Abstract
Methylotrophs grow on reduced single-carbon compounds like methylamine as the sole source of carbon and energy. In Methylobacterium extorquens AM1, the best-studied aerobic methylotroph, a periplasmic methylamine dehydrogenase that catalyzes the primary oxidation of methylamine to formaldehyde has been examined in great detail. However, recent metagenomic data from natural ecosystems are revealing the abundance and importance of lesser-known routes, such as the N-methylglutamate pathway, for methylamine oxidation. In this study, we used M. extorquens PA1, a strain that is closely related to M. extorquens AM1 but is lacking methylamine dehydrogenase, to dissect the genetics and physiology of the ecologically relevant N-methylglutamate pathway for methylamine oxidation. Phenotypic analyses of mutants with null mutations in genes encoding enzymes of the N-methylglutamate pathway suggested that γ-glutamylmethylamide synthetase is essential for growth on methylamine as a carbon source but not as a nitrogen source. Furthermore, analysis of M. extorquens PA1 mutants with defects in methylotrophy-specific dissimilatory and assimilatory modules suggested that methylamine use via the N-methylglutamate pathway requires the tetrahydromethanopterin (H4MPT)-dependent formaldehyde oxidation pathway but not a complete tetrahydrofolate (H4F)-dependent formate assimilation pathway. Additionally, we present genetic evidence that formaldehyde-activating enzyme (FAE) homologs might be involved in methylotrophy. Null mutants of FAE and homologs revealed that FAE and FAE2 influence the growth rate and FAE3 influences the yield during the growth of M. extorquens PA1 on methylamine.
INTRODUCTION
Methylated amines are a large group of biologically, industrially, and environmentally relevant compounds. The simplest methylated amine, (mono-)methyl amine (MA), is produced by anaerobic bacteria that decarboxylate nitrogenous organic matter in the gastrointestinal tract or animal waste and decompose methylated osmolytes, such as glycine betaine in the marine environment (1, 2). In addition, a growing fraction of MA is being released during the degradation of nitrogen-containing herbicides and pesticides (3), biomass combustion (1), and industrial processes, such as fish processing. MA also plays an important role in the global carbon and nitrogen budget (2, 4). However, MA does not accumulate in aerobic environments (4) because it gets rapidly consumed by methylotrophs that oxidize reduced single-carbon (C1) substrates to produce biomass and CO2 (5) and serves as a nitrogen source for several classes of aerobic microorganisms (6, 7).
There are three well-characterized enzymes/pathways used by methylotrophs to oxidize MA. In many Gram-negative methylotrophs, such as Methylobacterium extorquens AM1, MA is oxidized by a periplasmic methylamine dehydrogenase (MaDH) (8, 9) to formaldehyde. Methylamine oxidase, a Cu-containing enzyme present in Gram-positive methylotrophs (10) and methylotrophic yeast strains (11), oxidizes MA to formaldehyde (12) in a single step as well. In contrast, the N-methylglutamate (NMG) pathway, originally described in Pseudomonas spp. (now Aminobacter spp. [13]), is a complex, multistep pathway in which the methyl group from MA is transferred to glutamate to form two novel amino acid derivatives, NMG and γ-glutamylmethylamide (GMA), which are then oxidized to release formaldehyde (14). The NMG pathway involves at least three unique enzymes: N-methylglutamate synthase (NMGS), N-methylglutamate dehydrogenase (NMGDH), and γ-glutamylmethylamide synthetase (GMAS) (3, 15–17). While the enzymes involved in the NMG pathway were purified and characterized over 3 decades ago (18–23), the genes were discovered very recently (3, 15, 17). Genes encoding the enzymes of the NMG pathway are often clustered in an ∼10-kb genomic region (Fig. 1). A sarcosine oxidase-like cluster of four genes encodes NMGDH (mgdABCD), a glutamate synthase-like cluster of three genes encodes NMGS (mgsABC), and a glutamine synthetase-like gene (gmaS) encodes GMAS. Since the discovery of the NMG pathway gene cluster, sequence-based analysis has revealed that the NMG pathway is present in a large fraction of methylotrophs (even those containing MaDH) as well as nonmethylotrophs, where it serves to extract the nitrogen from MA (6).
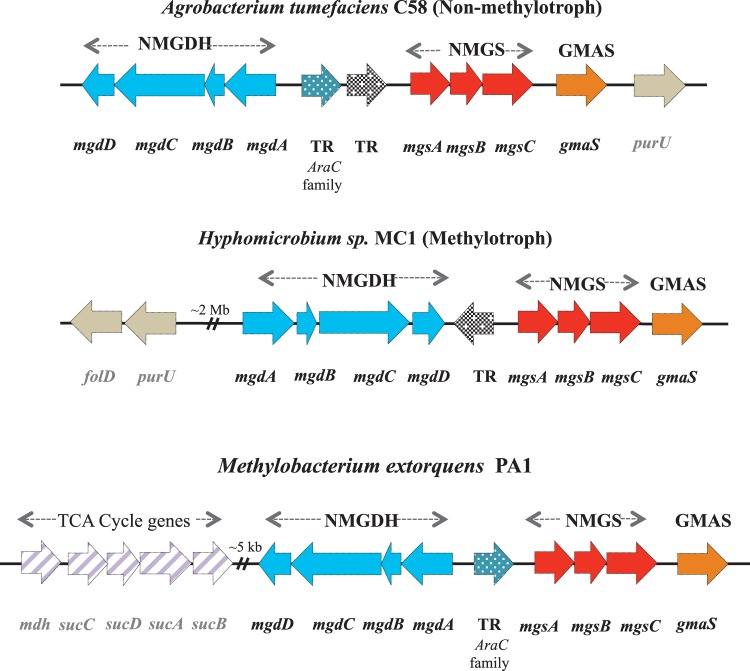
FIG 1.
(A) Arrangement of genes encoding the N-methylglutamate pathway on the chromosome of nonmethylotrophic proteobacteria (like Agrobacterium tumefaciens) and methylotrophic proteobacteria (like Hyphomicrobium sp. strain MC1 and Methylobacterium extorquens PA1). Blue, genes encoding the four subunits of NMGDH (mgdABCD); red, genes encoding the three subunits of NMGS (mgsABC); orange, a single gene encoding GMAS (gmaS). TR, transcriptional regulator; purU, gene encoding formyl-tetrahydrofolate hydrolase; folD, gene encoding bifunctional methylene tetrahydrofolate dehydrogenase and methenyl tetrahydrofolate cyclohydrolase; TCA, tricarboxylic acid.
Recent genetic analyses of the NMG pathway have produced somewhat contradictory results in terms of its topology. A study using Methyloversatilis universalis FAM5 (a facultative methylotroph of the betaproteobacteria) (15) showed that the enzyme γ-glutamylmethylamide synthetase was not essential during growth on low concentrations of MA. This observation supported a notion that γ-glutamylmethylamide synthetase is a bidirectional enzyme that is essential only at high concentrations of MA because it acts as a gatekeeper and keeps the flux of methylamine under control (15). However, in Methylocella silvestris BL2 (a facultative methanotroph of the alphaproteobacteria) (3) and, very recently, Methylobacterium extorquens DM4 (17), γ-glutamylmethylamide synthetase was shown to be essential for growth on MA. These studies supported a topology for the NMG pathway in which γ-glutamylmethylamide synthetase plays an essential role, directly or indirectly, in the formation of N-methylglutamate (24).
Additionally, uncertainty surrounding the end product of methylamine oxidation via the NMG pathway has occluded our understanding of how the NMG pathway feeds into the C1 assimilatory and dissimilatory modules. For a long time, it was assumed that methylamine oxidation mediated by the NMG pathway led to the production of formaldehyde (20). Then, as observed during methanol growth in M. extorquens species (25), the tetrahydromethanopterin (H4MPT)-dependent formaldehyde oxidation pathway (26, 27) would serve as the primary dissimilatory module. Formate, thus produced, would be incorporated into biomass through the tetrahydrofolate (H4F)-dependent C1 assimilation pathway (28–31) and the serine cycle (32, 33) (see Fig. S1 in the supplemental material). Of late, genes encoding a dissimilatory version of the H4F-dependent C1 transfer pathway (34) (characterized by the presence of folD and purU instead of mtdA, fch, and ftfL) have been found in the vicinity of the NMG pathway gene cluster (15) (Fig. 1). Additionally, N-methylglutamate dehydrogenase (mgdC) contains an H4F binding domain and produces methylene-H4F (CH2=H4F) in the presence of H4F in vitro (15). In light of these recent findings, CH2=H4F, instead of formaldehyde, has been proposed to be the end product of the NMG pathway. Like in the case of chloromethane metabolism by M. extorquens CM4 (34, 35), CH2=H4F as the end product of primary oxidation would seem to circumvent the need for an H4MPT-dependent formaldehyde oxidation pathway. A portion of the CH2=H4F would get assimilated via the serine cycle, while the rest would be oxidized by the dissimilatory branch of the H4F-dependent C1 transfer pathway (see Fig. S2 in the supplemental material). However, with burgeoning sequence data, we now know that methylotrophs that encode the NMG pathway often do not contain either folD or purU (data not shown). How do these methylotrophs oxidize CH2=H4F to formate, then? Or does MA oxidation via the NMG pathway lead to the production of some free formaldehyde, instead?
In order to better understand how the NMG pathway operates in vivo, we genetically dissected its physiology in M. extorquens PA1 (36). Although PA1 utilizes methanol in the same way as the better-characterized AM1 strain (25), here we find that it lacks methylamine dehydrogenase and possesses only the NMG pathway for MA use. By creating mutant strains with lesions in one or more genes of the NMG pathway and assaying the growth patterns of each of these strains on MA, we uncovered that the NMG pathway has a linear topology in PA1 akin to that very recently suggested for DM4 (17) and M. silvestris BL2 (3). Methylamine growth of null mutants with mutations in genes encoding enzymes of modules involved in C1 transfer pathways revealed that the H4MPT-dependent formaldehyde oxidation pathway was required for C1 dissimilation, whereas an intact H4F-mediated C1 transfer pathway was not required for either formate assimilation or dissimilation of CH2=H4F. Further, we unveiled that FAE homologs, often found in the genomes of methylotrophs (FAE2, FAE3) (37, 38), play a functional role during methylamine growth using the NMG pathway. The presence of either FAE or FAE2 significantly increased the growth rate but not the yield on methylamine, whereas the presence of FAE3 increased the yield on methylamine.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains relevant to this study included the following: the Δcel strain of the pink-pigmented wild-type (WT) stock of M. extorquens AM1 (CM2720) and the Δcel strain of the pink-pigmented wild-type stock of M. extorquens PA1 (CM2730), as described elsewhere (39). Unless specified, all growth conditions utilized a modified version of Hypho minimal medium, consisting of 100 ml phosphate salts solution (25.3 g of KH2PO4 plus 22.5 g Na2HPO4 in 1 liter deionized water), 100 ml sulfate salts solution [5 g of (NH4)2SO4 and 2 g of MgSO4·7 H2O in 1 liter deionized water], 799 ml of deionized water, and 1 ml of trace metal solution (40). A nitrogen-free version of the modified Hypho minimal medium used the same recipe described above but with a nitrogen-free sulfate salts solution (7 g of MgSO4·7 H2O in 1 liter deionized water). All components were autoclaved separately before mixing under sterile conditions. Filter-sterilized carbon sources were added just prior to inoculation in liquid minimal medium with (final concentrations) 3.5 mM sodium succinate, 15 mM methylamine hydrochloride, 15 mM methanol, and 3.5 mM sodium succinate and with 7.66 mM methylamine as a nitrogen source.
Growth rate measurements.
Apart from growth on methylamine as a carbon source, all other growth measurements were conducted using an automated growth platform (41). All growth regimes consisted of three cycles consisting of inoculation, acclimation, and growth measurement. All strains were stored in vials at −80°C in 10% dimethyl sulfoxide; growth was initiated by transferring 10 μl freezer stock into 10 ml of Hypho medium with 3.5 mM succinate in 50-ml flasks (covered with a 50-ml plastic beaker) in an incubator shaking at 225 rpm, and the strains were maintained at 30°C. Upon reaching stationary phase (∼2 days), the cultures were transferred 1:64 into fresh medium (in 48-well microtiter plates [CoStar 3548]) with the carbon source to be tested to a final volume of 640 μl, allowed to reach saturation in this acclimation phase, and again diluted 1:64 into fresh medium for the measured (experimental) growth. The 48-well microtiter plates were incubated in a room maintained at 30°C and 80% humidity in an incubation tower (Liconic USA LTX44 with custom-fabricated cassettes) shaking at 650 rpm (39). The increase in the optical density at 600 nm (OD600) for strains grown in 48-well microtiter plates was measured using an automated, robotic culturing and monitoring system (41). A series of robotic instruments (including a shovel, a transfer station, and a twister arm), all controlled by the Clarity manager, were used to move the 48-well plates from the incubation tower to a Perkin-Elmer Victor2 plate reader for OD600 measurements. The dynamics and specific growth rates of the cultures were calculated from the log-linear growth phase using open-source, custom-designed growth analysis software called CurveFitter (available at http://www.evolvedmicrobe.com/CurveFitter/).
Growth on methylamine as a carbon source was below the detection threshold of the automated growth platform described above. Growth from freezer stocks at −80°C was initiated by transferring 10 μl freezer stock into 10 ml of Hypho medium with 3.5 mM succinate in 50-ml flasks (covered with a 50-ml plastic beaker) in an incubator shaking at 225 rpm, and the culture was maintained at 30°C. Upon reaching stationary phase (∼2 days), cultures were transferred 1:16 into 9.4 ml fresh medium with 15 mM methylamine hydrochloride, allowed to reach saturation in this acclimation phase (∼3 days), and again diluted 1:16 into 9.4 ml fresh medium with 15 mM methylamine for the measured (experimental) growth (∼3 days). A 50-μl aliquot of three replicate cultures for each strain was sampled every 8 to 10 h during the growth phase. The OD600 of the culture was measured using a spectrophotometer (Bio-Rad). The dynamics and specific growth rates of the cultures were calculated from the log-linear growth phase. The yield was measured as the maximum OD600 during the growth phase. The growth rate and yield reported for each strain and condition are the means calculated from three biological replicates, unless otherwise noted.
Methanol and methylamine shock experiments.
Ten microliters of the freezer stock of strains CM3803 (CM2730 ΔmptG) and CM2730 were grown in 48-well microtiter plates with 3.5 mM succinate as the carbon source. Upon reaching stationary phase (∼2 days), CM3803 and CM2730 were transferred 1:64 into 630 μl of fresh medium with 3.5 mM succinate in two 48-well microtiter plates. After 12 h of incubation, a pulse of methanol resulting in final concentrations of (i) 1 mM methanol, (ii) 5 mM methanol, (iii) 10 mM methanol, and (iv) 50 mM methanol was added to triplicate cultures of CM3803 and CM2730 in one 48-well plate. Similarly, after 12 h of incubation, a pulse of methylamine resulting in final concentrations of (i) 1 mM methylamine, (ii) 5 mM methylamine, (iii) 10 mM methylamine, and (iv) 50 mM methylamine was added to triplicate cultures of CM3803 and CM2730 in the other 48-well plate. Triplicate control wells of CM2730 and CM3803 (to which no methanol and methylamine were added) were maintained in each 48-well plate as well. The increase in the OD600 was measured as described earlier.
Generation of mutant strains.
M. extorquens PA1 deletion mutants lacking mgsABC, fae2, fae3, gmaS, and mgdABCD were generated on the genetic background of CM2730 using the allelic exchange vector pCM433 (44). The double-deletion mutants lacking fae and fae2 or fae3 were generated on the genetic background of CM3753 (CM2730 Δfae) using the allelic exchange vector pCM433 (44). The double-deletion mutants lacking fae2 and fae3 were generated on the genetic background of CM3757 (CM2730 Δfae2) using the allelic exchange vector pCM433 (44). The double-deletion mutants lacking mgsABC and gmaS were generated on the genetic background of CM3733 (CM2730 ΔmgsABC) using the allelic exchange vector pCM433 (44).The triple-deletion mutants lacking fae, fae2, and fae3 were generated on the genetic background of CM4208 (CM2730 Δfae Δfae3) using the allelic exchange vector pCM433 (44). A region upstream and downstream of each of these genes or operons of ∼0.5 kb was amplified using PCR. The forward primer for the upstream flank was designed to have a 30-bp-long sequence at the 5′ end homologous to the sequence upstream of the NotI cut site in pCM433. The reverse primer for the upstream flank was designed to have a 30-bp sequence at the 5′ end homologous to the first 30 bp of the downstream flank. The reverse primer for the downstream flank was designed to have a 30-bp-long sequence at the 5′ end homologous to the sequence downstream of the NotI cut site in pCM433. The PCR products representing the upstream and downstream flanks were simultaneously ligated into the pCM433 vector cut with NotI using a three-part Gibson assembly protocol described elsewhere (45). Cloning of the upstream and downstream flanks for fae2, fae3, mgsABC, gmaS, and mgdABCD in pCM433 resulted in pDN51, pDN52, pDN53, pDN54, and pDN55, respectively. Mutant strains of M. extorquens PA1 were made by introducing the appropriate donor constructs through conjugation by a triparental mating between NEB 10β competent cells (New England BioLabs, Ipswich, MA) containing the donor construct, an Escherichia coli strain containing the conjugative plasmid pRK2073, and PA1, as described elsewhere (44). All mutant strains were confirmed by a diagnostic PCR analysis and validated by Sanger sequencing of the mutant locus. All strains and plasmids used and generated for this study are listed in Table 1.
TABLE 1.
M. extorquens strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| CM2730 | M. extorquens PA1 Δcel | 39 |
| CM3733 | CM2730 ΔmgsABC | This study |
| CM3753 | CM2730 Δfae | 25 |
| CM3757 | CM2730 Δfae2 | This study |
| CM3761 | CM2730 Δfae3 | This study |
| CM3765 | CM2730 ΔgmaS | This study |
| CM3769 | CM2730 ΔmgdABCD | This study |
| CM3773 | CM2730 ΔftfL | 25 |
| CM3799 | CM2730 ΔglyA | 25 |
| CM3803 | CM2730 ΔmptG | 25 |
| CM3835 | CM2730 ΔmgsABC ΔgmaS | This study |
| CM4208 | CM2730 Δfae Δfae3 | This study |
| CM4230 | CM2730 Δfae Δfae2 | This study |
| CM4232 | CM2730 Δfae2 Δfae3 | This study |
| CM4244 | CM2730 Δfae Δfae2 Δfae3 | This study |
| Plasmids | ||
| pCM433 | Allelic exchange vector (Tetr Sucs) | 44 |
| pDN50 | pCM433 with Δfae upstream and downstream flanks | 25 |
| pDN51 | pCM433 with Δfae2 upstream and downstream flanks | This study |
| pDN52 | pCM433 with Δfae3 upstream and downstream flanks | This study |
| pDN53 | pCM433 with ΔmgsABC upstream and downstream flanks | This study |
| pDN54 | pCM433 with ΔgmaS upstream and downstream flanks | This study |
| pDN55 | pCM433 with ΔmgdABCD upstream and downstream flanks | This study |
| pRK2073 | Helper plasmid (IncP tra) | 53 |
RESULTS
γ-Glutamylmethylamide synthetase is conditionally essential during methylamine growth in M. extorquens PA1.
Our strain of PA1 lacking the cel locus (encoding cellulose biosynthesis), to prevent clumping and permit accurate measurement of growth characteristics, is referred to here as the WT (39) and could grow on 15 mM MA with an average growth rate of 0.042 h−1 and an average final OD600 of 0.712 (three biological replicates were used throughout, unless otherwise noted). A 10-kb genomic region encoding enzymes of the NMG pathway was recently characterized in DM4 (17). In PA1, a completely syntenic cluster of nine genes (of which eight had >95% amino acid identity and one had ∼93% amino acid identity to the corresponding genes in DM4) encoding the enzymes of the NMG pathway was observed as well (Fig. 1). To determine if the NMG pathway is active and involved in methylamine oxidation, we made and tested the growth of null mutants lacking individual enzymes of the NMG pathway on MA. No measurable change in the OD600 was observed for the ΔmgdABCD mutant, the ΔmgsABC mutant, or the ΔgmaS mutant on MA as the sole carbon source. Contrary to the findings of studies in other methylotrophs (15), we did not detect growth for the ΔgmaS mutant on any concentration of MA ranging from 1 to 25 mM after 7 days or after a 14-day incubation on 15 mM MA. The essential role of all three enzymes (especially GMAS) for methylamine oxidation supported a linear topology for the NMG pathway in PA1.
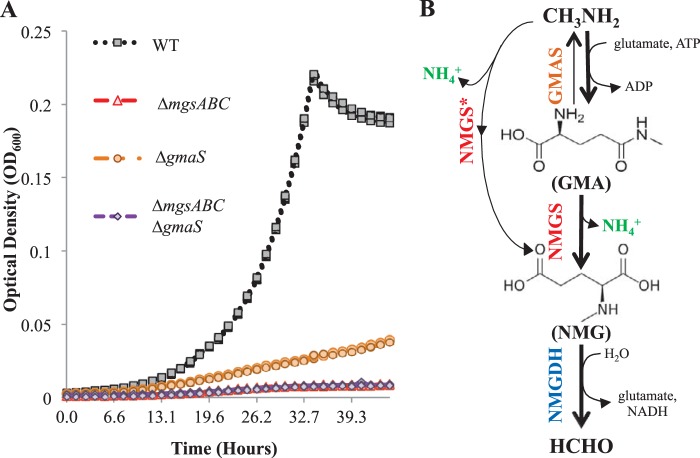
In order to even more sensitively test whether MA could be converted by strains with defects in different enzymes of the NMG pathway, we took advantage of the fact that MA can also be used as a nitrogen source, as ammonia (NH4+) ions are a by-product of MA oxidation (Fig. 2B). When MA is used only as a nitrogen source, the stoichiometric ratio of biomass (46) and carbon yield of the serine cycle (43) indicates that cells need to take in 15- to 20-fold more carbon than nitrogen. Therefore, growth on succinate with MA as a nitrogen source is 5-fold faster than that on MA as a carbon source but is still contingent on a functional NMG pathway. We leveraged these fast growth conditions to detect subtle phenotypes for NMG pathway-null mutants and further verify the linear topology of the NMG pathway. While the ΔmgsABC mutant and the ΔmgsABC ΔgmaS double mutant could not use MA as a nitrogen source (Fig. 2A), the ΔgmaS mutant grew poorly in succinate with MA as the nitrogen source, lacking the typical log-linear growth characteristics (Fig. 2A). The marginal ability of the ΔgmaS mutant to use MA as a nitrogen source indicated that the conversion of MA to NMG (with the concomitant release of NH4+) catalyzed by NMGS is physiologically relevant only when GMAS is absent. Additionally, the inability of the ΔmgsABC mutant to use MA as a nitrogen source corroborated the lack of a GMA-dissimilating enzyme in PA1. Altogether, these data suggest an essential role for GMAS during growth on MA as a carbon source but not during growth on MA as a nitrogen source in PA1, as indicated in Fig. 2B.
FIG 2.
(A) OD600-versus-time plots for three replicate cultures of (i) the Δcel wild-type strain of M. extorquens PA1, (ii) the ΔmgsABC mutant, (iii) the ΔgmaS mutant, and (iv) the ΔmgsABC ΔgmaS double mutant in nitrogen-free medium with 3.5 mM succinate and 7.66 mM methylamine. (B) The topology of the N-methylglutamate pathway in M. extorquens PA1 is linear; NMGS is capable of synthesizing NMG from GMA as well as methylamine, albeit only in the ΔgmaS mutant.
The H4MPT-dependent formaldehyde oxidation pathway is required during growth on MA with the NMG pathway.
It was recently proposed that, instead of formaldehyde, the oxidized C1 compound emerging from the NMG pathway might actually be CH2=H4F (15). If so, this end product should obviate the H4MPT-mediated formaldehyde oxidation pathway during growth on MA. We tested the hypothesis that methylamine growth mediated by the NMG pathway avoids formaldehyde production by examining a mutant lacking mptG (encoding β-ribofuranosylaminobenzene 5′-phosphate synthase, the first enzyme of the H4MPT biosynthesis pathway [42]). Previous work with AM1 has shown that methanol oxidation leads to formaldehyde buildup and rapid cell death in the ΔmptG mutant and that MA has the same effect, albeit to a lesser extent (26). We measured the capacity of the ΔmptG mutant of PA1 to withstand conditions that might lead to the production of variable amounts of formaldehyde at different rates. First, we tested the capacity of the ΔmptG mutant to tolerate different doses of methanol shock and MA shock during growth on a multicarbon substrate (succinate). As had been seen with AM1, even a pulse of 1 mM methanol completely inhibited the growth of the ΔmptG mutant of PA1 on succinate (Fig. 3A). In contrast, a pulse of 50 mM MA did not impede the growth of the ΔmptG mutant in succinate (Fig. 3B). Since the resilience of the ΔmptG mutant to MA shock might also be an artifact of tight regulation of the NMG pathway (17), we then tested whether this mutant could use MA as the sole nitrogen source. The WT, the ΔmptG mutant, and the ΔmgsABC mutant were grown to saturation on succinate with NH4+ as the nitrogen source and were then diluted 64-fold in medium with succinate as the carbon source and MA as the sole nitrogen source. After ∼16 h, the ΔmgsABC mutant could no longer grow, whereas the WT and the ΔmptG mutant continued to grow (Fig. 3C). These growth data suggested that once the internal nitrogen reservoir ran out, the ΔmptG mutant could still use MA as a nitrogen source; growth was moderately compromised (the growth rate was 23% lower on succinate with MA as the nitrogen source than with NH4+ as the nitrogen source; P < 0.001 for the difference in the mean growth rate of three biological replicates compared using the Student t test). The growth defect incurred by using MA versus NH4+ as a nitrogen source was more severe for the ΔmptG mutant than for the WT, where the growth rate on succinate with MA as the nitrogen source was merely 11% lower than that on succinate with NH4+ as the nitrogen source (P = 0.001) (see Table S1 in the supplemental material).
FIG 3.

(A) OD600-versus-time plots for three replicate cultures of the Δcel wild-type strain of PA1 and the ΔmptG mutant in medium with 3.5 mM succinate. A pulse of 1 mM methanol was added to the growth medium 12 h after incubation (as indicated by the arrow). (B) OD600-versus-time plots for three replicate cultures of the Δcel wild-type strain of PA1 and the ΔmptG mutant in medium with 3.5 mM succinate. A pulse of 50 mM methylamine was added to the growth medium 12 h after incubation (as indicated by the arrow). (C) Three replicate cultures of the Δcel wild-type strain of PA1, the ΔmptG mutant, and the ΔmgsABC mutant, grown to saturation in medium with 3.5 mM succinate, were transferred (1/64-fold dilution) to nitrogen-free medium with 3.5 mM succinate and 50 mM methylamine (as indicated by the arrow). The OD600-versus-time plots follow the growth of each of these three genotypes in nitrogen-free medium with 3.5 mM succinate and 50 mM methylamine.
Next, we examined the ability of the ΔmptG mutant strain to grow on MA as the sole carbon source. No observable growth or significant change in the OD600 was detected for the ΔmptG mutant strain on MA as a carbon source (Fig. 4). In order to quantify the extent of H4MPT-dependent formaldehyde oxidation required during growth on MA, we used a strain lacking fae (encoding a formaldehyde-activating enzyme that catalyzes the condensation of free formaldehyde with H4MPT [47]). A marginal rate of CH2=H4MPT production can be sustained by the spontaneous condensation of formaldehyde and H4MPT in a Δfae mutant (47). Therefore, the fae deletion only partially abolishes the H4MPT-dependent formaldehyde oxidation pathway and leads to an intermediate mutant phenotype in terms of methanol sensitivity (26). Unlike the ΔmptG strain, the strain with the fae deletion showed WT-like growth on MA (Fig. 4). During growth on MA, the absolute requirement for the H4MPT-mediated formaldehyde oxidation pathway indicates that at least some of the carbon from MA is ending up as formaldehyde, but the low rate of formaldehyde oxidation needed can be sustained in the absence of FAE.
FIG 4.
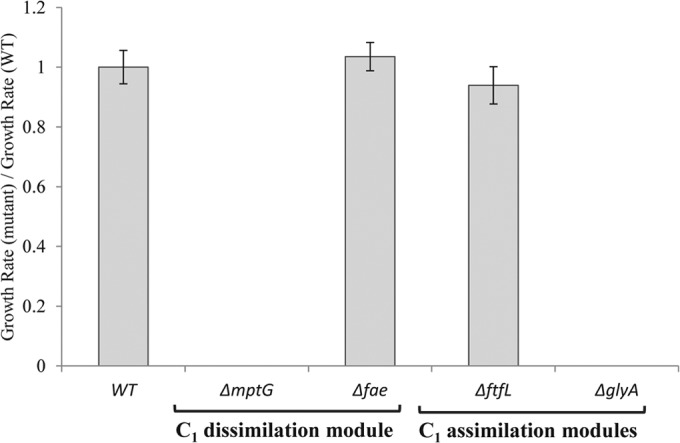
Ratios of growth rates of mutants with lesions in various methylotrophy-specific modules to the growth rate of the Δcel wild-type strain PA1 on 15 mM methylamine. The Δfae and ΔmptG mutants represent strains with partial and complete lesions in the H4MPT-dependent pathway for formaldehyde oxidation (or C1 dissimilation), respectively. The ΔftfL mutant represents a strain with a lesion in the H4F-dependent pathway for formate assimilation, and the ΔglyA mutant represents a strain with a lesion in the serine cycle for C1 assimilation. Error bars represent the 95% confidence intervals of the mean ratio for triplicate cultures for each condition.
An intact H4F pathway is not required for assimilation or dissimilation during methylamine growth of M. extorquens PA1.
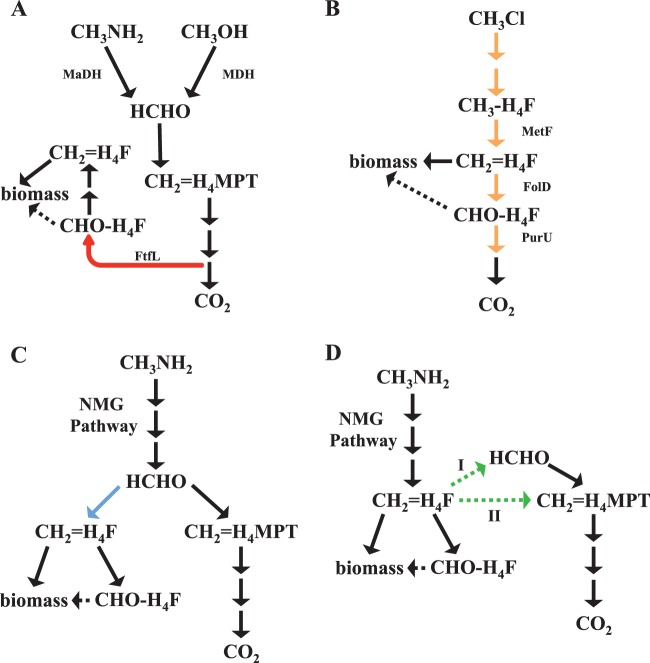
The growth of M. extorquens on any methylotrophic compound yet tested requires an intact H4F pathway for either assimilation (i.e., growth on methanol) or dissimilation (i.e., growth on chloromethane by CM4). Given that at least some of the carbon from MA appears to require the H4MPT pathway for dissimilation, we tested whether a complete H4F-dependent C1 transfer pathway was also required for growth on MA (Fig. 4). A strain lacking ftfL (encoding formyl-tetrahydrofolate ligase, which catalyzes the reduction of formate and free H4F to formyl-H4F in an ATP-dependent manner [29]) cannot grow on C1 compounds like methanol and formate (25) because of an early block in assimilation (29). We recently reported that a ΔftfL mutant of PA1 has the same phenotype as the ΔftfL mutant of AM1 (25). Contrary to the findings for other C1 compounds, the ΔftfL mutant could grow on MA at a rate indistinguishable from that of the WT (P = 0.8887). In contrast, a mutant lacking glyA (encoding serine hydroxymethyltransferase [48]) was incapable of growth on MA, confirming that C1 units are still assimilated via the serine cycle. These data, especially the nonessentiality of ftfL, indicate that use of the NMG pathway for growth on MA involves the flow of carbon through the C1-specific metabolic pathways such that the H4MPT-dependent pathway handles oxidation and CH2=H4F enters directly into the serine cycle. This observation is consistent with either formaldehyde or CH2=H4F (or both) being a branch point of metabolism during growth on MA, as depicted in Fig. 5C and D, in contrast to formate, which is the branch point of metabolism during growth on methanol (30, 31), as depicted in Fig. 5A.
FIG 5.
(A) Schematic representation of methanol dehydrogenase (MDH)- and MaDH-mediated methylamine metabolism in M. extorquens. Primary oxidation generates free formaldehyde, which is oxidized to formate by the H4MPT-dependent C1 dissimilation pathway. Formate is reduced by the H4F-dependent C1 assimilation pathway and assimilated using the serine cycle. The reaction highlighted in red, mediated by FtfL (formyl-tetrahydrofolate ligase), is unique to this topology. (B) Schematic representation of chloromethane metabolism in M. extorquens CM4. Primary oxidation produces H4F derivatives, using an H4F-dependent C1 dissimilation pathway, and completely bypasses formaldehyde production. Methylene tetrahydrofolate (CH2=H4F) is assimilated using the serine cycle. The reactions highlighted in orange, which are catalyzed by enzymes of the H4F-dependent C1 dissimilatory pathway (MetF, FolD, and PurU), are unique to this topology. (C) Schematic representation of the metabolic network involved in methylamine growth if the end product of the N-methylglutamate pathway is formaldehyde. The condensation of formaldehyde and H4F to form CH2=H4F, highlighted in blue, is unique to this topology. (D) Schematic representation of the metabolic network involved in methylamine growth if the end product of the N-methylglutamate pathway is CH2=H4F. CH2=H4F enters the H4MPT-mediated C1 assimilation pathway either by dissociation to formaldehyde (I) or by a direct cofactor switch (II). These two alternate reactions, indicated by green arrows, are unique to this topology.
FAE homologs play a functional role during growth on MA in PA1.
Do any unique gene products play a role in rerouting carbon through the C1-specific metabolic pathways when the NMG pathway operates? Many methylotrophs, including PA1, have two distant FAE homologs, fae2 (Mext_3143) and fae3 (Mext_1450), in the genome, yet their role in methylotrophy, if any, remains unknown (37, 49). We hypothesized that these gene products may play a role during growth on MA, perhaps catalyzing some of the novel reactions in Fig. 5C and D. To test our hypothesis, we deleted fae, fae2, and fae3 individually and in all possible combinations (Fig. 6A and B) and tested the growth of each of these mutants on 15 mM MA. Neither fae2 nor fae3 was essential for growth on MA since all the single-, double-, and triple-knockout mutants could grow on MA. The growth rates of strains with either fae or fae2 were mostly indistinguishable from the growth rate of PA1 or significantly higher in the case of the Δfae2 mutant (12%; P = 0.0108). However, mutant strains lacking both fae and fae2 showed a significant growth defect on MA (Fig. 6A). In addition, all mutant strains lacking fae3 showed a significant yield (maximum OD600) defect relative to the yield of the WT on MA (Fig. 6B).
FIG 6.
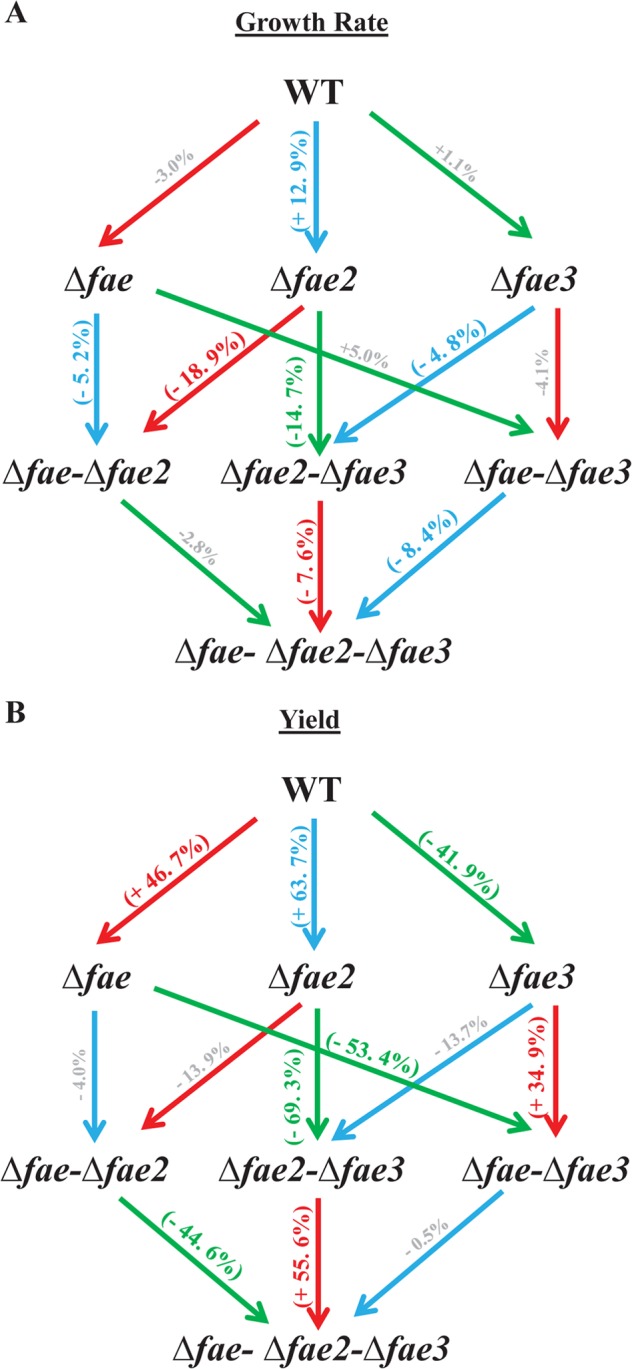
(A) Red, blue, and green arrows represent the deletion of fae, fae2, and fae3, respectively, in the genomic background from which the arrow originates. The numbers and signs on the arrows represent the percent increase/decrease in growth rate as a result of the gene deletion. For percentage values shown in bold and parentheses, the P value is <0.05 (with the difference in the growth rates of three replicate cultures of the two strains being compared using the Student t test). For percentage values shown in gray, the difference in growth rate between the two strains is not significant (P > 0.05). (B) Red, blue, and green arrows represent the deletion of fae, fae2, and fae3, respectively, in the genomic background from which the arrow originates. The numbers and signs on the arrows represent the percent increase/decrease in yield as a result of the gene deletion. For percentage values shown in bold and parentheses, the P value is <0.05 (with the difference in the maximum OD600 of three replicate cultures of the two strains being compared using the Student t test). For percentage values shown in gray, the difference in yield between the two strains is not significant (P > 0.05).
To determine whether the growth characteristics of the fae-, fae2-, and fae3-null mutants described in Fig. 6A and B are limited to growth on MA via the NMG pathway, we tested the growth of each of these mutants on another C1 compound (methanol), a multi-C compound (succinate), and succinate with MA as the nitrogen source (see Fig. S3 in the supplemental material). As expected, strains with a fae deletion did not grow on methanol. Deletion of either fae2 or fae3 led to a 5 to 10% growth rate defect on methanol (with no significant change in yield), but the Δfae2 Δfae3 mutant recovered to be indistinguishable in terms of growth rate (P = 0.644) and yield (P = 0.6889) from the WT (see Fig. S3 in the supplemental material). All mutants grew significantly slower than the WT on succinate, whereas only the double- and triple-knockout mutants grew significantly slower than the WT on succinate while using MA as a nitrogen source (see Fig. S3 in the supplemental material). No significant change in yield (relative to that of the WT) was observed for any mutant during growth on succinate (with either NH4+ or MA as the nitrogen source). FAE and FAE homologs influence a combination of growth and yield on many different compounds, but the phenotypic effect of each mutant varies on the basis of the growth conditions tested.
DISCUSSION
In this study, we dissected the genetics of the NMG pathway mediating slow growth on MA in PA1. All three enzymes of the NMG pathway—NMGS, NMGDH, and GMAS—were essential for using MA as a carbon source, but only NMGDH and NMGS were absolutely essential for using MA as a nitrogen source. These results corroborate the findings of a recent study of the NMG pathway in DM4 (17). In DM4, though, it was suggested that a distant gmaS homolog (found in all sequenced M. extorquens species) partially compensates for a gmaS deletion and enables the ΔgmaS mutant to use MA as a nitrogen source, albeit at much lower rates than the WT. We observed that the slow, steadily decelerating growth of the ΔgmaS mutant (Fig. 2A) in succinate with MA as the nitrogen source was completely abolished in a ΔgmaS ΔmgsABC mutant. Our data support the notion that NMGS has a physiologically relevant, albeit low, activity for the conversion of MA to NMG that manifests only in the absence of GMAS. In the presence of GMAS, instead of MA, GMA might serve as the true substrate for NMGS. The precision of our automated growth measurement platform (41), along with a screen that coupled fast growth to a reduced demand upon the NMG pathway (using MA as a nitrogen source in the presence of an alternate carbon source), revealed that the NMG pathway has a linear topology in PA1 akin to that proposed for M. silvestris BL2 (3) and, very recently, DM4 (17). This genetic evidence for a linear topology for the NMG pathway (Fig. 2B) might also explain (i) the extremely low specific activity observed for the conversion of MA and glutamate to NMG by NMGS in vitro (24) and (ii) the accumulation of GMA in Δmgs mutants (15).
While it has been demonstrated in other systems (15) that the NMG pathway produces CH2=H4F, neither FolD nor PurU, enzymes known to work in the dissimilatory direction of the H4F-dependent C1 transfer pathway to generate formate, are encoded by the PA1 genome. We also observed that the H4MPT-dependent C1 dissimilatory module was essential but that deletion of a complete H4F-dependent C1 assimilation pathway had no effect upon growth on MA (Fig. 4). This result led us reevaluate if CH2=H4F really was the end product of the NMG pathway in PA1. The extreme formaldehyde sensitivity of a mutant incapable of synthesizing H4MPT (the ΔmptG mutant), a cofactor involved in formaldehyde oxidation (26, 42), was leveraged to identify whether formaldehyde is generated and oxidized by the NMG pathway. The ΔmptG mutant could not grow on MA as a carbon source but could use MA as a nitrogen source (Fig. 3C and 4A). Additionally, a Δfae strain with a partial lesion in the H4MPT-mediated formaldehyde oxidation pathway could still grow on MA. These data suggest that at least some formaldehyde is generated during growth on MA, but unlike growth on methanol (where the Δfae mutant cannot grow), it is likely that either (i) less than 100% of MA is oxidized to formaldehyde or (ii) the rate of formaldehyde production is low enough to be sustained by a spontaneous reaction with H4MPT and H4F. Additionally, the rate of formaldehyde production is likely to be even lower when MA is used as a nitrogen source, perhaps low enough that the flux can be handled by nonspecific aldehyde dehydrogenases. However, given the reactive nature of formaldehyde, it is almost impossible to resolve whether formaldehyde is generated as the direct end product (Fig. 5C) or by the dissociation of CH2=H4F (Fig. 5D). Alternately, we cannot rule out a third scenario where CH2=H4F is the end product of the NMG pathway and a methylene transfer occurs to generate CH2=H4MPT. In such a scenario, mptG but not fae would be essential for growth on MA, which is consistent with our data as well.
Typically, on the basis of whether formaldehyde or CH2=H4F is produced as the end product of primary oxidation, one of two distinct topologies is used for methylotrophic metabolism in M. extorquens species (Fig. 5A and B). The key difference between the two topologies is whether C1 flux is channeled through an assimilatory or dissimilatory branch of the H4F-dependent C1 transfer pathway. In this study, we have uncovered a novel topology of C1 use in PA1 that completely avoids the H4F-dependent C1 pathway for net dissimilation or assimilation (Fig. 5C and D).
Finally, this paper is the first to report functional roles for FAE homologs (FAE2 and FAE3) that are often found in the genomes of methylotrophs. FAE2 and FAE appear to play somewhat redundant roles during growth on MA, positively reinforcing growth rates but not yield (Fig. 6A). Three of the four key residues that make and stabilize contact with the methyl groups of H4MPT in FAE (50) are conserved or similar in FAE2 (see Fig. S4 in the supplemental material). Therefore, FAE2 could be either an enzyme like FAE but with different kinetic parameters or perhaps a regulator that changes the expression of the pathway, sensing H4MPT, CH2=H4MPT, or both. FAE3 appears to play a role distinct from that of FAE and FAE2 by impacting yield only during growth on MA (Fig. 6B) and thus likely has a different biochemical function. Specific residues that allow FAE to distinguish H4MPT and H4F (50) are not conserved in FAE3. Therefore, FAE3 is unlikely to be involved in the H4MPT-dependent formaldehyde oxidation pathway and may work as either an enzyme or a regulator of the H4F pathway. Further investigation will be required to uncover the precise physiological roles and underlying biochemistry of FAE2 and FAE3.
The extremely slow growth on MA (doubling time, ∼1 day) when MA oxidation is mediated by the NMG pathway had occluded genetic and physiological analysis, until recently. Of late, though, the NMG pathway is going through a phase of rediscovery, in light of its importance in several environmental regimes (7, 51, 52). While various studies with different model systems (15–17) have dissected the genomics, genetics, and physiology of the NMG pathway in isolation, we extended this genetic approach to understand how NMG-dependent MA metabolism links to central C1-specific metabolic pathways in PA1. Unlike all previously documented instances of methylotrophy, we found that cells simultaneously require the H4MPT-dependent formaldehyde oxidation pathway and the serine cycle but do not need to move C1 units through an intact H4F-dependent C1 transfer pathway for either assimilation or dissimilation. Our work highlights that a single set of metabolic modules can be used in distinct configurations to route C1 flux in different ways, depending upon the growth substrate in question.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Marx lab for feedback on the manuscript.
C.J.M. acknowledges financial support from the NIH (GM078209).
Footnotes
Published ahead of print 15 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02026-14.
REFERENCES
- 1.Ge X, Wexler AS, Clegg SL. 2011. Atmospheric amines. Part I. A review. Atmos. Environ. 45:524–546. 10.1016/j.atmosenv.2010.10.012. [DOI] [Google Scholar]
- 2.Oremland RS, Marsh LM, Polcin S. 1982. Methane production and simultaneous sulphate reduction in anoxic, salt marsh sediments. Nature 296:143–145. 10.1038/296143a0. [DOI] [Google Scholar]
- 3.Chen Y, Scanlan J, Song L, Crombie A, Rahman MT, Schafer H, Murrell JC. 2010. γ-Glutamylmethylamide is an essential intermediate in the metabolism of methylamine by Methylocella silvestris. Appl. Environ. Microbiol. 76:4350–4357. 10.1128/AEM.00739-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neff JC, Holland EA, Dentener FJ, McDowell WH, Russell KM. 2002. The origin, composition and rates of organic nitrogen deposition: a missing piece of the nitrogen cycle? Biogeochemistry 57-58:99–136. 10.1023/A:1015791622742. [DOI] [Google Scholar]
- 5.Anthony C. 1982. The biochemistry of methylotrophs. Academic Press Ltd., London, United Kingdom. [Google Scholar]
- 6.Chen Y, McAleer KL, Murrell JC. 2010. Monomethylamine as a nitrogen source for the nonmethylotrophic bacterium, Agrobacterium tumefaciens. Appl. Environ. Microbiol. 76:4102–4104. 10.1128/AEM.00469-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y. 2012. Comparative genomics of methylated amine utilization by marine Roseobacter clade bacteria and development of functional gene markers (tmm, gmaS). Environ. Microbiol. 14:2308–2322. 10.1111/j.1462-2920.2012.02765.x. [DOI] [PubMed] [Google Scholar]
- 8.Eady RR, Large PJ. 1968. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem. J. 106:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chistoserdov AY, Tsygankov YD, Lidstrom ML. 1991. Genetic organization of methylamine utilization genes from Methylobacterium extorquens AM1. J. Bacteriol. 173:5901–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Iersel J, van der Meek RA, Duine JA. 1986. Methylamine oxidase from Arthrobacter P1. A bacterial copper-quinoprotein amine oxidase. Eur. J. Biochem. 161:415–419. [DOI] [PubMed] [Google Scholar]
- 11.Cai D, Klinman JP. 1994. Copper amine oxidase: heterologous expression, purification, and characterization of an active enzyme in Saccharomyces cerevisiae. Biochemistry 33:7647–7653. 10.1021/bi00190a019. [DOI] [PubMed] [Google Scholar]
- 12.Dooley DM, McIntire WS, McGuirl MA, Cote CE, Bates JL. 1990. Characterization of the active site of Arthrobacter P1 methylamine oxidase: evidence for copper-quinone interactions. J. Am. Chem. Soc. 112:2782–2789. 10.1021/ja00163a047. [DOI] [Google Scholar]
- 13.Shaw WV, Tsai L, Stadtman RR. 1966. The enzymatic synthesis of N-methylglutamic acid. J. Biol. Chem. 241:935–945. [PubMed] [Google Scholar]
- 14.Bamforth CW, O'Connor ML. 1979. The isolation of pleiotropic mutants of Pseudomonas aminovorans deficient in the ability to grow on methylamine and an examination of their enzymic constitution. Microbiology 110:143–149. [Google Scholar]
- 15.Latypova E, Yang S, Wang YS, Wang T, Chavkin TA, Hackett M, Schafer H, Kalyuzhnaya MG. 2010. Genetics of the glutamate-mediated methylamine utilization pathway in the facultative methylotrophic beta-proteobacteria Methyloversatilis universalis FAM5. Mol. Microbiol. 75:426–439. 10.1111/j.1365-2958.2009.06989.x. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Gomez NC, Nguyen S, Lidstrom ME. 2013. Elucidation of the role of methylene-tetrahydromethanopterin dehydrogenase MtdA in the tetrahydromethanopterin-dependent oxidation pathway in Methylobacterium extorquens AM1. J. Bacteriol. 195:2359–2367. 10.1128/JB.00029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruffaz C, Muller EEL, Louhichi-Jelail Y, Nelli YR, Guichard G, Bringel F. 2014. Genes of the N-methylglutamate pathway are essential for growth of Methylobacterium extorquens DM4 on monomethylamine. Appl. Environ. Microbiol. 80:3541–3550. 10.1128/AEM.04160-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hersh LB, Peterson JA, Thompson AA. 1971. An N-methylglutamate dehydrogenase from Pseudomonas M.A. Arch. Biochem. Biophys. 145:115–120. 10.1016/0003-9861(71)90016-6. [DOI] [PubMed] [Google Scholar]
- 19.Pollock RJ, Hersh LB. 1973. N-Methylglutamate synthetase. The use of flavin mononucleotide oxidative catalysis. J. Biol. Chem. 248:6724–6733. [PubMed] [Google Scholar]
- 20.Bamforth CW, Large PJ. 1977. Solubilization, partial purification, and properties of N-methylglutamate dehydrogenase from Pseudomonas aminovorans. Biochem. J. 161:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bamforth CW, Large PJ. 1977. The molecular size of N-methylglutamate dehydrogenase of Pseudomonas aminovorans. Biochem. J. 167:509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kung H-F, Wagner C. 1969. Γ-Glutamylmethylamide. A new intermediate in the metabolism of methylamine. J. Biol. Chem. 244:4136–4140. [PubMed] [Google Scholar]
- 23.Boulton CA, Haywood GW, Large PJ. 1979. N-Methylglutamate dehydrogenase, a flavoprotein purified from a new pink trimethylamine-utilizing bacterium. Microbiology 117:293–304. [Google Scholar]
- 24.Pollock RJ, Hersh LB. 1971. N-Methylglutamate synthetase. Purification and properties of the enzyme. J. Biol. Chem. 246:4737–4743. [PubMed] [Google Scholar]
- 25.Nayak DD, Marx CJ. 2014. Genotypic and phenotypic comparison of facultative methylotrophy between Methylobacterium extorquens strains PA1 and AM1. PLoS One 9:e107887. 10.1371/journal.pone.0107887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marx CJ, Chistoserdova L, Lidstrom ME. 2003. Formaldehyde-detoxifying role of the tetrahydromethanopterin-linked pathway in Methylobacterium extorquens AM1. J. Bacteriol. 185:7160–7168. 10.1128/JB.185.23.7160-7168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chistoserdova L, Vorholt JA, Thauer RK, Lidstrom ME. 1998. C1 transfer enzymes and coenzymes linking methylotrophic bacteria and methanogenic archaea. Science 281:99–102. 10.1126/science.281.5373.99. [DOI] [PubMed] [Google Scholar]
- 28.Pomper BK, Saurel O, Milon A, Vorholt JA. 2002. Generation of formate by the formyltransferase/hydrolase complex (Fhc) from Methylobacterium extorquens AM1. FEBS Lett. 523:133–137. 10.1016/S0014-5793(02)02962-9. [DOI] [PubMed] [Google Scholar]
- 29.Marx CJ, Laukel M, Vorholt JA, Lidstrom ME. 2003. Purification of the formate-tetrahydrofolate ligase from Methylobacterium extorquens AM1 and demonstration of its requirement for methylotrophic growth. J. Bacteriol. 185:7169–7175. 10.1128/JB.185.24.7169-7175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marx CJ, Van Dien SJ, Lidstrom ME. 2005. Flux analysis uncovers key role of functional redundancy in formaldehyde metabolism. PLoS Biol. 3:e16. 10.1371/journal.pbio.0030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crowther GJ, Kosaly G, Lidstrom ME. 2008. Formate as the main branch point for methylotrophic metabolism in Methylobacterium extorquens AM1. J. Bacteriol. 190:5057–5062. 10.1128/JB.00228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chistoserdova L, Chen SW, Lapidus A, Lidstrom ME. 2003. Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J. Bacteriol. 185:2980–2987. 10.1128/JB.185.10.2980-2987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quayle JR. 1972. The metabolism of one-carbon compounds in micro-organisms. Adv. Microb. Physiol. 7:119–203. 10.1016/S0065-2911(08)60078-8. [DOI] [Google Scholar]
- 34.Vannelli T, Messmer M, Studer A, Vuilleumier S, Leisinger T. 1999. A corrinoid-dependent catabolic pathway for growth of a Methylobacterium strain with chloromethane. Proc. Natl. Acad. Sci. U. S. A. 96:4615–4620. 10.1073/pnas.96.8.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Studer A, McAnulla C, Buchele R, Leisinger T, Vuilleumier S. 2002. Chloromethane-induced genes define a third C1 utilization pathway in Methylobacterium chloromethanicum CM4. J. Bacteriol. 184:3476–3484. 10.1128/JB.184.13.3476-3484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knief C, Frances L, Vorholt JA. 2010. Competitiveness of diverse Methylobacterium strains in the phyllosphere of Arabidopsis thaliana and identification of representative models, including M. extorquens PA1. Microb. Ecol. 60:440–452. 10.1007/s00248-010-9725-3. [DOI] [PubMed] [Google Scholar]
- 37.Kalyuzhnaya MG, Korotkova N, Crowther G, Marx CJ, Lidstrom ME, Chistoserdova L. 2005. Analysis of gene islands involved in methanopterin-linked C1 transfer reactions reveals new functions and provides evolutionary insights. J. Bacteriol. 187:4607–4614. 10.1128/JB.187.13.4607-4614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chistoserdova L, Kalyuzhnaya MG, Lidstrom ME. 2009. The expanding world of methylotrophic metabolism. Annu. Rev. Microbiol. 63:477–499. 10.1146/annurev.micro.091208.073600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delaney NF, Kaczmarek ME, Ward LM, Swanson PK, Lee M-C, Marx CJ. 2013. Development of an optimized medium, strain, and high-throughput culturing methods for Methylobacterium extorquens. PLoS One 8:e62957. 10.1371/journal.pone.0062957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agashe D, Martinez-Gomez NC, Drummond DA, Marx CJ. 2013. Good codons, bad transcript: large reductions in gene expression and fitness arising from synonymous mutations in a key enzyme. Mol. Biol. Evol. 30:549–560. 10.1093/molbev/mss273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delaney NF, Rojas Echenique JI, Marx CJ. 2013. Clarity: an open-source manager for laboratory automation. J. Lab Autom. 18:171–177. 10.1177/2211068212460237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasche ME, Havemann SA, Rosenzvaig M. 2004. Characterization of two methanopterin biosynthesis mutants of Methylobacterium extorquens AM1 by use of a tetrahydromethanopterin bioassay. J. Bacteriol. 186:1565–1570. 10.1128/JB.186.5.1565-1570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Dien SJ, Lidstrom ME. 2002. Stoichiometric model for evaluating the metabolic capacities of the facultative methylotroph Methylobacterium extorquens AM1, with applications to reconstruction of C3 and C4 metabolism. Biotechnol. Bioeng. 78:296–312. 10.1002/bit.10200. [DOI] [PubMed] [Google Scholar]
- 44.Marx CJ. 2008. Development of a broad-host-range sacB-based vector for unmarked allelic exchange. BMC Res. Notes 1:1. 10.1186/1756-0500-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, III, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6:343–345. 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 46.Redfield AC. 1958. The biological control of chemical factors in the environment. Am. Sci. 46:205–221. [PubMed] [Google Scholar]
- 47.Vorholt JA, Marx CJ, Lidstrom ME, Thauer RK. 2000. Novel formaldehyde-activating enzyme in Methylobacterium extorquens AM1. J. Bacteriol. 182:6645–6650. 10.1128/JB.182.23.6645-6650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chistoserdova L, Lidstrom ME. 1994. Genetics of the serine cycle in Methylobacterium extorquens AM1: cloning, sequence, mutation and physiological effect of glyA, the gene for serine hydroxymethyltransferase. J. Bacteriol. 176:6759–6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chistoserdova L, Jenkins C, Kalyuzhnaya MG, Marx CJ, Lapidus A, Vorholt JA, Staley JA, Lidstrom ME. 2004. The enigmatic Planctomycetes may hold a key to the origins of methanogenesis and methylotrophy. Mol. Biol. Evol. 21:1234–1241. 10.1093/molbev/msh113. [DOI] [PubMed] [Google Scholar]
- 50.Acharya P, Goenrich M, Hagemeier CH, Demmer U, Vorholt JA, Thauer RK, Ermler U. 2005. How an enzyme binds the C1 carrier tetrahydromethanopterin. Structure of the tetrahydromethanopterin-dependent formaldehyde-activating enzyme (Fae) from Methylobacterium extorquens AM1. J. Biol. Chem. 280:13712–13719. 10.1074/jbc.M412320200. [DOI] [PubMed] [Google Scholar]
- 51.Vorobev A, Beck DAC, Kalyuzhnaya MG, Lidstrom ME, Chistoserdova L. 2013. Comparative transcriptomics in three Methylophilaceae species uncover different strategies for environmental adaptation. PeerJ 1:e115. 10.7717/peerj.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chistoserdova L. 2011. Methylotrophy in a lake: from metagenomics to single-organism physiology. Appl. Environ. Microbiol. 77:4705–4711. 10.1128/AEM.00314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U. S. A. 76:1648–1652. 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.