Abstract
Clinical immunity to pregnancy associated-malaria (PAM) in multigravida women has been attributed to antibodies that recognize VAR2CSA on the infected erythrocyte (IE) surface. The size and complexity of VAR2CSA have focused efforts on selecting one or more of its six Duffy binding-like (DBL) domains for vaccine development. Presently, however, there is no consensus as to which DBL domain(s) would be most effective in eliciting immunity. This is because antibodies to a number of the DBL domains have been found to block the adhesion of VAR2CSA-expressing erythrocytes to chondroitin sulfate A (CSA)—a major criterion for evaluating vaccine candidacy. Opsonization of IEs by cytophilic antibodies that recognize VAR2CSA represents an important yet understudied effector mechanism in acquired immunity to PAM. To date, no studies have sought to determine the targets of those antibodies. In this study, we found that IgGs from multigravida Malian women showed (i) higher reactivity to recombinant DBL domains by enzyme-linked immunosorbent assay (ELISA), (ii) more binding to VAR2CSA-expressing IEs, and (iii) greater opsonization of these IEs by human monocytic cells than IgGs from malaria-exposed Malian men and malaria-naive American adults. Preincubation of IgGs from multigravida women with recombinant DBL2χ, DBL3χ, or DBL5ε domains significantly diminished opsonization of VAR2CSA-expressing IEs by human monocytes. These data identify the DBL2χ, DBL3χ, and DBL5ε domains as the primary targets of opsonizing IgGs for the first time. Our study introduces a new approach to determining the antigenic targets of opsonizing IgGs in phagocytosis assays.
INTRODUCTION
More than 100 million pregnant women in areas of malaria endemicity are at risk of developing pregnancy-associated malaria (PAM), a potentially severe consequence of Plasmodium falciparum infection in mothers and their unborn children and infants. It has been estimated that at least 10,000 women and 100,000 infants die each year from PAM-associated complications (1). Afflicted women suffer from the extensive accumulation of parasitized erythrocytes and leukocytes in the intervillous spaces of the placenta, which increases their risk of anemia, hypertension, premature delivery, and the potential death of low-birth-weight infants (2, 3). Trophozoite-infected erythrocytes (IEs) that express VAR2CSA, a member of the ∼350-kDa P. falciparum erythrocyte membrane protein 1 (PfEMP1) family, bind to chondroitin sulfate A (CSA) on the surface of syncytiotrophoblasts, the placental cells that mediate nutrient transfer between mother and fetus (4).
Women from areas of endemicity show decreased susceptibility to PAM after successive pregnancies, suggesting that they acquire immunity through repeated exposure to PAM-specific antigens. Indeed, previous studies have shown that sera or plasma from multigravida women recognize IEs collected from placenta (5, 6) as well as in vitro-selected, CSA-binding parasite lines (7). These findings, along with observations that IgGs of multigravida women recognize placental parasite isolates from different areas (8) and recombinant VAR2CSA proteins (7), support the existence of conserved domains of VAR2CSA targeted by protective antibodies. Identifying these putative domains would greatly facilitate the generation of a PAM vaccine (9), as the use of full-length VAR2CSA is challenging due to its large size and numerous disulfide bonds (10, 11). Hence, there is substantial impetus for utilizing one or more of the six cysteine-rich DBL domains (DBL1χ, DBL2χ, DBL3χ, DBL4ε, DBL5ε, and DBL6ε) in a vaccine.
To date, evaluating immunity to PAM has primarily involved measures of antibody capacity to block the binding of VAR2CSA-expressing IEs to CSA (12, 13). While studies have shown evidence of DBL domain-specific antibody binding to placental parasite isolates and blocking of IE adhesion to CSA, nearly all six DBL domains have been implicated to some extent (11, 14–19). This lack of consensus on which DBL domains are the most critical to the development of PAM immunity has slowed progress in developing a practical vaccine. The accumulation of circulating monocytes and tissue-resident macrophages within the placenta during PAM (20) underscores the importance of antibody-mediated phagocytosis (i.e., opsonization), an often-overlooked aspect of the acquired immune response (21, 22). Numerous in vitro studies have shown that human monocytes readily phagocytose IEs (23–27) and VAR2CSA-expressing IEs specifically (28–30). Keen et al. introduced opsonic phagocytosis as a novel correlate of protection against PAM (31). Subsequent studies by Ataide et al., using a flow cytometry-based phagocytosis assay, found that opsonizing antibodies to VAR2CSA-expressing IEs correlated positively with infant birth weight in secundigravidae with placental malaria infection and were decreased in those with HIV infection; in contrast, this correlation was not seen in primigravidae (32, 33). Nonetheless, no studies have sought to determine the targets of these opsonizing antibodies (34).
To identify these targets, we measured the binding of IgG from 10 malaria-exposed multigravida Malian women to all six DBL domains of VAR2CSA. IgGs from these women recognized several VAR2CSA DBL domains and mediated the phagocytosis of VAR2CSA-expressing IEs significantly better than IgGs from malaria-exposed Malian men and malaria-naive American adults. Importantly, preincubation of IgGs from multigravida women with recombinant DBL3χ, DBL5ε, and to a lesser extent DBL2χ significantly diminished the opsonization of VAR2CSA-expressing IEs by human monocytes. Our results support these VAR2CSA DBL domains as primary targets of IgG-mediated phagocytosis in multigravida women.
MATERIALS AND METHODS
Ethics statement.
Blood samples obtained from Malian adults were collected after written informed consent under a protocol (08-I-N120) approved by the Institutional Review Board (IRB) at the National Institute of Allergy and Infectious Diseases and the Ethics Committee of the Faculty of Medicine, Pharmacy, and Odontostomatology at the University of Bamako, Bamako, Mali.
Buffy coat cells were harvested from units of blood obtained from healthy American adult volunteers after written informed consent at the Department of Transfusion Medicine under a protocol approved by the IRB at the NIH Clinical Center (Bethesda, MD); monocytes were isolated from the buffy coat cells as indicated below.
Human antibodies.
In brief, sera were collected during the malaria transmission season in August 2010 from six healthy men and 10 healthy multigravida women living permanently in an area of Kenieroba, Mali, where P. falciparum is endemic. Women were not pregnant, and none of the Malian adults had symptoms of malaria at the time of blood collection. Sera pooled from 40 healthy malaria-naive American adults were purchased from the Interstate Blood Bank (Memphis, TN). IgGs were purified from serum samples using protein G Sepharose 4 Fast Flow columns (GE Healthcare, Laurel, MD).
P. falciparum antigens.
From the genomic DNA of the P. falciparum FCR3 cell line, DBL (GenBank AAQ73926) domains DBL1χ (residues 44 to 452), DBL2χ (residues 518 to 979), DBL3χ (residues 1209 to 1580), DBL4ε (residues 1587 to 1988), DBL5ε (residues 1989 to 2306), and DBL6ε (residues 2309 to 2632) were cloned in the pLM1 expression plasmid and later transformed into Escherichia coli BL21(DE3)-RIL cells. For expression, cells were cultured at 37°C to an optical density of 0.8 at 600 nm and induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h. Cells were harvested and lysed. Recovered inclusion bodies were solubilized in 6 M guanidine-HCl. All six DBL domains were refolded and purified as previously described (35). Purified recombinant DBL domains were dialyzed against 1× phosphate-buffered saline (PBS), pH 7.4, at 4°C and concentrated to 5 mg/ml. All constructs were confirmed by DNA sequencing. The identity of each DBL domain was confirmed by N-terminal sequencing. Molecular masses of constructs were confirmed by 4 to 20% SDS-PAGE using standard procedures. Merozoite apical membrane antigen 1 (AMA1), produced from recombinant E. coli and based on the genomic DNA of the P. falciparum FVO line, was a gift from David Narum (36).
ELISA.
Binding of IgGs in sera from individual Malian adults and the pool from American adult sera to P. falciparum antigens was measured by enzyme-linked immunosorbent assay (ELISA) as previously described (37).
Parasites.
P. falciparum 3D7 and FCR3 lines were cultured in human type O+ (38) erythrocytes (Interstate Blood Bank, Memphis, TN) at 2% hematocrit in parasite growth medium consisting of RPMI 1640 medium supplemented with l-glutamine, 25 mM HEPES, 50 mg/ml hypoxanthine (KD Medical, Columbia, MD), 28.5 mM sodium bicarbonate (Gibco, Grand Island, NY), 50 μg/ml gentamicin (Gibco), and 0.5% (wt/vol) Albumax II (Gibco), pH 7.2, in an atmosphere of 5% O2, 5% CO2, and 90% N2 at 37°C. FCR3 in vitro CSA binding selection was performed as previously described (39, 40) to establish FCR3-CSA. To limit variation in surface protein expression on IEs between experiments, parasites were cultured to a high volume, aliquoted, frozen, and later thawed when needed for experimentation. All cultures tested negative for Mycoplasma contamination.
Phagocytes.
THP-1 leukemia-derived monocytic cells (ATCC, Manassas, VA) were cultured between 1 × 105 and 5 × 105 cells/ml in monocyte growth medium consisting of RPMI 1640 (ATCC) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; Gibco) and 2% (vol/vol) β-mercaptoethanol (Sigma-Aldrich) in an atmosphere of 20% O2, 5% CO2, and 75% N2 at 37°C.
Human primary monocytes were isolated from the buffy coats of healthy American adults by negative selection with Ficoll diatrizoate density centrifugation as previously described (41). Briefly, donor buffy coats were collected by the NIH Clinical Center Department of Transfusion Medicine in the early morning. Within 1 h, 5 to 10 ml of buffy coat packed cell volume was spin-washed three times with 1× PBS and resuspended to 30 ml with 1× PBS. For negative selection of primary monocytes, cells were then incubated for 20 min with RosetteSep human monocyte enrichment cocktail (Stemcell Technologies, Vancouver, Canada) according to the manufacturer's specifications. Subsequently, the cell suspension was carefully layered over 15 ml of Ficoll-Paque Plus (GE Healthcare) and centrifuged for 20 min at 600 × g. Purified monocytes were collected from the interface between 1× PBS and Ficoll, spin-washed with 1× PBS twice, and maintained at 1 × 105 cells/ml in primary monocyte growth medium consisting of RPMI 1640 with Glutamax (Gibco) supplemented with 10% (vol/vol) FBS and 1% (vol/vol) penicillin-streptomycin (Sigma-Aldrich) in an atmosphere of 20% O2, 5% CO2, and 75% N2 at 37°C. Monocytes were maintained in culture for no more than 4 h following blood collection.
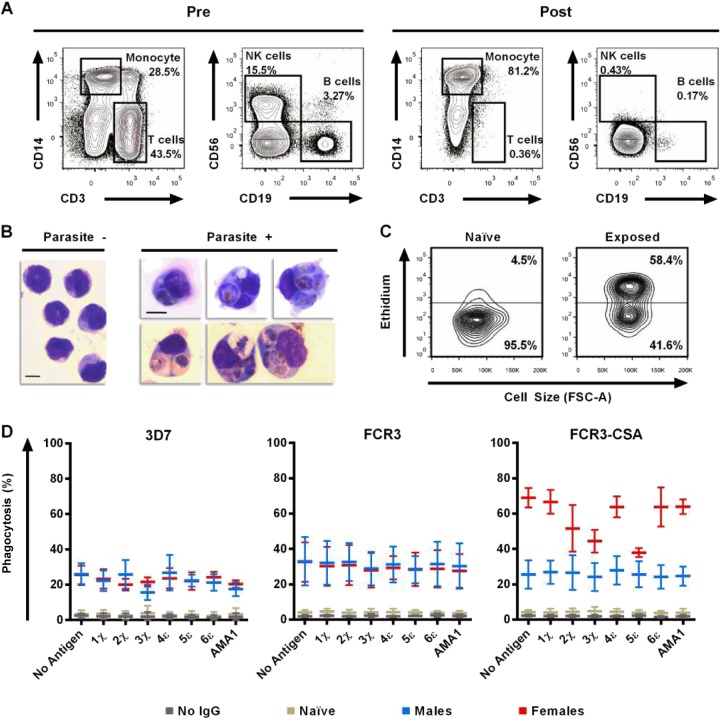
Enrichment was validated by flow cytometry using antibodies against cluster of differentiation (CD) antigens for monocytes (CD14), T cells (CD3), B cells (CD19), and natural killer cells (CD56). Briefly, 1 × 106 leukocytes sampled before or after the aforementioned primary monocyte enrichment were spin-washed and resuspended in 1 ml of 1× PBS in polypropylene fluorescence-activated cell sorter (FACS) tubes (BD Falcon, Franklin Lakes, NJ). Cells were then incubated with 5 μl of either mouse anti-CD14 Pacific Blue (clone M5E2; BD PharMingen), mouse anti-CD3 Alexa Fluor 700 (clone OKT3; eBioscience, San Diego, CA), mouse anti-CD56 PE (clone B159; BD PharMingen), and mouse anti-CD19 PE-Cy5 (clone SJ25C1; BD Biosciences) antibodies. Following three spin-washes, at least 250,000 leukocyte events were assessed by flow cytometry with the MACSQuant analyzer (Miltenyi Biotec, Inc., Auburn, CA). Leukocyte events were gated by size and granularity on forward scatter—area (FSC-A) and side scatter—area (SSC-A). For compensation, single-color-stained cells and unstained cells were analyzed using FlowJo software (Tree Star, Ashland, OR).
Antibody binding to IE surface.
3D7, FCR3, and FCR3-CSA parasite lines were cultured to the late trophozoite stage at 2 to 4% parasitemia. Erythrocytes were stained with 50 μg/ml hydroethidine (dihydroethidium bromide) (42) for 30 min at 37°C, spin-washed twice, and aliquoted at 5 × 106 erythrocytes/well into 96-well V-bottom plates. Cells were incubated with 2.5 mg/ml of purified adult IgG in RPMI 1640 at a total volume of 200 μl for 1 h at 37°C. After three spin-washes, cells were incubated with 5 μg/ml of either mouse anti-human IgG1 (BD PharMingen), IgG2 (BD PharMingen), IgG3 (SouthernBiotech), or IgG4 (SouthernBiotech) at a total volume of 100 μl for 1 h at 37°C. Following three spin-washes, cells were incubated with 100 μl of 6 μg/ml Alexa Fluor 647-conjugated goat anti-mouse antibody (Molecular Probes) for 1 h at 37°C. Samples were then washed thoroughly and immediately analyzed by flow cytometry. Erythrocytes were gated by forward and side scatter dot plot, with IEs subsequently gated by elevated ethidium fluorescence signal. A minimum of 5,000 IE events were collected. Surface binding was measured as the median fluorescence intensity (MFI) of the IE population.
Phagocytosis imaging.
Giemsa smears of cells were viewed using the Olympus CX41 microscope using an Olympus PLCN 100×/1.25 oil immersion objective. Photography was performed with the Lumenera Infinity3-1R research grade 1.4 megapixel cooled digital camera and software.
For live-cell imaging, 1 × 105 THP-1 cells were plated onto Lab-Tek polylysine-coated chambered slides (NalgeneNunc Thermo Fisher Scientific, Rochester, NY) for 24 h to allow adhesion. Late-stage FCR3-CSA IEs were magnetically enriched to >90% purity with QuadroMACs using Miltenyi LS columns (Miltenyi Biotec Inc.). IEs were stained with 2 drops/ml of Hoechst NucClue live-cell stain (Molecular Probes) for 20 min at room temperature, spin-washed four times, stained with 10 μg/ml of pHrodo succinimidyl ester (Molecular Probes) for 30 min at 37°C, spin-washed four times, and incubated with 10 mg/ml of purified IgG. Following three spin-washes, stained IEs were added to plated THP-1 cells and immediately imaged on a Leica TCS SP5 II confocal microscope (Leica, Heidelberg, Germany) using a Leica HCX Plan Apo 63×/1.4 to 0.6 oil immersion objective, equipped with a temperature- and atmosphere-controlled incubator for live-cell imaging. For 1 h, cells were viewed (original magnification, ×630) every 5 min at five 1-μm depths for z-stack analysis with Imaris 7.5 (Bitplane Scientific Software, Zurich, Switzerland).
In vitro phagocytosis assay.
The procedure was adapted from an assay previously described (29). Briefly, late-stage IEs were magnetically enriched to >90% purity, stained with 50 μg/ml hydroethidine for 30 min at 37°C, and suspended to 3 × 107 cells/ml. Purified IgGs were titrated in RPMI 1640 into a sterile 96-well V-bottomed plate (Corning) to a volume of 50 μl. For IgG binding, 50 μl of IE suspension was added to the 96-well plates containing IgG (total volume 100 μl) and left in the dark for 60 min at 37°C. Subsequently, IEs were spin-washed twice and then added to 1 × 105 THP-1 cells (IE-to-monocyte ratio of 15:1) in 1 ml of monocyte growth medium for coincubation in a sterile 24-well polypropylene plate (Thomson, Oceanside, CA) for 2 h in an atmosphere of 20% O2, 5% CO2, and 75% N2 at 37°C. Following coincubation, nonphagocytosed erythrocytes were lysed by the addition of ammonium-chloride-potassium (ACK) lysis buffer (Lonza) with debris spin-washed away. THP-1 cells were then fixed in 2% (vol/vol) paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) at 4°C, spin-washed, and stored at 4°C for ≤2 h until analysis. At least 5,000 THP-1 events were collected and analyzed for each sample. The level of phagocytosis was measured as the proportion of THP-1 events positive for ethidium fluorescence, denoted “Phagocytosis (%).”
For experiments with primary monocytes, procedures were performed as described above with the following modifications. Following incubation with IgGs, IEs were added to 1 × 105 primary monocytes (IE-to-monocyte ratio of 15:1) in 1 ml of primary monocyte growth medium. Following 2 h of phagocytosis challenge, lysis of nonphagocytosed erythrocytes, and two spin-washes, cells were stained with 5 μl of Pacific Blue-conjugated mouse anti-CD14 (BD PharMingen) for 30 min at 4°C. Subsequently, primary monocytes were spin-washed twice, fixed, spin-washed again, and analyzed. CD14+ primary monocytes were gated initially by forward and side scatter dot plot and subsequently identified as the Pacific Blue-positive subpopulation. A minimum of 5,000 CD14+ primary monocyte events were collected, with Phagocytosis (%) calculated as the percentage of events that were ethidium fluorescence positive.
Antigen reversal of phagocytosis.
For antigen reversal studies, test IgGs at 1.25 mg/ml were incubated with 100 μg of the recombinant P. falciparum antigen of interest for 1 h at 37°C prior to incubation with hydroethidine-labeled IEs.
Statistical data analysis.
Comparisons between unpaired data sets (i.e., gender groups) were analyzed by a two-tailed Mann-Whitney U test. Comparisons between paired data sets were analyzed by a two-tailed Wilcoxon signed-rank test. Correlations were examined by Spearman's rank sum tests. The significance limit was set at P values of <0.05. Calculations were performed using GraphPad Prism (Graph Pad Prism Inc., San Diego, CA).
RESULTS
IgGs from multigravida Malian women recognize recombinant VAR2CSA domains.
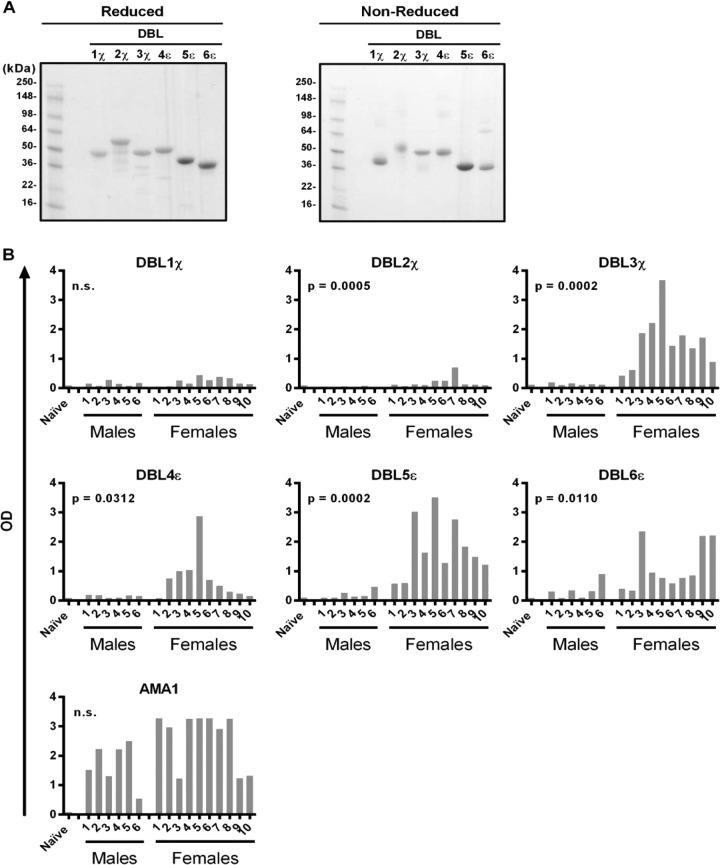
The six recombinant DBL domains of VAR2CSA showed appropriate molecular masses when analyzed by reducing and nonreducing SDS-PAGE (Fig. 1A). We performed ELISAs to assess IgG recognition of DBL domains in malaria-exposed multigravida Malian women, malaria-exposed Malian men, and malaria-naive American adults (see Table S1 in the supplemental material). Compared to Malian men, Malian women showed significantly higher IgG reactivity to five DBL domains (DBL2χ, P = 0.0005; DBL3χ, P = 0.0002; DBL4ε, P = 0.0312; DBL5ε, P = 0.0002; DBL6ε, P = 0.0110) (Fig. 1B). Malian men and women showed no significant differences in IgG reactivities to DBL1χ, which were low for both genders, or to merozoite apical membrane antigen 1 (AMA1), which were high for both genders. American adult IgG showed limited reactivity to all of these antigens (“Naive” in Fig. 1B). IgG1 was the predominant IgG subclass involved in DBL binding, with IgG2, IgG3, IgG4, and IgM exhibiting low recognition (see Fig. S1 in the supplemental material). The greater IgG reactivity to five VAR2CSA DBLs in multigravida Malian women than in Malian men suggests that the recombinant DBL proteins express epitopes of the native parasite protein.
FIG 1.
Malian adult IgG reactivity to P. falciparum antigens by ELISA. (A) Coomassie blue-stained reducing and nonreducing 4 to 20% SDS-PAGE gels indicating the correct mass for each of the six recombinant VAR2CSA DBL domains produced from E. coli. The molecular mass markers are shown in the leftmost lane. (B) Adult serum IgG reactivity to VAR2CSA DBL domains and P. falciparum merozoite protein AMA1 as measured by ELISA. Antigen-coated plates were incubated with a 1:200 dilution of serum. Optical density (OD) responses ranged from 0 to 4. For each antigen, P values refer to comparisons between Malian men and women (n.s., not significant). “Naive” samples refer to pooled IgGs from American adults. Bars show the means of results from one to four independent experiments run at least in triplicate.
CSA-selected IEs highly express VAR2CSA.
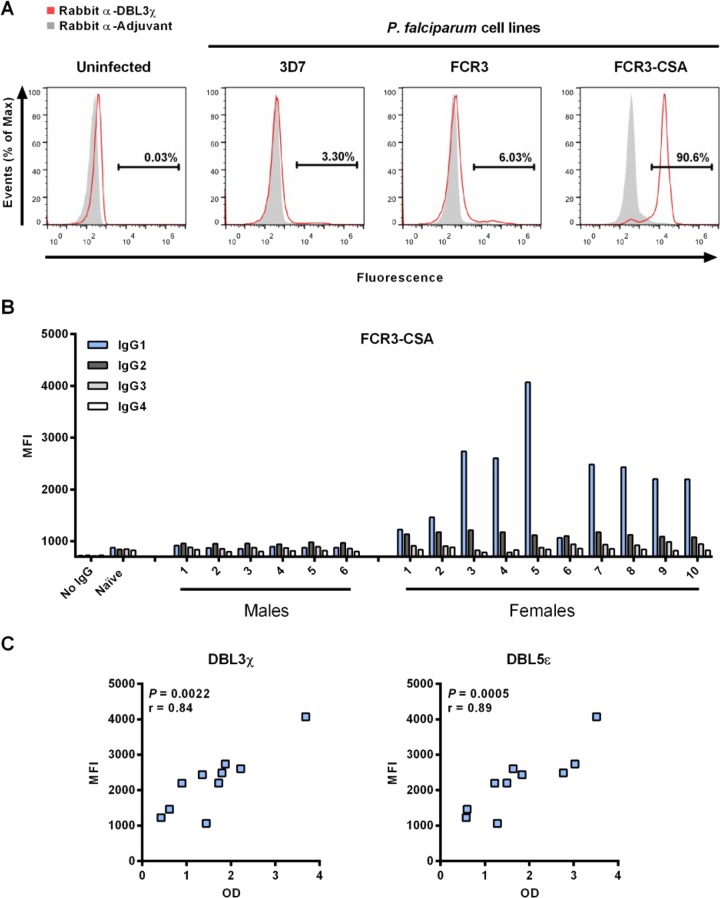
Our studies required comparisons between trophozoites expressing high levels of VAR2CSA and trophozoites expressing little or no VAR2CSA. To generate these parasite populations, we established a CSA-binding P. falciparum line, FCR3-CSA, by repeatedly selecting for FCR3 trophozoite-IEs that adhered to CSA-coated plastic plates in vitro. Nonselected parental FCR3 and 3D7 lines served as controls. To confirm whether FCR3-CSA had increased VAR2CSA expression levels relative to nonselected FCR3 and 3D7 parasite lines, we labeled cells with purified IgG from a rabbit immunized with DBL3χ (43). Rabbit anti-DBL3χ IgGs labeled FCR3-CSA IEs specifically and minimally labeled nonselected FCR3 or 3D7 IEs (Fig. 2A). Control IgGs from a rabbit immunized with adjuvant alone showed minimal labeling of FCR3-CSA, FCR3, and 3D7 IEs, similar to that of uninfected erythrocytes (Fig. 2A).
FIG 2.
FCR3-CSA IgG1 recognition by Malian women correlates with DBL3χ and DBL5ε ELISA reactivity. (A) Uninfected erythrocytes and magnetically enriched P. falciparum 3D7, FCR3, and FCR3-CSA IEs were incubated with purified IgGs from rabbits immunized with DBL3χ or adjuvant alone. Cells were then incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG for fluorescence detection (x axis). Each experiment was performed at least twice, with >50,000 IE events collected per experiment. Representative histograms are shown. (B) Human IgG subclass binding to FCR3-CSA late-stage IEs was detected by flow cytometry using mouse anti-human IgG subclass-specific antibodies and Alexa Fluor 647-conjugated goat anti-mouse antibodies. At least 5,000 IE events were analyzed for median fluorescence intensity (MFI). Bars show the means of duplicates. Malian women showed significantly higher IgG1 binding than Malian men (P = 0.0002). (C) For Malian women, IgG1 binding to FCR3-CSA IEs is shown in relation to IgG recognition of DBL3χ and DBL5ε by ELISA. The P value and Spearman's r coefficient are reported for each antigen.
IgGs from multigravida Malian women bind to the surface of FCR3-CSA IEs.
To confirm that IgGs from multigravida Malian women also bind specifically to native VAR2CSA on the trophozoite-IE surface, we first purified IgGs from sera to control for IgG concentration differences between serum samples and to avoid the possibility of IgM interfering with the binding of IgG to VAR2CSA (28, 44). Using flow cytometry, we found that IgG1 from Malian women showed significantly higher recognition of FCR3-CSA IEs than did IgG1 from Malian men (P = 0.0002) (Fig. 2B). This was also true for IgG2 (P = 0.0002), but the reactivity was low (MFI, ∼1,000). IgG3 and IgG4 from Malian women and men showed no differences in reactivity to FCR3-CSA (Fig. 2B). No significant differences in the binding of any IgG subclasses to FCR3 or 3D7 IEs were observed between Malian women and men (MFIs were consistently <1,000 units). In Malian women, IgG1 binding to FCR3-CSA IEs showed a significant positive correlation to DBL3χ (P = 0.0022, r = 0.84) and DBL5ε (P = 0.005, r = 0.89) recognition by ELISA (Fig. 2C).
IgG- and FcγR-dependent phagocytosis of IEs by THP-1 cells.
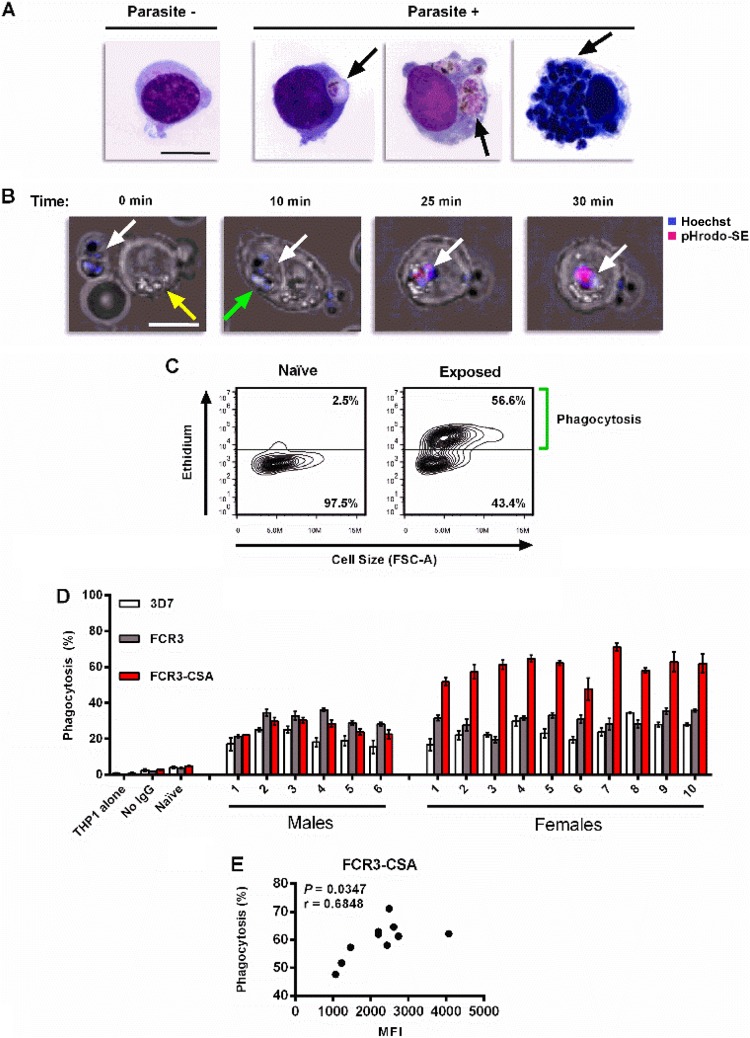
FCR3-CSA IEs incubated with purified IgGs pooled from Malian women and men were efficiently internalized by THP-1 cells (Fig. 3A). Acidification of IEs, as revealed by pHrodo staining, occurred within 25 min of monocyte uptake (Fig. 3B; see Movie S1 in the supplemental material). To assess quantitatively the internalization of IEs, we used a flow cytometry-based phagocytosis assay (Fig. 3C). IEs were first highly enriched by magnetic selection and then stained with hydroethidine to identify viable parasites. In metabolically active cells, nonfluorescent hydroethidine is converted to fluorescent ethidium, which intercalates with cellular nucleic acids (42). Thus, hydroethidine distinguishes highly fluorescent viable parasites from autofluorescent dead parasites and uninfected erythrocytes.
FIG 3.
Infected erythrocytes incubated with IgGs from Malians are phagocytosed by monocytic THP-1 cells. (A) Giemsa-stained smears contrasting a THP-1 cell that did not internalize FCR3-CSA IEs (Parasite −) and the parasite-filled phagosomes (black arrows) seen in THP-1 cells that internalized parasites (Parasite +). Bar, 10 μm. (B) Confocal images of a THP-1 cell acidifying a Hoechst- and pHrodo-SE-labeled FCR3-CSA IE at four time points during coincubation. An IE (white arrow) adjacent to a THP-1 cell (yellow arrow) is subsequently engulfed (green arrow) and acidified, as depicted in Movie S1 in the supplemental material. Bar, 10 μm. (C) Representative flow plots of THP-1 cells after coincubation with ethidium-fluorescent FCR3-CSA IEs preincubated with pooled IgG either from malaria-naive Americans (left) or from malaria-exposed multigravida Malian women (right). Following a 2-h coincubation with IEs and ammonium-chloride-potassium buffer (ACK) lysis treatment to remove noninternalized erythrocytes, THP-1 cells that phagocytosed IEs exhibited high ethidium fluorescence. Phagocytosis was measured as the proportion of THP-1 cells above the autofluorescent level (horizontal bar) as denoted by the green bracket. Each flow plot shows 5,000 events. (D) Phagocytosis of P. falciparum 3D7, FCR3, and FCR3-CSA IEs preincubated with 10 mg/ml IgGs from malaria-exposed Malian men and multigravida women, pooled IgGs from malaria-naive American adults, or no IgGs. A sample containing only THP-1 cells served as a negative control. Bars represent means ± standard errors of the means (SEM) from three independent experiments. IgGs from Malian women mediated significantly higher phagocytosis of FCR3-CSA IEs than IgGs from Malian men (P = 0.0002). Also, IgGs from Malian women mediated significantly greater phagocytosis of FCR3-CSA IEs than either FCR3 (P = 0.002) or 3D7 (P = 0.002) IEs. (E) For Malian women, IgG1 binding to the surface of FCR3-CSA IEs (Fig. 2B) is shown in relation to IgG-mediated phagocytosis of FCR3-CSA IEs. The P value and Spearman's r coefficient are shown.
IgGs from Malian women efficiently opsonize FCR3-CSA IEs.
IgGs from Malian men and women did not differ in their opsonization of FCR3 or 3D7 IEs (Fig. 3D). In contrast, IgGs from multigravida Malian women mediated higher phagocytosis of FCR3-CSA IEs than did IgGs from Malian men (Fig. 3D, P = 0.0002). Also, IgGs from Malian women mediated significantly greater phagocytosis of FCR3-CSA IEs than either FCR3 (P = 0.002) or3D7 (P = 0.002) IEs. Such a difference was not observed with IgGs from Malian men. Note that purified IgGs from American adults had very low opsonic activity for FCR3-CSA, FCR3, and 3D7 IEs, similar to results without IgG (Fig. 3D, “Naive” and “No IgG”). In Malian women, only IgG1 binding to the surface of FCR3-CSA IEs showed a significant positive correlation to IgG-mediated phagocytosis (P = 0.0347, r = 0.6848) (Fig. 3E).
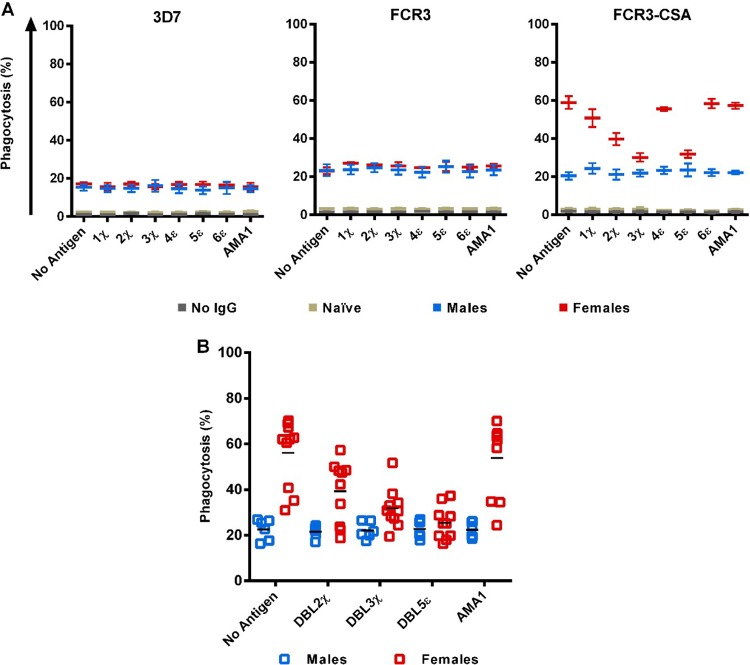
DBL2χ, DBL3χ, and DBL5ε are the targets of IgG-mediated phagocytosis of FCR3-CSA IEs.
As an initial step to determine the targets of opsonizing antibodies from multigravida women, we prepared pools of IgGs purified from multigravida Malian women, Malian men, or American adults and exposed them to individual DBL domains. These IgG pools were then incubated with IEs at 1.25 mg/ml, an IgG concentration that mediated slightly less than the maximum response on average for most individual samples to FCR3-CSA IEs (see Fig. S2 in the supplemental material). In this antigen reversal assay, using pooled IgG from Malian women without preincubation with antigen, the extent of phagocytosis was about 60% (Fig. 4A, FCR3-CSA, “no antigen”). When the same pooled IgG was preincubated with recombinant DBL domains, we found that phagocytosis of FCR3-CSA IEs was substantially reduced by 30 to 50% with DBL2χ (P = 0.001), DBL3χ (P = 0.0005), and DBL5ε (P = 0.0005), but not with DBL1χ, DBL4ε, DBL6ε, or AMA1 (Fig. 4A). In contrast, prior exposure to each of these antigens did not alter IgG-mediated phagocytosis of FCR3 or 3D7 IEs (Fig. 4A). Antigen reversal also had no effect on the IgG-mediated phagocytosis of FCR3-CSA, FCR3, or 3D7 IEs in assays using pooled IgGs from Malian men and American adults.
FIG 4.
Antigen reversal with DBL2χ, DBL3χ, and DBL5ε lowers opsonization of FCR3-CSA for THP-1 cells. (A) Purified IgG pools (1.25 mg/ml) from Malian women, Malian men, and American adults were exposed to 100 μg of the recombinant P. falciparum antigen of interest, as noted on the x axis, for 1 h. 3D7, FCR3, and FCR3-CSA IEs were incubated with the pretreated IgGs for 1 h and then incubated with THP-1 cells. Means ± SEM of phagocytosis from three independent experiments are shown. (B) IgGs (1.25 mg/ml) from all 16 malaria-exposed Malian adults were independently exposed to 100 μg parasite antigen (or no antigen) and coincubated with FCR3-CSA IEs and THP-1 cells as above. For IgGs from Malian women, significant antigen reversal of phagocytosis was seen with DBL2χ (P = 0.001), DBL3χ (P = 0.0005), and DBL5ε (P = 0.0005) but not with AMA1. For IgGs from Malian men, antigen reversal of phagocytosis was not seen with any antigen. Phagocytosis mediated by pooled IgGs from American adults was <4% (data not shown) and not impacted by antigen reversal. Black bar, mean of the group.
Building on this result, we evaluated DBL2χ, DBL3χ, and DBL5ε antigen reversal of FCR3-CSA IE opsonization for each of the 16 Malian adults. In antigen reversal assays using individual IgG samples from Malian women, phagocytosis of FCR3-CSA IEs was significantly reduced by 30 to 55% with DBL2χ (P = 0.001), DBL3χ (P = 0.0005), and DBL5ε (P = 0.0005), but not with AMA1 (Fig. 4B). Similar reductions were not observed in antigen reversal assays using individual IgGs from Malian men (Fig. 4B). We then sought to determine if the magnitude of phagocytosis reduction due to antigen reversal would correlate to antigen recognition; interestingly, ELISA responses showed no significant correlation with antigen reversal (data not shown).
Anti-DBL2χ, DBL3χ, and DBL5ε IgGs mediate phagocytosis of FCR3-CSA IEs by primary monocytes.
IgG has high affinity for FcγRI, which is present on undifferentiated THP-1 cells. However, the lack of FcγRIII expression by THP-1 cells (45) suggested that the in vitro phagocytosis of IEs by THP-1 cells might not be fully representative of the in vivo phagocytosis of IEs by monocytes. To address this possibility, we repeated some experiments using human CD14+ primary monocytes enriched from peripheral blood mononuclear cells (PBMCs) by negative selection (Fig. 5A). Primary monocytes efficiently phagocytosed FCR3-CSA IEs in the presence of purified IgGs pooled from Malian women (Fig. 5B). Phagocytosis was higher in the presence of IgGs from malaria-exposed Malian women than from malaria-naive American adults (Fig. 5C). In antigen reversal assays using pooled IgGs from Malian women, phagocytosis of FCR3-CSA IEs was substantially decreased by 25 to 45% with DBL2χ, DBL3χ, and DBL5ε, but not with DBL1χ, DBL4ε, DBL6ε, or AMA1 (Fig. 5D). Prior exposure to any of these antigens did not affect IgG-mediated phagocytosis of FCR3 or 3D7 IEs. Also, similar reductions were not observed in antigen reversal assays using pooled IgG samples from Malian men or American adults (Fig. 5D).
FIG 5.
DBL2χ, DBL3χ, and DBL5ε antigen reversal lowers opsonization of FCR3-CSA for primary monocytes. (A) Representative flow plots of PBMCs collected from malaria-naive Americans before (Pre) and after (Post) depletion of T cells, B cells, and NK cells to negatively select for primary monocytes. Propidium iodide labeling confirmed ≥99% viability of monocytes. Each plot shows at least 50,000 events. (B) Giemsa smears of enriched primary monocytes before (Parasite −) and after (Parasite +) phagocytosis of IEs. Bar, 10 μm. Photomicrographs were collected as stated in the legend for Fig. 3A. (C) Representative flow plots of enriched primary monocytes after coincubation with ethidium-labeled FCR3-CSA IEs preincubated with pooled IgGs from either malaria-naive Americans (left) or malaria-exposed multigravida Malian women (right). Following a 2-h coincubation with IEs and ACK lysis treatment to remove noninternalized erythrocytes, CD14+ monocytes that phagocytosed IEs showed high ethidium fluorescence. Phagocytosis was measured as the proportion of CD14+ monocytes above the autofluorescence level (horizontal bar). Each flow plot shows 5,000 events. (D) Purified IgG pools (1.25 mg/ml) from Malian women, Malian men, and American adults were pretreated with 100 μg of recombinant antigens (or no antigen) for 1 h. 3D7, FCR3, and FCR3-CSA IEs were incubated with pretreated IgGs for 1 h and then incubated with CD14+ monocytes. Means ± SEM of phagocytosis from three independent experiments (using monocytes from three separate donors) are shown.
DISCUSSION
P. falciparum infection during pregnancy remains a threat to the health of women and their children in areas of endemicity. Vaccination to induce immunity against placental parasitemia represents an attractive long-term, cost-effective strategy to protect women from PAM. However, challenges in developing this and other malaria vaccines include identifying protective antibody specificities and antigenic epitopes and establishing assays that predict clinical protection in humans living in areas of endemicity. In this study, we identified the DBL domain targets of IgGs that mediate the phagocytosis of VAR2CSA-expressing IEs by human monocytes, an essential yet often-overlooked effector mechanism against PAM. We demonstrate that in contrast to malaria-exposed Malian men, IgGs from malaria-exposed multigravida Malian women highly recognize five recombinant DBL domains of VAR2CSA as well as the surface of FCR3-CSA IEs. In addition, IgGs from Malian multigravida women more efficiently opsonize FCR3-CSA IEs than IgGs from Malian men, using either THP-1 cells or primary human monocytes. We performed the first application of antigen reversal of IgG-mediated phagocytosis of P. falciparum-infected erythrocytes. In doing so, we demonstrate that DBL3χ, DBL5ε, and to a lesser degree DBL2χ are the primary targets of opsonizing antibodies from multigravida women.
Consistent with previous observations (46, 47), the serum IgGs of malaria-exposed multigravida women predominantly recognized the DBL3χ and DBL5ε domains of VAR2CSA by ELISA. Sequencing and structural modeling have characterized DBL3χ and DBL5ε as relatively conserved (48) due to stabilizing cysteine residues and DBL4ε and DL6ε as heterogeneous by way of variable loops (49). While the structure of full-length VAR2CSA has yet to be resolved, one study proposed a compact structure whereby the extracellular region of VAR2CSA radiates outwardly from the cell surface, starting with DBL6ε, with the large and aromatic residue-rich interdomain region between DBL2χ and DBL3χ forming a structural turn that causes the protein to bend upon itself (50). Previous studies have generally used binding to CSA as a means to determine which domains might be responsible for the placental accumulation of IEs. Investigators have reported that DBL2χ (19, 51) and DBL3χ (19, 35, 43, 52) are involved in VAR2CSA binding to CSA. Conversely, while DBL4ε and DBL6ε have both been implicated in binding to CSA or placental tissue (17, 18, 52), they did not reverse IgG-mediated opsonization of VAR2CSA-expressing IEs. Moreover, our results show that DBL5ε was recognized by IgGs from multigravida women and induced antigen reversal. While this domain has previously been shown to be immunogenic (53–55), it has not been conclusively shown to be essential for CSA binding despite showing some affinity to negatively charged glycans (56). Nonetheless, DBL3χ and DBL5ε are considered highly homologous (10), possibly expressing the same epitopes. This redundancy may explain the similar degree to which DBL3χ and DBL5ε antigen reversal reduces the IgG-mediated phagocytosis of FCR3-CSA in samples from Malian women. According to this model, DBL2χ and DBL3χ are located at the apex, a practical location for opsonizing antibodies to attach. It may be that reactivity to DBL5ε is primarily due to its similarity to DBL3χ.
IgG1 was the major IgG subclass involved in recognizing VAR2CSA DBL domains by ELISA as well as binding to the surface of FCR3-CSA IEs by flow cytometry. This result is in agreement with a previous study that found that IgGs from multigravida Cameroonian women reactive to placental parasite isolates were mainly IgG1 and to a lesser extent IgG3 (57). In addition, IgG1 and IgG3 have been reported to bind to or opsonize VAR2CSA-expressing IEs (31, 58), and IgG3 from multigravida women has been shown to recognize VAR2CSA-DBL5ε (59). The human IgG1 and IgG3 subclasses are recognized as efficient mediators of opsonization, attributable to their high affinities for FcγRs (60).
We utilized phagocytosis by human monocytes (either undifferentiated THP-1 cells or primary monocytes) as a functional assay to assess the uptake of VAR2CSA-expressing IEs. Imaging of pHrodo-labeled IEs confirmed that human monocytes internalize and subsequently acidify parasites. Further, the use of hydroethidine to label erythrocytes ensured that the flow cytometry assay was reflecting phagocytosis of infected, viable erythrocytes (42). While we observed clear differences between IgGs from Malian men and multigravida Malian women, when we focus only on IgGs from multigravida women and compare results from different assays, there are several inconsistencies in the data. For example, IgGs from female 5, who showed the highest IgG ELISA titers to nearly all VAR2CSA domains as well as IgG1 binding to the surface of FCR3-CSA IEs, mediated only the fourth highest level of phagocytosis of these cells. Alternatively, women with relatively low IgG reactivity to DBL domains (females 1, 2, and 10) showed levels of opsonization and antigen reversal comparable to those of multigravida women with higher reactivity to DBL domains. Moreover, despite showing substantial reactivity to DBL domains, female 6 consistently showed the lowest levels of IgG1 surface binding to and opsonization of FCR3-CSA IEs among women. These seemingly discordant results may arise from differences in the fine specificity or avidity of the antibody repertoires in the individual women.
We reasoned that preincubation of IgGs from multigravida Malian women with specific recombinant DBL domains would reduce IgG opsonization of FCR3-CSA IEs. Any resulting decreases in phagocytosis, termed “antigen reversal,” would reflect the degree to which particular DBL domains are targeted by opsonizing IgGs. Given that antigen reversal of phagocytosis was typically highest for those domains with high antibody responses from multigravida women (DBL3χ and DBL5ε), our assay may reflect the higher depletion of antibodies after incubation with the recombinant antigens.
We did not have full clinical histories for the multigravidae who participated in this study (i.e., whether they suffered from PAM in one or more pregnancies, and when they last delivered). Therefore, it is difficult to assess how strongly the phagocytosis activity measured in this study associates with clinical protection against PAM. Previous studies have linked IgG recognition of placental parasite isolates and VAR2CSA during pregnancy with good birth outcomes at delivery (61, 62). Moreover, we considered only HIV-negative volunteers. It would be interesting to observe how HIV coinfection influences domain targeting. Ataide and colleagues recently showed that coinfection with HIV in primigravida women was associated with the worst outcomes (32) and that HIV impairs the development of IgG subclasses that principally mediate phagocytosis (33). However, the authors suggest that constant exposure to VAR2CSA-expressing IEs may override the immunosuppressive effects of HIV. Evaluating primigravidae in the opsonization assays and monitoring women through serial pregnancies is beyond the scope of the present study. However, a longitudinal study monitoring young women from nonpregnant to multigravida status would permit us to define relationships between different types of assays as well as between in vitro opsonization and in vivo resistance to PAM. Such a longitudinal study in a larger group of women would facilitate identification of assays that could be used to evaluate clinical trials of vaccine candidates directed to PAM. Further, it will be important to determine whether the opsonization patterns observed with these cultured parasites are similar to those using parasites derived from the placenta.
Despite a limited number of samples, our investigation sheds light onto important areas of interest in the field of acquired immunity to PAM. We provide the first evidence that the DBL2χ, DBL3χ, and DBL5ε domains of VAR2CSA are targeted by the opsonizing IgGs of P. falciparum-exposed multigravida women. These findings demonstrate that antigen reversal of antibody-mediated phagocytosis is a practical tool for identifying specific targets of functional polyclonal antibodies in studies of PAM and potentially other infectious diseases.
Supplementary Material
ACKNOWLEDGMENTS
We thank the volunteers of Kenieroba, Mali, David Narum (LMIV, NIAID, NIH) for kindly providing AMA1-FVO, Hong Zhou, Ababacar Diouf, Bingbing Deng, Sundar Ganesan, Juraj Kabat, Lubin Jiang, Julia Knoeckel, Simon Metenou, and Kevin L. Holmes (NIH) for technical assistance, and Diane W. Taylor (University of Hawaii), Eric B. Nelson, and Marshall L. Hayes (Cornell University) for their helpful discussions.
This work was supported by the Intramural Research Program of NIAID, NIH.
Footnotes
Published ahead of print 25 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02097-14.
REFERENCES
- 1.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7:93–104. 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2011. World malaria report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Hartman TK, Rogerson SJ, Fischer PR. 2010. The impact of maternal malaria on newborns. Ann. Trop. Paediatr. 30:271–282. 10.1179/146532810X12858955921032. [DOI] [PubMed] [Google Scholar]
- 4.Fried M, Duffy PE. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502–1504. 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 5.Ricke CH, Staalsoe T, Koram K, Akanmori BD, Riley EM, Theander TG, Hviid L. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165:3309–3316. 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 6.Maubert B, Fievet N, Tami G, Cot M, Boudin C, Deloron P. 1999. Development of antibodies against chondroitin sulfate A-adherent Plasmodium falciparum in pregnant women. Infect. Immun. 67:5367–5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K, Hviid L, Theander TG. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200:1197–1203. 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. 1998. Maternal antibodies block malaria. Nature 395:851–852. 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 9.Staalsoe T, Jensen AT, Theander TG, Hviid L. 2002. Novel Plasmodium falciparum malaria vaccines: evidence-based searching for variant surface antigens as candidates for vaccination against pregnancy-associated malaria. Immunol. Lett. 84:133–136. 10.1016/S0165-2478(02)00159-1. [DOI] [PubMed] [Google Scholar]
- 10.Andersen P, Nielsen MA, Resende M, Rask TS, Dahlback M, Theander T, Lund O, Salanti A. 2008. Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog. 4:e42. 10.1371/journal.ppat.0040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez P, Viebig NK, Dechavanne S, Lepolard C, Gysin J, Scherf A, Gamain B. 2008. Var2CSA DBL6-epsilon domain expressed in HEK293 induces limited cross-reactive and blocking antibodies to CSA binding parasites. Malar. J. 7:170. 10.1186/1475-2875-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy MF, Maier AG, Byrne TJ, Marty AJ, Elliott SR, O'Neill MT, Payne PD, Rogerson SJ, Cowman AF, Crabb BS, Brown GV. 2006. VAR2CSA is the principal ligand for chondroitin sulfate A in two allogeneic isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 148:117–124. 10.1016/j.molbiopara.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Duffy PE, Fried M. 2003. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 71:6620–6623. 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barfod L, Nielsen MA, Turner L, Dahlback M, Jensen AT, Hviid L, Theander TG, Salanti A. 2006. Baculovirus-expressed constructs induce immunoglobulin G that recognizes VAR2CSA on Plasmodium falciparum-infected erythrocytes. Infect. Immun. 74:4357–4360. 10.1128/IAI.01617-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto VV, Ditlev SB, Jensen KE, Resende M, Dahlback M, Andersen G, Andersen P, Theander TG, Salanti A, Nielsen MA. 2011. Differential induction of functional IgG using the Plasmodium falciparum placental malaria vaccine candidate VAR2CSA. PLoS One 6:e17942. 10.1371/journal.pone.0017942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen MA, Pinto VV, Resende M, Dahlback M, Ditlev SB, Theander TG, Salanti A. 2009. Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA. Infect. Immun. 77:2482–2487. 10.1128/IAI.00159-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magistrado PA, Minja D, Doritchamou J, Ndam NT, John D, Schmiegelow C, Massougbodji A, Dahlback M, Ditlev SB, Pinto VV, Resende M, Lusingu J, Theander TG, Salanti A, Nielsen MA. 2011. High efficacy of anti DBL4varepsilon-VAR2CSA antibodies in inhibition of CSA-binding Plasmodium falciparum-infected erythrocytes from pregnant women. Vaccine 29:437–443. 10.1016/j.vaccine.2010.10.080. [DOI] [PubMed] [Google Scholar]
- 18.Saveria T, Oleinikov AV, Wiliamson K, Chaturvedi R, Lograsso J, Keitany G, Fried M, Duffy P. 2013. Antibodies to Escherichia coli-expressed C-terminal domains of Plasmodium falciparum VAR2CSA inhibit binding of CSA-adherent parasites to placental tissue. Infect. Immun. 81:1031–1039. 10.1128/IAI.00978-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahlback M, Jorgensen LM, Nielsen MA, Clausen TM, Ditlev SB, Resende M, Pinto VV, Arnot DE, Theander TG, Salanti A. 2011. The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J. Biol. Chem. 286:15908–15917. 10.1074/jbc.M110.191510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Moraes LV, Tadokoro CE, Gomez-Conde I, Olivieri DN, Penha-Goncalves C. 2013. Intravital placenta imaging reveals microcirculatory dynamics impact on sequestration and phagocytosis of Plasmodium-infected erythrocytes. PLoS Pathog. 9:e1003154. 10.1371/journal.ppat.1003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua CL, Brown G, Hamilton JA, Rogerson S, Boeuf P. 2013. Monocytes and macrophages in malaria: protection or pathology? Trends Parasitol. 29:26–34. 10.1016/j.pt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. 2003. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am. J. Trop. Med. Hyg. 68:115–119. [PubMed] [Google Scholar]
- 23.Celada A, Cruchaud A, Perrin LH. 1982. Opsonic activity of human immune serum on in vitro phagocytosis of Plasmodium falciparum infected red blood cells by monocytes. Clin. Exp. Immunol. 47:635–644. [PMC free article] [PubMed] [Google Scholar]
- 24.Chan JA, Howell KB, Reiling L, Ataide R, Mackintosh CL, Fowkes FJ, Petter M, Chesson JM, Langer C, Warimwe GM, Duffy MF, Rogerson SJ, Bull Cowman PCAF, Marsh K, Beeson JG. 2012. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J. Clin. Invest. 122:3227–3238. 10.1172/JCI62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stubbs J, Olugbile S, Saidou B, Simpore J, Corradin G, Lanzavecchia A. 2011. Strain-transcending Fc-dependent killing of Plasmodium falciparum by merozoite surface protein 2 allele-specific human antibodies. Infect. Immun. 79:1143–1152. 10.1128/IAI.01034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tebo AE, Kremsner PG, Luty AJ. 2002. Fcgamma receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clin. Exp. Immunol. 130:300–306. 10.1046/j.1365-2249.2002.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tippett E, Fernandes LA, Rogerson SJ, Jaworowski A. 2007. A novel flow cytometric phagocytosis assay of malaria-infected erythrocytes. J. Immunol. Methods 325:42–50. 10.1016/j.jim.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Barfod L, Dalgaard MB, Pleman ST, Ofori MF, Pleass RJ, Hviid L. 2011. Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1. Proc. Natl. Acad. Sci. U. S. A. 108:12485–12490. 10.1073/pnas.1103708108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barfod L, Dobrilovic T, Magistrado P, Khunrae P, Viwami F, Bruun J, Dahlback M, Bernasconi NL, Fried M, John D, Duffy PE, Salanti A, Lanzavecchia A, Lim CT, Ndam NT, Higgins MK, Hviid L. 2010. Chondroitin sulfate A-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants. J. Immunol. 185:7553–7561. 10.4049/jimmunol.1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng G, Aitken E, Yosaatmadja F, Kalilani L, Meshnick SR, Jaworowski A, Simpson JA, Rogerson SJ. 2009. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes are associated with protection from treatment failure and the development of anemia in pregnancy. J. Infect. Dis. 200:299–306. 10.1086/599841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keen J, Serghides L, Ayi K, Patel SN, Ayisi J, van Eijk A, Steketee R, Udhayakumar V, Kain KC. 2007. HIV impairs opsonic phagocytic clearance of pregnancy-associated malaria parasites. PLoS Med. 4:e181. 10.1371/journal.pmed.0040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ataide R, Hasang W, Wilson DW, Beeson JG, Mwapasa V, Molyneux ME, Meshnick SR, Rogerson SJ. 2010. Using an improved phagocytosis assay to evaluate the effect of HIV on specific antibodies to pregnancy-associated malaria. PLoS One 5:e10807. 10.1371/journal.pone.0010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ataide R, Mwapasa V, Molyneux ME, Meshnick SR, Rogerson SJ. 2011. Antibodies that induce phagocytosis of malaria infected erythrocytes: effect of HIV infection and correlation with clinical outcomes. PLoS One 6:e22491. 10.1371/journal.pone.0022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kane EG, Taylor-Robinson AW. 2011. Prospects and pitfalls of pregnancy-associated malaria vaccination based on the natural immune response to Plasmodium falciparum VAR2CSA-expressing parasites. Malar. Res. Treat. 10.4061/2011/764845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh K, Gitti RK, Diouf A, Zhou H, Gowda DC, Miura K, Ostazeski SA, Fairhurst RM, Garboczi DN, Long CA. 2010. Subdomain 3 of Plasmodium falciparum VAR2CSA DBL3x is identified as a minimal chondroitin sulfate A-binding region. J. Biol. Chem. 285:24855–24862. 10.1074/jbc.M110.118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giersing B, Miura K, Shimp R, Wang J, Zhou H, Orcutt A, Stowers A, Saul A, Miller LH, Long C, Singh S. 2005. Posttranslational modification of recombinant Plasmodium falciparum apical membrane antigen 1: impact on functional immune responses to a malaria vaccine candidate. Infect. Immun. 73:3963–3970. 10.1128/IAI.73.7.3963-3970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. 2008. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine 26:193–200. 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolofsky KT, Ayi K, Branch DR, Hult AK, Olsson ML, Liles WC, Cserti-Gazdewich CM, Kain KC. 2012. ABO blood groups influence macrophage-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes. PLoS Pathog. 8:e1002942. 10.1371/journal.ppat.1002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49:179–191. 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 40.Noviyanti R, Brown GV, Wickham ME, Duffy MF, Cowman AF, Reeder JC. 2001. Multiple var gene transcripts are expressed in Plasmodium falciparum infected erythrocytes selected for adhesion. Mol. Biochem. Parasitol. 114:227–237. 10.1016/S0166-6851(01)00266-3. [DOI] [PubMed] [Google Scholar]
- 41.Semnani RT, Keiser PB, Coulibaly YI, Keita F, Diallo AA, Traore D, Diallo DA, Doumbo OK, Traore SF, Kubofcik J, Klion AD, Nutman TB. 2006. Filaria-induced monocyte dysfunction and its reversal following treatment. Infect. Immun. 74:4409–4417. 10.1128/IAI.01106-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Heyde HC, Elloso MM, vande Waa J, Schell K, Weidanz WP. 1995. Use of hydroethidine and flow cytometry to assess the effects of leukocytes on the malarial parasite Plasmodium falciparum. Clin. Diagn. Lab. Immunol. 2:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh K, Gittis AG, Nguyen P, Gowda DC, Miller LH, Garboczi DN. 2008. Structure of the DBL3x domain of pregnancy-associated malaria protein VAR2CSA complexed with chondroitin sulfate A. Nat. Struct. Mol. Biol. 15:932–938. 10.1038/nsmb.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semblat JP, Raza A, Kyes SA, Rowe JA. 2006. Identification of Plasmodium falciparum var1CSA and var2CSA domains that bind IgM natural antibodies. Mol. Biochem. Parasitol. 146:192–197. 10.1016/j.molbiopara.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleit HB, Kobasiuk CD. 1991. The human monocyte-like cell line THP-1 expresses Fc gamma RI and Fc gamma RII. J. Leukoc. Biol. 49:556–565. [DOI] [PubMed] [Google Scholar]
- 46.Barfod L, Bernasconi NL, Dahlback M, Jarrossay D, Andersen PH, Salanti A, Ofori MF, Turner L, Resende M, Nielsen MA, Theander TG, Sallusto F, Lanzavecchia A, Hviid L. 2007. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Mol. Microbiol. 63:335–347. 10.1111/j.1365-2958.2006.05503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tutterrow YL, Avril M, Singh K, Long CA, Leke RJ, Sama G, Salanti A, Smith JD, Leke RG, Taylor DW. 2012. High levels of antibodies to multiple domains and strains of VAR2CSA correlate with the absence of placental malaria in Cameroonian women living in an area of high Plasmodium falciparum transmission. Infect. Immun. 80:1479–1490. 10.1128/IAI.00071-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avril M, Cartwright MM, Hathaway MJ, Smith JD. 2011. Induction of strain-transcendent antibodies to placental-type isolates with VAR2CSA DBL3 or DBL5 recombinant proteins. Malar. J. 10:36. 10.1186/1475-2875-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bockhorst J, Lu F, Janes JH, Keebler J, Gamain B, Awadalla P, Su XZ, Samudrala R, Jojic N, Smith JD. 2007. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol. Biochem. Parasitol. 155:103–112. 10.1016/j.molbiopara.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Clausen TM, Christoffersen S, Dahlback M, Langkilde AE, Jensen KE, Resende M, Agerbaek MO, Andersen D, Berisha B, Ditlev SB, Pinto VV, Nielsen MA, Theander TG, Larsen S, Salanti A. 2012. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J. Biol. Chem. 287:23332–23345. 10.1074/jbc.M112.348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bigey P, Gnidehou S, Doritchamou J, Quiviger M, Viwami F, Couturier A, Salanti A, Nielsen MA, Scherman D, Deloron P, Tuikue Ndam N. 2011. The NTS-DBL2X region of VAR2CSA induces cross-reactive antibodies that inhibit adhesion of several Plasmodium falciparum isolates to chondroitin sulfate A. J. Infect. Dis. 204:1125–1133. 10.1093/infdis/jir499. [DOI] [PubMed] [Google Scholar]
- 52.Gamain B, Trimnell AR, Scheidig C, Scherf A, Miller LH, Smith JD. 2005. Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites. J. Infect. Dis. 191:1010–1013. 10.1086/428137. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez P, Petres S, Mecheri S, Gysin J, Scherf A. 2010. Strain-transcendent immune response to recombinant Var2CSA DBL5-epsilon domain block P. falciparum adhesion to placenta-derived BeWo cells under flow conditions. PLoS One 5:e12558. 10.1371/journal.pone.0012558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brolin KJ, Persson KE, Wahlgren M, Rogerson SJ, Chen Q. 2010. Differential recognition of P. falciparum VAR2CSA domains by naturally acquired antibodies in pregnant women from a malaria endemic area. PLoS One 5:e9230. 10.1371/journal.pone.0009230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srivastava A, Gangnard S, Dechavanne S, Amirat F, Lewit Bentley A, Bentley GA, Gamain B. 2011. Var2CSA minimal CSA binding region is located within the N-terminal region. PLoS One 6:e20270. 10.1371/journal.pone.0020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gnidehou S, Jessen L, Gangnard S, Ermont C, Triqui C, Quiviger M, Guitard J, Lund O, Deloron P, Ndam NT. 2010. Insight into antigenic diversity of VAR2CSA-DBL5epsilon domain from multiple Plasmodium falciparum placental isolates. PLoS One 5:e13105. 10.1371/journal.pone.0013105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Megnekou R, Staalsoe T, Taylor DW, Leke R, Hviid L. 2005. Effects of pregnancy and intensity of Plasmodium falciparum transmission on immunoglobulin G subclass responses to variant surface antigens. Infect. Immun. 73:4112–4118. 10.1128/IAI.73.7.4112-4118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elliott SR, Brennan AK, Beeson JG, Tadesse E, Molyneux ME, Brown GV, Rogerson SJ. 2005. Placental malaria induces variant-specific antibodies of the cytophilic subtypes immunoglobulin G1 (IgG1) and IgG3 that correlate with adhesion inhibitory activity. Infect. Immun. 73:5903–5907. 10.1128/IAI.73.9.5903-5907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guitard J, Cottrell G, Magnouha NM, Salanti A, Li T, Sow S, Deloron P, Tuikue Ndam N. 2008. Differential evolution of anti-VAR2CSA-IgG3 in primigravidae and multigravidae pregnant women infected by Plasmodium falciparum. Malar. J. 7:10. 10.1186/1475-2875-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. 2009. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 113:3716–3725. 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 61.Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. 2004. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363:283–289. 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 62.Tutterrow YL, Salanti A, Avril M, Smith JD, Pagano IS, Ako S, Fogako J, Leke RG, Taylor DW. 2012. High avidity antibodies to full-length VAR2CSA correlate with absence of placental malaria. PLoS One 7:e40049. 10.1371/journal.pone.0040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.