57 genes and 3OHC8-HSL regulated 72 genes, of which 44 are the same. Comparison of the AHL-negative mutant to the Bp82 parent or to itself with all three AHLs added together showed that 41 and 175 genes were differentially regulated, respectively. Twenty of these were differentially regulated in both comparisons (see Fig. 1 for an overview).
The B. mallei QS regulon.
We identified a total of 43 QS-controlled genes in B. mallei. This is considerably fewer than the hundreds regulated by QS in B. pseudomallei and B. thailandensis. The vast majority of the QS-controlled genes in B. mallei showed QS activation (Table 1). The QS-1 signal C8-HSL positively regulated 14 genes. The QS-3 signal 3OHC8-HSL induced five of those activated by C8-HSL, as well as an additional nine genes (Fig. 1). When both AHLs were added together (C8-HSL and 3OHC8-HSL), 32 genes were activated and 1 was repressed. B. mallei does not possess a QS-2 system, and therefore we did not use 3OHC10-HSL in our analyses. When we compared wild-type B. mallei to the AHL-negative strain, we observed that 16 genes were differentially regulated. Fourteen genes showed higher activity in the wild-type strain, indicative of QS activation, and two were QS repressed. Seven genes identified as QS controlled by comparing the wild type to the AHL-negative strain were also QS controlled by the addition of both C8-HSL and 3OHC8-HSL together (Fig. 1).
DISCUSSION
The Bptm group is an interesting triad. Each species has a different lifestyle: B. thailandensis is a saprophyte, B. pseudomallei is an opportunistic pathogen, and B. mallei is a host-restricted pathogen. Yet, these bacteria share a high degree of genetic similarity. The Bptm group affords us an opportunity to explore how homologous genetic regulatory circuits may be used in different ways. We are interested in QS, and we identified QS-controlled genes in each species during the transition from exponential growth to stationary-phase growth (T phase) in LB broth. Recently, we published a comprehensive survey of QS-controlled factors in B. thailandensis and found that T-phase cells exhibited the largest number of QS-controlled genes, with 65% showing QS activation (34). For this reason and because QS is generally thought to regulate important factors as bacteria transition through high-population density growth to stationary phase, we focused our B. pseudomallei and B. mallei QS analyses on T-phase cells. However, we acknowledge that our analysis was limited, and additional QS-controlled factors would likely be identified under different growth conditions.
The largest set of QS-controlled genes was in the saprophyte B. thailandensis (>500 genes). The QS regulon of the opportunistic pathogen B. pseudomallei included between 200 and 300 genes, and that of the host-adapted B. mallei contained only about 40 genes. With this information, we asked which genes had QS-controlled orthologs in all three species. Is there a core set of QS-controlled genes in the Bptm group? Seven orthologs showed QS control in all members of the Bptm group. We refer to the set of seven as the core QS regulon. These orthologs code for a predicted CBP, two products involved with malleilactone synthesis, the Obc1 enzyme for oxalate biosynthesis, and three hypothetical proteins of unknown function (Table 2 and Fig. 3).
The predicted CBP orthologs (Fig. 3A) contain a chitin-binding 3 family domain (which is often associated with cellulose and chitin binding) and are predicted to be membrane associated and located primarily on the outside of the cell. The B. mallei putative CBP ortholog BMAA1785 is a virulence factor in a wax moth larva infection model (36). Malleilactone is a polyketide synthase-derived product that shows iron-binding activity and acts as a virulence factor for B. thailandensis in a Caenorhabditis elegans infection model (37). We recently reported that AHLs differentially regulate the mal genes in B. thailandensis (34). In B. pseudomallei, malB and malF showed QS activation when all three AHLs were added to an AHL-negative mutant; in B. mallei, many mal genes (malA to malM) were QS activated by single or combined additions of AHLs to an AHL-negative mutant. The obc1 genes code for an oxalate biosynthetic enzyme. Oxalate is an anion made by many species in several domains of life. This anion serves diverse functions; it is a virulence factor for some pathogenic fungi, it can act as a chelator for certain metals, it is an end product of metabolism in many animal and plant tissues, and it can serve as a carbon source for some bacteria (38–40). Oxalate production is QS dependent and serves an important role in pH homeostasis of B. thailandensis and B. pseudomallei (33, 41). These bacteria activate obc1 via the QS-1 system, and the consequent oxalate production serves to counter ammonia-induced base toxicity and prevent cell death in stationary phase (33). The observation that obc1 is QS activated in B. mallei raises the possibility that B. mallei also uses oxalate to prevent base-induced toxicity.
The remaining three QS-controlled core orthologs code for uncharacterized hypothetical proteins. One set (BP1026B_II0841, BTH_II1638, and BMAA0609) is found only in closely related Burkholderia species, including B. oklahomensis, and codes for a protein with an H-type lectin domain (Table 2 and Fig. 3D). H-type lectin domains are frequently involved with carbohydrate binding and cell recognition or adhesion (42). Another of the core orthologs is encoded by BP1026B_I2932, BTH_I0515, and BMA0021 (Table 2 and Fig. 3E). This ortholog is predicted to code for a polypeptide with a domain characteristic of thiazole/oxazole-modified microcins (TOMMs). TOMMs are ribosomally produced peptides that contain posttranslationally installed heterocycles. TOMMs have diverse activities (antibacterial, antitumor, or morphogenic) (43). Finally, BP1026B_II0970, BTH_II1513, and BMAA1348 are members of the QS-controlled core. These genes code for proteins with a hydroxymethylglutaryl-coenzyme A reductase (HMGR) domain (Table 2 and Fig. 3F). HMGR enzymes are involved in mevalonate and ultimately isoprenoid synthesis. These products are used in signal transduction networks or lipid synthesis (44). In all species, the gene for this hypothetical HMGR protein lies immediately downstream of the QS-1 luxI homolog (bpsI1, btaI1, or bmaI1) (Fig. 3F). Though B. thailandensis is considered nonpathogenic, it is able to replicate in cultured mammalian cells and is able to resist predation by amoeba (45, 46). B. thailandensis and B. pseudomallei mutants in this ortholog (BP1026B_II0970/BTH_II1513) showed reduced survival in Dictyostelium discoideum amoeba but were able to replicate to wild type-levels during intracellular infection assays in RAW 264.7 cells (46). In Burkholderia cenocepacia, an orthologous gene acts as an enhancer of AHL-mediated phenotypes (47). The organization of the QS-1 system and the QS-enhancer genes shows a high degree of synteny among B. cenocepacia and the Bptm group, raising the possibility that this gene has a broad role in the QS-controlled phenotypes of related Burkholderia.
Are there QS-controlled genes common to the two species that can live as saprophytes? We found a large group of QS-controlled factors (71 orthologs) unique to the QS regulons of B. pseudomallei and B. thailandensis (see Table S5 in the supplemental material). A striking trend is that many of these genes are absent from the B. mallei genome. They include the gene clusters for capsule polysaccharide synthesis II (CPS II) (Fig. 4A), several secondary metabolites (including the PQS-like signal 2-alkyl-4-quinolone [Fig. 3A]), bactobolin, and an additional uncharacterized product made by genes upstream of the bactobolin cluster (Table 3).
TABLE 3.
QS-controlled genes associated with secondary metabolite production
| Secondary metabolite | Locus tag(s) for production of the metabolite bya: |
||
|---|---|---|---|
| B. pseudomallei | B. thailandensis | B. mallei | |
| 2-Alkyl-4-quinolone | BP1026B_II0535-II0541 | BTH_II1929-II1935 | |
| Bactobolin | BP1026B_II1232-II1254 | BTH_II1223-II1242 | |
| Burkholdac | BTH_I2357-I2369 | ||
| Isonitrile | BP1026B_II0180-II0185 | BTH_II0229-I2357 | BMAA1919-A1924 |
| Maleobactin | BP1026B_I1731-I1736 | BTH_I2414-I2419 | BMA1177-1183 |
| Malleilactone | BP1026B_II0328-II0340 | BTH_II2088-II2099 | BMAA1446-A1459 |
| Pyochelin | BP1026B_II0641-II0648 | BTH_II1826-II1833 | |
| Rhamnolipid 1 | BP1026B_II0593-II0598 | BTH_II1075-II1081 | BMAA0459-A0464 |
| Rhamnolipid 2 | BP1026B_II1432-II1437 | BTH_II1875-II1881 | BMAA0919-A0925 |
| Terphenyl | BP1026B_II0147 | BTH_II0204 | |
| Thailandamide | BTH_II1662-II1681 | ||
| Unknown | BP1026B_I1157-I1176 | BTH_I1952-I1971 | BMA1620-1639 |
| Unknown | BP1026B_II1935-II1945 | BTH_II0562-II0572 | |
| Unknown | BP1026B_II1250-II1267 | BTH_II1209-II1218 | |
| Unknown | BP1026B_II2504-II2509 | BTH_II2344-II2349 | BMAA2085-A2090 |
| Unknown | BP1026B_I1663-I1681 | BMA1122*-1038 | |
| Unknown | BP1026B_II1103-II1108 | BMAA1200-A1206* | |
| Syrbactin | BP1026B_II1345-II1353 | BMAA1016-A1021*; BMAA1117-A1119* | |
| Malleipeptin | BP1026B_II1742-II1746 | BMAA1642-A1647 | |
Genetic determinants for predicted and characterized secondary metabolites are indicated by locus tags for B. pseudomallei 1026b, B. thailandensis E264, and B. mallei ATCC 23344. Orthologous regions are shown for each metabolite across species columns. Underlined text corresponds to loci that showed QS activation. Italics correspond to loci that showed both positive and negative regulation by QS under different conditions. An asterisk indicates that a B. mallei cluster is interrupted by an insertion sequence, compared to the orthologous B. pseudomallei cluster.
We also observed instances where gene clusters conserved in the saprophytic QS regulons were present but showed degeneration in the B. mallei genome. In B. pseudomallei and B. thailandensis, QS controls genes for an uncharacterized secondary metabolite (BP1026B_I1157 and -I1158/BTH_I1950 to -I1970) (Table 3). Genes in the orthologous B. mallei cluster are not QS controlled. Interestingly, one of these orthologs in the B. mallei cluster (BMA1365) is a predicted nonfunctional pseudogene. Another example is an operon that encodes predicted histidine transport functions (BP1026B_I0929 to BP1026B_I0932 [BP1026B_I0929-I0932]/BTH_I1772-I1774). This operon was QS repressed in B. pseudomallei and B. thailandensis (see Table S5 in the supplemental material). The orthologous region in B. mallei is not QS controlled, and the first gene in the operon is a pseudogene. A final example involves a small uncharacterized operon (BP1026B_II1878-II1880/BTH_II0626-BTH_II0627) that shows QS activation in both B. pseudomallei and B. thailandensis but not B. mallei. The BP1026B_II1880/BTH_II0626 ortholog codes for an acetyltransferase family protein with 10 transmembrane domains. The B. mallei ortholog (BMAA0415) is a pseudogene. We suggest that perhaps functional degeneration as well as gene loss have driven a reduction in the QS-controlled genes in B. mallei.
Finally, there are genes that are QS controlled in only B. pseudomallei and B. thailandensis yet have B. mallei orthologs. We cannot exclude the possibility that they are QS controlled in B. mallei under conditions that we did not test or that they are not QS controlled in B. mallei for another reason. B. mallei has lost over 20% of its ancestral genome, and this may have pleotropic regulatory impacts.
Our cross-species analysis also identified two orthologs unique to the genomes and QS regulons of B. pseudomallei and B. mallei, BP1026B_I1678/BMA1121 and BP1026B_I1564/BMA1011. BP1026B_I1678/BMA1121 each code for JmjC domain-containing polypeptides. JmjC domains are found in members of the cupin metalloenzyme superfamily. The function of BP1026B_I1678/BMA1121 remains to be determined. In eukaryotes, Jumonji (jmj) family proteins are involved in histone modification by methylation. Jmj domains are present in bacterial proteins but remain uncharacterized (48). In B. pseudomallei, BP1026B_I1678 is flanked by numerous QS-controlled genes, including a gene cluster for a predicted secondary metabolite found only in this species (Fig. 4B). Examination of the orthologous region in B. mallei showed that a large IS-mediated deletion event likely occurred near BMA1121 and that two neighboring pseudogenes showed QS activation (Fig. 4B). The second ortholog that is uniquely QS controlled by B. pseudomallei and B. mallei is BP1026B_I1564/BMA1011. Genes neighboring BP1026B_I1564 are also QS activated in B. pseudomallei and code for uncharacterized hypothetical proteins, one of which contains an LpqC (poly-3-hydroxybutyrate depolymerase) domain and a signal peptide, suggesting a role as a secreted hydrolase.
We identified a group of nine orthologs controlled by QS in B. mallei and B. thailandensis but not B. pseudomallei. All nine factors have orthologs in the B. pseudomallei genome. A close look at these factors showed that the majority of them actually group to genes associated with the core Bptm QS regulon. Four of the nine are malleilactone biosynthesis genes (Fig. 3B), and two map to a TOMM gene cluster (Fig. 3E).
QS contributes to B. pseudomallei virulence (21, 26, 29), yet it is unknown which QS-controlled factors are important in the host. The most strongly QS-controlled B. pseudomallei genes code for CPS II (also QS controlled in B. thailandensis), a predicted CBP (which is part of the core regulon), and the production of secondary metabolites. The observation that QS strongly activates the CPS II genes (BP106B_II0468 to -II0473) is consistent with the observation that QS promotes biofilm formation in B. pseudomallei (24). However, B. pseudomallei produces four CPS or exopolysaccharide clusters, and we do not know which of these contribute to cell aggregation or surface adherence.
As is true for B. thailandensis (34), QS controls many B. pseudomallei genes involved in secondary metabolite production. These B. pseudomallei secondary metabolite genes include those coding for bactobolin, malleilactone, 2-alkyl-4-quinolone, and two uncharacterized products (Table 3), all of which are also regulated by QS in B. thailandensis. The B. pseudomallei QS regulon also contains a predicted secondary metabolite gene cluster unique to this species (Table 3 and Fig. 4B). Genes involved in production of several factors previously associated with B. pseudomallei virulence showed complex QS regulation. The type III secretion system effector genes, bopE, bipD, and bsaM, were repressed by QS, while the genes for Burkholderia lethal factor 1 (BP1026B_1486) and wbiD for lipopolysaccharide biosynthesis were QS activated.
How do our results compare to those from other studies of QS gene regulation in the Bptm group? The best-studied member of the group is B. pseudomallei. Thus, we compared our B. pseudomallei findings to previous reports on QS-controlled factors in B. pseudomallei (see Table S6 in the supplemental material). Two groups identified QS-1-controlled factors in different B. pseudomallei isolates (Bp008 and PP844) (49, 50), and a third group used microarrays to study yet another B. pseudomallei strain (K96243) (unpublished data, available at http://www.melioidosis.info/about.aspx). Nearly half, 97, of the 216 B. pseudomallei QS-controlled genes we identified were also identified in other studies. This overlap provides a validation of the results reported by the different groups, and QS-controlled genes uniquely found by the different groups may be due to strain-to-strain variations, sampling differences, or other variations in methodology and analysis.
The relatively small B. mallei QS regulon included genes for the predicted CBP discussed above, malleilactone biosynthesis, and the JmjC domain-containing protein, also discussed above. Additionally, the benABC operon was QS activated. It is unclear where B. mallei, which is a host-restricted bacterium, might come into contact with benzoate. However, the BenB ortholog in B. pseudomallei was identified as an antigen in human sera, suggesting that this factor is produced in vivo during melioidosis (51). We note that we did not see the ben operon in our B. pseudomallei QS regulon.
Comparison of the QS regulons of the Bptm group affords the opportunity to begin to address how a QS regulon might evolve. There is suggestive evidence that some QS-controlled factors in B. mallei are actively being maintained and some are not. For example, the region coding for the predicted CBP (Fig. 3A) shows conservation and divergence among the QS regulons and genomes of the Bptm group; the predicted CBP genes in each species are orthologous, but the neighboring genes in B. mallei are divergent. The genes for the predicted CBP orthologs are among the most highly QS-activated genes in each species. In B. mallei, the CBP is a virulence factor in an insect infection model (36). This highlights the fact that this protein is functional in B. mallei. However, the role of this protein in an insect (which has a high composition of chitin) might be very different than its role in a mammal or in the environment. The region coding for the putative CBP ortholog also codes for a PQS-like cluster found in B. thailandensis and B. pseudomallei but not B. mallei. The PQS cluster in B. thailandensis and in B. pseudomallei is QS regulated (Fig. 3A). Presumably, the PQS cluster was eliminated from the B. mallei genome by IS element-mediated gene loss, a driving force for B. mallei genome erosion (8). It seems significant that the QS-activated CBP gene is retained in B. mallei.
There also appear to be regions of the B. mallei genome and QS regulon that are decaying remnants of the ancestral B. pseudomallei isolate from which B. mallei evolved. The synteny map of such a region (Fig. 4B) has been discussed previously, as it contains B. pseudomallei sequence for a predicted B. pseudomallei-unique secondary metabolite. In this region, there is considerable divergence among the three Burkholderia species. The B. pseudomallei region shows extensive regulation by QS. Many of the QS-controlled orthologs are absent from the B. mallei genome (there is a large deletion and a number of pseudogenes), but some that remain show QS control. It seems unlikely that this region is functional in B. mallei. As discussed above, there are multiple examples of QS-controlled orthologs in B. pseudomallei and B. thailandensis that are pseudogenes in B. mallei and also not regulated by QS. Such observations represent other instances of functional degeneration in the B. mallei genome that are associated with loss of QS control.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Northwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (U54AI057141) and by U.S. Public Health Service grant GM-59026.
Footnotes
Published ahead of print 2 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01974-14.
REFERENCES
- 1.Majerczyk C, Greenberg EP, Chandler JR. 2012. Quorum sensing in Burkholderia, p 40–57 In Vasil M, Darwin A. (ed), Regulation of bacterial virulence. ASM Press, Washington, DC. [Google Scholar]
- 2.Brett PJ, DeShazer D, Woods DE. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48:317–320. 10.1099/00207713-48-1-317. [DOI] [PubMed] [Google Scholar]
- 3.Dance DA. 2000. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 74:159–168. 10.1016/S0001-706X(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AC, Currie BJ. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383–416. 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitlock GC, Estes DM, Torres AG. 2007. Glanders: off to the races with Burkholderia mallei. FEMS Microbiol. Lett. 277:115–122. 10.1111/j.1574-6968.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 6.Yu Y, Kim HS, Chua HH, Lin CH, Sim SH, Lin D, Derr A, Engels R, Deshazer D, Birren B, Nierman WC, Tan P. 2006. Genomic patterns of pathogen evolution revealed by comparison of Burkholderia pseudomallei, the causative agent of melioidosis, to avirulent Burkholderia thailandensis. BMC Microbiol. 6:46. 10.1186/1471-2180-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holden MTG, Titball RW, Peacock SJ, Cerdeño-Tárraga AM, Atkins T, Crossman LC, Pitt T, Churcher C, Mungall K, Bentley SD, Sebaihia M, Thomson NR, Bason N, Beacham IR, Brooks K, Brown KA, Brown NF, Challis GL, Cherevach I, Chillingworth T, Cronin A, Crossett B, Davis P, Deshazer D, Feltwell T, Fraser A, Hance Z, Hauser H, Holroyd S, Jagels K, Keith KE, Maddison M, Moule S, Price C, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Simmonds M, Songsivilai S, Stevens K, Tumapa S, Vesaratchavest M, Whitehead S, Yeats C, Barrell BG, Oyston PCF, Parkhill J. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. U. S. A. 101:14240–14245. 10.1073/pnas.0403302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nierman WC, Deshazer D, Kim HS, Tettelin H, Nelson KE, Feldblyum T, Ulrich RL, Ronning CM, Brinkac LM, Daugherty SC, Davidsen TD, Deboy RT, Dimitrov G, Dodson RJ, Durkin AS, Gwinn ML, Haft DH, Khouri H, Kolonay JF, Madupu R, Mohammoud Y, Nelson WC, Radune D, Romero CM, Sarria S, Selengut J, Shamblin C, Sullivan SA, White O, Yu Y, Zafar N, Zhou L, Fraser CM. 2004. Structural flexibility in the Burkholderia mallei genome. Proc. Natl. Acad. Sci. U. S. A. 101:14246–14251. 10.1073/pnas.0403306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song H, Hwang J, Yi H, Ulrich RL, Yu Y, Nierman WC, Kim HS. 2010. The early stage of bacterial genome-reductive evolution in the host. PLoS Pathog. 6:e1000922. 10.1371/journal.ppat.1000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Losada L, Ronning CM, Deshazer D, Woods D, Fedorova N, Kim HS, Shabalina SA, Pearson TR, Brinkac L, Tan P, Nandi T, Crabtree J, Badger J, Beckstrom-Sternberg S, Saqib M, Schutzer SE, Keim P, Nierman WC. 2010. Continuing evolution of Burkholderia mallei through genome reduction and large-scale rearrangements. Genome Biol. Evol. 2:102–116. 10.1093/gbe/evq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuqua WC, Winans SC, Greenberg EP. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsek MR, Greenberg EP. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. U. S. A. 97:8789–8793. 10.1073/pnas.97.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loh J, Pierson EA, Pierson LS, Stacey G, Chatterjee A. 2002. Quorum sensing in plant-associated bacteria. Curr. Opin. Plant Biol. 5:285–290. 10.1016/S1369-5266(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 14.Dunn AK. 2012. Vibrio fischeri metabolism: symbiosis and beyond. Adv. Microb. Physiol. 61:37–68. 10.1016/B978-0-12-394423-8.00002-0. [DOI] [PubMed] [Google Scholar]
- 15.Fuqua C, Winans SC, Greenberg EP. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50:727–751. 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 16.Rutherford ST, Bassler BL. 2012. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2:a012427. 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Straight PD, Kolter R. 2009. Interspecies chemical communication in bacterial development. Annu. Rev. Microbiol. 63:99–118. 10.1146/annurev.micro.091208.073248. [DOI] [PubMed] [Google Scholar]
- 18.Chandler JR, Heilmann S, Mittler JE, Greenberg EP. 2012. Acyl-homoserine lactone-dependent eavesdropping promotes competition in a laboratory co-culture model. ISME J. 12:2219–2228. 10.1038/ismej.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulrich RL, Hines HB, Parthasarathy N, Jeddeloh JA. 2004. Mutational analysis and biochemical characterization of the Burkholderia thailandensis DW503 quorum-sensing network. J. Bacteriol. 186:4350–4360. 10.1128/JB.186.13.4350-4360.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulrich RL, Deshazer D, Hines HB, Jeddeloh JA. 2004. Quorum sensing: a transcriptional regulatory system involved in the pathogenicity of Burkholderia mallei. Infect. Immun. 72:6589–6596. 10.1128/IAI.72.11.6589-6596.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulrich RL, Deshazer D, Brueggemann EE, Hines HB, Oyston PC, Jeddeloh JA. 2004. Role of quorum sensing in the pathogenicity of Burkholderia pseudomallei. J. Med. Microbiol. 53:1053–1064. 10.1099/jmm.0.45661-0. [DOI] [PubMed] [Google Scholar]
- 22.Chandler JR, Duerkop BA, Hinz A, West TE, Herman JP, Churchill MEA, Skerrett SJ, Greenberg EP. 2009. Mutational analysis of Burkholderia thailandensis quorum sensing and self-aggregation. J. Bacteriol. 191:5901–5909. 10.1128/JB.00591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duerkop BAB, Ulrich RLR, Greenberg EPE. 2007. Octanoyl-homoserine lactone is the cognate signal for Burkholderia mallei BmaR1-BmaI1 quorum sensing. J. Bacteriol. 189:5034–5040. 10.1128/JB.00317-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamage AM, Shui G, Wenk MR, Chua KL. 2011. N-Octanoylhomoserine lactone signalling mediated by the BpsI-BpsR quorum sensing system plays a major role in biofilm formation of Burkholderia pseudomallei. Microbiology (Reading, Engl.) 157:1176–1186. 10.1099/mic.0.046540-0. [DOI] [PubMed] [Google Scholar]
- 25.Lumjiaktase P, Diggle SP, Loprasert S, Tungpradabkul S, Daykin M, Cámara M, Williams P, Kunakorn M. 2006. Quorum sensing regulates dpsA and the oxidative stress response in Burkholderia pseudomallei. Microbiology (Reading, Engl.) 152:3651–3659. 10.1099/mic.0.29226-0. [DOI] [PubMed] [Google Scholar]
- 26.Song Y, Xie C, Ong Y-M, Gan Y-H, Chua KL. 2005. The BpsIR quorum-sensing system of Burkholderia pseudomallei. J. Bacteriol. 187:785–790. 10.1128/JB.187.2.785-790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duerkop BA, Varga J, Chandler JR, Peterson SB, Herman JP, Churchill MEA, Parsek MR, Nierman WC, Greenberg EP. 2009. Quorum-sensing control of antibiotic synthesis in Burkholderia thailandensis. J. Bacteriol. 191:3909–3918. 10.1128/JB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duerkop BA, Herman JP, Ulrich RL, Churchill MEA, Greenberg EP. 2008. The Burkholderia mallei BmaR3-BmaI3 quorum-sensing system produces and responds to N-3-hydroxy-octanoyl homoserine lactone. J. Bacteriol. 190:5137–5141. 10.1128/JB.00246-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valade E, Thibault FM, Gauthier YP, Palencia M, Popoff MY, Vidal DR. 2004. The PmlI-PmlR quorum-sensing system in Burkholderia pseudomallei plays a key role in virulence and modulates production of the MprA protease. J. Bacteriol. 186:2288–2294. 10.1128/JB.186.8.2288-2294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton RE, Grant GD, Matthews B, Batzloff M, Owen SJ, Kyan S, Flegg CP, Clark AM, Ulett GC, Morrison N, Peak IR, Beacham IR. 2013. Quorum sensing negatively regulates multinucleate cell formation during intracellular growth of Burkholderia pseudomallei in macrophage-like cells. PLoS One 8:e63394. 10.1371/journal.pone.0063394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majerczyk C, Kinman L, Han T, Bunt R, Greenberg EP. 2013. Virulence of Burkholderia mallei quorum sensing mutants. Infect. Immun. 81:1471–1478. 10.1128/IAI.00048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López CM, Rholl DA, Trunck LA, Schweizer HP. 2009. Versatile dual-technology system for markerless allele replacement in Burkholderia pseudomallei. Appl. Environ. Microbiol. 75:6496–6503. 10.1128/AEM.01669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goo E, Majerczyk CD, An JH, Chandler JR, Seo Y-S, Ham H, Lim JY, Kim H, Lee B, Jang MS, Greenberg EP, Hwang I. 2012. Bacterial quorum sensing, cooperativity, and anticipation of stationary-phase stress. Proc. Natl. Acad. Sci. U. S. A. 109:19775–19780. 10.1073/pnas.1218092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majerczyk C, Brittnacher M, Jacobs M, Armour CD, Radey M, Schneider E, Phattarasokul S, Bunt R, Greenberg EP. 2014. Global analysis of the Burkholderia thailandensis quorum sensing-controlled regulon. J. Bacteriol. 196:1412–1424. 10.1128/JB.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brittnacher MJ, Fong C, Hayden HS, Jacobs MA, Radey M, Rohmer L. 2011. PGAT: a multistrain analysis resource for microbial genomes. Bioinformatics 27:2429–2430. 10.1093/bioinformatics/btr418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schell MA, Lipscomb L, Deshazer D. 2008. Comparative genomics and an insect model rapidly identify novel virulence genes of Burkholderia mallei. J. Bacteriol. 190:2306–2313. 10.1128/JB.01735-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biggins JB, Ternei MA, Brady SF. 2012. Malleilactone, a polyketide synthase-derived virulence factor encoded by the cryptic secondary metabolome of Burkholderia pseudomallei group pathogens. J. Am. Chem. Soc. 134:13192–13195. 10.1021/ja3052156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Kan JAL. 2006. Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 11:247–253. 10.1016/j.tplants.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Dehner CA, Awaya JD, Maurice PA, DuBois JL. 2010. Roles of siderophores, oxalate, and ascorbate in mobilization of iron from hematite by the aerobic bacterium Pseudomonas mendocina. Appl. Environ. Microbiol. 76:2041–2048. 10.1128/AEM.02349-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahin N. 2003. Oxalotrophic bacteria. Res. Microbiol. 154:399–407. 10.1016/S0923-2508(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 41.Rogul M, Carr SR. 1972. Variable ammonia production among smooth and rough strains of Pseudomonas pseudomallei: resemblance to bacteriocin production. J. Bacteriol. 112:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, Esko JD, Sharon N. 2009. Microbial lectins: hemagglutinins, adhesins, and toxins, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- 43.Melby JO, Nard NJ, Mitchell DA. 2011. Thiazole/oxazole-modified microcins: complex natural products from ribosomal templates. Curr. Opin. Chem. Biol. 15:369–378. 10.1016/j.cbpa.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Istvan ES. 2001. Bacterial and mammalian HMG-CoA reductases: related enzymes with distinct architectures. Curr. Opin. Struct. Biol. 11:746–751. 10.1016/S0959-440X(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 45.Harley VS, Dance DA, Drasar BS, Tovey G. 1998. Effects of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios 96:71–93. [PubMed] [Google Scholar]
- 46.Hasselbring BM, Patel MK, Schell MA. 2011. Dictyostelium discoideum as a model system for identification of Burkholderia pseudomallei virulence factors. Infect. Immun. 79:2079–2088. 10.1128/IAI.01233-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Grady EP, Viteri DF, Sokol PA. 2012. A unique regulator contributes to quorum sensing and virulence in Burkholderia cenocepacia. PLoS One 7:e37611. 10.1371/journal.pone.0037611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeuchi T, Watanabe Y, Takano-Shimizu T, Kondo S. 2006. Roles of jumonji and jumonji family genes in chromatin regulation and development. Dev. Dyn. 235:2449–2459. 10.1002/dvdy.20851. [DOI] [PubMed] [Google Scholar]
- 49.Wongtrakoongate P, Tumapa S, Tungpradabkul S. 2012. Regulation of a quorum sensing system by stationary phase sigma factor RpoS and their co-regulation of target genes in Burkholderia pseudomallei. Microbiol. Immunol. 56:281–294. 10.1111/j.1348-0421.2012.00447.x. [DOI] [PubMed] [Google Scholar]
- 50.Ooi WF, Ong C, Nandi T, Kreisberg JF, Chua HH, Sun G, Chen Y, Mueller C, Conejero L, Eshaghi M, Ang RML, Liu J, Sobral BW, Korbsrisate S, Gan Y-H, Titball RW, Bancroft GJ, Valade E, Tan P. 2013. The condition-dependent transcriptional landscape of Burkholderia pseudomallei. PLoS Genet. 9:e1003795. 10.1371/journal.pgen.1003795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puah SM, Puthucheary SD, Chua KH. 2013. Potential immunogenic polypeptides of Burkholderia pseudomallei identified by shotgun expression library and evaluation of their efficacy for serodiagnosis of melioidosis. Int. J. Med. Sci. 10:539–547. 10.7150/ijms.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.