Abstract
Bacteriophage J-1 was isolated in 1965 from an abnormal fermentation of Yakult using Lactobacillus casei strain Shirota, and a related phage, PL-1, was subsequently recovered from a strain resistant to J-1. Complete genome sequencing shows that J-1 and PL-1 are almost identical, but PL-1 has a deletion of 1.9 kbp relative to J-1, resulting in the loss of four predicted gene products involved in immunity regulation. The structural proteins were identified by mass spectrometry analysis. Similarly to phage A2, two capsid proteins are generated by a translational frameshift and undergo proteolytic processing. The structure of gene product 16 (gp16), a putative tail protein, was modeled based on the crystal structure of baseplate distal tail proteins (Dit) that form the baseplate hub in other Siphoviridae. However, two regions of the C terminus of gp16 could not be modeled using this template. The first region accounts for the differences between J-1 and PL-1 gp16 and showed sequence similarity to carbohydrate-binding modules (CBMs). J-1 and PL-1 GFP-gp16 fusions bind specifically to Lactobacillus casei/paracasei cells, and the addition of l-rhamnose inhibits binding. J-1 gp16 exhibited a higher affinity than PL-1 gp16 for cell walls of L. casei ATCC 27139 in phage adsorption inhibition assays, in agreement with differential adsorption kinetics observed for both phages in this strain. The data presented here provide insights into how Lactobacillus phages interact with their hosts at the first steps of infection.
INTRODUCTION
Biotechnological processes employing bacterial cultures are subject to interference by infection with bacteriophages (1). The deleterious impact of phages in fermentations is common in the dairy industry, where the organoleptic properties of cheese and other dairy products rely on the use of starter cultures with combinations of specific strains. Phage infection of these carefully selected bacteria can substantially delay the process, alter the quality of the product, and in the worst-case scenario abort the entire fermentation batch (2).
Contamination in the dairy industry is common, as phages can reside in the substrates for fermentation and on surfaces of vessels and other equipment (3–6). Strategies to control this contamination include milk pasteurization and sanitation of equipment (6–9), but phages are rarely completely eliminated. Cultures of starter strains themselves can be phage contaminated, likely due to induction of resident prophages and concomitant cell lysis (10–13). In general, it is helpful to avoid lysogenic lactic acid bacterium (LAB), but the nature of starter strains is not always well defined, and in some strains multiple prophages may be present (14). Rotation of starter strains generally helps to minimize the negative impact of phages on fermentation (1). The increasing number of phage and bacterial genomes sequenced has helped to elucidate phage-host interactions and the engineering of resistant strains that are more robust during fermentation (15, 16).
Lactobacillus casei is widely employed in fermentation of vegetables, meat, and dairy products, including cheese and fermented milk. This bacterium is of interest because in addition to its organoleptic properties in fermentation, some strains may have probiotic properties (17, 18). Lactobacillus casei can tolerate the low pH of the stomach and colonize the gastrointestinal tract (GI) (19), with potential beneficial outcomes (20, 21).
Forty-three phages that infect L. casei have been reported, 16 of which morphologically belong to the Myoviridae, 21 are Siphoviridae, and the other six have not been morphologically classified (22). Among L. casei phages, A2 and phiAT3 are the best characterized, and the complete genome sequences have been determined. A2 was isolated in Spain from the whey of a failed homemade blue cheese product using L. casei ATCC 393, and phiAT3 was recovered following induction from L. casei 393 using mitomycin C (23, 24). Both phages belong to the Siphoviridae family and are temperate, although they share little nucleotide sequence similarity (23, 24).
Among the other phages of L. casei, J-1 was isolated in 1965 in association with fermentation failures during the production of Yakult, a Japanese beverage fermented from skimmed milk and Chlorella extracts (25). Upon isolation, J-1 was shown to behave as a virulent phage in the L. casei Shirota strain used for manufacturing of Yakult. An L. casei strain resistant to J-1 infection was isolated, but after 2 years of use, a second phage designated PL-1 was isolated that infected the resistant strain (26). J-1 and PL-1 were characterized extensively (reviewed by Sechaud et al. [27]) and shown to be serologically related (28–30). Conditions for transfection of protoplasts with phage DNA (31–34), phage inactivation (35–38), and characterization of DNA content (39, 40) have been reported. The lysis cassette of PL-1 encoding a holin and an endolysin—an N-acetylmuramoyl l-alanine amidase—has been characterized (41), but the complete genome sequences of J-1 and PL-1 were reported only recently (42).
Here, we describe the genomic and structural features of phages J-1 and PL-1. Interestingly, PL-1 and J-1 genomes are almost identical and among sequenced phages are most closely related to phage A2. Compared to J-1, PL-1 has a deletion of 1.9 kbp comprising 4 genes associated with the immunity cassette. PL-1 also differs from J-1 in a gene (16) whose product has a predicted structure similar to distal tail proteins that form the baseplate hub in other Siphoviridae. J-1 gene product 16 (gp16) recognizes sugar motifs and specifically binds to Lactobacillus casei/paracasei cells, blocking phage adsorption, suggesting that gp16 is involved in host recognition.
MATERIALS AND METHODS
Strains, bacteriophages, and growth conditions.
Lactobacillus paracasei subsp. paracasei ATCC 27092 and Lactobacillus casei subsp. casei ATCC 27139 were grown in MRS medium (Difco, USA) at 37°C under static conditions. Escherichia coli DH5α was used for cloning, and E. coli BL21(DE3)pLysS (Invitrogen, USA) was used for protein expression. E. coli strains were grown in LB broth or nutritive medium (Difco, USA) at 37°C under moderate shaking. When appropriate, antibiotics were added at the following concentrations: kanamycin, 15 μg/ml (Sigma, USA); chloramphenicol, 34 μg/ml (Sigma, USA).
Lactobacillus phages J-1 and PL-1 used in this study were a kind gift from Andrea Quiberoni from INLAIN (Instituto de Lactología Industrial). Phage J-1 was propagated on L. casei subsp. casei ATCC 27139 and PL-1 on L. paracasei subsp. paracasei ATCC 27092 in MRS broth. Bacteriophage stocks were stored at 4°C in phage buffer (20 mM Tris-HCl, 100 mM NaCl, and 10 mM MgSO4).
Electron microscopy.
A total of 5 μl of Lactobacillus phage J-1 and Lactobacillus phage PL-1 lysates (1011 PFU/ml) was allowed to sit on freshly glow-discharged 400-mesh carbon-coated Formvar copper grids for approximately 15 s. The grids were then rinsed with distilled water and stained with 1% uranyl acetate. Virus particles were imaged on an FEI Morgagni transmission electron microscope at 80 kV at a magnification of ×56,000.
Identification of PL-1 and J-1 proteins.
Approximately 50 μl (a total of 5 × 1010 PFU) of CsCl-purified J-1 and PL-1 particles was collected by centrifugation at 20,000 × g for 30 min. The pellet was resuspended in 37.5 μl of distilled water, frozen at −70°C, and then mixed by vortexing. This cycle was repeated three times, and the solution was then heated to 75°C for 4 min. DNase (2 U) (Fermentas, USA) was added and incubated for 30 min to reduce viscosity. Finally, 4× SDS sample buffer was added and boiled for 2.5 min. Approximately 25 μl was loaded onto a 12% SDS-polyacrylamide gel and electrophoresed at 90 V until the dye ran off the gel. The gel was stained with Coomassie blue. The visible bands were compared to a protein standard to determine the approximate molecular mass.
For protein identification by mass spectrometry (MS), the same protocol described above was applied but the gel was stained with colloidal Coomassie blue. The protein bands were excised and digested in situ with trypsin or Lys-C followed by peptide elution, chromatography, and tandem mass spectrometry (MS/MS) on an LTQ Velos Orbitrap mass spectrometer and a matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometer. Peptides were matched against predicted J-1 and PL-1 phage proteins.
Computational methods.
A Lactobacillus phage database was created, and genome map diagrams were drawn using Phamerator (43), with the threshold parameters of 32.5% identity with ClustalW and a BLASTP E value of 10−50, as described previously.
Protein domains in gp16 and gp17 of J-1 and PL-1 were detected using the Pfam server (44). In gp16, a Sipho_tail domain (PF05709) was detected, achieving E values of 1.1E−72 and 4.6E−72 for J-1 and PL-1, respectively. The template for the comparative modeling of the Sipho_tail domain structure was that of PDB code 2X8K (chain A), which belongs to this family according to Pfam and was retrieved by BLAST. To align gp16 with the template sequence, we used the HMMER software and the HMM profile corresponding to the Sipho_tail family. For both J-1 and PL-1, two regions (Dom1 and Dom2) were not covered with this template structure, corresponding to an insertion of high probability in the Pfam HMM logo. Dom1 and Dom2 were submitted separately to the HHpred server (45) in order to detect known structures with remote homology. Dom1 resulted in a significant hit against a carbohydrate-binding module (PDB code 2XON, chain L) with 98.4 and 93.2 probability values for J-1 and PL-1, respectively. In the case of J-1, alignment presents an E value of 4.1E−06, a P value of 1.3E−10, an identity of 15%, and a coverage of 80%. For PL-1, Dom1 achieved an E value of 1.1, a P value of 3.5E−05, an identity of 14%, and a coverage of 61%. Dom2 does not result in any significant hit for both J-1 and PL-1, so no further analysis was done.
No hits were found when submitting gp17 of J-1 and PL-1 to the Pfam server. Both sequences were further submitted to the HHpred server, achieving a high-probability hit (97.8%) against chain A in PDB code 1WRU (E value of 0.0055 and P value of 1.7E−07), a sequence that belongs to the Prophage_tail (PF06605) family. The corresponding HMM profile was used to align gp17 sequences with the template structure sequence. Sequence identities between the template structure (PDB code 1WRU, chain A) and both PL-1 and J-1 in the context of the Pfam family (Prophage_tail) cover only about 40% of the sequences for the two of them, achieving 7% identity each. In this case, HHpred alignment was preferred to use for comparative modeling, which presents 8% identity and 35% coverage. MODELLER (46) was used to build models based on mentioned alignments over the aligned regions. For each target sequence, 10 different models were built, and their quality measures were assigned using the GA341 when globular domains were analyzed.
Phage adsorption assays.
For kinetics of adsorption assays, L. casei subsp. casei ATCC 27139 or L. paracasei subsp. paracasei ATCC 27092 were grown till an optical density at 600 nm (OD600) of 1, and 150 μl of cells was infected with 150 μl of J-1 or PL-1 at a multiplicity of infection of 0.005. One tube per time point was prepared, and phages were allowed to adsorb from 5 min to 1 h at 37°C in the presence of 10 mM CaCl2. Cells were removed by centrifugation, and unadsorbed phages in the supernatant were measured by using the double-agar method.
Cell wall purification was done essentially as previously described (47). Briefly, bacterial cells were centrifuged at 3,200 × g and resuspended in 50 mM Tris-HCl (pH 7.5). Cells were disrupted by sonication in the presence of glass beads (10 cycles of 20 s followed by intervals of 1 min on ice). Whole extracts were incubated with DNase (30 μg/ml) (Thermo Scientific, USA) and RNase (5 μg/ml) (Thermo Scientific, USA) at 37°C for 1 h, and nonlysed cells and debris were removed by a short centrifugation step (1,500 × g for 5 min). Cell walls were recovered from the supernatant by centrifugation at 20,000 × g for 20 min, and the pellet was washed three times with distilled water and stored at −20°C. One part of this sample was lyophilized, and the weight (μg) was measured. Phage adsorption assays using cell walls were done as described above but using 100 μg of cell walls instead of whole cells.
Molecular cloning.
DNA sequences were amplified by PCR using Go Taq DNA polymerase (Promega, USA) by following the manufacturer's instructions. An N-terminal green fluorescent protein (GFP) fusion expression vector was constructed, amplifying egfp from pMP14 (48) with the following primers: ED74 (TGACTcatatgGTGAGCAAGGGCGAGGAGC) and ED75 (TACCAggatccCTTGTACAGCTCGTCCATGCC) (lowercase letters correspond to restriction sites). The PCR product was digested with NdeI and BamHI and cloned into pET28b (Novagen, Merck Millipore, USA) to render pET28-GFP. Genes 16 and 17 were amplified using J-1 or PL-1 DNA as templates. Gene 16 was amplified using ED76 (TAGCAgaattcGCAAATTTAATATTTGGAGG) and ED77 (TGAACgagctcTCATAGCCATGCCTCCT), and gene 17 was amplified using ED78 (GCTAAgaattcAAGGATTTTTATTTTGTGGA) and ED79 (GATCCaagcttCTAAACTGCGTATACCTCA). Amplicons were digested with the appropriate restriction enzymes, EcoRI/SacI or EcoRI/HindIII (Promega, USA), and cloned into pET28-GFP. Plasmids were named pET28-J-1 GFP-gp16, pET28-PL-1 GFP-gp16, pET28-J-1 GFP-gp17, and pET28-PL-1 GFP-gp17.
Protein purification.
Plasmids described above were transformed into E. coli BL21(DE3) cells (Invitrogen, USA) by electroporation for protein expression and further purification. Transformed cells were grown at 37°C to an OD600 of 0.5 in nutrient broth, and expression was induced by the addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cells were left overnight at 21°C before being harvested. Cell pellets were resuspended in binding buffer (50 mM Tris-HCl [pH 8], 300 mM NaCl, and 1 mM phenylmethylsulfonyl fluoride [PMSF]) and disrupted by sonication (4 cycles of 15 s). The lysate was centrifuged at 16,000 × g for 20 min, and the supernatants were incubated with 300 μl of preequilibrated Ni-agarose resin (Novagen, USA) for 2 h at 4°C. The matrix was washed with 10 volumes of binding buffer, 10 volumes of the same buffer containing 25 mM imidazole, and 5 volumes containing 60 mM imidazole. Elution was done with 4 volumes of buffer with 120 mM imidazole. Eluted samples were dialyzed twice against protein buffer (50 mM Tris-HCl [pH 8], 150 mM NaCl, 1 mM dithiothreitol [DTT]) and once again in the same buffer with 30% glycerol and then stored at −20°C.
gp16 fluorescence binding assays.
Cell binding assays using purified J-1 GFP-gp16 and PL-1 GFP-gp16 and GFP proteins were carried out as described before (49) with some modifications. Briefly, 0.3 ml of exponentially growing bacterial cells was centrifuged and resuspended in 100 μl of modified phage buffer (20 mM Tris-HCl, 100 mM NaCl, 10 mM MgSO4, 0.1% Tween 20, 10 mM CaCl2) and incubated with 1 μg of protein fusions for 20 min at room temperature. Cells were washed twice with phosphate-buffered saline (PBS) buffer, and binding to the bacterial cells was detected by fluorescence microscopy (Axiostar Plus; Carl Zeiss) with a 100× objective with oil immersion and phase contrast. When binding in the presence of sugars was tested, J-1 GFP-gp16 and PL-1 GFP-gp16 were preincubated with 0.25 M l-rhamnose, d-glucose, or N-acetylglucosamine for 30 min at room temperature, and the protocol was followed as described above.
Inhibition of adsorption assays.
The inhibition of adsorption assay is an adaptation of the adsorption assay using cell walls. In brief, 50 μl of cell walls (100 μg) were incubated with 50 μl of buffer (control), J-1 GFP-gp16, PL-1 GFP-gp16, or GFP at different concentrations at room temperature for 30 min. Then, 50 μl of phage was added (1 × 106 PFU/ml). The mixture was incubated at 37°C for 1 h, and cell walls were removed by centrifugation at 3,200 × g for 10 min. The unadsorbed phage in the supernatant was measured using the double agar method. To test inhibition of adsorption by sugars, the same protocol was followed using the indicated carbohydrates instead of proteins. All sugars were used at 0.25 M.
Nucleotide sequence accession numbers.
The GenBank accession numbers for J-1 and PL-1 are KC171646 and KC171647, respectively.
RESULTS
Genome sequencing of bacteriophages J-1 and PL-1.
The J-1 and PL-1 genome sequences were determined by pyrosequencing. J-1 and PL-1 virion DNAs are 40,931 bp and 38,880 bp long, respectively, and their G+C contents are 44.8 and 44.9%, respectively. Both phages have cohesive ends with 10-base, single-strand 3′ extensions (left end, 3′-CGGTCGGCCT), 4 bases shorter than the GAACGGTCGGCCTC sequence previously described by Nakashima et al. (50). Dot plot analysis shows that J-1 and PL-1 are closely related (Fig. 1), with PL-1 having a 1.9-kbp deletion corresponding to coordinates 23513 to 25418 in J-1.
FIG 1.
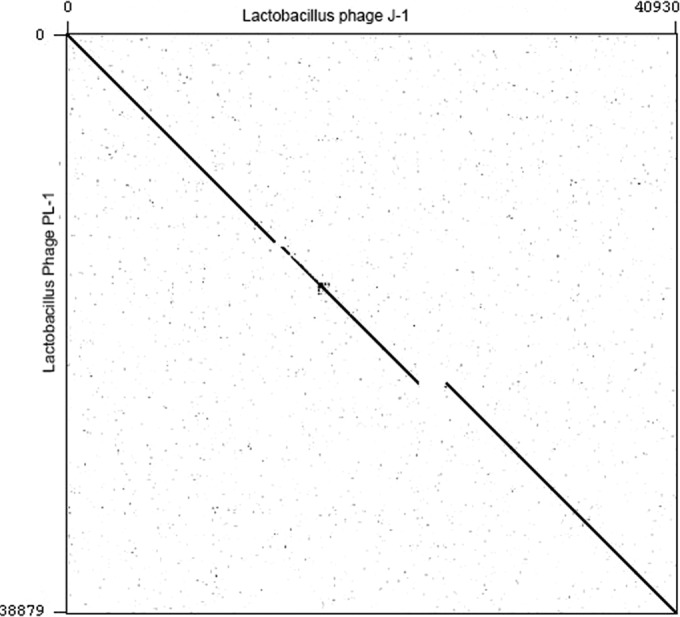
Dot plot comparison of Lactobacillus phages J-1 and PL-1. A sequence file containing J-1 was compared against a file containing PL-1 using Gepard (91).
Organization of J-1 and PL-1 genomes.
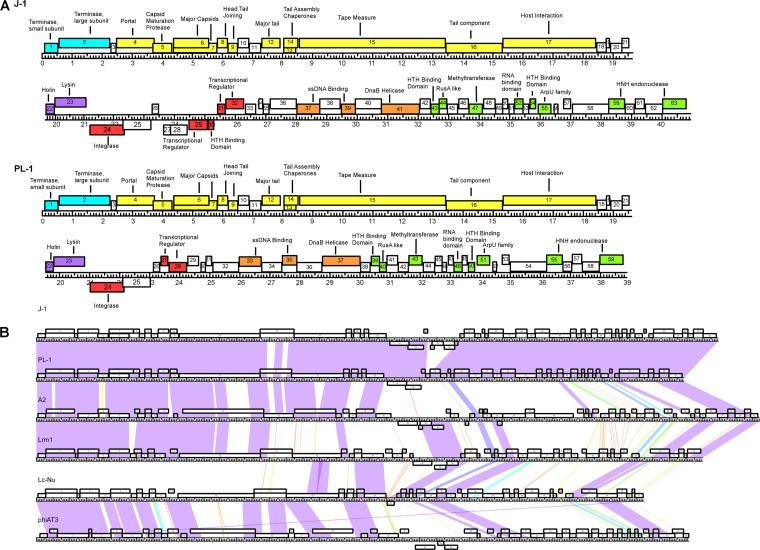
Analysis of the J-1 genome revealed 63 potential open reading frames (ORFs) and no tRNA genes. The most commonly used start codon is ATG (73.5%), with lower usage of GTG (17%) and TTG (9.5%). In J-1, 57 ORFs are transcribed rightward, and 6 are transcribed leftward, and the deletion in PL-1 results in loss or alteration of four leftward transcribed genes, 27 to 30 (Fig. 2A). The genome organizations can be divided into the following modules: DNA packaging, virion structure, lysis, integration, immunity, and replication. The presence of integrase genes (24) of the tyrosine recombinase family suggests a temperate origin of these phages.
FIG 2.
(A) Annotated genome maps of bacteriophages J-1 and PL-1. The viral genomes of J-1 and PL-1 are represented in four tiers, with markers spaced at 1-kbp and 100-bp intervals. The predicted genes are shown as boxes either above or below the genome, depending on whether they are rightward or leftward transcribed, respectively. Gene numbers are shown within each box. Putative genes can be divided in the following six modules: packaging (light blue), virion structure (yellow), lysis (purple), integration and immunity (red), and replication (orange). The putative proteins found in the extreme right region are colored in green, while the ORFs lacking function are white. (B) Global comparison of Lactobacillus phages. Two pairwise nucleotide alignment of phages J-1, PL-1, and related Lactobacillus phages (Lrm1, A2, phiAT3, and Lc-Nu) using Phamerator (43). The genomes are represented by horizontal lines, with putative genes shown as boxes above (transcribed rightward) or below (transcribed leftward) each genome; the number of each gene is shown within each box.
Nucleotide sequence comparison with other LAB phages shows that J-1 and PL-1 are most closely related to phages A2 (23, 51–54) and Lrm1, especially in the DNA packaging and virion structure modules (Fig. 2B). Because the J-1 and PL-1 genomes are similar, we will describe the functional assignments for J-1 and indicate where they differ for PL-1 (Table 1).
TABLE 1.
Lactobacillus phage J-1 and PL-1 predicted genes and gene products
| J-1 gene strand | Start–stop positions (length in aa) | PL-1 gene strand | Start–stop positions (length in aa) | Best database match (organism, gene) | % identity | Predicted function |
|---|---|---|---|---|---|---|
| 1F | 90–545 (152) | 1F | 90–545 (152) | Lactobacillus phage Lrm1, 1 | 97 | Terminase, small subunit |
| 2F | 567–2279 (571) | 2F | 567–2279 (571) | Lactobacillus phage A2, 2 | 96 | Terminase, large subunit |
| 3F | 2291–2482 (64) | 3F | 2291–2482 (64) | Lactobacillus phage Lrm1, 3 | 96 | |
| 4F | 2488–3741 (418) | 4F | 2488–3741 (418) | Lactobacillus phage A2, 3 | 96 | Portal |
| 5F | 3695–4324 (210) | 5F | 3695–4324 (210) | Lactobacillus phage A2, 4 | 85 | Capsid maturation protease |
| 6F | 4366–5568(401) | 6F | 4366–5568(401) | Lactobacillus phage A2, 5a | 99 | Major capsid |
| 7F | 4366–5825 (487) | 7F | 4366–5825 (487) | Lactobacillus phage A2, 5b | 99 | Major capsid |
| 8F | 5836–6195 (120) | 8F | 5836–6195 (120) | Lactobacillus phage A2, 6 | 75 | Head-tail joining |
| 9F | 6185–6514 (110) | 9F | 6185–6514 (110) | Lactobacillus phage A2, 7 | 92 | Head-tail joining |
| 10F | 6514–6900 (129) | 10F | 6514–6900 (129) | Lactobacillus phage Lrm1, 9 | 98 | |
| 11F | 6900–7286 (129) | 11F | 6900–7286 (129) | Lactobacillus phage Lrm1,10 | 98 | |
| 12F | 7320–7937 (206) | 12F | 7320–7937 (206) | Lactobacillus phage A2, 10 | 98 | Major tail |
| 13F | 8036–8449 (138) | 13F | 8036–8449 (138) | Lactobacillus phage Lrm1, 12 | 99 | Tail assembly chaperones |
| 14F | 8036–8550 (172) | 14F | 8036–8550 (172) | Lactobacillus phage Lrm1, 12 | 90 | Tail assembly chaperones |
| 15F | 8572–13434 (1621) | 15F | 8572–13434 (1621) | Lactobacillus phage Lrm1, 13 | 97 | Tape measure |
| 16F | 13435–15474 (680) | 16F | 13435–15327 (631) | Lactobacillus phage A2, 13 | 45 | Tail component |
| 17F | 15471–18590 (1040) | 17F | 15324–18443 (1040) | Lactobacillus phage Lrm1, 15 | 85 | Host interaction |
| 18F | 18600–18923 (108) | 18F | 18453–18782 (110) | Lactobacillus phage A2, 15 | 100 | |
| 19F | 18916–19047 (44) | 19F | 18769–18900 (44) | Lactobacillus rhamnosus Lc-Nu, 17 | 84 | |
| 20F | 19072–19464 (131) | 20F | 18925–19317 (131) | Lactobacillus phage Lc-Nu, 18 | 97 | |
| 21F | 19445–19687 (81) | 21F | 19298–19540 (81) | Lactobacillus phage Lc-Nu, 19 | 79 | |
| 22F | 19677–19949 (91) | 22F | 19530–19802 (91) | Lactobacillus phage PL1 | 99 | Holin |
| 23F | 19951–21003 (351) | 23F | 19804–20856 (351) | Lactobacillus phage PL1 | 100 | Lysin |
| 24R | 22375–21224 (384) | 24R | 22228–21077 (384) | Lactobacillus casei BL23 | 100 | Integrase |
| 25R | 23246–22311 (312) | 25R | 23099–22164 (312) | Lactobacillus casei BL23 | 96 | |
| 26F | 23265–23495 (77) | 26F | 23118–23348 (77) | Lactobacillus casei BL23 | 100 | |
| 27R | 23632–23564 (24) | Lactobacillus casei ATCC 334 | 95 | |||
| 28R | 23895–23656 (74) | Lactobacillus phage A2, 21 | 66 | |||
| 29R | 25117–24488 (210) | Lactobacillus phage phiAT3, 25 | 88 | Repressor | ||
| 30R | 25311–25105 (69) | Lactobacillus rhamnosus LMS2-1 | 100 | HTH binding domain | ||
| 31F | 25430–25684 (85) | 27F | 23379–23633 (85) | Lactobacillus rhamnosus LMS2-1 | 100 | Repressor |
| 32F | 25687–26298 (204) | 28F | 23636–24247 (204) | L. paracasei ATCC 25302 | 89 | Antirepressor |
| 33F | 26326–26685 (120) | 29F | 24275–24634 (120) | Lactobacillus phage phiAT3, 25 | 97 | |
| 34F | 26767–26919 (51) | 30F | 24716–24868 (51) | Lactobacillus phage Lrm1, 31 | 98 | |
| 35F | 26924–27127 (68) | 31F | 24873–25076 (68) | Lactobacillus phage phiAT3, 26 | 91 | |
| 36F | 27145–28032 (296) | 32F | 25094–25981 (296) | Enterococcus phage phiEf11 | 29 | |
| 37F | 28032–28787 (252) | 33F | 25981–26736 (252) | Lactobacillus phage LBR48 | 59 | ssDNA binding protein |
| 38F | 28784–29458 (225) | 34F | 25094–25981 (225) | Lactobacillus rhamnosus HN001 | 94 | |
| 39F | 29473–29958 (162) | 35F | 27422–27907 (162) | L. paracasei subsp. paracasei 8700:2 | 88 | ssDNA binding protein |
| 40F | 29936–30808 (290) | 36F | 27888–28757 (290) | Lactobacillus phage Lc-Nu, 34 | 78 | HTH binding domain |
| 41F | 30805–32067 (421) | 37F | 28754–30016 (421) | Lactobacillus phage Lc-Nu, 35 | 98 | DnaB helicase |
| 42F | 32069–32413 (115) | 38F | 30018–30362 (115) | Lactobacillus phage Lc-Nu, 36 | 90 | |
| 43F | 32426–32713 (96) | 39F | 30375–30662 (96) | Lactobacillus phage Lc-Nu, 37 | 91 | HTH binding domain |
| 44F | 32700–32954 (85) | 40F | 30649–30903 (85) | Lactobacillus phage Lc-Nu, 38 | 93 | RusA like |
| 45F | 32951–33316 (122) | 41F | 30900–31265 (122) | Lactobacillus phage Lc-Nu, 39 | 99 | |
| 46F | 33328–33666 (113) | 42F | 31277–31615 (113) | Lactobacillus phage A2, 43 | 96 | |
| 47F | 33678–34133 (152) | 43F | 31627–32082 (152) | Lactobacillus phage Lc-Nu, 40 | 96 | Methyltransferase |
| 48F | 34144–34551 (136) | 44F | 32093–32500 (136) | Lactobacillus phage Lrm1, 48 | 57 | |
| 49F | 34544–34789 (82) | 45F | 32493–32738 (82) | L. paracasei ATCC 25302 | 94 | |
| 50F | 34782–34952 (57) | 46F | 32731–32901 (57) | L. paracasei ATCC 25302 | 56 | |
| 51F | 34977–35174 (66) | 47F | 32926–33123 (66) | L. paracasei ATCC 25302 | 88 | |
| 52F | 35164–35457 (98) | 48F | 33113–33406 (98) | Lactobacillus phage Lb338-1 | 81 | RNA binding domain |
| 53F | 35450–35641 (64) | 49F | 33399–33590 (64) | Haemophilus parasuis SH0165 | 38 | |
| 54F | 35662–35880 (73) | 50F | 33611–33829 (73) | Lactobacillus phage Lc-Nu, 44 | 90 | HTH binding domain |
| 55F | 35945–36382 (146) | 51F | 33894–34331 (146) | Lactobacillus phage Lrm1, 49 | 96 | ArpU family |
| 56F | 36471–36617 (50) | 52F | 34417–34566 (50) | Lactobacillus phage A2, 54 | 98 | |
| 57F | 36779–37033 (85) | 53F | 34728–34982 (85) | Lactobacillus phage A2, 57 | 89 | |
| 58F | 37058–38275 (406) | 54F | 35007–36224 (406) | Lactobacillus phage Lc-Nu, 47 | 96 | |
| 59F | 38262–38792 (177) | 55F | 36211–36741 (177) | Lactobacillus phage A2, 60 | 97 | HNH endonuclease |
| 60F | 38796–39116 (107) | 56F | 36745–37065 (107) | Lactobacillus phage phi AT3, 52 | 78 | |
| 61F | 39119–39442 (108) | 57F | 37068–37391 (108) | Lactobacillus phage A2, 61 | 89 | |
| 62F | 39455–40036 (194) | 58F | 37404–37985 (194) | Lactobacillus phage A2, 62 | 95 | |
| 63F | 40026–40820 (265) | 59F | 37975–38769 (265) | Lactobacillus phage Lrm1, 54 | 98 | HNH endonuclease/P-loop NTPase domain |
DNA packaging and virion structure.
The DNA packaging module of J-1 contains genes 1 and 2, which are predicted to encode the terminase small and large subunits, respectively. J-1 gp1 is similar to the terminase small subunit of phage Lrm1 (Orf1) (12), but the organization differs from that of phage A2, where the terminase small subunit is encoded by gene 61, and biochemical evidence supports this functional assignment (55) (23). In J-1 and PL-1, a single orf at the right end of the genome (genes 63 and 59, respectively) corresponds to a fusion of A2 orf60 and orf61, similar to that of orf54 in phage Lrm1 (12). It is unclear whether J-1 gp63 and PL-1 gp59 play a role in DNA packaging in addition to gp1 and gp2.
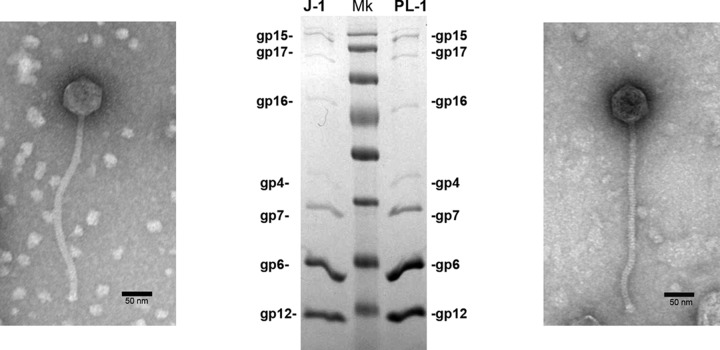
Examination by electron microscopy shows that J-1 and PL-1 have siphovirial morphologies. J-1 has an isometric head of 65 ± 4 nm in diameter and a tail length of 283 ± 8 nm. PL-1 also has an isometric head of 62 ± 4 nm in diameter, a tail length of 290 ± 7 nm, and a baseplate structure at the tip of the tail (Fig. 3); this is consistent with prior reports (26). These reflect the most frequently found morphologies among LAB phages (22).
FIG 3.
Analysis and functional assignment of J-1 and PL-1 structural proteins. Illustrated are electron micrographs of J-1 (left) and PL-1 (right) phage particles and SDS gel electrophoresis of virion proteins showing the predicted gene products. Molecular mass markers (Mk) are, from top to bottom, 170, 130, 100, 70, 55, 40, 35, and 25 kDa.
Genes 4 and 5 code for putative portal and protease proteins, respectively, and are closely related to the corresponding genes in phage A2. The major capsid gene is organized similarly to that in A2, where two types of capsid subunits are expressed: the product of gene 5 (gp5A) and a C-terminal 85-amino-acid (aa) extended form (gp5B) resulting from a −1 programmed translational frameshift at the 3′ end of gene 5. Both proteins appear to be essential for phage viability (53). In J-1 and PL-1, a slippery sequence (CCCAAAA) is present at the end of gene 6, and a −1 frameshift can facilitate expression of a longer form (gp7), 86 amino acids longer than gp6 (Fig. 4A). Expression of gp6 and gp7 is supported by SDS-PAGE of virion proteins (Fig. 3) and mass spectrometry (MS) (Table 2), including peptides unique to gp7 (Fig. 4A). Quantification suggests that the ratio of gp6 to gp7 is 2.5:1 (Fig. 3). J-1 gp6 and gp7 are also proteolytically processed, involving cleavage of the N-terminal 123 residues, similarly to that reported for the A2 capsid subunit. This is supported by the observation that cleavage with Lys-C (which cleaves after lysine residues) prior to MS/MS generates an AVPTDAS peptide, reflecting cleavage following an arginine residue, and correspondence to the sequence (N-AVPTADAS) of the mature form of the A2 capsid. The 123-residue peptide presumably acts as a scaffold for capsid assembly (56).
FIG 4.

Translational frameshift of capsid and chaperone mRNAs. A translational −1 frameshifting near the end of transcribed genes 6 and 13 results in synthesis of two different-length products, gp6-gp7 (A) and gp13-gp14 (B), respectively. The proposed slippery sequence is shaded in gray. Amino acid sequences depicted in bold letters correspond to peptides detected by MALDI-MS for the short and long forms of the protein. The underlined sequence in gp6-gp7 (A) corresponds to the N-terminal peptide detected by MALDI-MS and confirms the predicted proteolytical processing.
TABLE 2.
Identification of virion-associated proteins
| Protein (type) | J-1 |
PL-1 |
||||
|---|---|---|---|---|---|---|
| Molecular mass (kDa) | % coveragea | No. of PSMsb | Molecular mass (kDa) | % coveragea | No. of PSMsb | |
| gp4 (portal) | 46.3 | 51.91 | 45 | 46.3 | 52.39 | 51 |
| gp6 (major capsid) | 29.90 | 55.44 | 837 | 29.9 | 82.54 | 561 |
| gp7 (major capsid) | 38.3 | 69.61 | 644 | 38.3 | 74.33 | 548 |
| gp12 (major tail) | 22.1 | 74.27 | 220 | 22.1 | 80.58 | 337 |
| gp15 (TMP) | 173.2 | 60.27 | 882 | 173.2 | 59.04 | 476 |
| gp16 (tail component) | 75.1 | 51.91 | 91 | 69.2 | 25.99 | 29 |
| gp17 (host interaction) | 112.7 | 43.17 | 45 | 113.0 | 39.90 | 88 |
Percentage of predicted protein sequence identified in peptides.
PSMs, peptide spectrum matches.
J-1 genes 8 to 11 are organized similarly to the putative head-tail connector protein-encoding genes of both A2 and have partial sequence similarity to Staphylococcus aureus phage PVL head-tail connectors (23). We presume that they provide similar functions in J-1, but we did not identify the products by MS/MS analysis of intact virions, presumably because they are absent or present in low abundance.
Phages belonging to the Siphoviridae family have long, flexible tails containing many copies of the major tail subunit. J-1 gp12 has similarity to the major tail protein of A2 (orf10 [98% identity]), but J-1 and PL-1 lack the putative frameshifting sequence (CCCAAAA) present in the major tail subunit genes of phages A2 and Lrm1 (12, 57). J-1 genes 13 and 14 likely encode tail assembly chaperones expressed via a highly conserved (58) programmed translational frameshift (5′-AAAAAAAT; Fig. 4B). These assembly chaperones are not expected to be virion components but were detected by MS, perhaps as contaminants in the phage lysates. Peptides identified are consistent with frameshifting at the predicted sequence. J-1 gp15 is the putative tape measure protein based on its position and size (4,902 bp), as well as similarity with the putative tape measure proteins of Lrm1 and A2 phages. A large number of peptides derived from this protein were identified by MS (Table 2), and a product of the expected size was observed by SDS-PAGE (Fig. 3).
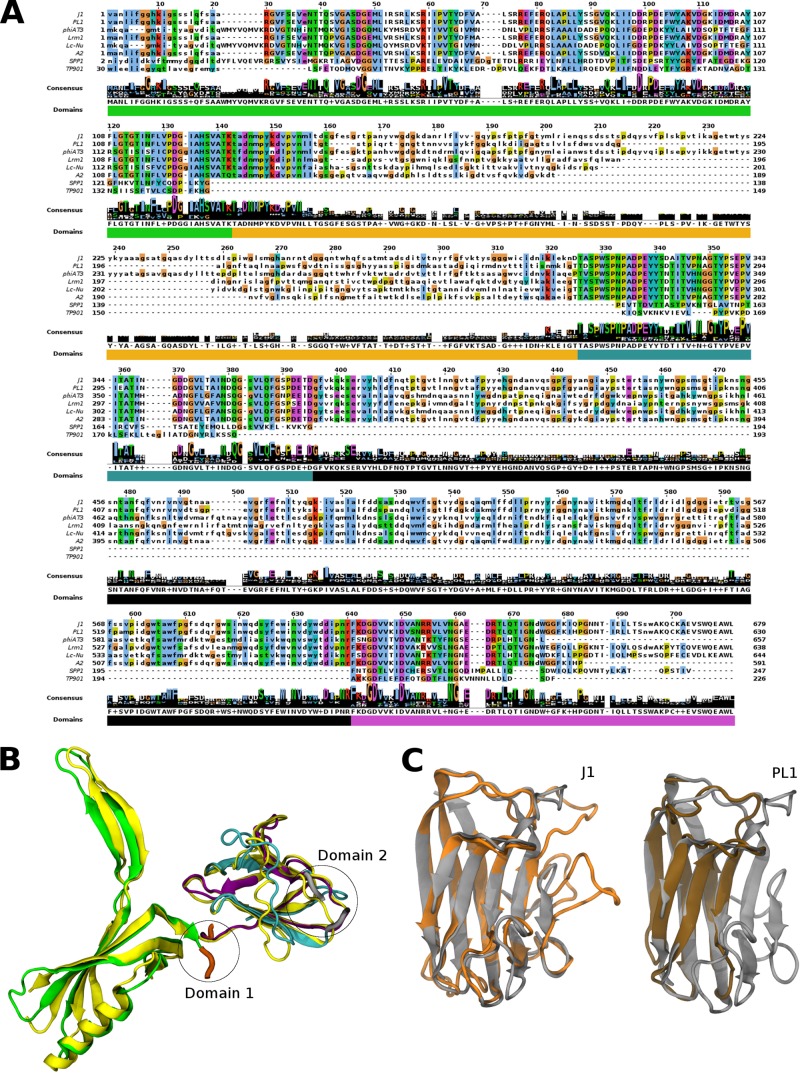
A notable difference between J-1 and PL-1 is found in genes 16 and 17. gp16 of J-1 and PL-1 was detected by MS analysis, and unique peptides for each protein were identified (Fig. 3 and Table 2). gp16 has similarity to A2 gp13 and several genes annotated as putative phage tail protein-encoding genes in the genomes of several Lactobacillus strains. Multiple-sequence alignment revealed three highly conserved regions and two variable regions (Fig. 5A). The N terminus of gp16 is identical in J-1 and PL-1 (residues 1 to 146) and conserved in the other analyzed phages. After that, regions of 171 aa in J-1 and 122 aa in PL-1 with low identity between each other are found. Divergent sequences were also identified in Lrm1, Lc-Nu, A2, and phiAT3. A short highly conserved region follows; then a 237-aa region that is similar between J-1 and PL-1 but with lower identity among the other phages was found, and finally at the C terminus the gene products are highly similar (Fig. 5A). A Sipho_tail Pfam domain that encompasses the whole gene 16 of J-1 and PL-1 was identified. However, the Sipho_tail HMM logo presents two long insertions with high probability. Further analysis by HHpred revealed similarity between the N terminus of gp16 and the distal tail proteins (Dit) of Bacillus subtilis SPP1 (59) and Lactococcus lactis TP901-1 (60, 61). J-1 gp16 may thus act like Dit in providing a hub for anchoring the tail tube, tail spike, and baseplate (62). Modeling of the J-1 gp16 N-terminal domain on SPP1 Dit (PDB code 2X8K, chain A) suggests that they fold into very similar structures (Fig. 5B). The first of the variable regions in gp16 (Dom1; ∼120 aa) is a predicted carbohydrate-binding module (CBM) present in enzymes that depolymerize plant cell wall polysaccharides into simple sugars (Fig. 5C) to increase the catalytic efficiency by targeting the enzymes to its substrate (63). J-1 and PL-1 gp16 Dom1 was modeled on the endo-β-1,4-galactanase from Thermotoga maritima (PDB code 2XON, chain L), revealing putative structural differences between the J-1 and PL-1 proteins that could reflect recognition of distinctive sugar motifs or a differential sugar binding affinity (Fig. 5C). The second variable region comprises 237 aa (Dom2) (Fig. 5B) and lacks a suitable template in the PDB for modeling. The predicted secondary structure contained mostly B sheets resembling galectin.
FIG 5.
gp16 alignment and structure prediction. (A) Amino acid sequence alignment of gp16 with similar proteins found in Lactobacillus phages, Orf19.1 of Spp1 and Orf46 of TP901-1. Uppercase letters correspond to aligned Pfam domain (Sypho_tail). (B) Predicted structure of gp16 of J-1 and PL-1 based on Spp1 Orf19.1 crystal structure (PDB code 2X8K, chain A). Dom1 and Dom2 correspond to the regions that could not be modeled with this PDB code. The template is shown in yellow, and the colors in the modeled structure correspond to the domains shown in panel A. (C) Predicted structure of Dom1 J-1 (orange) and Dom1 PL-1 (brown) of gp16 based on the carbohydrate-binding module (CBM) crystal structure of the endo-β-1,4-galactanase from Thermotoga maritima (PDB code 2XON, chain L) (gray).
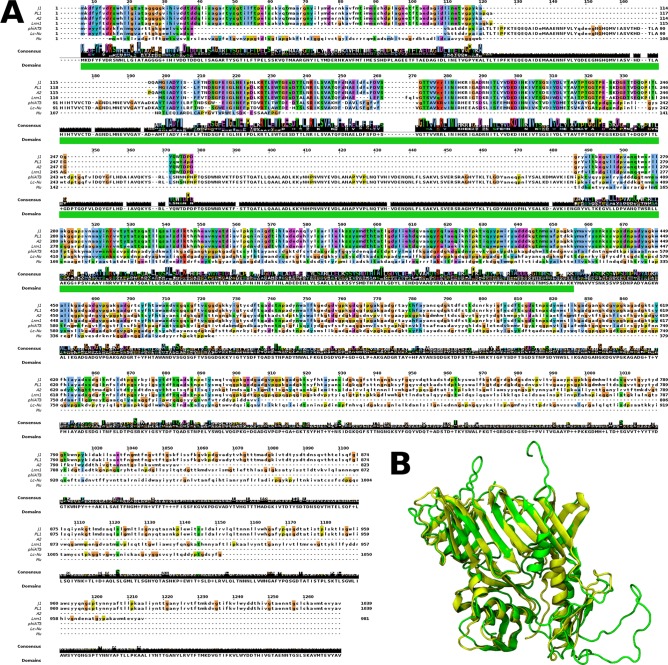
J-1 and PL-1 gp17 have similarity to proteins of phages Lrm1 and A2 that have been annotated as host specificity proteins as well as several host-encoded proteins (presumably prophage encoded in Lactobacillus and Streptococcus) annotated as phage-related tail-host interaction proteins. The J-1 and PL-1 gp17 proteins share 98% identity, and their N termini is shared with related proteins of other Lactobacillus phages (Fig. 6A) and contain the Prophage_tail Pfam family. HHpred analysis revealed that residues 1 to 400 share similarity to gp44 of phage Mu, and the structure was modeled using this as a template (PDB code 1WRU). The predicted structure of the N-terminal region can be superimposed on Mu gp44 (Fig. 6B) and is also similar to both T4 gp27 (PDB code 1K28) and Lactococcus phage p2 Orf16 (PDB codes 2WZP and 2X53). These proteins assemble as identical trimers but can adopt different architectural arrangements (62). Although the differences between J-1 gp17 and PL-1 gp17 (19 residues in total) are all in the N-terminal region, the predicted structures are nearly identical. The rest of gp17 resembles host recognition proteins in Streptococcus thermophilus phages (64, 65) and contains five collagen-like repeats (Gly-X-Y; Fig. 6A). The numbers of Gly-X-Y repeats differ in J-1, PL-1, and their homologues, perhaps through recombination or replication errors (64, 66). Taken together, the analyses of gp16 and gp17 suggest that they are tail components involved in host recognition, and the variation in the gp16 proteins and their homologues contributes to host specificity.
FIG 6.
gp17 alignment and structure prediction. (A) Amino acid sequence alignment of gp17 with similar proteins found in Lactobacillus phages and gp44 of phage Mu. Uppercase letters correspond to the aligned Pfam domain (Prophage_tail). (B) Predicted structure of the first 400 amino acids of gp17 of J-1 and PL-1 (green) based on Mu gp44 crystal structure (PDB code 1WRU) (yellow).
J-1 and PL-1 adsorption assays.
While propagating J-1 and PL-1, we observed that although J-1 forms large (∼2-mm) plaques on lawns of both L. casei subsp. casei ATCC 27139 (host to J-1) and L. paracasei subsp. paracasei ATCC 27092 (host to PL-1), PL-1 forms similarly sized plaques (∼1.5 mm) on lawns of strain 27092 but tiny pinprick plaques on strain 27139, so we decided to use these two strains for further experiments. Both J-1 and PL-1 adsorb with similar kinetics to L. paracasei subsp. paracasei ATCC 27092, with more than 90% of particles adsorbed after 5 min of incubation (Fig. 7A). However, the adsorption kinetics for both phages is different with L. casei subsp. casei ATCC 27139. Only 60% of particles were adsorbed after 5 min, and adsorption of all of the particles required 30 min of incubation for J-1 and 60 min for PL-1 (Fig. 7B). These results suggest that even though J-1 and PL-1 are very similar, the small structural differences between each other account for the disparities observed in this first step of infection.
FIG 7.
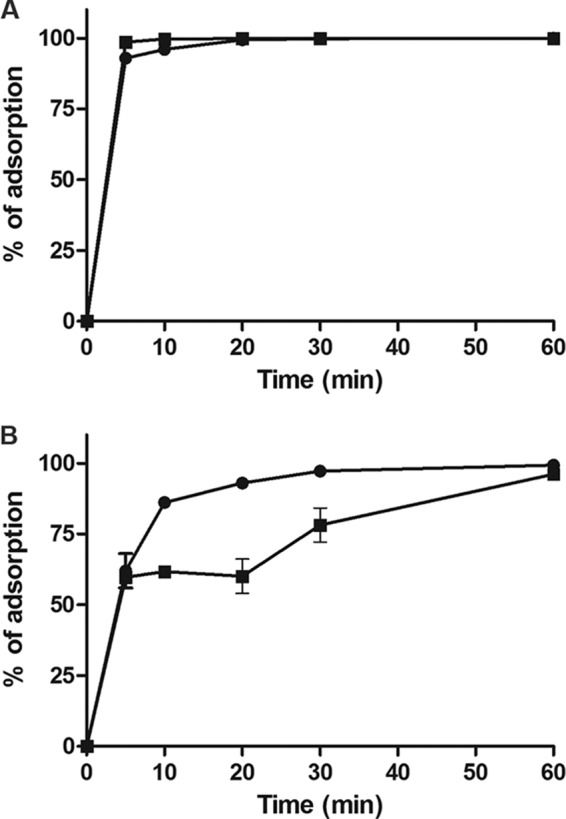
Kinetics of adsorption of J-1 and PL-1. L. paracasei subsp. paracasei ATCC 27092 (A) or L. casei subsp. casei ATCC 27139 (B) cells were incubated with J-1 (circles) or PL-1 (squares). At the indicated time points, PFU/ml in the supernatant was measured and the percentage of adsorption was calculated.
gp16 fluorescence binding assays.
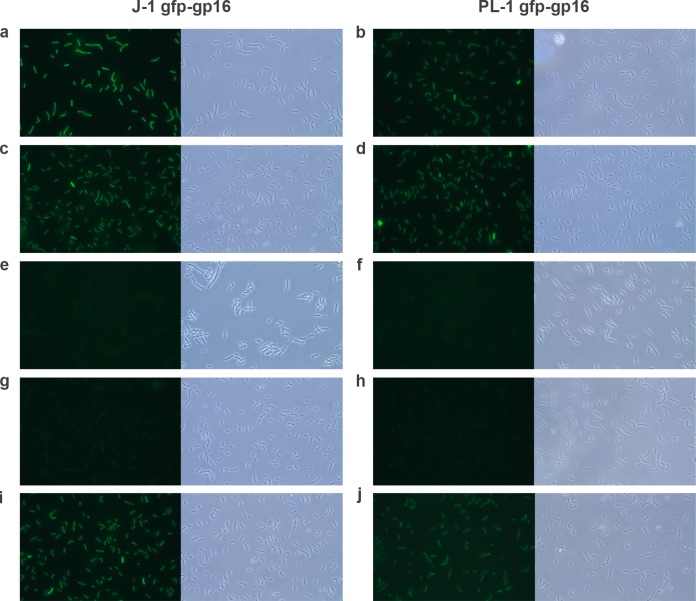
To test the roles of gp16 and gp17 in adsorption, we constructed and expressed GFP fusions of both proteins encoded by J-1 and PL-1. Both GFP-gp17 fusions were expressed but insoluble and were not studied further. Whole cells were incubated with the recombinant proteins and then visualized by fluorescence microscopy (Fig. 8). The fluorescent images showed that J-1 GFP-gp16 and PL-1 GFP-gp16 could bind to the cell surface of L. casei subsp. casei ATCC 27139 (Fig. 8a and b) as well as L. paracasei subsp. paracasei ATCC 27092 (Fig. 8c and d). Fluorescence was dependent on gp16 binding and specific to the strains tested in that no signal above the background level was observed with Lactobacillus acidophilus (Fig. 8e to f) or with GFP alone (not shown). The uniform distribution of fluorescence suggests the ligands for gp16 binding are not localized in any particular part of the cell but regularly distributed on the cell surface. These observations are consistent with J-1 and PL-1 recognizing saccharide-containing receptors within the outer layer of the cell wall, including a role for l-rhamnose as noted previously (67–69).
FIG 8.
Binding of GFP-gp16 to Lactobacillus cells. Recombinant proteins J-1 GFP-gp16 and PL-1 GFP-gp16 were incubated with Lactobacillus casei subsp. casei ATCC 27139 (a-b), L. paracasei subsp. paracasei ATCC 27092 (c-d), Lactobacillus acidophilus (e-f), and L. casei subsp. casei ATCC 27139 in the presence of l-rhamnose (g-h) or glucose (i-j). Cells were visualized by phase-contrast microscopy (left image) and fluorescence microscopy (right image). Magnification, 1,000×.
Examination of the effect of the addition of different monosaccharides to the GFP-gp16 binding assay is consistent with l-rhamnose being an important component of the receptor. Of all the sugars tested, only l-rhamnose showed strong interference with gp16 binding (Fig. 8g to j) and also reduced adsorption of whole phage particles by over 80% (Fig. 9). Similar results were observed with L. paracasei subsp. paracasei ATCC 27092 (data not shown). We also tested whether the GFP-gp16 fusions were able to specifically interfere with phage adsorption. We observed concentration-dependent inhibition of adsorption of both J-1 and PL-1 to L. casei subsp. casei ATCC 27139, although maximal adsorption inhibition of J-1 was 48% in the presence of J-1 gp16 but only 20% if PL-1 gp16 was used as a competitor (Fig. 10A). When adsorption inhibition of PL-1 was tested, these values were 39% and 25% for J-1 gp16 and PL-1 gp16, respectively (Fig. 10B). These observations are consistent with the sequence variations between J-1 gp16 and PL-1 gp16 being sufficient for a differential binding affinity to the cell surface and playing a role in host specificity.
FIG 9.
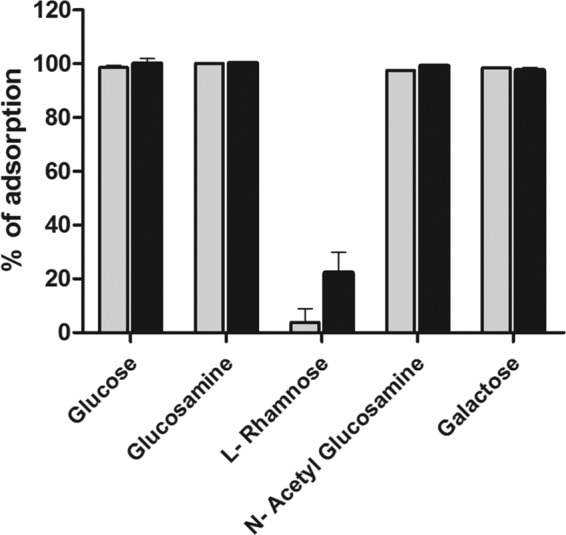
Adsorption of J-1 and PL-1 in the presence of sugars. J-1 (gray bars) and PL-1 (black bars) were preincubated with 0.25 M the indicated sugars and further incubated with cell walls of L. casei subsp. casei ATCC 27139. PFU/ml in the supernatant was measured, and the percentage of adsorption compared to the control was calculated. The error bars represent the standard deviations from experiments done in triplicate.
FIG 10.
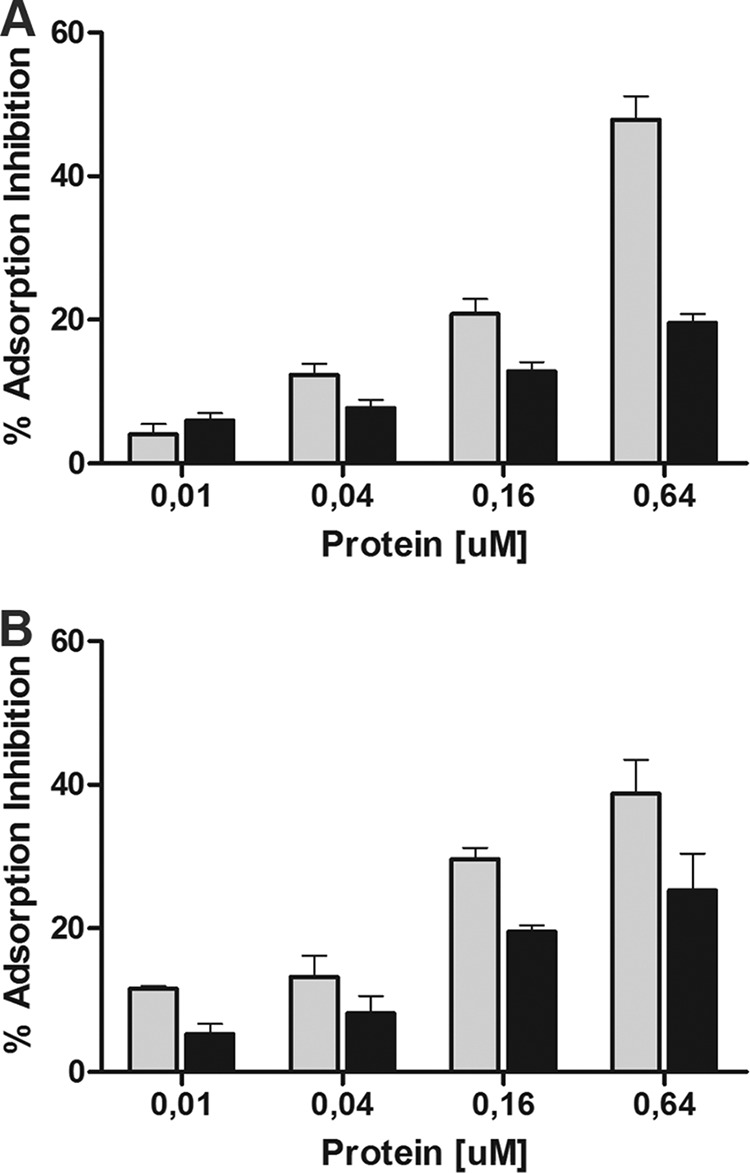
Adsorption inhibition assays. Adsorption inhibition was determined when L. casei subsp. casei ATCC 27139 cell walls were incubated with increasing amounts of J-1 gp16 (gray bars) or PL-1 gp16 (black bars), followed by adsorption assays using phage J-1 (A) or PL-1 (B). The error bars represent the standard deviations from experiments done in triplicate.
Lysis.
The lysis cassette follows the virion structural gene products, with gp22 being the putative holin containing a predicted signal sequence, two putative transmembrane helices, and a highly charged C terminus. J-1 gp23 is the endolysin with N-acetylmuramoyl-l-alanine amidase activity that hydrolyzes the amide linkage in the peptidoglycan of L. casei, as has been shown previously (41).
Integration and immunity.
J-1 gene 24 is identical in nucleotide sequence to that of a putative tyrosine-integrase gene present in the genome of L. casei BL23 (LCABL_10790), and gp24 is also related at the amino acid sequence level to the phage Lrm1 integrase. At the 5′ side of the integrase gene, there is a region of approximately 221 bp with no coding potential that is the likely location of attP. Comparison of this region using BLASTN revealed a short segment of sequence identity (49/50) in an intergenic region between an endolysin gene and a putative uncharacterized protein in L. casei BL23, BDII, LC2W, and ATCC 334 strains. This sequence is partially present in phages AT3, FSW, and Lrm1, temperate phages induced from L. casei ATCC 393, L. casei subsp. casei ATCC 27139, and Lactobacillus rhamnosus strain M1, respectively (12, 24, 70). We were not able to assign any function to genes 25 and 26, and there are no homologous phage proteins to the correspondent gene products in the protein database.
Genes 27 and 28 of J-1 are transcribed leftward and are similar to orf21 and orf22 of phage A2. It is noteworthy that the deletion of 1.9 kbp in the PL-1 genome spans positions 23513 to 25418 in J-1 and includes J-1 genes 27 to 30 (see below). The N terminus of J-1 gp27 shares similarity to gp21 of phage A2, which is predicted to be an excise, although this has not been experimentally validated. J-1 gp28 is similar to Orf25 of Lrm1, and these are related to a negative regulator of the tcd operon (TcdC) found in several Gram-positive bacteria. This regulator has been extensively studied in Clostridium difficile, where it regulates expression of toxin genes tcdA and tcdB (71–73).
Using HHpred and the Conserved Domains Database (CDD) of NCBI (74), it was found that the products of gene 29 of J-1, which is transcribed leftward, and of gene 31 (gene 27 in PL-1), which is transcribed rightward, have maximal homology to CI and Cro proteins of lambda (75, 76). It is plausible that this organization corresponds to the synteny found in other lambda-like phages where CI is coded from right to left and Cro in the opposite direction. Genes 30 and 32 of J-1 (28 in PL-1) have no homology to any known sequence at the DNA level, but a helix-turn-helix (HTH) domain could be recognized, indicating that probably act as transcriptional regulators.
Replication module.
Genes 37 to 41 (33 to 37 in PL-1) are predicted to be involved in DNA replication and recombination. J-1 37 (33 in PL-1) encodes an Erf family protein involved in DNA single-stranded annealing (SSAPs) (77), and gp39 (gp35 in PL-1) is a putative single-stranded DNA (ssDNA) binding protein (78). J-1 genes 40 to 44 (37 to 40 in PL-1) are similar to orf34 to orf38 of phage Lc-Nu and likely perform similar functions. gp40 (gp36 of PL-1) has an HTH binding domain at the N terminus and is similar to several putative replication proteins. In gene 40, a 19-bp AT-rich region was observed flanked by several direct and inverted repeats, features common to phage replication origins (79). Upstream of this sequence, three directed repeats comprised of other directed and inverted repeats were found. The AT-rich region is followed by inverted repeats capable of forming a stem-loop structure similar to the one found in phage Lc-Nu (80). J-1 gp41 (gp37 in PL-1) is similar to DnaB helicases (81), and gp43 (gp39 in PL-1) is a putative DNA binding protein. J-1 gp44 (gp40 in PL-1) is a putative RusA-like Holliday junction resolvase (82).
Other genes at the extreme right end of the genome code for small proteins mostly of unknown function, although J-1 47 (43 in PL-1) encodes a putative cytosine DNA methyltransferase. J-1 genes 54 and 55 (50 and 51 in PL-1) both encode potential transcriptional regulators (83). J-1 gene 59 (55 in PL-1) codes for a putative HNH endonuclease, and the possible function of the product of gene 63 (59 in PL-1) has already been described.
DISCUSSION
We described here the genomic and structural analysis of Lactobacillus phages J-1 and PL-1. Although both phages were isolated about 50 years ago and extensively studied (especially PL-1), their genome sequences have only recently become publicly available (42). J-1 was isolated from a failed fermentation during manufacture of Yakult (25), and PL-1 was isolated subsequently when using a derived strain resistant to J-1 (26, 27). Both phages differ by only four gene products in the immunity region and in a tail protein (Fig. 2).
The deletion in the PL-1 genome removes genes corresponding to genes 27, 28, 29, and 30 of J-1. The presence of an integrase gene (24) strongly suggests that these phages are either temperate or derived from a temperate parent. Stetter (84) described the isolation of PL-1 lysogens in L. casei ATCC 334. A putative attB was found in the genome of L. casei BL23, and turbid plaques could be detected after infection with J-1 and PL-1. However, we were not able to isolate lysogens in this strain (data not shown). All the components of the module are currently being studied in order to propose a mechanism to regulate lysis-lysogeny in these phages.
J-1 and PL-1 are closely related to L. casei phage A2 among the packaging and structural genes (1 to 17). The predicted structural gene products could be detected by SDS-PAGE and identified by MS analysis as components of the virion (Fig. 3 and Table 2). Similar to A2, two capsid proteins are present in the viral particle. These two gene products are the result of a translational frameshift, so gp6 and gp7 share the amino termini but gp7 is 86 amino acids longer than gp6. In both proteins, the N terminus is proteolytically processed (Fig. 4).
The virion structural gene module encoding the tail components following the canonical organization depicted by Veesler and Cambillau (62), with the tail terminator, the major tail protein (MTP), the two chaperones (with the conserved translational frameshift), the tape measure protein (TMP), the baseplate hub (Dit), the gp27-like/tail-associated lysozyme or tail fiber (Tal), and baseplate/tip peripheral proteins, seems to be conserved but with some interesting differences. Only two high-molecular-mass proteins, gp16 (75 and 69 kDa in J-1 and PL-1, respectively) and gp17 (113 kDa), appear to be part of the baseplate and host recognition apparatus. The predicted structure of gp16 is similar to that of Dit proteins that form the baseplate hub. The N-domain and the belt present in Orf19.1 of Spp1 (62) could be superimposed with the N terminus of gp16. This conserved structure suggests that gp16 monomers, similarly to other phage Dit proteins, could connect to each other through this region to form a circular-shaped hexamer with a central wide channel that allows DNA traffic during phage infection (59, 61, 62, 85, 86). Also in analogy with Orf19.1 of Spp1, it could be expected that the C domain protrudes out of the cylinder core. The C terminus of gp16 is longer than that of other characterized Dit proteins, and a variable region between J-1, PL-1, and other analyzed Lactobacillus phages was recognized (Fig. 5A). The predicted structures of these variable regions (so called Dom1 of J-1 and PL-1 gp16) were similar to those of carbohydrate-binding modules (CBMs). After modeling was complete, the structural differences between both domains became more evident, suggesting a distinctive binding or affinity for sugars (Fig. 5B).
A GFP-gp16 fusion yielded a product able to bind to Lactobacillus casei/paracasei cells (Fig. 8). Binding of this protein was inhibited in the presence of l-rhamnose. This sugar also inhibited phage adsorption to purified cell walls (Fig. 9). The presence of l-rhamnose was demonstrated in the cell walls of L. casei subsp. casei ATCC 27139 (87). Data shown here strongly suggest that this carbohydrate is being used by the phage for host recognition, and gp16 is involved in this process. Adsorption inhibition assays indicate that affinity of binding of J-1 gp16 to the cell walls of strain 27139 is higher than that for PL-1 gp16 (Fig. 10). This is in agreement with results from whole-cell phage adsorption assays (Fig. 7) and the observation that fluorescence intensity with PL-1 gp16 in the decorating assays was consistently slightly dimmer than with J-1 gp16 in this strain (Fig. 8). We speculate that these differences might be sufficient for a distinctive host range between both phages in a suitable strain.
Based on the similarity to other phage proteins, gp17 has been annotated as the host interaction protein. The N terminus of gp17 has a similar structure to the gp27-like proteins that are part of baseplates and assemble as trimers (62). An alignment of the host interaction proteins of other Lactobacillus phages (Fig. 6A) showed a highly conserved N-terminal moiety, probably indicating its involvement in interaction with other phage proteins. Goulet et al. (88) have shown that in phage Spp1, gp19.1 (Dit) and the N terminus of gp21 (Tal) form a complex of one Dit hexamer with one gp21 N-terminal trimer, where gp21 can display a closed or an open conformation delineating a central channel to allow DNA passage during infection. The conservation of Dit and the N terminus of gp27-like proteins suggests that the Tal opening mechanism could be conserved in siphophages infecting Gram-positive bacteria (88). The C terminus of gp17 (even similar between J-1 and PL-1) is not conserved in the other analyzed Lactobacillus phages, and the presence of a number of collagen-like repeats resembles the host interaction proteins found in S. thermophilus phages. In these phages, it has been experimentally demonstrated that proteins carrying these motifs are responsible for host recognition, though probably not exclusively since mutants with an expanded host range also mapped in other tail genes (64, 66). In contrast with lactococcal phages, no baseplate/tip peripheral proteins (as receptor binding proteins [RBPs]) could be detected. The high molecular mass of gp16 and gp17 and the data presented here suggest the C terminus of these proteins could be playing that role in J-1 and PL-1 phages.
A peptidoglycan-digesting domain could not be identified as part of gp17 or located somewhere else in the genome. These activities digest the peptidoglycan to form a hole permitting the passage of the double-stranded DNA (dsDNA) at the beginning of infection. Tail-associated lysozymes have been recognized and characterized in Tuc2009 and TP901-1 lactococcal phages but not in p2 (89, 90).
High-resolution crystal structures of the baseplate proteins in addition to electron micrograph reconstruction of phage particles would provide extremely useful data to decipher the particular strategies of host recognition and infection used by these phages.
ACKNOWLEDGMENTS
This work was partially supported by ANPCYT PICT2008-0218 and UBACYT GEF 20020100200006 to M.P. M.E.D. and E.L. are doctoral fellows of CONICET (Consejo Nacional de Investigaciones Científicas y Tecnológicas, Argentina).
We thank Andrea Quiberoni (Instituto de Lactología Industrial, Argentina) for providing J-1 and PL-1 phages. We thank Pittsburgh Bacteriophage Genome Center and Madelon Portela from Institut Pasteur de Montevideo for assistance with MALDI experiments. We especially thank Carmen Sanchez-Rivas for helpful discussions.
Footnotes
Published ahead of print 12 September 2014
REFERENCES
- 1.Garneau JE, Moineau S. 2011. Bacteriophages of lactic acid bacteria and their impact on milk fermentations. Microb. Cell Fact. 10(Suppl 1):S20. 10.1186/1475-2859-10-S1-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brussow H. 2001. Phages of dairy bacteria. Annu. Rev. Microbiol. 55:283–303. 10.1146/annurev.micro.55.1.283. [DOI] [PubMed] [Google Scholar]
- 3.del Rio B, Binetti AG, Martin MC, Fernandez M, Magadan AH, Alvarez MA. 2007. Multiplex PCR for the detection and identification of dairy bacteriophages in milk. Food Microbiol. 24:75–81. 10.1016/j.fm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Madera C, Monjardin C, Suarez JE. 2004. Milk contamination and resistance to processing conditions determine the fate of Lactococcus lactis bacteriophages in dairies. Appl. Environ. Microbiol. 70:7365–7371. 10.1128/AEM.70.12.7365-7371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suarez VB, Quiberoni A, Binetti AG, Reinheimer JA. 2002. Thermophilic lactic acid bacteria phages isolated from Argentinian dairy industries. J. Food Prot. 65:1597–1604. [DOI] [PubMed] [Google Scholar]
- 6.Verreault D, Gendron L, Rousseau GM, Veillette M, Masse D, Lindsley WG, Moineau S, Duchaine C. 2011. Detection of airborne lactococcal bacteriophages in cheese manufacturing plants. Appl. Environ. Microbiol. 77:491–497. 10.1128/AEM.01391-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebrecht AC, Guglielmotti DM, Tremmel G, Reinheimer JA, Suarez VB. 2010. Temperate and virulent Lactobacillus delbrueckii bacteriophages: comparison of their thermal and chemical resistance. Food Microbiol. 27:515–520. 10.1016/j.fm.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Briggiler Marco M, De Antoni GL, Reinheimer JA, Quiberoni A. 2009. Thermal, chemical, and photocatalytic inactivation of Lactobacillus plantarum bacteriophages. J. Food Prot. 72:1012–1019. [DOI] [PubMed] [Google Scholar]
- 9.Suarez VB, Reinheimer JA. 2002. Effectiveness of thermal treatments and biocides in the inactivation of Argentinian Lactococcus lactis phages. J. Food Prot. 65:1756–1759. [DOI] [PubMed] [Google Scholar]
- 10.Lunde M, Aastveit AH, Blatny JM, Nes IF. 2005. Effects of diverse environmental conditions on {phi}LC3 prophage stability in Lactococcus lactis. Appl. Environ. Microbiol. 71:721–727. 10.1128/AEM.71.2.721-727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madera C, Garcia P, Rodriguez A, Suarez JE, Martinez B. 2009. Prophage induction in Lactococcus lactis by the bacteriocin Lactococcin 972. Int. J. Food Microbiol. 129:99–102. 10.1016/j.ijfoodmicro.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Durmaz E, Miller MJ, Azcarate-Peril MA, Toon SP, Klaenhammer TR. 2008. Genome sequence and characteristics of Lrm1, a prophage from industrial Lactobacillus rhamnosus strain M1. Appl. Environ. Microbiol. 74:4601–4609. 10.1128/AEM.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raya RR, Hebert EM. 2009. Isolation of phage via induction of lysogens. Methods Mol. Biol. 501:23–32. 10.1007/978-1-60327-164-6_3. [DOI] [PubMed] [Google Scholar]
- 14.Ventura M, Canchaya C, Bernini V, Altermann E, Barrangou R, McGrath S, Claesson MJ, Li Y, Leahy S, Walker CD, Zink R, Neviani E, Steele J, Broadbent J, Klaenhammer TR, Fitzgerald GF, O'Toole PW, van Sinderen D. 2006. Comparative genomics and transcriptional analysis of prophages identified in the genomes of Lactobacillus gasseri, Lactobacillus salivarius, and Lactobacillus casei. Appl. Environ. Microbiol. 72:3130–3146. 10.1128/AEM.72.5.3130-3146.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrangou R, Horvath P. 2012. CRISPR: new horizons in phage resistance and strain identification. Annu. Rev. Food Sci. Technol. 3:143–162. 10.1146/annurev-food-022811-101134. [DOI] [PubMed] [Google Scholar]
- 16.Sturino JM, Klaenhammer TR. 2006. Engineered bacteriophage-defence systems in bioprocessing. Nat. Rev. Microbiol. 4:395–404. 10.1038/nrmicro1393. [DOI] [PubMed] [Google Scholar]
- 17.Marranzino G, Villena J, Salva S, Alvarez S. 2012. Stimulation of macrophages by immunobiotic Lactobacillus strains: influence beyond the intestinal tract. Microbiol. Immunol. 56:771–781. 10.1111/j.1348-0421.2012.00495.x. [DOI] [PubMed] [Google Scholar]
- 18.Rochat T, Bermudez-Humaran L, Gratadoux JJ, Fourage C, Hoebler C, Corthier G, Langella P. 2007. Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependent catalase on DSS-induced colitis in mice. Microb. Cell Fact. 6:22. 10.1186/1475-2859-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene JD, Klaenhammer TR. 1994. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl. Environ. Microbiol. 60:4487–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawase M, He F, Kubota A, Harata G, Hiramatsu M. 2010. Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett. Appl. Microbiol. 51:6–10. 10.1111/j.1472-765X.2010.02849.x. [DOI] [PubMed] [Google Scholar]
- 21.Galdeano CM, de Moreno de LeBlanc A, Vinderola G, Bonet ME, Perdigon G. 2007. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin. Vaccine Immunol. 14:485–492. 10.1128/CVI.00406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villion M, Moineau S. 2009. Bacteriophages of lactobacillus. Front. Biosci. 14:1661–1683. [DOI] [PubMed] [Google Scholar]
- 23.Garcia P, Ladero V, Suarez JE. 2003. Analysis of the morphogenetic cluster and genome of the temperate Lactobacillus casei bacteriophage A2. Arch. Virol. 148:1051–1070. 10.1007/s00705-003-0008-x. [DOI] [PubMed] [Google Scholar]
- 24.Lo TC, Shih TC, Lin CF, Chen HW, Lin TH. 2005. Complete genomic sequence of the temperate bacteriophage PhiAT3 isolated from Lactobacillus casei ATCC 393. Virology 339:42–55. 10.1016/j.virol.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Hino M, Ikebe N. 1965. Lactic acid bacteria employed for beverage production. II. Isolation and some properties of a bacteriophage isolated during the fermentation of lactic acid beverage. J. Chem. Soc. Jpn. 39:472–476. [Google Scholar]
- 26.Watanabe K, Takesue S, Jin-Nai K, Yoshikawa T. 1970. Bacteriophage active against the lactic acid beverage-producing bacterium Lactobacillus casei. Appl. Microbiol. 20:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sechaud L, Cluzel PJ, Rousseau M, Baumgartner A, Accolas JP. 1988. Bacteriophages of lactobacilli. Biochimie 70:401–410. 10.1016/0300-9084(88)90214-3. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe K, Takesue S, Ishibashi K. 1977. Reversibility of the adsorption of bacteriophage PL-1 to the cell walls isolated from Lactobacillus casei. J. Gen. Virol. 34:189–194. 10.1099/0022-1317-34-1-189. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe K, Ishibashi K, Nakashima Y, Sakurai T. 1984. A phage-resistant mutant of Lactobacillus casei which permits phage adsorption but not genome injection. J. Gen. Virol. 65(Part 5):981–986. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe K, Hayashida M, Ishibashi K, Nakashima Y. 1984. An N-acetylmuramidase induced by PL-1 phage infection of Lactobacillus casei. J. Gen. Microbiol. 130:275–277. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe K, Kakita Y, Nakashima Y, Miake F. 1992. Calcium requirement for protoplast transfection mediated by polyethylene glycol of Lactobacillus casei by PL-1 phage DNA. Biosci. Biotechnol. Biochem. 56:1859–1862. 10.1271/bbb.56.1859. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe K, Kakita Y, Nakashima Y, Miake F. 1995. Involvement of host cell energy in the transfection of Lactobacillus casei protoplasts with phage PL-1 DNA. Curr. Microbiol. 30:39–43. 10.1007/BF00294522. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe K, Kakita Y, Nakashima Y, Sasaki T. 1990. Protoplast transfection of Lactobacillus casei by phage PL-1 DNA. Agric. Biol. Chem. 54:937–941. 10.1271/bbb1961.54.937. [DOI] [PubMed] [Google Scholar]
- 34.Kakita Y, Nakashima Y, Ono N, Miake F, Watanabe K. 1996. Effects of some calcium-related agents on the protoplast transfection of Lactobacillus casei with phage PL-1 DNA. Curr. Microbiol. 33:359–363. 10.1007/s002849900128. [DOI] [PubMed] [Google Scholar]
- 35.Kakita Y, Kashige N, Murata K, Kuroiwa A, Funatsu M, Watanabe K. 1995. Inactivation of Lactobacillus bacteriophage PL-1 by microwave irradiation. Microbiol. Immunol. 39:571–576. 10.1111/j.1348-0421.1995.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 36.Kashige N, Kakita Y, Nakashima Y, Miake F, Watanabe K. 2001. Mechanism of the photocatalytic inactivation of Lactobacillus casei phage PL-1 by Titania thin film. Curr. Microbiol. 42:184–189. 10.1007/s002840010201. [DOI] [PubMed] [Google Scholar]
- 37.Capra ML, del L Quiberoni A, Ackermann HW, Moineau S, Reinheimer JA. 2006. Characterization of a new virulent phage (MLC-A) of Lactobacillus paracasei. J. Dairy Sci. 89:2414–2423. 10.3168/jds.S0022-0302(06)72314-1. [DOI] [PubMed] [Google Scholar]
- 38.Kakita Y, Kashige N, Miake F, Watanabe K. 1997. Photocatalysis-dependent inactivation of Lactobacillus phage PL-1 by a ceramics preparation. Biosci. Biotechnol. Biochem. 61:1947–1948. 10.1271/bbb.61.1947. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe K, Takesue S, Ishibashi K. 1980. DNA of phage PL-1 active against Lactobacillus casei ATCC 27092. Agric. Biol. Chem. 44:453–455. 10.1271/bbb1961.44.453. [DOI] [Google Scholar]
- 40.Khosaka T. 1977. Physicochemical properties of a virulent Lactobacillus phage containing DNA with cohesive ends. J. Gen. Virol. 37:209–214. 10.1099/0022-1317-37-1-209. [DOI] [Google Scholar]
- 41.Kashige N, Nakashima Y, Miake F, Watanabe K. 2000. Cloning, sequence analysis, and expression of Lactobacillus casei phage PL-1 lysis genes. Arch. Virol. 145:1521–1534. 10.1007/s007050070073. [DOI] [PubMed] [Google Scholar]
- 42.Dieterle ME, Jacobs-Sera D, Russell D, Hatfull G, Piuri M. 2014. Complete genome sequences of Lactobacillus phages J-1 and PL-1. Genome Announc. 2(1):e00998-13. 10.1128/genomeA.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cresawn SG, Bogel M, Day N, Jacobs-Sera D, Hendrix RW, Hatfull GF. 2011. Phamerator: a bioinformatic tool for comparative bacteriophage genomics. BMC Bioinformatics 12:395. 10.1186/1471-2105-12-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, Heger A, Hetherington K, Holm L, Mistry J, Sonnhammer EL, Tate J, Punta M. 2014. Pfam: the protein families database. Nucleic Acids Res. 42:D222–D230. 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soding J, Biegert A, Lupas AN. 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33:W244–W248. 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb B, Sali A. 2014. Protein structure modeling with MODELLER. Methods Mol. Biol. 1137:1–15. 10.1007/978-1-4939-0366-5_1. [DOI] [PubMed] [Google Scholar]
- 47.Palomino MM, Allievi MC, Grundling A, Sanchez-Rivas C, Ruzal SM. 2013. Osmotic stress adaptation in Lactobacillus casei BL23 leads to structural changes in the cell wall polymer lipoteichoic acid. Microbiology 159:2416–2426. 10.1099/mic.0.070607-0. [DOI] [PubMed] [Google Scholar]
- 48.Piuri M, Jacobs WR, Jr, Hatfull GF. 2009. Fluoromycobacteriophages for rapid, specific, and sensitive antibiotic susceptibility testing of Mycobacterium tuberculosis. PLoS One 4:e4870. 10.1371/journal.pone.0004870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habann M, Leiman PG, Vandersteegen K, Van den Bossche A, Lavigne R, Shneider MM, Bielmann R, Eugster MR, Loessner MJ, Klumpp J. 2014. Listeria phage A511, a model for the contractile tail machineries of SPO1-related bacteriophages. Mol. Microbiol. 92:84–99. 10.1111/mmi.12539. [DOI] [PubMed] [Google Scholar]
- 50.Nakashima Y, Ikeda H, Kakita Y, Miake F, Watanabe K. 1994. Restriction map of the genomic DNA of Lactobacillus casei bacteriophage PL-1 and nucleotide sequence of its cohesive single-stranded ends. J. Gen. Virol. 75(Pt 9):2537–2541. [DOI] [PubMed] [Google Scholar]
- 51.Ladero V, Garcia P, Bascaran V, Herrero M, Alvarez MA, Suarez JE. 1998. Identification of the repressor-encoding gene of the Lactobacillus bacteriophage A2. J. Bacteriol. 180:3474–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia P, Ladero V, Alonso JC, Suarez JE. 1999. Cooperative interaction of CI protein regulates lysogeny of Lactobacillus casei by bacteriophage A2. J. Virol. 73:3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia P, Rodriguez I, Suarez JE. 2004. A −1 ribosomal frameshift in the transcript that encodes the major head protein of bacteriophage A2 mediates biosynthesis of a second essential component of the capsid. J. Bacteriol. 186:1714–1719. 10.1128/JB.186.6.1714-1719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moscoso M, Suarez JE. 2000. Characterization of the DNA replication module of bacteriophage A2 and use of its origin of replication as a defense against infection during milk fermentation by Lactobacillus casei. Virology 273:101–111. 10.1006/viro.2000.0382. [DOI] [PubMed] [Google Scholar]
- 55.Garcia P, Alonso JC, Suarez JE. 1997. Molecular analysis of the cos region of the Lactobacillus casei bacteriophage A2. Gene product 3, gp3, specifically binds to its downstream cos region. Mol. Microbiol. 23:505–514. [DOI] [PubMed] [Google Scholar]
- 56.Benevides JM, Bondre P, Duda RL, Hendrix RW, Thomas GJ., Jr 2004. Domain structures and roles in bacteriophage HK97 capsid assembly and maturation. Biochemistry 43:5428–5436. 10.1021/bi0302494. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez I, Garcia P, Suarez JE. 2005. A second case of −1 ribosomal frameshifting affecting a major virion protein of the Lactobacillus bacteriophage A2. J. Bacteriol. 187:8201–8204. 10.1128/JB.187.23.8201-8204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J, Hendrix RW, Duda RL. 2004. Conserved translational frameshift in dsDNA bacteriophage tail assembly genes. Mol. Cell 16:11–21. 10.1016/j.molcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Veesler D, Robin G, Lichiere J, Auzat I, Tavares P, Bron P, Campanacci V, Cambillau C. 2010. Crystal structure of bacteriophage SPP1 distal tail protein (gp19.1): a baseplate hub paradigm in gram-positive infecting phages. J. Biol. Chem. 285:36666–36673. 10.1074/jbc.M110.157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bebeacua C, Bron P, Lai L, Vegge CS, Brondsted L, Spinelli S, Campanacci V, Veesler D, van Heel M, Cambillau C. 2010. Structure and molecular assignment of lactococcal phage TP901-1 baseplate. J. Biol. Chem. 285:39079–39086. 10.1074/jbc.M110.175646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veesler D, Spinelli S, Mahony J, Lichiere J, Blangy S, Bricogne G, Legrand P, Ortiz-Lombardia M, Campanacci V, van Sinderen D, Cambillau C. 2012. Structure of the phage TP901-1 1.8 MDa baseplate suggests an alternative host adhesion mechanism. Proc. Natl. Acad. Sci. U. S. A. 109:8954–8958. 10.1073/pnas.1200966109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veesler D, Cambillau C. 2011. A common evolutionary origin for tailed-bacteriophage functional modules and bacterial machineries. Microbiol. Mol. Biol. Rev. 75:423–433. 10.1128/MMBR.00014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shoseyov O, Shani Z, Levy I. 2006. Carbohydrate binding modules: biochemical properties and novel applications. Microbiol. Mol. Biol. Rev. 70:283–295. 10.1128/MMBR.00028-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duplessis M, Moineau S. 2001. Identification of a genetic determinant responsible for host specificity in Streptococcus thermophilus bacteriophages. Mol. Microbiol. 41:325–336. 10.1046/j.1365-2958.2001.02521.x. [DOI] [PubMed] [Google Scholar]
- 65.Dupont K, Vogensen FK, Neve H, Bresciani J, Josephsen J. 2004. Identification of the receptor-binding protein in 936-species lactococcal bacteriophages. Appl. Environ. Microbiol. 70:5818–5824. 10.1128/AEM.70.10.5818-5824.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duplessis M, Levesque CM, Moineau S. 2006. Characterization of Streptococcus thermophilus host range phage mutants. Appl. Environ. Microbiol. 72:3036–3041. 10.1128/AEM.72.4.3036-3041.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yokokura T. 1971. Phage receptor material in Lactobacillus casei cell wall. I. Effect of l-rhamnose on phage adsorption to the cell wall. Jpn. J. Microbiol. 15:457–463. [DOI] [PubMed] [Google Scholar]
- 68.Yokokura T. 1977. Phage receptor material in Lactobacillus casei. J. Gen. Microbiol. 100:139–145. 10.1099/00221287-100-1-139. [DOI] [PubMed] [Google Scholar]
- 69.Ishibashi K, Takesue S, Watanabe K, Oishi K. 1982. Use of lectins to characterize the receptor sites for bacteriophage PL-1 of Lactobacillus casei. J. Gen. Microbiol. 128:2251–2259. [Google Scholar]
- 70.Shimizu-Kadota M, Sakurai T. 1982. Prophage curing in Lactobacillus casei by isolation of a thermoinducible mutant. Appl. Environ. Microbiol. 43:1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dupuy B, Govind R, Antunes A, Matamouros S. 2008. Clostridium difficile toxin synthesis is negatively regulated by TcdC. J. Med. Microbiol. 57:685–689. 10.1099/jmm.0.47775-0. [DOI] [PubMed] [Google Scholar]
- 72.Carter GP, Douce GR, Govind R, Howarth PM, Mackin KE, Spencer J, Buckley AM, Antunes A, Kotsanas D, Jenkin GA, Dupuy B, Rood JI, Lyras D. 2011. The anti-sigma factor TcdC modulates hypervirulence in an epidemic BI/NAP1/027 clinical isolate of Clostridium difficile. PLoS Pathog. 7:e1002317. 10.1371/journal.ppat.1002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matamouros S, England P, Dupuy B. 2007. Clostridium difficile toxin expression is inhibited by the novel regulator TcdC. Mol. Microbiol. 64:1274–1288. 10.1111/j.1365-2958.2007.05739.x. [DOI] [PubMed] [Google Scholar]
- 74.Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. 2011. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229. 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hochschild A, Lewis M. 2009. The bacteriophage lambda CI protein finds an asymmetric solution. Curr. Opin. Struct. Biol. 19:79–86. 10.1016/j.sbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hall BM, Roberts SA, Heroux A, Cordes MH. 2008. Two structures of a lambda Cro variant highlight dimer flexibility but disfavor major dimer distortions upon specific binding of cognate DNA. J. Mol. Biol. 375:802–811. 10.1016/j.jmb.2007.10.082. [DOI] [PubMed] [Google Scholar]
- 77.Iyer LM, Koonin EV, Aravind L. 2002. Classification and evolutionary history of the single-strand annealing proteins, RecT, Redbeta, ERF and RAD52. BMC Genomics 3:8. 10.1186/1471-2164-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arcus V. 2002. OB-fold domains: a snapshot of the evolution of sequence, structure and function. Curr. Opin. Struct. Biol. 12:794–801. 10.1016/S0959-440X(02)00392-5. [DOI] [PubMed] [Google Scholar]
- 79.Schnos M, Zahn K, Blattner FR, Inman RB. 1989. DNA looping induced by bacteriophage lambda O protein: implications for formation of higher order structures at the lambda origin of replication. Virology 168:370–377. 10.1016/0042-6822(89)90278-X. [DOI] [PubMed] [Google Scholar]
- 80.Tuohimaa A, Riipinen KA, Brandt K, Alatossava T. 2006. The genome of the virulent phage Lc-Nu of probiotic Lactobacillus rhamnosus, and comparative genomics with Lactobacillus casei phages. Arch. Virol. 151:947–965. 10.1007/s00705-005-0672-0. [DOI] [PubMed] [Google Scholar]
- 81.Stephens KM, McMacken R. 1997. Functional properties of replication fork assemblies established by the bacteriophage lambda O and P replication proteins. J. Biol. Chem. 272:28800–28813. 10.1074/jbc.272.45.28800. [DOI] [PubMed] [Google Scholar]
- 82.Mahdi AA, Sharples GJ, Mandal TN, Lloyd RG. 1996. Holliday junction resolvases encoded by homologous rusA genes in Escherichia coli K-12 and phage 82. J. Mol. Biol. 257:561–573. 10.1006/jmbi.1996.0185. [DOI] [PubMed] [Google Scholar]
- 83.Lleo MM, Fontana R, Solioz M. 1995. Identification of a gene (arpU) controlling muramidase-2 export in Enterococcus hirae. J. Bacteriol. 177:5912–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stetter KO. 1977. Evidence for frequent lysogeny in lactobacilli: temperate bacteriophages within the subgenus Streptobacterium. J. Virol. 24:685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sciara G, Bebeacua C, Bron P, Tremblay D, Ortiz-Lombardia M, Lichiere J, van Heel M, Campanacci V, Moineau S, Cambillau C. 2010. Structure of lactococcal phage p2 baseplate and its mechanism of activation. Proc. Natl. Acad. Sci. U. S. A. 107:6852–6857. 10.1073/pnas.1000232107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flayhan A, Vellieux FM, Lurz R, Maury O, Contreras-Martel C, Girard E, Boulanger P, Breyton C. 2014. Crystal structure of pb9, the distal tail protein of bacteriophage T5: a conserved structural motif among all siphophages. J. Virol. 88:820–828. 10.1128/JVI.02135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yasuda E, Tateno H, Hirabayashi J, Iino T, Sako T. 2011. Lectin microarray reveals binding profiles of Lactobacillus casei strains in a comprehensive analysis of bacterial cell wall polysaccharides. Appl. Environ. Microbiol. 77:4539–4546. 10.1128/AEM.00240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goulet A, Lai-Kee-Him J, Veesler D, Auzat I, Robin G, Shepherd DA, Ashcroft AE, Richard E, Lichiere J, Tavares P, Cambillau C, Bron P. 2011. The opening of the SPP1 bacteriophage tail, a prevalent mechanism in Gram-positive-infecting siphophages. J. Biol. Chem. 286:25397–25405. 10.1074/jbc.M111.243360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stockdale SR, Mahony J, Courtin P, Chapot-Chartier MP, van Pijkeren JP, Britton RA, Neve H, Heller KJ, Aideh B, Vogensen FK, van Sinderen D. 2013. The lactococcal phages Tuc2009 and TP901-1 incorporate two alternate forms of their tail fiber into their virions for infection specialization. J. Biol. Chem. 288:5581–5590. 10.1074/jbc.M112.444901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spinelli S, Veesler D, Bebeacua C, Cambillau C. 2014. Structures and host-adhesion mechanisms of lactococcal siphophages. Front. Microbiol. 5:3. 10.3389/fmicb.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krumsiek J, Arnold R, Rattei T. 2007. Gepard: a rapid and sensitive tool for creating dot plots on genome scale. Bioinformatics 23:1026–1028. 10.1093/bioinformatics/btm039. [DOI] [PubMed] [Google Scholar]