Abstract
Tedizolid, the active moiety of tedizolid phosphate, is a recently approved oxazolidinone antibacterial with activity against a wide range of Gram-positive pathogens, including resistant strains such as methicillin-resistant Staphylococcus aureus. To date, 6 days of 200 mg tedizolid once daily has been shown to be noninferior to 10 days of 600 mg linezolid twice daily in two randomized, double-blind phase 3 trials (ESTABLISH-1 and ESTABLISH-2) for the treatment of patients with acute bacterial skin and skin structure infections (ABSSSIs). The intent of this study was to characterize the platelet profiles of patients receiving tedizolid relative to linezolid over the course of treatment using pooled data from these two trials. The occurrences of clinically defined and statistical analysis plan–specified reduced platelet counts were assessed at the study days 7 to 9 visit, the study days 11 to 13 visit, and the posttherapy evaluation (PTE) visit. At the study days 7 to 9 visit, incidences of reduced platelet counts were low and largely similar between the groups. The only notable difference was a lower incidence of thrombocytopenia (platelet counts, <150,000 cells/mm3) among patients who received tedizolid (3.2%) relative to those who received linezolid (5.6%). At the study days 11 to 13 visit, patients who received tedizolid had lower incidences of platelet counts of <150,000 cells/mm3 (−5.9%), <112,500 cells/mm3 (−2.4%), and <100,000 cells/mm3 (−1.9%) than patients in the linezolid group. Similar differences were noted at the PTE visit. Findings across the two phase 3 ABSSSI trials suggest that 6 days of 200 mg tedizolid daily confers a low potential for reduced platelet counts among patients with ABSSSIs. (The ESTABLISH-1 and ESTABLISH-2 trials have been registered at ClinicalTrials.gov under registration numbers NCT01170221 and NCT01421511, respectively.)
INTRODUCTION
Tedizolid, the active moiety of tedizolid phosphate, is a recently approved oxazolidinone (available in oral and intravenous formulations) with in vitro activity against a wide range of Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (1–4). Tedizolid has been compared with linezolid in two phase 3 randomized, double-blind, double-dummy, multicenter, multinational, noninferiority trials (5, 6). These trials were designed to evaluate the efficacy and safety of 200 mg tedizolid once daily for 6 days compared with 600 mg linezolid twice daily for 10 days for treatment of patients with acute bacterial skin and skin structure infections (ABSSSIs) (7, 8). In both phase 3 clinical trials, 6 days of 200 mg tedizolid daily (followed by 4 days of placebo) demonstrated noninferior efficacy to 600 mg linezolid twice daily for the primary endpoint of early clinical response, and similar efficacies for patients who received tedizolid and linezolid were noted for all secondary endpoints (5, 6). Furthermore, incidences of severe and serious treatment-emergent adverse events (TEAEs) were infrequent (≤2%) and comparable between the patients who received tedizolid and those who received linezolid. Collectively, these findings help to establish tedizolid as a new treatment option for ABSSSIs.
Although efficacy results of phase 3 ABSSSI trials were favorable, it is critical to consider the safety profile of any new agent to assess its usefulness in clinical practice. With oxazolidinones, an important safety concern is hematologic toxicity, particularly thrombocytopenia, with prolonged therapy (9–12). Although the duration of therapy was limited to ≤10 days, the intent of this report is to begin to characterize the platelet profile of 6 days of 200 mg tedizolid daily relative to 10 days of 600 mg linezolid twice daily using pooled data from the two phase 3 ABSSSI clinical trials (5, 6).
MATERIALS AND METHODS
Phase 3 ABSSSI clinical trials ESTABLISH-1 and ESTABLISH-2.
The ESTABLISH-1 and ESTABLISH-2 studies (Efficacy and Safety of 6-Day Oral and/or Intravenous Tedizolid in Acute Bacterial Skin and Skin Structure Infections vs. 10-Day Oral and/or Intravenous Linezolid Therapy) (registered at ClinicalTrials.gov under registration numbers NCT01170221 and NCT01421511, respectively) were randomized double-blind multicenter phase 3 trials conducted to assess the efficacy and safety of oral or intravenous tedizolid doses of 200 mg once daily for 6 days compared with those of oral or intravenous linezolid doses of 600 mg twice daily for 10 days in the management of ABSSSI (5, 6).
Statistical analysis and outcomes.
For the purpose of this integrated platelet analysis, data were pooled for all patients treated with at least one dose of the study drug (safety analysis population) from the ESTABLISH-1 and ESTABLISH-2 trials. Among patients who received at least one dose of the study drug, platelet counts were compared between patients who received tedizolid and those who received linezolid. In addition, platelet counts were evaluated only in patients in the safety analysis population with a baseline platelet count above the lower limit of normal (LLN), defined as 150,000 cells/mm3. To assess the platelet profile over time, the incidence of reduced platelet counts was assessed at the visits on study days 7 to 9 and study days 11 to 13 and at the posttreatment evaluation (PTE) visit.
Platelet counts were evaluated by prespecified commonly accepted clinical and statistical analysis plan–specified thresholds. The statistical analysis plan–specified reduced platelet counts considered in this analysis included the occurrence of platelet counts below the LLN (150,000 cells/mm3) and occurrence of platelet counts <75% of the LLN (112,500 cells/mm3) (13). The evaluated clinically defined reduced platelet counts included three clinically relevant cutoffs: ≤100,000 cells/mm3, ≤50,000 cells/mm3, and ≤20,000 cells/mm3. These absolute platelet thresholds were evaluated because they, particularly the latter two, have been implicated as carrying an increased risk for spontaneous bleeding (14–18). In addition, decreases of ≤50% from baseline in patients with normal baseline platelet counts were evaluated because the occurrence of this endpoint has been identified as a meaningful exposure-response reduced-platelet-count outcome (19–21). Furthermore, platelet counts were categorized into toxicity grades according to the Division of Microbiology and Infectious Diseases (DMID) adult toxicity table (22), and shifts from baseline in toxicity grades at the study days 7 to 9 visit, the study days 11 to 13 visit, and the PTE visit were summarized by treatment group. Platelet counts of 75,000 to 99,999 cells/mm3 are categorized as grade 1, 50,000 to 74,999 cells/mm3 as grade 2, 20,000 to 49,999 cells/mm3 as grade 3, and <20,000 cells/mm3 as grade 4.
Bivariate associations between the treatment and occurrence of reduced platelet counts at defined thresholds were assessed using Fisher's exact test. The relative risk of a reduced platelet count was calculated for patients receiving tedizolid compared with patients receiving linezolid, and the corresponding 95% confidence interval was calculated for each relative risk.
RESULTS
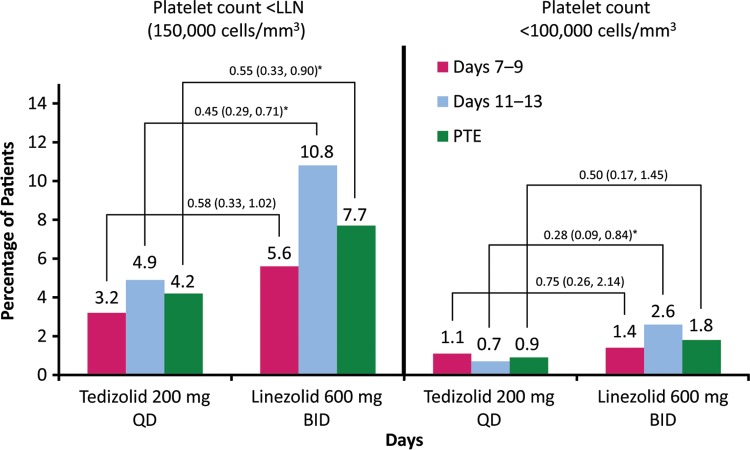
Of 1,333 enrolled patients (intent-to-treat [ITT] analysis population), 1,324 received at least one dose of the study drug and were included in the safety analysis population (662 patients in each treatment group) (see Table S1 in the supplemental material). Results of the comparative platelet count analyses from the integrated ESTABLISH phase 3 studies are given in the tables and Fig. 1. Platelet counts for 555 patients in the tedizolid group and 552 patients in the linezolid were assessed at the visit on study days 7 to 9. The incidences of reduced platelet counts at that visit were low and largely similar between the treatment groups (Table 1; Fig. 1). A lower incidence of thrombocytopenia in the tedizolid group (3.2%) than in the linezolid group (5.6%) was observed, although this was not a statistically significant difference. Further differences in reduced platelet counts were observed between treatment groups at the end-of-treatment (EOT) visit on study days 11 to 13 (Table 2, Fig. 1), when platelet counts of 552 patients in the tedizolid group and 538 patients in the linezolid group were assessed. Patients who received tedizolid had lower incidences of statistical analysis plan–defined platelet outcomes (<LLN and <75% LLN) and platelet counts of <100,000 cells/mm3. Similarly, patients who received tedizolid had a lower percentage of platelet counts of <150,000 cells/mm3, <112,500 cells/mm3, and <100,000 cells/mm3 at the PTE visit than those who received linezolid (545 patients were assessed in each group) (Table 3). Similar to the overall reduced-platelet-count analyses (Tables 1 to 3), a low occurrence of negative toxicity grade shifts was reported at study days 7 to 9 for both treatment groups (Table 4). At the EOT visit, three (0.6%) patients in the tedizolid group experienced a negative toxicity grade shift (one patient shifted from grade 0 to grade 2, one patient shifted from grade 0 to grade 3, and one patient shifted from grade 1 to grade 2), whereas negative toxicity grade shifts were noted in seven (1.4%) patients who received linezolid (all shifted from grade 0 to grade 1; P = 0.22). Similar findings for toxicity grade shifts were noted at the PTE visit. No patient in either treatment group experienced bleeding-related adverse events (AEs). Moreover, although none of the patients experiencing abnormal platelet counts or toxicity grade ≥3 during the course of the study discontinued therapy because of TEAEs, two patients in the tedizolid group discontinued therapy for other reasons (one refused to take study medication, and one did not have adequate medication after leaving town for a family emergency). In addition, one patient in the linezolid group who discontinued the study was lost to follow-up.
FIG 1.
Incidence of platelet counts of <150,000 and <100,000 cells/mm3 at the visit on study days 7 to 9, at end of therapy (EOT) on study days 11 to 13, and at posttherapy evaluation (PTE) (7 to 14 days after EOT visit). BID, twice daily; LLN, lower limit of normal; PTE, posttherapy evaluation; QD, once daily. Treatment differences (shown over the connecting lines) reflect relative risk (RR) (95% CI). *, P < 0.05 (Fisher's exact test).
TABLE 1.
Platelet parameters at visit on days 7 to 9, safety analysis population from ESTABLISH-1 and ESTABLISH-2a
| Patient group | Platelet count (cells/mm3), outcome | Data (no. [%]) for patients taking: |
Relative risk (95% CId) | |
|---|---|---|---|---|
| 200 mg tedizolid QD | 600 mg linezolid BID | |||
| Received ≥1 dose of study drugb | <150,000, <LLN | 18 (3.2) | 31 (5.6) | 0.58 (0.33–1.02) |
| <112,500, <75% LLN | 8 (1.4) | 12 (2.2) | 0.66 (0.27–1.61) | |
| <100,000 | 6 (1.1) | 8 (1.4) | 0.75 (0.26–2.14) | |
| <50,000 | 1 (0.2) | 1 (0.2) | 1.00 (0.06–15.86) | |
| <20,000 | 0 (0.0) | 0 (0.0) | NA | |
| Received ≥1 dose of study drug and had baseline platelet count of >150,000 cells/mm3c | 150,000, <LLN | 9 (1.9) | 9 (1.9) | 0.98 (0.39–2.45) |
| <112,500, <75% LLN | 4 (0.8) | 2 (0.4) | 1.96 (0.36–10.66) | |
| <100,000 | 3 (0.6) | 0 (0.0) | NA | |
| <50,000 | 1 (0.2) | 0 (0.0) | NA | |
| <20,000 | 0 (0.0) | 0 (0.0) | NA | |
| ≥50% decrease from baseline | 3 (0.6) | 3 (0.6) | 0.98 (0.20–4.84) | |
Patients were randomly assigned to receive once-daily (QD) dosing of tedizolid for 6 days or twice-daily (BID) dosing of linezolid for 10 days.
n = 555 tedizolid and 552 linezolid.
n = 471 tedizolid and 462 linezolid.
CI, confidence interval; NA, not applicable.
TABLE 2.
Platelet parameters at end-of-therapy visit on study days 11 to 13, safety analysis population from ESTABLISH-1 and ESTABLISH-2a
| Patient group | Platelet count (cells/mm3), outcome | Data (no. [%]) for patients taking: |
Relative risk (95% CId) | |
|---|---|---|---|---|
| 200 mg tedizolid QD | 600 mg linezolid BID | |||
| Received ≥1 dose of study drugb | <150,000, <LLN | 27 (4.9) | 58 (10.8) | 0.45 (0.29–0.71) |
| <112,500, <75% LLN | 7 (1.3) | 20 (3.7) | 0.34 (0.15–0.80) | |
| <100,000 | 4 (0.7) | 14 (2.6) | 0.28 (0.09–0.84) | |
| <50,000 | 1 (0.2) | 0 (0.0) | NA | |
| <20,000 | 0 (0.0) | 0 (0.0) | NA | |
| Received ≥1 dose of study drug and had baseline platelet count of >150,000 cells/mm3c | 150,000, <LLN | 16 (3.4) | 31 (6.8) | 0.50 (0.28–0.90) |
| <112,500, <75% LLN | 4 (0.9) | 8 (1.8) | 0.49 (0.15–1.60) | |
| <100,000 | 2 (0.4) | 3 (0.7) | 0.65 (0.11–3.85) | |
| <50,000 | 1 (0.2) | 0 (0.0) | NA | |
| <20,000 | 0 (0.0) | 0 (0.0) | NA | |
| ≥50% decrease from baseline | 4 (0.9) | 7 (1.5) | 0.55 (0.16–1.88) | |
Patients were randomly assigned to receive once-daily (QD) dosing of tedizolid for 6 days or twice-daily (BID) dosing of linezolid for 10 days.
n = 552 tedizolid and 538 linezolid.
n = 467 tedizolid and 453 linezolid.
CI, confidence interval; NA, not applicable.
TABLE 3.
Platelet parameters at the posttherapy evaluation, safety analysis population from ESTABLISH-1 and ESTABLISH-2a
| Patient group | Platelet count (cells/mm3), outcome | Data (no. [%]) for patients taking: |
Relative risk (95% CId) | |
|---|---|---|---|---|
| Tedizolid 200 mg QD (n [%]) | Linezolid 600 mg BID (n [%]) | |||
| Received ≥1 dose of study drugb | <150,000, <LLN | 23/545 (4.2) | 42/545 (7.7) | 0.55 (0.33–0.90) |
| <112,500, <75% LLN | 7/545 (1.3) | 13/545 (2.4) | 0.54 (0.22–1.34) | |
| <100,000 | 5/545 (0.9) | 10/545 (1.8) | 0.50 (0.17–1.45) | |
| <50,000 | 2/545 (0.4) | 1/545 (0.2) | 2.00 (0.18–21.99) | |
| <20,000 | 0/545 (0.0) | 0/545 (0.0) | NA | |
| Received ≥1 dose of study drug and had baseline platelet count of >150,000 cells/mm3c | 150,000, <LLN | 14/466 (3.0) | 21/464 (4.5) | 0.66 (0.34–1.29) |
| <112,500, <75% LLN | 4/466 (0.9) | 2/464 (0.4) | 1.99 (0.37–10.82) | |
| <100,000 | 3/466 (0.6) | 1/464 (0.2) | 2.99 (0.31–28.61) | |
| <50,000 | 2/466 (0.4) | 0/464 (0.0) | NA | |
| <20,000 | 0/466 (0.0) | 0/464 (0.0) | NA | |
| ≥50% decrease from baseline | 6/466 (1.3) | 6/464 (1.3) | 1.00 (0.32–3.07) | |
Patients were randomly assigned to receive once-daily (QD) dosing of tedizolid for 6 days or twice-daily (BID) dosing of linezolid for 10 days.
n = 545 for both groups.
n = 466 tedizolid and 464 linezolid.
CI, confidence interval; NA, not applicable.
TABLE 4.
Incidence of platelet toxicity shifts, safety analysis population from ESTABLISH-1 and ESTABLISH-2
| Study drug and visita | Baseline gradeb | Incidencec at postbaseline grade ofb: |
||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| 200 mg tedizolid QD | ||||||
| Days 7–9 | 0 | 501/505 (99.2) | 3/4 (75.0) | 0 | 0 | 0 |
| 1 | 2/505 (0.4) | 1/4 (25.0) | 0 | 0 | 0 | |
| 2 | 1/505 (0.2) | 0 | 1/1 (100) | 0 | 0 | |
| 3 | 1/505 (0.2) | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | |
| EOT | 0 | 493/495 (99.6) | 3/4 (75.0) | 1/2 (50.0) | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 1/495 (0.2) | 1/4 (25.0) | 1/2 (50.0) | 0 | 0 | |
| 3 | 1/495 (0.2) | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | |
| PTE | 0 | 494/497 (99.4) | 2/3 (66.7) | 1/2 (50.0) | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 1/497 (0.2) | 1/3 (33.3) | 1/2 (50.0) | 0 | 0 | |
| 3 | 2/497 (0.4) | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | |
| 600 mg linezolid BID | ||||||
| Days 7–9 | 0 | 495/496 (99.8) | 3/4 (75.0) | 0 | 0 | 1/1 (100) |
| 1 | 0 | 1/4 (25.0) | 3/4 (75.0) | 0 | 0 | |
| 2 | 1/496 (0.2) | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 1/4 (25.0) | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | |
| EOT | 0 | 477/484 (98.6) | 2/4 (50.0) | 0 | 0 | 1/1 (100) |
| 1 | 7/484 (1.4) | 2/4 (50.0) | 1/3 (33.3) | 0 | 0 | |
| 2 | 0 | 0 | 2/3 (66.7) | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | |
| PTE | 0 | 490/494 (99.2) | 3/4 (75.0) | 0 | 0 | 1/1 (100) |
| 1 | 3/494 (0.6) | 0 | 0 | 0 | 0 | |
| 2 | 1/494 (0.2) | 1/4 (25.0) | 2/3 (66.7) | 0 | 0 | |
| 3 | 0 | 0 | 1/3 (33.3) | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | |
QD, once daily; EOT, end of therapy (days 11 to 13); PTE, posttherapy evaluation; BID, twice daily.
Toxicity shifts are defined by the Division of Microbiology and Infectious Diseases adult toxicity table as follows (22): grade 1, mild—transient or mild discomfort (<48 h), no medical intervention/therapy required; grade 2, moderate—mild to moderate limitation in activity, some assistance may be needed, no or minimal medical intervention/therapy required; grade 3: severe—marked limitation in activity, some assistance usually required, medical intervention/therapy required, hospitalization possible; grade 4: life-threatening—extreme limitation in activity, significant assistance required, significant medical intervention/therapy required, hospitalization or hospice care probable.
Values shown are the number of toxicity shifts over the number of patients with platelet data at the baseline and postbaseline visits (%).
DISCUSSION
The primary goal of this analysis was to quantify the platelet profile of patients who received tedizolid across the two phase 3 ABSSSI studies. Given the short duration of drug administration, we did not expect to see demonstrable changes in the platelet count. Our approach was aimed at detecting an early signal of a drug effect on the platelet count. Consistent with the findings in the earlier phase 1 and 2 clinical trials, a low incidence of reduced platelet count was observed with tedizolid in ESTABLISH-1 and ESTABLISH-2 (23, 24). Among all of the patients enrolled in these two studies, even among those with platelet counts below LLN (<150,000 cells/mm3) at baseline, the occurrences of statistical analysis plan–defined reduced platelet counts were low (<5%) at the study days 7 to 9 visit, the study days 11 to 13 visit, and the PTE visit. Only a few instances of reduced platelet counts, as defined by the commonly accepted clinical thresholds, occurred during the study period among patients who received tedizolid. It is critical to determine the number of individuals who experience a platelet toxicity grade shift when quantifying a platelet profile for a drug regimen, because the platelet count is often abnormal in a number of patients at study entry. Less than 1% of patients who received tedizolid experienced a platelet toxicity grade shift over the study period (i.e., study days 7 to 9, study days 11 to 13, or PTE), and only two patients who received tedizolid had negative grade toxicity shifts of ≥2. None of the patients in the tedizolid treatment arm who experienced a reduced platelet count discontinued treatment because of an AE. Collectively, findings across ESTABLISH-1 and ESTABLISH-2 suggest that treatment for 6 days with 200 mg tedizolid daily confers a low risk for reduced platelet counts among patients with ABSSSI.
Tedizolid (200 mg) was also associated with numerically lower incidences of platelet parameters falling below clinically defined and statistical analysis plan–defined thresholds relative to linezolid across the two phase 3 ABSSSI studies. As anticipated, differences in the occurrence of statistical analysis plan–defined reduced-platelet-count outcomes were most pronounced at the study days 11 to 13 visit and the PTE visit. However, the proportion of patients with substantially reduced platelet counts relative to baseline readings was low in each group at these observation points (e.g., 0.9% of tedizolid and 1.5% of linezolid patients with normal platelet counts at baseline had dropped by ≥50% at the study days 11 to 13 visit). Although the different treatment durations for tedizolid and linezolid likely contributed to these findings, the studied durations of therapies were consistent with those demonstrated to be effective for management of ABSSSIs (i.e., 6 days of treatment for tedizolid and 10 days of treatment for linezolid). Although the most pronounced differences were observed at later time points, statistical analysis plan–defined reduced platelet counts were noted even at the visit on study days 7 to 9 for both drugs (after completion of active drug in the tedizolid arm and before completion of active drug in the linezolid arm). The clinical relevance of these findings is unknown; however, observations suggest possible differences in platelet profiles between 200 mg tedizolid daily and 600 mg linezolid twice daily. Some of the low platelet counts may not directly reflect a pharmacological effect of the drug but rather some artifact caused by the blood sampling technique, shipping of samples from the clinical site and the central laboratory, or measurement techniques used at the central laboratory. However, if such an artifact is introduced, it should be evenly distributed between the two groups of patients through randomization and should be independent of the time of sampling. Additional study is warranted to determine whether observed differences in platelet profiles persist or even worsen with longer treatment.
The potential differential AE profile findings between 200 mg tedizolid once daily for 6 days and 600 mg linezolid twice daily for 10 days are biologically plausible. These changes could be ascribed to the higher total daily dose, the higher systemic free drug exposure, and the longer duration of linezolid treatment. Additionally, the differences in platelet profiles between tedizolid and linezolid may be a function of their differential potential to inhibit mitochondrial protein synthesis. In a preclinical study, tedizolid and linezolid were compared for their abilities to inhibit protein synthesis (incorporation of 35S-methionine) in intact rat heart mitochondria (Edward E. McKee, unpublished data). Tedizolid demonstrated a half-maximal inhibitory concentration (IC50) of ∼0.3 μM, whereas linezolid demonstrated an IC50 of ∼6 μM. When the IC50s for tedizolid and linezolid are viewed in the context of the unbound or free minimum plasma concentration (Cmin) levels observed in patients, there is potential for support of hematologic toxicity differences between tedizolid and linezolid. Tedizolid achieves a free maximum serum concentration (Cmax) of ∼0.3 μg/ml (0.8 μM) and has a half-life of ∼12 h. In a typical 24-h period, this will result in free drug levels below the mitochondrial IC50 for a period of ∼8 h with concentrations never exceeding 2 to 3 times the IC50 (25, 26). In contrast, for linezolid, the free Cmax is ∼15 μg/ml (50 μM), and the half-life is ∼5 h, meaning the free drug levels of linezolid are predicted to exceed the mitochondrial IC50 for the entire 24-h dosing period, never falling below 2 to 3 times the IC50 (25, 26). Therefore, this degree of intracellular exposure to linezolid may not allow adequate mitochondrial inhibition recovery and could contribute to hematologic toxicity.
Several limitations should be noted when these findings are interpreted. The data pertain to phase 3 clinical trial patients, a population that may not reflect the breadth of diversity and risk for reduced platelet counts that might be seen in the general population with ABSSSIs. Although clinical trials provide valuable information on efficacy, the AE profile of patients enrolled in clinical trials might not be fully reflective of the diverse patient populations seen in practice. This is especially true when AEs such as thrombocytopenia are evaluated. As such, the real-world safety profile must be established as the drug is used in clinical practice. As part of these real-world evaluations, attention should be given to studying the hematologic toxicity profile of tedizolid in patients with chronic kidney disease (CKD) because of the increased risk for linezolid-induced thrombocytopenia in this patient population (27–30). For linezolid, this risk for thrombocytopenia might arise from its increased exposure in patients with renal insufficiency (28, 29) The accumulation of the two primary metabolites of linezolid also increases with the severity of renal dysfunction, resulting in metabolite levels 7- to 8-fold higher than those in patients with normal kidney function; however, their involvement in thrombocytopenia remains unclear (31). Because patients with CKD were excluded from tedizolid phase 3 trials, as is frequently the case when evaluating an antibacterial drug for a new indication, little is known about how tedizolid might affect platelet levels in this specific patient population. Tedizolid does not undergo renal elimination, and results from phase 1 studies suggest that its pharmacokinetics and safety profile are not altered in patients with severe renal dysfunction or those requiring long-term hemodialysis (33). Therefore, although tedizolid was approved in such patients, monitoring its potential to cause thrombocytopenia in the CKD population is warranted.
Another consideration is that the duration of tedizolid therapy was limited to 6 days in the phase 3 studies. Although this duration has proved effective and safe in ABSSSI, it is unclear whether the platelet findings observed in the phase 3 studies can be extended to longer durations of therapy. Findings from a 21-day phase 1 study in healthy volunteers suggest that at therapeutic doses, longer durations of tedizolid therapy might have better tolerability in regard to platelet profiles than longer durations of linezolid therapy (24, 34). However, longer durations of therapy must be more extensively studied in a controlled fashion before definitive conclusions regarding the long-term efficacy and safety of tedizolid can be made. We recognize that concerns associated with myelosuppression are not limited solely to platelets. Based on the extremely low frequency of AEs across other cell lineages in the phase 3 studies, we limited this analysis to platelets because this is the most common hematologic toxicity noted with linezolid. Hematologic toxicities other than low platelet count occurred even more rarely and will be reported in full in a pooled report of phase 3 safety and efficacy (A. F. Shorr, T. P. Lodise, G. R. Corey, C. De Anda, E. Fang, A. Das, P. Prokocimer, unpublished data).
The collective findings across ESTABLISH-1 and ESTABLISH-2 suggest that 200 mg tedizolid daily for 6 days is associated with a low proportion of patients with platelet counts below clinically accepted and statistical analysis plan–defined thresholds for toxicity. The clinical data suggest potential differences in the platelet profile between 6 days of 200 mg tedizolid once daily and 10 days of 600 mg linezolid twice daily. The differences in adverse platelet outcomes between tedizolid and linezolid were most pronounced at study days 11 to 13 and the PTE, which makes sense given that linezolid was administered for 4 more days than tedizolid. However, subtle differences in platelet outcomes were noted at study days 7 to 9, a point at which patients in both groups were still receiving active treatment. Although these early changes might suggest a potentially clinically relevant pharmacological difference, additional studies with longer durations of drug administration must be conducted to determine whether this early signal translates into a longer-term benefit of tedizolid compared with that of linezolid on hematologic parameters. Further study is warranted, and use should be closely monitored because real-world experience can differ from findings in the controlled setting of clinical trials.
Supplementary Material
ACKNOWLEDGMENTS
T.P.L. is a Cubist Pharmaceuticals consultant and grant recipient and received financial compensation in connection with the design of the study and interpretation of the results. S.L.M. is a Cubist Pharmaceuticals consultant and was compensated for this work. P.P. is an employee of Cubist Pharmaceuticals. E.F. was previously an employee of Cubist Pharmaceuticals.
Editorial support for the manuscript was provided by ApotheCom ScopeMedical, Yardley, PA, and funded by Cubist Pharmaceuticals, Lexington, MA.
Footnotes
Published ahead of print 22 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03509-14.
REFERENCES
- 1.Shaw KJ, Poppe S, Schaadt R, Brown-Driver V, Finn J, Pillar CM, Shinabarger D, Zurenko G. 2008. In vitro activity of TR-700, the antibacterial moiety of the prodrug TR-701, against linezolid-resistant strains. Antimicrob. Agents Chemother. 52:4442–4447. 10.1128/AAC.00859-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louie A, Liu W, Kulawy R, Drusano GL. 2011. In vivo pharmacodynamics of torezolid phosphate (TR-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains in a mouse thigh infection model. Antimicrob. Agents Chemother. 55:3453–3460. 10.1128/AAC.01565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prokocimer P, Bien P, Deanda C, Pillar CM, Bartizal K. 2012. In vitro activity and microbiological efficacy of tedizolid (TR-700) against Gram-positive clinical isolates from a phase 2 study of oral tedizolid phosphate (TR-701) in patients with complicated skin and skin structure infections. Antimicrob. Agents Chemother. 56:4608–4613. 10.1128/AAC.00458-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke JB, Finn J, Hilgers M, Morales G, Rahawi S, G CK, Picazo JJ, Im W, Shaw KJ, Stein JL. 2010. Structure-activity relationships of diverse oxazolidinones for linezolid-resistant Staphylococcus aureus strains possessing the cfr methyltransferase gene or ribosomal mutations. Antimicrob. Agents Chemother. 54:5337–5343. 10.1128/AAC.00663-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prokocimer P, De Anda C, Fang E, Mehra P, Das A. 2013. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 309:559–569. 10.1001/jama.2013.241. [DOI] [PubMed] [Google Scholar]
- 6.Moran GJ, Fang E, Corey RG, Das AF, De Anda C, Prokocimer P. 2014. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect. Dis. 14:696–705. 10.1016/S1473-3099(14)70737-6. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. 2010. Draft guidance for industry: acute bacterial skin and skin structure infections: developing drugs for treatment. Food and Drug Administration, Silver Spring, MD: http://www.regulations.gov/#!documentDetail;D=FDA-2010-D-0433-0002. [Google Scholar]
- 8.US Food and Drug Administration. 2013. Guidance for industry: acute bacterial skin and skin structure infections: developing drugs for treatment. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/downloads/Drugs/./Guidances/ucm071185.pdf. [Google Scholar]
- 9.Shaw KJ, Barbachyn MR. 2011. The oxazolidinones: past, present, and future. Ann. N. Y. Acad. Sci. 1241:48–70. 10.1111/j.1749-6632.2011.06330.x. [DOI] [PubMed] [Google Scholar]
- 10.Attassi K, Hershberger E, Alam R, Zervos MJ. 2002. Thrombocytopenia associated with linezolid therapy. Clin. Infect. Dis. 34:695–698. 10.1086/338403. [DOI] [PubMed] [Google Scholar]
- 11.Orrick JJ, Johns T, Janelle J, Ramphal R. 2002. Thrombocytopenia secondary to linezolid administration: what is the risk? Clin. Infect. Dis. 35:348–349. 10.1086/341310. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y, Takesue Y, Nakajima K, Ichiki K, Ishihara M, Wada Y, Tsuchida T, Uchino M, Ikeuchi H. 2011. Risk factors associated with the development of thrombocytopenia in patients who received linezolid therapy. J. Infect. Chemother. 17:382–387. 10.1007/s10156-010-0182-1. [DOI] [PubMed] [Google Scholar]
- 13.Pfizer, Inc. 2013. Zyvox package insert. Pfizer Inc., New York, NY. [Google Scholar]
- 14.Wazny LD, Ariano RE. 2000. Evaluation and management of drug-induced thrombocytopenia in the acutely ill patient. Pharmacotherapy 20:292–307. 10.1592/phco.20.4.292.34883. [DOI] [PubMed] [Google Scholar]
- 15.Newland AC. 1995. The problem of thrombocytopenia and its management. Anticancer Drugs 6(Suppl 5):S65–S73. [DOI] [PubMed] [Google Scholar]
- 16.Visentin GP, Liu CY. 2007. Drug-induced thrombocytopenia. Hematol. Oncol. Clin. North Am. 21:685–696, vi. 10.1016/j.hoc.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy JC, Shuman MA, Aster RH. 2004. Quinine/quinidine-induced thrombocytopenia: a great imitator. Arch. Intern. Med. 164:218–220. 10.1001/archinte.164.2.218. [DOI] [PubMed] [Google Scholar]
- 18.Aster RH, Bougie DW. 2007. Drug-induced immune thrombocytopenia. N. Engl. J. Med. 357:580–587. 10.1056/NEJMra066469. [DOI] [PubMed] [Google Scholar]
- 19.Warkentin TE, Roberts RS, Hirsh J, Kelton JG. 2003. An improved definition of immune heparin-induced thrombocytopenia in postoperative orthopedic patients. Arch. Intern. Med. 163:2518–2524. 10.1001/archinte.163.20.2518. [DOI] [PubMed] [Google Scholar]
- 20.Warkentin TE, Greinacher A, Koster A, Lincoff AM, American College of Chest Physicians 2008. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133(Suppl):340S–380S. 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- 21.Patel N, VanDeWall H, Tristani L, Rivera A, Woo B, Dihmess A, Li HK, Smith R, Lodise TP. 2012. A comparative evaluation of adverse platelet outcomes among Veterans' Affairs patients receiving linezolid or vancomycin. J. Antimicrob. Chemother. 67:727–735. 10.1093/jac/dkr522. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health. 2007. Adult toxicity table. National Institutes of Health, Division of Microbiology and Infectious Diseases, Bethesda, MD: http://www.niaid.nih.gov/LabsAndResources/resources/DMIDClinRsrch/Documents/dmidadulttox.pdf. [Google Scholar]
- 23.Prokocimer P, Bien P, Surber J, Mehra P, DeAnda C, Bulitta JB, Corey GR. 2011. Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob. Agents Chemother. 55:583–592. 10.1128/AAC.00076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prokocimer P, Bien P, Muñoz KA, Aster R. 2008. Hematological effects of TR-701, linezolid and placebo administered for 21 days in healthy subjects, poster F1–2069a Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother.-Infect. Dis. Soc. Am. 46th Annu. Meet., Washington, DC. [Google Scholar]
- 25.Flanagan S, Passarell J, Lu Q, Fiedler-Kelly J, Prokocimer P. 2012. Tedizolid population pharmacokinetics, exposure-response, and target attainment, poster A-017c Abstr. 2nd Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Food and Drug Administration. 2014. Briefing document: tedizolid phosphate tablets and injection, p 11 Anti-Infect. Drug Advisory Comm. Meet., 31 March 2014. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM390789.pdf. [Google Scholar]
- 27.Lin Y-H, Wu V-C, Tsai I-J, Ho Y-L, Hwang J-J, Tsau Y-K, Wu C-Y, Wu K-D, Hsueh P-R. 2006. High frequency of linezolid-associated thrombocytopenia among patients with renal insufficiency. Int. J. Antimicrob. Agents 28:345–351. 10.1016/j.ijantimicag.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K, Takeshita A, Ikawa K, Shigemi A, Yaji K, Shimodozono Y, Morikawa N, Takeda Y, Yamada K. 2010. Higher linezolid exposure and higher frequency of thrombocytopenia in patients with renal dysfunction. Int. J. Antimicrob. Agents 36:179–181. 10.1016/j.ijantimicag.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Nukui Y, Hatakeyama S, Okamoto K, Yamamoto T, Hisaka A, Suzuki H, Yata N, Yotsuyanagi H, Moriya K. 2013. High plasma linezolid concentration and impaired renal function affect development of linezolid-induced thrombocytopenia. J. Antimicrob. Chemother. 68:2128–2133. 10.1093/jac/dkt133. [DOI] [PubMed] [Google Scholar]
- 30.Cossu AP, Musu M, Mura P, De Giudici LM, Finco G. 2014. Linezolid-induced thrombocytopenia in impaired renal function: is it time for a dose adjustment? A case report and review of literature. Eur. J. Clin. Pharmacol. 70:23–28. 10.1007/s00228-013-1585-6. [DOI] [PubMed] [Google Scholar]
- 31.Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK, O'Grady M, Hopkins NK, Jungbluth GL. 2003. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob. Agents Chemother. 47:2775–2780. 10.1128/AAC.47.9.2775-2780.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dryden MS. 2011. Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J. Antimicrob. Chemother. 66(Suppl 4):S7–S15. [DOI] [PubMed] [Google Scholar]
- 33.Cubist Pharmaceuticals. 2014. Sivextro (tedizolid phosphate) package insert. Cubist Pharmaceuticals US, Lexington, MA. [Google Scholar]
- 34.Bien P, Prokocimer P, Muñoz KA, Bohn J. 2009. The safety of 21-day multiple ascending oral doses of TR-701, a novel oxazolidinone prodrug antibiotic, poster P1089 Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis., Helsinki, Finland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.