Abstract
Detailed mechanisms of WhiB-like (Wbl) proteins involved in antibiotic biosynthesis and morphological differentiation are poorly understood. Here, we characterize the role of WblAch, a Streptomyces chattanoogensis L10 protein belonging to this superfamily. Based on DNA microarray data and verified by real-time quantitative PCR (qRT-PCR), the expression of wblAch was shown to be positively regulated by AdpAch. Gel retardation assays and DNase I footprinting experiments showed that AdpAch has specific DNA-binding activity for the promoter region of wblAch. Gene disruption and genetic complementation revealed that WblAch acts in a positive manner to regulate natamycin production. When wblAch was overexpressed in the wild-type strain, the natamycin yield was increased by ∼30%. This provides a strategy to generate improved strains for natamycin production. Moreover, transcriptional analysis showed that the expression levels of whi genes (including whiA, whiB, whiH, and whiI) were severely depressed in the ΔwblAch mutant, suggesting that WblAch plays a part in morphological differentiation by influencing the expression of the whi genes.
INTRODUCTION
The Gram-positive filamentous soil bacterial genus Streptomyces is characterized by its complex life cycle, which involves the formation of a substrate mycelium that goes on to develop aerial hyphae, the tips of which ultimately coil and septate into spores. These bacteria are well known for the ability to produce a variety of commercially valuable antibiotics and other secondary metabolites (1, 2). Previous investigations suggested that the triggering of antibiotic biosynthesis is closely coordinated with the initiation of morphological differentiation in Streptomyces species (1, 3, 4), and both processes have been shown to be stringently controlled via a hierarchical regulatory network involving the integration of various physiological and environmental signals (5, 6). Although a significant number of regulatory genes and mechanisms involved in the regulatory networks governing morphological development and antibiotic production in Streptomyces have been elucidated, relatively little is known about the correlation of these two physiological processes.
The whiB-like (wbl) genes, which encode homologues of WhiB, have received attention because of their important roles in diverse aspects of actinobacterial biology, such as morphological differentiation and antibiotic production (7, 8). The gene products are small cytoplasmic proteins that contain four conserved cysteine residues able to coordinate an Fe-S cluster and are found largely in actinomycetes, including some mycobacteria (9). It has been reported that wbl genes are induced by various stimuli, especially oxidative stress, suggesting a unique role in maintaining redox homeostasis (10–12). In Mycobacterium tuberculosis, WhiB3 acts as a metabolic regulator that binds to the promoters of polyketide biosynthetic genes (13), while WhiB6 acts as an initial phagosomal signal receptor (14). It has also been shown that WhiB7 is a critical protein for generating resistance to antibiotics in M. tuberculosis (15). Recently, whiB3 (16), whiB4 (17), whiB5 (18), and whiB2 (19) were shown to play essential roles in the virulence of M. tuberculosis during progressive infection.
In Streptomyces coelicolor M145, there are 11 WhiB-like proteins, among which WblA was reported to act as an important transcriptional regulator involved in antibiotic production and morphological differentiation (7, 20, 21). WblA and its orthologues have been described as crucial antibiotic downregulators for the biosynthesis of various antibiotics such as actinorhodin (21), doxorubicin (22), tautomycetin (23), and moenomycin (24). Moreover, it was discovered that wblA is negatively regulated by the pleiotropic regulator AdpA in S. coelicolor (25). However, in our study, we revealed differing functions of wblAch in antibiotic biosynthesis and in the AdpA-WblA regulatory relationship in Streptomyces chattanoogensis L10, an industrial strain used for natamycin production. We demonstrate that wblAch is an AdpAch regulon that is under general direct positive control of AdpAch. We also find that WblAch acts as a pivotal activator for natamycin biosynthesis and morphological differentiation in S. chattanoogensis L10.
MATERIALS AND METHODS
Media, plasmids, strains, and growth conditions.
The strains used in the present study are listed in Table 1. General techniques for bacterial growth and isolation and manipulation of nucleic acids were carried out according to standard protocols for Escherichia coli and S. coelicolor, respectively (26). S. chattanoogensis L10 strains were grown at 28°C on YMG agar (1% malt extract, 0.4% yeast extract, 0.4% glucose, 0.2% CaCO3, and 2% agar, pH 7.2) for sporulation and at 30°C in YEME medium (0.3% yeast extract, 0.3% malt extract, 0.5% tryptone, 1% glucose) for natamycin production.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| S. chattanoogensis | ||
| WT | S. chattanoogensis L10 wild type | 30 |
| ΔwblAch | wblAch-disrupted mutant | This study |
| ΔadpAch | adpAch-disrupted mutant | 28 |
| YC1 | wblAch-disrupted mutant complemented with wblAch and its own promoter | This study |
| YC2 | Wild-type strain carrying wblAch overexpression | This study |
| YC3 | Wild-type strain carrying an empty vector | This study |
| Saccharomyces cerevisiae BY4741 | Indicator organism for natamycin bioassays | Laboratory stock |
| E. coli | ||
| TG1 | General cloning host | Novagen |
| ET12567/pUZ8002 | Methylation-deficient E. coli strain for conjugation with the helper plasmid | 41 |
| BL21(DE3) | Host for protein expression | Novagen |
| BW25113/pIJ790 | Strain used for PCR-targeted mutagenesis | 27 |
| Plasmids | ||
| pMD19-T vector | General cloning vector | TaKaRa |
| pT-wblAch | pUC18 containing the promoter of wblAch | This study |
| pSET152 | Integrative shuttle vector | 26 |
| pIJ8630 | Integrative shuttle vector | 42 |
| pIJ773 | Source of apramycin resistance cassette; aac(3)IV oriT | 27 |
| pMRD312 | Cosmid containing wblAch | This study |
| pMRD313 | wblAch replaced by aac(3)IV cassette in pMRD312 | This study |
| pYP1 | wblAch replaced by an 81-bp scar in pMRD312 | This study |
| pYP2 | pSET152 carrying wblAch and its promoter | This study |
| pYP3 | pIJ8630 carrying wblAch and ermE* promoter | This study |
In-frame deletion and complementation of wblAch.
Disruption of wblAch was performed by gene replacement according to a modified PCR targeting system (27). First, the cosmid pMRD312, containing the wblAch open reading frame (ORF) fragment, was introduced into E. coli BW25113/pIJ790. Second, the wblAch::FRT (FLP recombination target)-oriT-aac(3)IV-FRT disruption cassette was amplified from pIJ773 with primer pair wblAch-del-F/wblAch-del-R and electrotransformed into E. coli BW25113/pIJ790/cosmid pMRD312 to generate the disruption cosmid pMRD313, which replaced most of the wblAch coding region (amino acids 50 to 112). Third, the targeted cosmid pMRD313 was transformed into E. coli DH5α/BT340 in order to excise the aac(3)IV cassette. The resulting cosmid, pYP1, was conjugated by E. coli ET12567/pUZ8002 into S. chattanoogensis L10. The wblAch disruption mutant was selected by replica plating for thiostrepton-sensitive colonies and confirmed by PCR amplification with primer pair wblAch-F/wblAch-R.
For complementation, the integrative vector pSET152 was used. Primer pair wblAch-BamHI-F1/wblAch-EcoRV-R1 was used to amplify a 750-bp DNA fragment containing the wblAch ORF and its own promoter. The amplified PCR products were then inserted into pSET152, which was digested with BamHI and EcoRV, and the resultant plasmid, pYP2, was integrated into the chromosome of the wblAch deletion mutant at the phage ΦC31 attB site for complementation.
Overexpression of wblAch.
For wblAch overexpression, a 342-bp DNA fragment containing the complete wblAch gene was amplified by using primers wblAch-NdeI-F and wblAch-NotI-F. Afterwards, this PCR product was subcloned into the pMD19 vector (TaKaRa) after dA addition and then digested with NdeI and NotI to give a NdeI-NotI DNA fragment containing wblAch. This fragment was ligated into the same sites of pIJ8630, which contained the ermE* promoter inserted into the BamHI site. The resulting plasmid was then introduced into E. coli ET12567/pUZ8002 for E. coli-Streptomyces conjugation.
Microarray analysis.
Mycelia of S. chattanoogensis L10 wild-type (WT) and mutant strains grown in YEME medium were collected at 16 h, 24 h, and 36 h. Total RNA was isolated by using the RNA Extract kit (Qiagen, Germany) according to the manufacturer's instructions and checked for an RNA integrity number (RIN) to inspect RNA integration by using an Agilent Bioanalyzer 2100 instrument (Agilent Technologies, Santa Clara, CA, USA). Microarray assays, including primer design, labeling, hybridization, and washing, and microarray data normalization were performed by Shanghai Biotechnology Corporation (China) according to standard protocols.
Electrophoretic mobility shift assays.
Expression of the recombinant AdpAch protein with a His tag at its C terminus was performed with E. coli BL21, and the protein was purified by using Ni-nitrilotriacetic acid (NTA) His Bind resin (Novagen), as previously described (28). For probe preparation, probe A, probe B, and probe C, covering each binding site of the wblAch promoter, were amplified with the primers listed in Table 2. The promoter region (384 bp) of wblAch was amplified with primer pair wblAch-EMSA-F/wblAch-EMSA-R. Afterwards, the PCR products were cloned into the pMD19 vector (TaKaRa). The biotin-labeled probes were then prepared by PCR amplification with 5′-biotin-labeled M13 universal primers. About 1 ng of probe was incubated with purified His6-AdpAch at 25°C for 30 min in buffer (20 mM Tris [pH 7.5], 0.01% bovine serum albumin [BSA], 5% glycerol, 50 μg ml−1 sheared salmon sperm DNA); the protein concentrations used are indicated in Fig. 2. For the competition assay, 100-fold molar excesses of unlabeled probe and nonspecific DNA were each added to 0.2 μg purified His6-AdpAch. Reactions were displayed on 5% acrylamide gels for separation in 0.5× Tris-borate-EDTA (TBE) buffer. Electrophoretic mobility shift assay (EMSA) gels were then electroblotted onto a nylon membrane and UV fixed by using a UV cross-linker. Labeled DNA was detected with streptavidin-horseradish peroxidase (HRP) and BeyoECL Plus (Beyotime, China) according to the manufacturer's instructions.
TABLE 2.
Oligonucleotides used in this study

Sequences representing restriction sites and mutagenesis are underlined.
FIG 2.
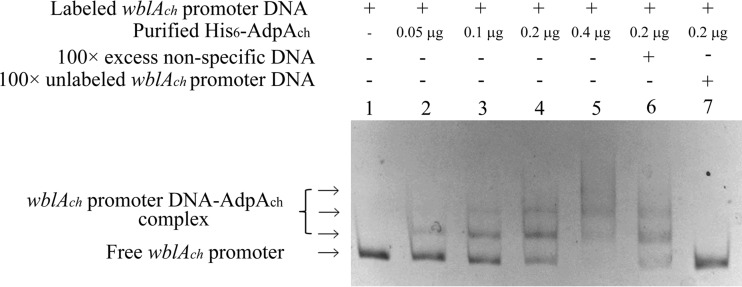
Gel mobility shift assays using a labeled DNA fragment containing the wblAch promoter region and the His6-AdpAch protein. Lane 1, labeled fragment; lanes 2 to 5, labeled fragment with 0.05 μg, 0.1 μg, 0.2 μg, and 0.4 μg of purified His6-AdpAch protein, respectively; lane 6, labeled fragment with a 100-fold excess of nonspecific DNA and 0.2 μg purified His6-AdpAch protein; lane 7, labeled fragment with a 100-fold excess of unlabeled wblAch promoter DNA and 0.2 μg purified His6-AdpAch protein.
DNase I footprinting assay.
A DNase I footprinting assay was performed as previously described (29). First, the 6-carboxyfluorescein (FAM)-labeled wblAch probe was amplified by PCR using 5′-FAM-labeled M13 universal primers from plasmid pT-wblAch, followed by gel recovery. About 50 ng of fluorescently labeled probe was then added to the reaction mixture to a final volume of 50 μl together with the AdpAch protein, which was ultrafiltered with a centrifugal ultrafiltration device (YM-10; Millipore) for a 10-kDa cutoff and eluted in 20 mM Tris buffer (pH 7.5). After binding of the AdpAch protein to the wblAch probe (30°C for 30 min), 0.01 U of DNase I (Promega) was added for 1 min at 30°C. The reactions were then stopped with an equal volume of 100 mM EDTA, and samples were extracted by using phenol-chloroform. After precipitation with a combination of 10 μl 7.5 M ammonium acetate (NH4Ac), 40 μg glycogen, and 100 μl 100% ethanol, the digested DNA mixture was loaded into an ABI 3130 DNA sequencer with the Liz-500 DNA marker (MCLAB). The DNA sequencing ladder was prepared according to instructions provided with the Thermo Sequenase dye primer manual cycle sequencing kit (USB).
Real-time quantitative PCR analysis.
Mycelium was harvested from strains grown in YEME medium or on YMG agar medium overlaid with cellophane discs for different times. Total RNAs were then isolated by using TRIzol reagent (Bio Basic Inc.) according to the manufacturer's guidelines. After treatment with DNase I (TaKaRa), PCR was carried out to ensure that there was no genomic DNA. The quality and quantity of RNA samples were then determined by UV spectroscopy and checked by agarose gel electrophoresis. A primeScript First Strand cDNA synthesis kit (TaKaRa) was used to generate cDNA from 2 μg of total RNAs according to the manufacturer's protocol. Quantitative real-time PCR of selected genes was performed on a Roche LightCycler 480 instrument (Roche), and ∼1 ng of synthesized cDNA was used as a DNA template in a 20-μl final reaction mixture volume for real-time quantitative PCR (qRT-PCR) with 10 μl Power SYBR green PCR master mix. hrdB was used as an internal control, and all experiments were done in triplicate.
Natamycin bioassay and HPLC analysis.
Natamycin produced by S. chattanoogensis on YMG agar was measured by bioassays using Saccharomyces cerevisiae as an indicator organism. Agar plugs were cut from the lawns of S. chattanoogensis that had been grown on YMG medium for 10 days by using a core borer (about 0.8 cm in diameter) and were placed onto a lawn of freshly plated Saccharomyces cerevisiae cells. After incubation at 30°C for 18 h, the inhibition zones of the bioassay plates were recorded. Natamycin production was further confirmed by high-performance liquid chromatography (HPLC) analysis using the Agilent 1100 HPLC system. An Agilent HC-C18 column (5 μm, 4.6 by 250 mm) was used, and the UV detector was set at 303 nm. The mobile phase and gradient elution processes were previously described (30).
Scanning electron microscopy.
For scanning electron microscopy, agar plugs ∼1 cm in diameter were cut from the lawns of S. chattanoogensis strains grown on YMG medium for 10 days and then plunged into liquid nitrogen. The cut agar blocks were observed by scanning electron microscopy (S-3000N; Hitachi) after sputter coating with gold. The detailed process was performed as previously described (28).
Accession numbers.
The GenBank accession numbers for the sequences of wblAch, whi genes, and other genes in the microarray analysis are KM264326 to KM264342. Experimental details and data from the microarrays have been deposited in the Gene Expression Omnibus (GEO) under accession no. GSE59806.
RESULTS
Identification of wblAch as a member of the AdpAch regulon by transcriptional analysis.
AdpA is an important pleiotropic regulator in the A-factor regulatory cascade in Streptomyces griseus (31). Induction of adpA requires the dissociation of ArpA from the adpA promoter, and the members of the AdpA regulon are then activated to participate in several important cellular functions (32, 33). In our previous study, we showed that AdpAch plays an important role in natamycin biosynthesis and morphological differentiation in S. chattanoogensis L10 (28). For a more detailed analysis of AdpAch, we compared the pattern of gene expression of the wild-type (WT) strain to that of the ΔadpAch mutant strain. Mycelium was harvested after 16 h, 24 h, and 36 h of growth, and RNA was isolated from three independent cultures of each strain.
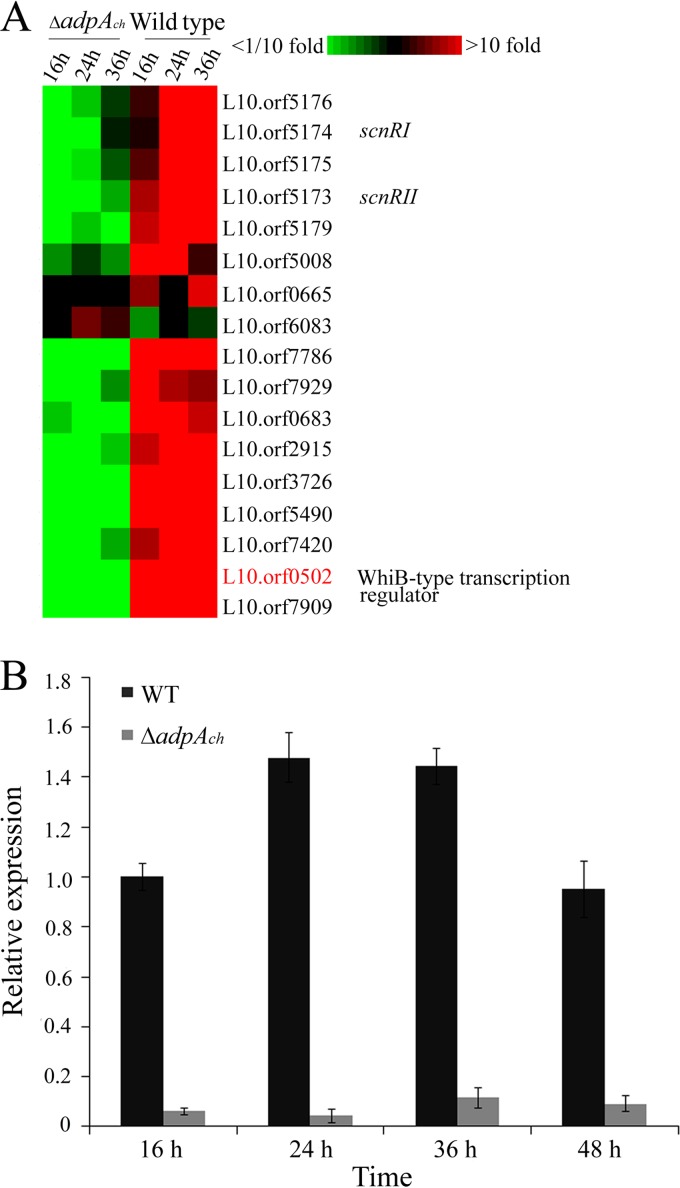
In the DNA microarray analysis, we used the criterion of a fold change of ≥2 and ≤0.5 (P < 0.05) to discriminate AdpAch-responsive genes. According to this criterion, approximately 809 and 431 genes were transcriptionally downregulated and upregulated, respectively, in the adpAch mutant strain compared to the WT strain at all tested time points. Among these genes, we noticed that L10.orf0502, which encodes a WhiB-type transcription regulator, was downregulated 79-fold at 16 h, 23-fold at 24 h, and 6.4-fold at 36 h in the ΔadpAch mutant strain (Fig. 1A). These results suggested that L10.orf0502 is positively regulated by AdpAch. A sequence similarity search showed that L10.orf0502 is an orthologous protein of the WblA protein (GenBank accession no. NP_627776.1) (90% identity) of Streptomyces coelicolor A3(2). Thus, it was designated wblAch. To further verify the microarray data and clarify the relationship between the wblAch and AdpAch in S. chattanoogensis L10, we carried out real-time quantitative PCR (qRT-PCR) to test the expression of the wblAch gene in the ΔadpAch mutant and in the WT strain. As expected, the transcript levels of wblAch in the ΔadpAch disruption mutant had decreased significantly in contrast to that of the WT strain at all tested time points (Fig. 1B). These results further confirmed that AdpAch acts as an activator of wblAch transcription in S. chattanoogensis L10.
FIG 1.
Expression profiles of wblAch differentially expressed during growth of the WT strain and the ΔadpAch mutant. (A) Microarray analysis of the transcription profiles of selected genes. RNA samples were isolated at 16 h, 24 h, and 36 h during growth on YEME medium. (B) RT-PCR analysis of wblAch transcript levels in the WT strain and the ΔadpAch mutant. The expression level of wblAch is presented relative to the wild-type sample at 16 h, which was arbitrarily assigned a value of 1. The transcription level of hrdB was assayed as an internal control. Error bars were calculated by measuring the standard deviations among data from three replicates of each sample.
Interaction of AdpAch with the promoter region of wblAch.
To determine whether AdpAch plays a direct role in the regulation of the wblAch gene, electrophoretic mobility shift assays (EMSAs) were performed. For this purpose, AdpAch was overexpressed in the E. coli BL21 strain as a recombinant His6-tagged protein and purified by Ni-NTA agarose chromatography. A biotin-labeled wblAch probe covering the wblAch promoter region from positions −321 to +63 was prepared. Results from EMSAs showed that purified His6-AdpAch can bind to the upstream regions of wblAch to form a complex (Fig. 2). There was only one shifted band for the wblAch probe at a low His6-AdpAch protein concentration. However, when the protein concentration was increased, two other shifted bands were observed, accompanied by the disappearance of the lower band (Fig. 2, lanes 2 to 5). It is therefore likely that there is more than one His6-AdpAch-binding site located on the wblAch promoter region. In order to examine binding specificity, EMSAs with an excess of unlabeled specific and nonspecific competitor DNA were performed. As shown in Fig. 2, the retarded bands (lanes 6 and 7) disappeared in the presence of excess unlabeled wblAch probe but not in the presence of nonspecific DNA. These results suggest that purified AdpAch regulates the transcription of wblAch by specifically binding to the upstream regions of wblAch.
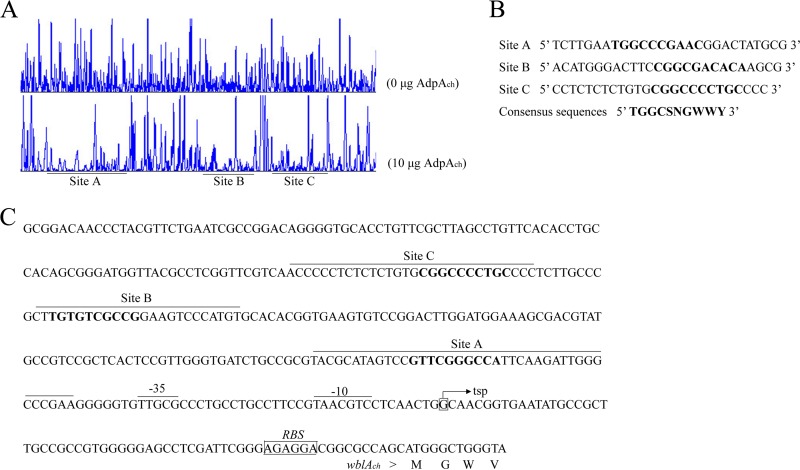
To further define the accurate binding sites of AdpAch in the upstream region of wblAch, DNase I footprinting assays were carried out. The experiments revealed that His6-AdpAch protected three regions, from nucleotides −83 to −44, −182 to −160, and −226 to −192 relative to the wblAch transcription start point (Fig. 3A and C). After further analysis of the protected DNA regions, we found that there was a highly conserved AdpAch-binding sequence in each binding site, which is identical to that found in S. griseus (Fig. 3B) (34). Therefore, series of mutations were constructed to determine the actual AdpAch-binding sites. As shown in Fig. S4 in the supplemental material, no binding shift was detected for mutated sites A to C compared with their corresponding wild-type targets. Thus, these consensus sequences are essential for the binding activity of AdpAch.
FIG 3.
DNase I footprinting assay for determination of the AdpAch-binding site. (A) A 5′-FAM-labeled wblAch probe was used in the DNase I footprinting assay without or with purified AdpAch (10 μg). The protected regions are underlined. (B) Sequences of the determined AdpAch-binding sites and the consensus AdpAch-binding sequence (in boldface type). (C) Nucleotide sequences of the wblAch promoter region and the predicted AdpAch-binding sites. The transcription start point (tsp) is marked by a bent arrow, the AdpAch-binding sites are overlined, and the ribosome-binding site (RBS) is boxed.
wblAch is a pivotal activator of natamycin biosynthesis.
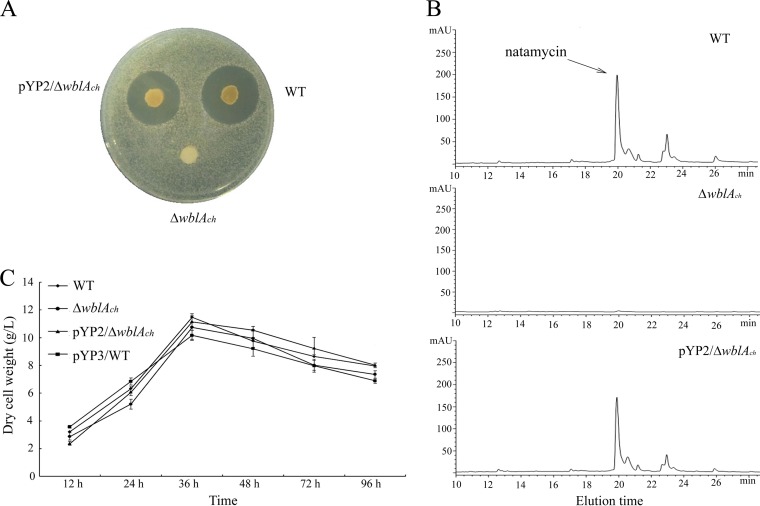
Previous reports have shown that WblA and its orthologues act as negative regulators for antibiotic biosynthesis in various Streptomyces species and that wblA deletion mutants lead to overproduction of the corresponding antibiotics (21–24). In order to identify the function of wblAch in natamycin biosynthesis in S. chattanoogensis L10, a wblAch deletion mutant was constructed via homologous recombination, as described Materials and Methods. The disruption mutant was confirmed by PCR analysis and Southern blot analysis (see Fig. S1 in the supplemental material). Meanwhile, the complemented strain was constructed by reintroducing a 750-bp DNA fragment containing wblAch and its own promoter into the mutant strain. To assess natamycin production, Saccharomyces cerevisiae was used as an indicator organism for the bioassay. As shown in Fig. 4A, no inhibition was observed with the agar plug from the wblAch deletion mutant, whereas the growth-inhibiting activity against S. cerevisiae was restored when plasmid pYP2 was reintroduced into the ΔwblAch mutant. To further confirm this phenotype, high-performance liquid chromatography (HPLC) analysis was carried out. No peak of natamycin was present in the culture filtrates of the wblAch deletion mutant, in contrast to the culture filtrates of the WT strain, and the complemented strain had restored the production of natamycin to a level similar to that of the WT strain (Fig. 4B). Nevertheless, these three strains had comparable growth rates and similar biomasses (Fig. 4C). These results indicate that WblAch may act as a positive regulator for natamycin biosynthesis.
FIG 4.
Effect of wblAch disruption on growth and production of natamycin. (A and B) Natamycin bioassay (A) and HPLC analysis of fermentation filtrates (B) from the WT strain, the wblAch deletion mutant, and the wblAch-complemented mutant. The peak of natamycin is marked by an arrow. mAU, milli-absorbance units. (C) Growth of the wild-type strain and mutant strains.
To test this hypothesis, we carried out qRT-PCR to evaluate the effects of WblAch on the transcription of scnRI and scnRII, pathway-specific regulators for natamycin biosynthesis (28). As expected, the transcript levels of scnRI and scnRII were lower in the wblAch mutant strain than in the WT strain at all tested time points from 16 h to 48 h (Fig. 5). Moreover, WblAch was expressed as an N-terminally His6-tagged protein in order to test whether WblAch directly regulates natamycin biosynthesis by its binding activity. Unfortunately, the EMSA result showed that WblAch cannot directly bind to the promoters of scnRI, scnRII, or any of the other structural genes in the cluster (data not shown).
FIG 5.
Quantitative RT-PCR analysis of scnRI (A) and scnRII (B) in the WT strain and the wblAch deletion mutant. The RNA samples were obtained from cultures grown in YEME medium for 16 h, 24 h, 36 h, and 48 h. The expression levels of scnRI and scnRII are presented relative to the levels in the wild-type sample at 16 h, which was arbitrarily assigned a value of 1. The transcription level of hrdB was assayed as an internal control, and error bars were calculated by measuring the standard deviations among data from three replicates of each sample.
Overexpression of wblAch results in increased natamycin production.
Previous investigations indicated that overexpression of wblA resulted in decreased production of the corresponding antibiotic (21–24). To evaluate the function of wblAch in S. chattanoogensis L10, pIJ8630::wblAch was constructed, in which wblAch was under the control of the strong constitutive ermE* promoter, and this recombinant plasmid was then introduced into the WT strain. As shown in Fig. 6, the level of natamycin production of the resulting mutant (YC2) was increased ∼1.3-fold compared to that of the WT strain after 96 h of incubation, although the biomasses of these strains were similar (Fig. 4C). This result reinforced the evidence that wblAch is a pivotal activator for natamycin production.
FIG 6.
Effect of wblAch overexpression on production of natamycin. Natamycin production of fermentation filtrates in the wild-type strain (WT), the wild-type strain with pIJ8630 (pIJ8630/WT), and the wblAch overexpression strain (pYP3/WT) at different incubation times is shown.
Pleiotropic effects of wblAch on morphology differentiation.
To investigate the effect of a deletion of wblAch on development, the mutant was compared to the wild-type strain on YMG medium. As shown in Fig. S2A in the supplemental material, disruption of wblAch caused a whi phenotype, being able to erect aerial hyphae but defective in sporulation. Scanning electron microscopy of the wblAch mutant grown on YMG medium revealed thin, sparse aerial hyphae, with characteristic aberrant sporulation septation (see Fig. S2B in the supplemental material). The morphological deficiency in the mutant strain was fully complemented by the pSET152 derivative carrying a 750-bp DNA fragment containing wblAch and its own promoter. In addition, we further evaluated the transcription of whi genes, which are related to spore formation in Streptomyces. Our data showed that the transcription levels of the whi genes were severely decreased in the ΔwblAch mutant, with the exception of whiD, which exhibited a slight increase (Fig. 7). Other whi genes, such as whiA and whiE, showed low transcriptional activity in the ΔwblAch mutant, ∼20% of the wild-type levels, while transcriptional activities of whiB, whiH, and whiI were almost absent in the mutant. These results implied that WblAch may play a part in morphological differentiation through interacting with other whi genes.
FIG 7.
Effect of wblAch disruption on the transcription of whi genes. The RNA samples were harvested from strains grown on YMG agar medium overlaid with cellophane discs for 4 days. The expression level of whiA is presented relative to the wild-type sample, which was arbitrarily assigned a value of 1. The transcription level of hrdB was assayed as an internal control, and error bars were calculated by measuring the standard deviations among data from three replicates of each sample.
DISCUSSION
The biochemical and genetic mechanisms of WblA with regard to secondary metabolite and morphological differentiation have not been determined previously. In this study, we found that AdpAch, a pleiotropic regulator in S. chattanoogensis L10, acts as a positive regulator of the expression of wblAch. However, wblA was previously reported to be negatively regulated by AdpA in S. coelicolor, a model organism for studying bacterial differentiation (25). This finding is directly contradictory to our own findings. AdpAch shares 92% sequence identity with AdpAsc (28), and WblAch shares 90% sequence identity with WblAsc. Although these two types of protein share good amino acid sequence homology, different regulatory patterns are observed in the AdpA-WblA regulatory relationship in these two strains. The host-specific characteristics of the WblA orthologues in different strain backgrounds seem inconceivable because of the important roles of these proteins in controlling antibiotic production and morphological differentiation in Streptomycetes. Notably, results of microarray and chromatin immunoprecipitation (ChIP)/chromatin affinity precipitation (ChAP)-seq analysis of Streptomyces griseus coincide with our results (35, 36). Moreover, wblAch was shown to encode a positive regulator participating in natamycin biosynthesis in S. chattanoogensis L10, whereas wblA and its orthologues were previously determined to be novel antibiotic downregulators in various Streptomyces species. Based on these cumulative evidences, WblAch in S. chattanoogensis L10, an industrial strain for natamycin production, is suggested to be involved in a number of functions that differ from those observed in other Streptomyces species in previous reports.
The Wbl proteins, which harbor a predicted helix-turn-helix motif, are supposed to be able to bind DNA as transcription factors. A series of Wbl proteins, including WhiB1 (37), WhiB2 (38), WhiB3 (13), and WhiB4 (17), have been experimentally demonstrated to bind with DNA. However, the EMSA results did not support the hypothesis that WblAch has the binding ability to regulate the expression of genes involved in natamycin biosynthesis directly. Similar to our results, previous genetic studies on WblA orthologues did not reveal the mechanisms by which they regulate antibiotic production in Streptomyces. Previous reports showed that Wbl proteins in general may change their regulatory properties in response to dormancy signals, including O2 and nitric oxide (NO), via their iron-sulfur cluster (39). It was also shown that the Wbl proteins can bind specific target proteins as ligands to modify their activity (40). Thus, it is likely that the binding activity of WblA orthologues may require a redox state or interaction with other cellular proteins.
Natamycin is the major secondary metabolite of S. chattanoogensis L10 in YEME medium; simultaneously, a yellow pigment is generated in the same medium. Several lines of evidence imply that these two pathways compete for precursors originating from primary metabolism. In our study, overexpression of wblAch resulted in increased production of natamycin, whereas the production of yellow pigment had decreased severely (see Fig. S3 and Table S1 in the supplemental material). Notably, the available literature reported that Wbl proteins are involved in the metabolic switchover, such as WhiB3, which was shown to act as an intracellular redox sensor and regulates fatty acid metabolism by binding directly to its promoter sequence in M. tuberculosis (13), and WhiB5, which was shown to be involved in metabolic regulation during starvation in M. tuberculosis (18). WblA was reported to be able to sense and respond to the intracellular redox environment in a manner considered to be coupled to central metabolism (7, 39). Based on this characteristic of the Wbl protein, we speculated that WblAch may act as a metabolic regulator where overexpression of wblAch results in a redirection of the flux toward the biosynthesis of natamycin. Thus, it could provide a strategy for engineering new overproducer strains of natural products where regulation of the enzymes involved in metabolic flux becomes a realistic way to generate improved strains. In sum, our findings in this study should lead to an increased understanding of the biological function of the WhiB-like protein WblA, and further experiments will provide more accurate information on the biological functions of these proteins.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Chris Wood for critical reading and scientific editing.
This work was supported by the Key Program of Zhejiang Provincial Natural Science Foundation of China (grant no. LZ12C01001), the National Basic Research Program of China (973 Program) (grant no. 2012CB721005), the National High-Technology Research and Development Program of China (863 Program) (grant no. 2012AA02A706), the National Key Technology R and D Program (grant no. 2011BAD23B05-2), and the Specialized Research Fund for the Doctoral Program of Higher Education (grant no. 20120101110143).
Footnotes
Published ahead of print 29 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01849-14.
REFERENCES
- 1.Hopwood DA. 2007. Streptomyces in nature and medicine: the antibiotic makers. Oxford University Press, New York, NY. [Google Scholar]
- 2.Demain AL. 1999. Pharmaceutically active secondary metabolites of microorganisms. Appl. Microbiol. Biotechnol. 52:455–463. 10.1007/s002530051546. [DOI] [PubMed] [Google Scholar]
- 3.van Wezel GP, McDowall KJ. 2011. The regulation of the secondary metabolism of Streptomyces: new links and experimental advances. Nat. Prod. Rep. 28:1311–1333. 10.1039/c1np00003a. [DOI] [PubMed] [Google Scholar]
- 4.Chater KF. 1993. Genetics of differentiation in Streptomyces. Annu. Rev. Microbiol. 47:685–713. 10.1146/annurev.mi.47.100193.003345. [DOI] [PubMed] [Google Scholar]
- 5.Liu G, Chater KF, Chandra G, Niu GQ, Tan HR. 2013. Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol. Mol. Biol. Rev. 77:112–143. 10.1128/MMBR.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takano E. 2006. γ-Butyrolactones: Streptomyces signaling molecules regulating antibiotic production and differentiation. Curr. Opin. Microbiol. 9:287–294. 10.1016/j.mib.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Soliveri JA, Gomez J, Bishai WR, Chater KF. 2000. Multiple paralogous genes related to the Streptomyces coelicolor developmental regulatory gene whiB are present in Streptomyces and other actinomycetes. Microbiology 146:333–343. [DOI] [PubMed] [Google Scholar]
- 8.Zheng F, Long QX, Xie JP. 2012. The function and regulatory network of WhiB and WhiB-like protein from comparative genomics and systems biology perspectives. Cell Biochem. Biophys. 63:103–108. 10.1007/s12013-012-9348-z. [DOI] [PubMed] [Google Scholar]
- 9.Geiman DE, Raghunand TR, Agarwal N, Bishai WR. 2006. Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB-like genes. Antimicrob. Agents Chemother. 50:2836–2841. 10.1128/AAC.00295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Den Hengst CD, Buttner MJ. 2008. Redox control in actinobacteria. Biochim. Biophys. Acta 1780:1201–1216. 10.1016/j.bbagen.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Rustad TR, Harrell MI, Liao R, Sherman DR. 2008. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One 3:e1502. 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson C, Luna B, Ammerman NC, Maiga M, Agarwa N, Bishai WR. 2012. Gene expression of Mycobacterium tuberculosis putative transcription factors whiB1-7 in redox environments. PLoS One 7:e37516. 10.1371/journal.pone.0037516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh A, Crossman DK, Mai D, Guidry L, Voskui MI, Renfrow MB, Steyn AJC. 2009. Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response. PLoS Pathog. 5:e1000545. 10.1371/journal.ppat.1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohde K, Yates RM, Purdy GE, Russell DG. 2007. Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 219:37–54. 10.1111/j.1600-065X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 15.Burian J, Yim G, Hsing M, Axerio-Cilies P, Cherkasov A, Spiegelman GB, Thompson CJ. 2013. The mycobacterial antibiotic resistance determinant WhiB7 acts as a transcriptional activator by binding the primary sigma factor SigA (RpoV). Nucleic Acids Res. 41:10062–10076. 10.1093/nar/gkt751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steyn AJC, Collins DM, Hondalus MK, Jacobs WR, Kawakami RP, Bloom BR. 2002. Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth. Proc. Natl. Acad. Sci. U. S. A. 99:3147–3152. 10.1073/pnas.052705399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawla M, Parikh P, Saxena A, Munshi M, Mehta M, Mai D, Srivastava AK, Narasimhulu KV, Redding KE, Vashi N, Kumar D, Steyn AJC, Singh A. 2012. Mycobacterium tuberculosis WhiB4 regulates oxidative stress response to modulate survival and dissemination in vivo. Mol. Microbiol. 85:1148–1165. 10.1111/j.1365-2958.2012.08165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casonato S, Sánchez AC, Haruki H, González MR, Provvedi R, Dainese E, Jaouen T, Gola S, Bini E, Vicente M, Johnsson K, Ghisotti D, Palù G, Hernández-Pando R, Manganelli R. 2012. WhiB5, a transcriptional regulator that contributes to Mycobacterium tuberculosis virulence and reactivation. Infect. Immun. 80:3132–3144. 10.1128/IAI.06328-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konar M, Alam MS, Arora C, Agrawal P. 2012. WhiB2/Rv3260c, a cell division-associated protein of Mycobacterium tuberculosis H37Rv, has properties of a chaperone. FEBS J. 279:2781–2792. 10.1111/j.1742-4658.2012.08662.x. [DOI] [PubMed] [Google Scholar]
- 20.Fowler-Goldsworthy K, Gust B, Mouz S, Chandra G, Findlay KC, Chater KF. 2011. The actinobacteria-specific gene wblA controls major developmental transitions in Streptomyces coelicolor A3(2). Microbiology 157:1312–1328. 10.1099/mic.0.047555-0. [DOI] [PubMed] [Google Scholar]
- 21.Kang SH, Huang J, Lee HN, Hur YA, Cohen SN, Kim ES. 2007. Interspecies DNA microarray analysis identifies WblA as a pleiotropic down-regulator of antibiotic biosynthesis in Streptomyces. J. Bacteriol. 189:4315–4319. 10.1128/JB.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noh JH, Kim SH, Lee HN, Lee SY, Kim ES. 2010. Isolation and genetic manipulation of the antibiotic down-regulatory gene, wblA ortholog for doxorubicin-producing Streptomyces strain improvement. Appl. Microbiol. Biotechnol. 86:1145–1153. 10.1007/s00253-009-2391-z. [DOI] [PubMed] [Google Scholar]
- 23.Nah JH, Park SH, Yoon HM, Choi SS, Lee CH, Kim ES. 2012. Identification and characterization of wblA-dependent tmcT regulation during tautomycetin biosynthesis in Streptomyces sp. CK4412. Biotechnol. Adv. 30:202–209. 10.1016/j.biotechadv.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Rabyk M, Ostash B, Rebets Y, Walker S, Fedorenko V. 2011. Streptomyces ghanaensis pleiotropic regulatory gene wblAgh influences morphogenesis and moenomycin production. Biotechnol. Lett. 33:2481–2486. 10.1007/s10529-011-0728-z. [DOI] [PubMed] [Google Scholar]
- 25.Lee HN, Kim JS, Kim P, Lee HS, Kim ES. 2013. Repression of antibiotic downregulator WblA by AdpA in Streptomyces coelicolor. Appl. Environ. Microbiol. 79:4159–4163. 10.1128/AEM.00546-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, United Kingdom. [Google Scholar]
- 27.Gust B, Challis GL, Fowler K, Kieser T, Chater KF. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. U. S. A. 100:1541–1546. 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du YL, Li SZ, Zhou Z, Chen SF, Fan WM, Li YQ. 2011. The pleitropic regulator AdpAch is required for natamycin biosynthesis and morphological differentiation in Streptomyces chattanoogensis. Microbiology 157:1300–1311. 10.1099/mic.0.046607-0. [DOI] [PubMed] [Google Scholar]
- 29.Mao XM, Zhou Z, Cheng LY, Hou XP, Guan WJ, Li YQ. 2009. Involvement of SigT and RstA in the differentiation of Streptomyces coelicolor. FEBS Lett. 583:3145–3150. 10.1016/j.febslet.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Du YL, Shen XL, Yu P, Bai LQ, Li YQ. 2011. Gamma-butyrolactone regulatory system of Streptomyces chattanoogensis links nutrient utilization, metabolism, and development. Appl. Environ. Microbiol. 77:8415–8426. 10.1128/AEM.05898-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohnishi Y, Yamazaki H, Kato JY, Tomono A, Horinouchi S. 2005. AdpA, a central transcriptional regulator in the A-factor regulatory cascade that leads to morphological development and secondary metabolism in Streptomyces griseus. Biosci. Biotechnol. Biochem. 69:431–439. 10.1271/bbb.69.431. [DOI] [PubMed] [Google Scholar]
- 32.Onaka H, Horinouchi S. 1997. DNA-binding activity of the A-factor receptor protein and its recognition DNA sequences. Mol. Microbiol. 24:991–1000. 10.1046/j.1365-2958.1997.4081772.x. [DOI] [PubMed] [Google Scholar]
- 33.Kato JY, Miyahisa I, Mashiko M, Ohnishi Y, Horinouchi S. 2004. A single target is sufficient to account for the biological effects of the A-factor receptor protein of Streptomyces griseus. J. Bacteriol. 186:2206–2211. 10.1128/JB.186.7.2206-2211.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamazaki H, Tomono A, Ohnishi Y, Horinouchi S. 2004. DNA-binding specificity of AdpA, a transcriptional activator in the A-factor regulatory cascade in Streptomyces griseus. Mol. Microbiol. 53:555–572. 10.1111/j.1365-2958.2004.04153.x. [DOI] [PubMed] [Google Scholar]
- 35.Higo A, Hara H, Horinouchi S, Ohnishi Y. 2012. Genome-wide distribution of AdpA, a global regulator for secondary metabolism and morphological differentiation in Streptomyces, revealed the extent and complexity of the AdpA regulatory network. DNA Res. 19:259–273. 10.1093/dnares/dss010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hara H, Ohnishi Y, Horinouchi S. 2009. DNA microarray analysis of global gene regulation by A-factor in Streptomyces griseus. Microbiology 155:2197–2210. 10.1099/mic.0.027862-0. [DOI] [PubMed] [Google Scholar]
- 37.Smith LJ, Stapleton MR, Buxton RS, Green J. 2012. Structure-function relationships of the Mycobacterium tuberculosis transcription factor WhiB1. PLoS One 7:e40407. 10.1371/journal.pone.0040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rybniker J, Nowag A, van Gumpel E, Nissen N, Robinson N, Plum G, Hartmann P. 2010. Insights into the function of the WhiB-like protein of mycobacteriophage TM4—a transcriptional inhibitor of WhiB2. Mol. Microbiol. 77:642–657. 10.1111/j.1365-2958.2010.07235.x. [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Guidry L, Narasimhulu KV, Mai D, Trombley J, Redding KE, Giles GI, Lancaster JR, Steyn AJC. 2007. Mycobacterium tuberculosis WhiB3 responds to O2 and nitric oxide via its [4Fe-4S] cluster and is essential for nutrient starvation survival. Proc. Natl. Acad. Sci. U. S. A. 104:11562–11567. 10.1073/pnas.0700490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garg S, Alam MS, Bajpai R, Kishan KR, Agrawal P. 2009. Redox biology of Mycobacterium tuberculosis H37Rv: protein-protein interaction between GlgB and WhiB1 involves exchange of thiol-disulfide. BMC Biochem. 10:1. 10.1186/1471-2091-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacNeil DJ, Klapko LM. 1987. Transformation of Streptomyces avermitilis by plasmid DNA. J. Ind. Microbiol. 2:209–218. 10.1007/BF01569542. [DOI] [Google Scholar]
- 42.Sun J, Kelemen GH, Fernandez-Abalos JM, Bibb MJ. 1999. Green fluorescent protein as a reporter for spatial and temporal gene expression in Streptomyces coelicolor A3(2). Microbiology 145:2221–2227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.