Abstract
The rewarding properties of drugs contribute to the development of abuse and addiction. Here we present a new assay to investigate the motivational properties of ethanol in the genetically tractable model, Drosophila melanogaster. Flies learn to associate cues with ethanol intoxication and, although transiently aversive, the experience leads to a long-lasting attraction for the ethanol-paired cue, implying that intoxication is rewarding. Temporally blocking transmission in dopaminergic neurons revealed that flies require activation of these neurons to express, but not develop, conditioned preference for ethanol-associated cues. Moreover, flies acquire, consolidate, and retrieve these rewarding memories using distinct sets of neurons of the mushroom body. Finally, mutations in scabrous, encoding a fibrinogen-related peptide that regulates Notch signaling, disrupt the formation of memories for ethanol reward. Our results thus establish that Drosophila can be useful in understanding the molecular, genetic and neural mechanisms underling the rewarding properties of ethanol.
The rewarding properties of abused drugs engage neural and molecular mechanisms that have evolved to promote the pursuit of natural rewards. However, drug rewards become overvalued at the expense of other rewards and, unlike natural rewards, do not serve a beneficial homeostatic or reproductive purpose1. Elucidating the mechanisms underlying the intense rewarding properties of abused drugs is therefore critical for understanding reward-seeking behaviors related to addiction. Despite recent progress, much remains to be learned about drug reward at the molecular and cellular level.
Humans and animals rapidly learn cues and contexts that predict the availability of highly rewarding drugs. One of the most popular rodent models for drug reward, conditioned place preference (CPP), measures preference for a cue that was previously associated with a drug2. Here we describe the development and characterization of a CPP-like assay to measure the rewarding properties of ethanol in the genetically tractable model organism Drosophila melanogaster.
Drosophila are remarkably similar to mammals in both their behavioral responses to acute ethanol exposure and the molecular pathways shown to regulate this response, including the cAMP, neuropeptide F (NPY in mammals), epidermal growth-factor (EGF) receptor, and dopamine pathways3. Drosophila also exhibit a natural preference for low, non-intoxicating concentrations of ethanol: they lay their eggs on ethanol-containing substrate, move towards the smell of ethanol, and show a preference for consuming ethanol-containing food3. It has not been shown, however, whether flies experience ethanol intoxication, which is only achieved by unnaturally high doses of ethanol, as rewarding. To investigate this, we developed an assay that assesses choice between two neutral cues following administration of an acute, intoxicating dose of ethanol associated with one of the cues. Thus, similar to CPP models in mammals, our assay ascertains the rewarding properties of ethanol intoxication by measuring preference for an associated cue.
In addition to establishing that flies perceive intoxicating levels of ethanol as rewarding, we investigated whether neurochemical systems important in reward perception in mammals, such as dopamine, play a similar role in flies. By temporally blocking neurotransmission in dopaminergic neurons, we found that dopamine release is required for expression but not acquisition or consolidation of conditioned preference. Moreover, the sequential activation of distinct neuronal subsets of the mushroom body, which is a target of dopaminergic neurons, is required for acquisition, consolidation, and retrieval of memory for ethanol reward.
Finally, in a screen for molecules required for memories of ethanol reward, we identified scabrous (sca), a gene related to mammalian microfibrillar-associated glycoproteins (MPAGs) that regulates the Notch signaling pathway4-7. Although Notch signaling is best known for its multiple roles in development of the nervous system8, its activation also influences structural and functional plasticity in the adult mouse CNS9, long-term spatial memory in mice10 and shock memory in flies11,12. Our results suggest that regulation of Notch signaling by sca may also be important for memories of ethanol reward.
RESULTS
Ethanol is both aversive and rewarding to flies
To establish whether flies experience ethanol intoxication as rewarding, we exposed flies to two attractive odor cues (isoamyl alcohol and an ethyl acetate mixture), one of which was associated with a moderately intoxicating dose of ethanol vapor. We then determined which odor the flies preferred to move towards in a Y-maze (Supplementary Fig. 1a). Although naïve flies had no significant preference for the odors used (Supplementary Fig. 1b), a reciprocal training procedure was used to control for any inherent odor preferences (Fig. 1a). Preference for the ethanol-associated odor was expressed as a conditioned preference index (CPI), where a positive CPI indicates attraction and a negative CPI implies repulsion to the ethanol-paired odor. Control odor tests, ethanol absorption assays performed for each fly strain, and detailed statistical analyses are listed in Supplementary Tables 1-5.
Figure 1. Ethanol is both aversive and rewarding to flies.
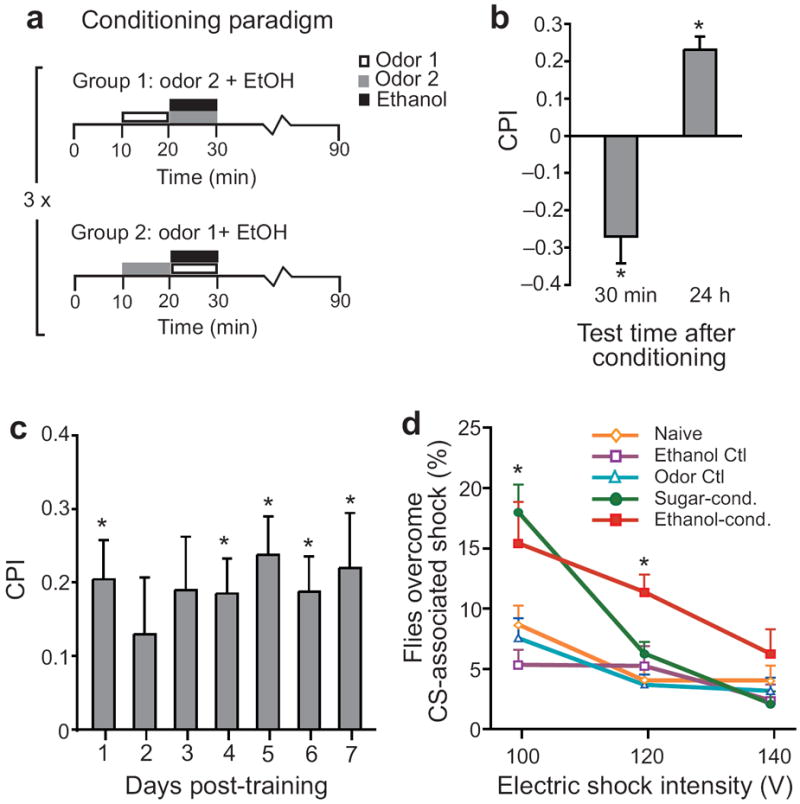
(a) Flies were trained by three spaced training sessions of a 10-min exposure to one odor followed by a 10-min exposure to the second odor paired with 53% ethanol vapor. During the test flies were given the choice of the two odors, and a preference index was calculated by subtracting the number of flies entering the Odor- vial from the Odor+ vial and dividing this number by the total number of flies. Conditioned preference index (CPI) was calculated by averaging the preference indexes of the two reciprocal groups. All values are reported as mean ± s.e.m. (b) Flies showed conditioned aversion when tested 30 min after training (N=8, p=0.006) and conditioned preference 24 hrs after training (N=8, p=0.02) compared to an unpaired control (Wilcoxon 2-sample). (c) Conditioned preference lasted for up to seven days if flies were left undisturbed (Wilcoxon 1-way, N=8, p=0.007 on day 7). (d) Compared to flies that received either odor or ethanol alone, flies conditioned with ethanol or sucrose (Dunnett’s, N=11/group, p=0.05 and p=0.006 respectively) walked over a 2 cm, 100V electric grid to attain the conditioned odor, whereas only flies conditioned with ethanol walked over a 120V electric grid to attain the conditioned odor (p=0.0004). Exposures to either odor alone or ethanol alone (p=0.99 and p=0.87 respectively) did not affect the likelihood to walk over an electric grid.
After an extensive search for ethanol doses and training procedures that produced an optimal conditioned response, we chose a protocol in which flies were given three training sessions spaced by one hour. Each training session consisted of a 10-min exposure to odor 1, followed by a 10-min exposure to odor 2 together with 53% ethanol vapor (and vice-versa; Fig. 1a). We observed conditioned aversion when flies were tested 30 min after training, but conditioned preference 24 hrs later (Fig. 1b). The transition from aversion to preference occurred between 12 and 15 hrs post-training (Supplementary Fig 1c). Remarkably, if the flies were left undisturbed after training, conditioned preference was maintained for at least 7 days (Fig. 1c). These results show that ethanol intoxication, while initially aversive, is associated with a long-lasting expression of reward.
Importantly, conditioned aversion or preference did not result from either repeated odor (Supplementary Fig. 2a) or ethanol exposure (Supplementary Fig. 2b) alone, or from repeated trials in which odors were presented 20 min following ethanol exposure (Supplementary Figs. 2c,d). Thus, both aversion and preference are contingent on the temporal association between odor and ethanol exposure.
Flies overcome a shock barrier to attain conditioned cue
A hallmark of addiction is continued drug use despite negative consequences. To examine if our assay could be used to test addiction-like behavior, we asked if flies would overcome an aversive stimulus to gain access to the odor previously associated with ethanol. Indeed, conditioned flies showed reduced aversion to both 100 V and 120 V electric shocks; an effect not observed in flies exposed to either odor or ethanol alone (Fig. 1d, Supplementary Fig. 3a).
We also trained flies to associate odors with sucrose (Fig. 1d, Supplementary Fig. 3b), a substance known to be rewarding to flies. Sucrose-conditioned flies showed reduced aversion to the 100 V but not the 120 V grid (Fig. 1d). Thus, flies tolerated punishment (electrical shock) in order to approach an odor cue predictive of ethanol or sucrose reward. Intriguingly however, ethanol-conditioned flies tolerated a larger electric shock than sucrose-conditioned flies.
Pharmacological properties of ethanol induce preference
Although previous studies have shown that flies display innate preference for low concentrations of ethanol, such as those found in fermenting fruit3 it is unlikely that flies become intoxicated under these conditions. In our assay, by contrast, we aimed to use a moderately intoxicating concentration of ethanol that would elicit hyperactivity, a behavior thought to model the disinhibiting and euphoric effects of ethanol in humans13.
To determine whether conditioned odor preference could be attributed to the pharmacological effects of ethanol, we first measured internal ethanol levels in conditioned flies. Indeed, extracts of conditioned flies contained significant levels of ethanol (Fig 2a, Supplementary Fig. 3c), which were sufficient to stimulate locomotion (Fig. 2b). We next asked if flies needed to be “under the influence” in order to form associations with odors by delivering the odor either immediately before (Fig. 2c) or after (Figs. 2d) the ethanol exposure. We found that conditioned preference was elicited only when the odor was presented immediately after the ethanol exposure, when flies still contained significant levels of ethanol (Fig. 2d). Thus, the formation of conditioned preference required the temporal coincidence of internal ethanol levels and the odor cue.
Figure 2. Pharmacological properties of ethanol induce preference.
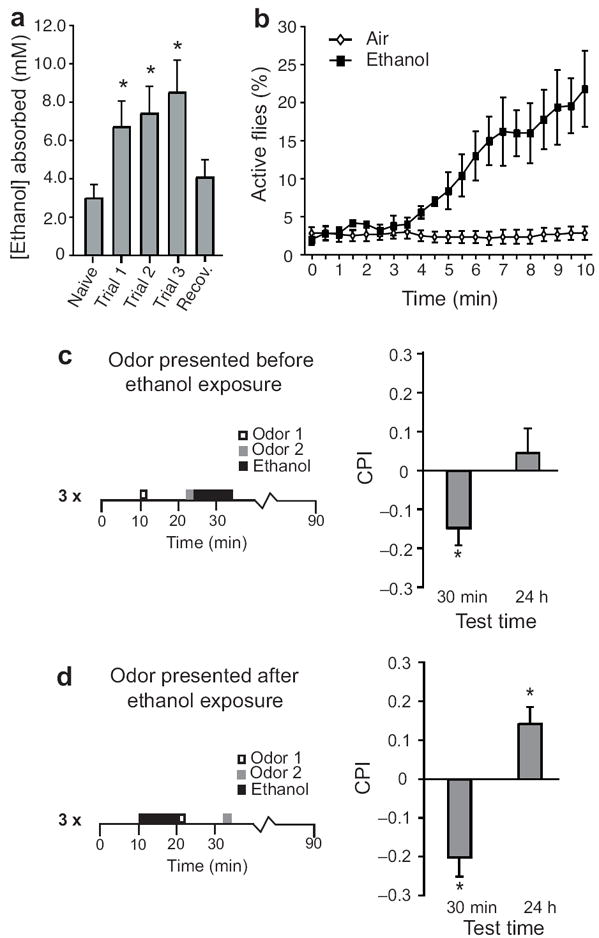
(a) Flies absorbed significant amounts of ethanol during conditioning (Student’s-t post-hoc, N=11/group, p=0.04, p=0.02, p=0.004 for trials 1, 2, and 3 respectively) and recovered within 30 min (p=0.54). (b) Ethanol absorbed during training induced a significant increase in locomotor activity characteristic of acute intoxication (Repeated Measures ANOVA, N=11/group, p=0.002). (c) An odor presented prior to ethanol resulted in significant conditioned aversion (Wilcoxon 1-way, N=8/group, p=0.007) but not conditioned preference (p=1.00) suggesting that an odor can predict onset of the aversive effects of ethanol. (d) An odor presented directly after ethanol resulted in significant conditioned aversion and significant conditioned preference (Wilcoxon 1-way, N=8, p=0.007 for both behaviors) suggesting that ethanol intoxication is required for conditioned preference to form. All data are shown as mean ± s.e.m.
Dopamine is required for expression of ethanol reward
In mammals, investigations using diverse methods have converged on the conclusion that natural rewards and addictive drugs alike influence behavior as a result of their ability to increase synaptic dopamine in the nucleus accumbens1,14. Although dopamine plays a role in the acute locomotor effects of cocaine and ethanol in flies15,16, it is unknown whether it also plays a role in ethanol reward. We therefore blocked dopaminergic neurotransmission with spatial and temporal resolution in behaving flies to investigate a potential role for dopamine in the aversive and rewarding effects of ethanol. To do this, we expressed the dominant-negative and temperature-sensitive variant of dynamin, Shibire temperature-sensitive (Shits), in dopaminergic neurons using the GAL4-UAS binary expression system17. At temperatures at or above 29°C, Shits rapidly impairs synaptic transmission in targeted neurons by inhibiting neurotransmitter vesicle recycling17. We silenced dopaminergic neurons using two different GAL4 drivers: one expressed under the control of the tyrosine hydroxylase promotor (TH-GAL4, expressed in most dopaminergic neurons18) and one under the control of dopamine decarboxylase (Ddc-GAL4, expressed in most dopaminergic and serotonergic neurons19). Perturbing neurotransmission in Ddc– or TH– expressing neurons using UAS-shits at the restrictive temperature (30°C) during both training and testing did not affect conditioned aversion (Fig. 3a), but blocked the formation of conditioned preference (Fig. 3b). No effects were observed at the permissive temperature (24°C) (Supplementary Figs. 4a,b). Note that testing flies at 29°C compared to 24°C generally increased their conditioned responses (Figs. 3a,b, Supplementary Figs. 4a,b), possibly due to enhanced locomotion or stronger perception of the training odors. These results indicate that distinct neuronal populations mediate conditioned aversion and preference for ethanol, where activity of dopaminergic neurons is required for flies to form and/or express the association between the attractive properties of ethanol and an odor, but not required for flies to form aversive memories.
Figure 3. Dopamine is required for conditioned preference.
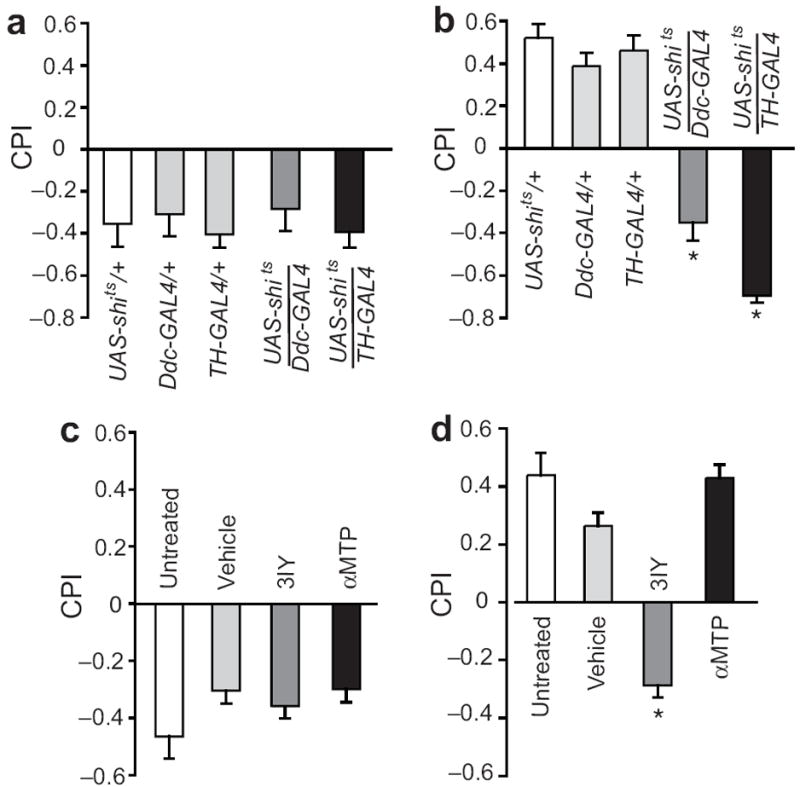
(a) Blocking synaptic transmission in dopaminergic neurons during both training and testing did not affect conditioned aversion tested 30 min after training (Kruskal-Wallis, N=8/group, p=0.84), but (b) blocked the formation of preference in both TH− and Ddc–expressing neurons tested 24 hrs later (Wilcoxon 1-way, N=8, p=0.0003 and p=0.007, respectively). (c) Conditioned aversion was not affected by decreasing serotonin levels in the brain using αMTP or dopamine levels using 3IY (Kruskal-Wallis, N=8/group, p=0.07). (d) Conditioned preference was not affected by αMTP (Student’s-t post-hoc, N=8/group, p=0.21) but was blocked by decreasing dopamine levels in the brain using 3IY (p=0.0002). All data are shown as mean ± s.e.m.
Serotonin has known roles in formation of memories of cues associated with abused drugs, and in modulating the behavioral effects induced by these drugs20. To distinguish between the contributions of dopamine and serotonin neurons that express Ddc-GAL4, we tested conditioned preference in flies that had been fed the TH inhibitor 3-iodo-tyrosine (3IY) or the serotonin synthesis inhibitor alpha-methoxytryptophan (∝MTP). As expected, 3IY and ∝MTP substantially reduced dopamine and serotonin levels, respectively, in the flies’ brain (Supplementary Figs. 4c,d). Neither drug had an effect on conditioned aversion (Fig. 3c). However, 3IY, but not ∝MTP, blocked the formation of conditioned preference (Fig. 3d), thus supporting a role for dopamine in the memory of the attractive properties of ethanol.
In mammals, drugs of abuse, including ethanol, amplify dopaminergic responses to natural rewards and reward-related environmental cues1,14. Consequently, reward-related cues become associated with the reinforcing effects induced by dopamine release in specific brain regions. To test whether activity of dopaminergic neurons was required during acquisition of conditioned preference, we used UAS-shits to impair transmission of TH-expressing neurons with temporal specificity. We shifted the temperature such that neural activity was silenced only during training, when ethanol was presented simultaneously with the odor cue (Fig. 4a). Intriguingly, we found that this manipulation had no effect on the development of conditioned preference (Fig. 4a). This result suggested that dopaminergic neurons might be recruited into the relevant neural circuit during the processes of memory consolidation and/or retrieval. We therefore suppressed neurotransmission in the 24 hrs between training and testing (presumed consolidation phase) or during preference testing (retrieval phase). While activity of dopaminergic neurons during consolidation was dispensable for conditioned preference to form (Fig. 4b), their activity was essential for expression of the memory (Fig. 4c).
Figure 4. Dopamine is required for expression of ethanol reward.

Transiently blocking neurotransmission of TH-expressing cells during (a) acquisition or (b) consolidation did not affect conditioned preference (Kruskal-Wallis N=8/group, p=0.06 and p=0.27, respectively). (c) Activity of TH-expressing cells was required for the retrieval or expression of conditioned preference (Kruskal-Wallis N=8/group, p=0.0005). All data are shown as mean ± s.e.m.
The mushroom body is required for ethanol reward memory
In mammals, discrete brain regions mediate the conditioned response to drug reward including the nucleus accumbens, prefrontal cortex, amygdala, and dorsal striatum1. In Drosophila, the regions of the brain mediating ethanol preference or reward are unknown. However, it is well established that discrete subsets of neurons within the fly mushroom body play a role in odor memory processing, including the conditioned response to sugar reward21. In addition, dopaminergic neurons known to play a role in modulating memories for sucrose reward project to the mushroom body22. Since the mushroom body is important for memories of sugar reward, and dopamine is required for expression of memories of ethanol reward, we hypothesized that the mushroom body may be required for conditioned preference.
The mushroom body consists of three major classes of neurons whose axonal branches occupy distinct subsets of lobes: the αβ, α’β’, and γ neurons. We investigated the role of different mushroom body neurons in the conditioned response to ethanol by suppressing synaptic transmission using UAS-shits in combination with a series of mushroom body GAL4 drivers (Fig. 5a, Supplementary Figs 5a-f). Impairing neurotransmission during training and testing in the entire mushroom body (OK107, Supplementary Fig. 5b), in a combination of γ and αβ neurons (MB247 and 201Y, Supplementary Fig. 5c,d), or in just αβ neurons (5-66a, Supplementary Fig. 5e) disrupted both conditioned aversion (Fig. 5b) and preference (Fig. 5c). There were no differences in conditioned responses in these groups at the permissive temperature with the exception of the highly expressed OK107 driver (Supplementary Figs. 5g,h), an effect that is likely due to low levels of expression at the permissive temperature. In addition, olfactory control tests showed that manipulating mushroom body neurotransmission did not significantly impair odor attraction (Supplementary Table 1). Intriguingly, the odor acuity showed more within-group variation than conditioned preference (Supplementary Table 1), suggesting that it may be sensitive to small fluctuations in ambient humidity, temperature, and fly activity.
Figure 5. The mushroom body is required for aversion and preference.

(a) Schematic of the subsets of mushroom body neurons: yellow = γ neurons, blue = αβ neurons, red = α’β’ neurons. (b) We transiently inactivated neurotransmission in selected sets of mushroom body neurons using the GAL4 drivers OK107, 201Y, MB247, 5-66a and 4-59. Blocking synaptic transmission of specific mushroom body neurons using the GAL4 drivers OK107 (Student’s-t post-hoc, N=8/group, p<0.0001), 201Y (p=0.003), MB247 (p<0.0001) and 5-66a (p=0.01) during training and testing disrupted conditioned aversion tested 30 min after training. Colored circles represent mushroom body neurons in which GAL4 drivers are expressed as defined above. (c) Inactivation of mushroom body using OK107 (p<0.0001), 201Y (p=0.003), MB247 (p<0.0001) and 5-66a (p=0.01) during both training and test disrupted conditioned preference 24 hrs after training. All data are shown as mean ± s.e.m.
To determine when each subset of mushroom body neurons was required for memories of ethanol reward, we blocked transmission specifically during acquisition, consolidation, or retrieval of conditioned preference. We found that silencing a combination of γ and αβ neurons (OK107, MB247, 201Y) during acquisition blocked conditioned preference (Fig. 6a). Because acquisition was not affected by silencing of αβ neurons alone (5-66a), we infer that the γ neurons rather than αβ neurons are important for acquisition (Fig. 6a, Supplementary Fig. 5a-e). In contrast, silencing the α’β’ neurons (OK107, 4-59) during consolidation or the αβ neurons (5-66a) during testing blocked conditioned preference (Figs. 6b,c). Thus, the function of different subsets of mushroom body neurons is needed for the distinct phases involved in forming conditioned preference. Our results thus reveal sequential use of the γ, α’β’, and then αβ neurons. Since our data show that neurotransmission of both dopaminergic and αβ neurons specifically affected conditioned preference expression, we hypothesize that the manifestation of ethanol reward memory may be mediated by dopaminergic innervation of the αβ neurons. This is in line with recent studies showing that dopaminergic innervation of the α lobe plays a role in conditioned response to shock and the effect of satiety on the conditioned response to sucrose22,23.
Figure 6. Sequential use of mushroom body neurons.
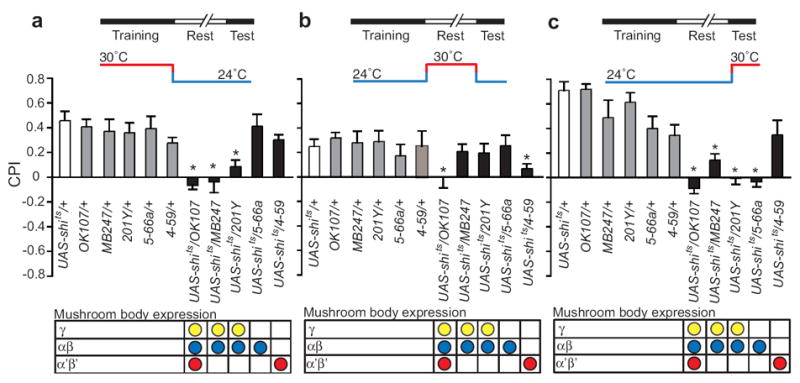
(a) Inactivation using drivers OK107 (Student’s-t post-hoc, N=8/group p<0.0001), 201Y (p<0.0001) and MB247 (p=0.006) but not 5-66a during training disrupted acquisition, implicating the γ neurons in acquisition of conditioned preference. (b) Inactivation using drivers OK107 (p=0.004), and 4-59 (p=0.02) disrupted stabilization, implicating the α’β’ neurons in consolidation of conditioned preference. (c) Inactivation using drivers OK107 (p<0.0001), 201Y (p=0.003), MB247 (p<0.0001) and 5-66a (p<0.0001) disrupted retrieval, implicating the αβ neurons in retrieval or expression of conditioned preference. All data are shown as mean ± s.e.m.
scabrous is required for ethanol reward memory
Since we showed that activity of mushroom body neurons was required for the memory of ethanol reward, we were interested in identifying genes that may act in the mushroom body to regulate these memories. To investigate this, we tested memory for ethanol reward 24 hrs after training in 160 strains with GAL4 reporter expression in the mushroom body. We found 3 mutations that resulted in persistent conditioned aversion, 54 mutations that resulted in lack of conditioned preference, and 3 mutations that resulted in enhanced conditioned preference (Fig. 7a).
Figure 7. scabrous (sca) affects memories for ethanol reward.

(a) A screen for conditioned ethanol preference of 160 P{GawB}-containing strains with known expression in the mushroom body identified 3 mutations in which conditioned aversion persisted 24 hrs after training (magenta), 54 mutations in which conditioned preference was not expressed (orange), and 3 mutations in which conditioned preference was enhanced (green). Values represent mean (N=8 per strain). (b) In the 5-120 mutant, the P{GawB} element was inserted 125 bp 5’ of exon 1 of scabrous. (c) qPCR showed that the 5-120 mutation decreased scabrous mRNA expression to 55% that of wild-type controls (mean ± s.e.m., N=6 independent samples). (d) – (g) CPI values represent mean ± s.e.m. (d) sca5-120 does not affect conditioned aversion for ethanol 30 min after training. (e) sca5-120 affects conditioned preference for ethanol 24 hrs after training. (f) Complementation analysis of conditioned preference 30 min after training with two independent sca alleles confirms that sca does not affect conditioned aversion. (g) sca5-120 fails to complement the sca1 and scaBP2 alleles for 24 hr conditioned preference. (h) The 5-120 GAL4 expression pattern suggests that sca is expressed in the mushroom body αβ and γ neurons, the antennal lobe (AL), eye, and a number of cell bodies near the ventrolateral protocerebrum and subesophageal ganglia (See also Supplementary Figure 7).
Of particular interest were the three mutants in which conditioned aversion persisted, since this phenotype resembled what was observed when dopaminergic neurotransmission was blocked. The mutant with the most severe phenotype, 5-120, carries a P{GawB} (GAL4-containing P-element) insertion 125 bp 5’ of exon 1 of scabrous (sca)24 (Fig 7b). Quantitative RT-PCR showed that the 5-120 mutation significantly decreased sca expression to 55% that of wild-type controls (Fig 7c). Although sca5-120 mutants show conditioned aversion to ethanol when tested 30 min after training (Fig. 7c), they failed to switch from conditioned aversion to preference (Fig. 7d). sca5-120 mutants show normal sensitivity to acute ethanol exposure, place-memory performance, and olfactory-shock memory performance24, indicating that their failure to form associations between odorants and the rewarding effects of ethanol is not a result of a general inability to learn. sca5-120 mutants also showed normal olfactory acuity to the odorants used in this assay (Table 5), and did not show any gross morphological abnormalities of the mushroom body (Supplementary Fig. 6). An attempt to rescue the sca5-120 phenotype by over-expressing UAS-sca in sca5-120 mutant flies resulted in lethality. However, complementation analysis with two independent amorphic sca alleles25, sca1 and scaBP2, revealed that both failed to complement the behavioral deficit of sca5-120 (Fig 7f,g). This confirmed that disruption of sca results in the failure to switch from conditioned aversion to conditioned preference. Expression of UAS-GFP in the 5-120-GAL4 pattern suggests that in the adult brain, sca is expressed in the mushroom body αβ and γ neurons, antennal lobe, eye, and a number of cell bodies near the ventrolateral protocerebrum and subesophageal ganglia (Fig 7h, Supplementary Fig. 7).
DISCUSSION
Here we provide evidence that ethanol intoxication is rewarding to flies, map this response to a discrete location in the fly brain, and describe a novel gene important for the formation of reward memory. Our model reveals that, like mammals, flies (1) show both conditioned aversion and preference to ethanol, (2) show long-lasting preference for a cue associated with ethanol intoxication, (3) will overcome an aversive stimulus to obtain a cue associated with ethanol, (4) use dopaminergic systems to express memories for a cue associated with ethanol intoxication, and (5) rely on sequential use of distinct brain circuits to acquire, consolidate, and express memories of reward. These similarities suggest that the neural and molecular pathways mediating ethanol’s rewarding properties are evolutionarily conserved.
A model for ethanol reward in Drosophila
Previous studies using Drosophila to model alcohol-related behaviors have concentrated either on the acute locomotor stimulatory and sedative effects of ethanol, or preference for low concentrations of alcohol3. Our work shows that flies develop long-lasting preference for an intoxication-associated cue, and that ethanol is rewarding due to its pharmacological properties. This strengthens a previous study showing that flies will develop preference for ethanol consumption even in the presence of an aversive tastant26. In addition, because the concentrations of ethanol required for conditioned preference to form are considerably higher than what Drosophila would encounter in nature, it is unlikely that our assay measures an innate adaptive attraction to ethanol that is specific to fruit flies.
Importantly, the conditioned preference we investigate here focuses specifically on the acute rewarding properties of ethanol. Our hope is that this assay will spur the development of more complex tests that model more advanced stages in the addiction cycle, such as withdrawal after chronic exposure and reinstatement of drug seeking after abstinence.
Conditioned aversion to conditioned preference
We show that the preference for a cue associated with ethanol spontaneously switches from negative (aversion) to positive (attraction) 12-15 hrs after conditioning. Opposing behavioral responses to an identical dose of a drug of abuse are surprisingly common. For example, acute exposure to nicotine in rats results in short-term conditioned place aversion that switches to longer-lasting conditioned place preference27. These opposing responses may be due to conflicting responses to the sensory and pharmacological effects of the drug. For example, infant rats exhibit aversive learning to ethanol’s orosensory effects, but show positive reinforcement to its post-ingestive effects28. We speculate that the initial aversive properties of ethanol in flies are not dependent on its intoxicating effects, but rather are a result of a negative physiological reaction or sensory response, similar to that observed in rats.
It is also possible that in addition to an associative process, aversion may also be dependent on non-associative mechanisms such as sensitization. This is supported by the observation that flies still develop conditioned aversion when an odor is presented immediately before ethanol exposure. Future genetic and neuroanatomical studies should allow us to clearly discern whether conditioned aversion and preference are interdependent or parallel processes.
Dopamine and ethanol reward
Our finding that blocking dopaminergic systems resulted in lack of conditioned preference concurs with data from a large body of existing literature demonstrating the requirement for dopamine in reward. Despite extensive investigation on the role of dopamine in reward however, surprisingly little is known about the dopaminergic mechanisms mediating ethanol reward. In fact, much of the evidence investigating the role of dopamine in ethanol reward in mammals is conflicting. We speculate that much of the variation between results of these studies potentially derives from compensatory mechanisms that occur throughout development in mutant animals, and non-specific or ineffective pharmacological manipulations. The genetic tools available in Drosophila allow us to avoid these caveats by permitting precise temporal control over neurotransmission of dopaminergic cells. Our data is consistent with the findings that mice lacking the D2 receptor29 or DARPP-3230 show decreased CPP, and that both fluphenazine administration31 and dopamine receptor blockade32 prevent expression of memory for ethanol reward. This suggests a conserved role for dopamine in expression of conditioned preference in flies and mice.
Our result that dopamine was involved specifically in expression of ethanol reward can be interpreted in two main ways: first, that dopamine is required for retrieval of the conditioned odor-ethanol association, and second, that dopamine is involved with the conditioned motivational effects of alcohol cues without affecting cue memory retrieval. Although our results cannot directly distinguish between these hypotheses, they are consistent with the prediction-error hypothesis that supports involvement of dopamine in the motivational effects of alcohol predictive cues. In mammals, it has been proposed that dopamine is released when the actual value of a reward differs from its predicted value14,33. We found that activation of dopamine systems is required only when the conditioned odors are presented in the absence of the ethanol reward: i.e., when the flies may expect ethanol yet receive only odor cues. We therefore speculate that dopamine may be involved in the assessment of differences in actual and predicted reward in flies.
Dissociating mechanisms underlying natural and drug reward
The molecular and cellular mechanisms that lead to compulsive drug use are believed to be similar to those involved normally in reward-related learning1. We found that although some of the neural substrates underlying both are conserved in flies, such as the mushroom body αβ neurons, there are distinct mechanisms that underlie the motivational properties of ethanol and sucrose as rewards. For example, whereas blocking output of dopaminergic neurotransmission does not directly affect 3 min memory for sucrose34, it disrupts 24 hr memory for ethanol reward. Intriguingly however, blocking output of the PPL1 cluster of TH+ neurons enhances 3 hr memory for sucrose reward in satiated flies22. This suggests that the neural substrates for memory of ethanol reward may overlap with the neural substrates that modify, but are not required for, memory of sucrose reward.
Comparisons between these studies need to be interpreted with caution, however, since the odors used and time of test differed for each of these experiments. Our data however, in combination with other evidence showing the dopamine receptor dDA1 mediates formation of memories associated with sucrose reward in Drosophila35 suggests that dopamine plays a more complex role in mediating reward in the fly than previously suggested.
Sequential use of mushroom body neurons
Experiments conducted with flies defective in learning and memory have led to the prevailing model in which mushroom body neurons associate the odor conditioned stimulus with a shock or sugar unconditioned stimulus using potential coincidence detecting molecules, such as the adenylyl cyclase rutabaga36, and store the associations within the specific neurons that are activated by a particular odor37,38,. Like previous studies, we found that the αβ and α’β’ neurons were required for retrieval and stabilization of memory, respectively21,37,38. Our results differ from previous work on memory that use pairings of aversive odors and electric shock, since we found a potential role for γ neurons in acquisition of the memory for ethanol reward. These results are intriguing and suggest that either the odors we employed (which are attractive) and/or the ethanol stimulus require γ neuron activation. Indeed, some combination of γ and/or αβ neuron activation is required for the acute locomotor stimulatory effects of ethanol39, suggesting that the acute response to the ethanol stimulus requires mushroom body neuron activation. Our results, however, must be interpreted with caution since we did not use a γ neuron-specific driver. It is possible that acquisition of memory may require non-overlapping subsets of αβ neurons defined by the 5-66a, 201Y, and MB247 GAL4 drivers.
Our results also differ from recent work describing a role for the α’β’ neurons in acquisition of memory for both aversive (shock) and appetitive (sucrose) conditioning21. This model suggests that olfactory information received from the second-order olfactory projection neurons is first processed in parallel by the αβ and α’β’ neurons during acquisition21. Subsequently, memories are stored in αβ neurons, whose activity is required during recall21. Our data suggests that for ethanol reward memories, olfactory information received from the second-order olfactory projection neurons is first processed in parallel by the αβ and γ neurons prior to activity of α’β’ neurons establishing consolidation of memory in the αβ neurons. Then, like memories for shock and sucrose, memories for ethanol reward are stored in αβ neurons, whose activity is required during recall.
An important difference between these studies is the time at which memory was tested. Shock-memory was tested 3, 5 or 30 min after training37,38, sucrose memory was tested 3 hrs after training21, and ethanol memory was tested 24 hrs after training. It is possible that output of distinct subsets of mushroom body neurons is required for different stages of memory. For example, recent work suggests that animals that receive multiple spaced odor-shock pairings form a memory trace in the αβ neurons between 9 and 24 hrs after training, and in the γ neurons between 18 and 48 hrs after training40.
scabrous is required for ethanol reward memory
Because activation of the mushroom body was required for the development of conditioned preference for ethanol, we assayed a collection of fly strains with known expression in the mushroom body to search for novel genes involved in ethanol reward. We show that sca is required for the formation of memories of ethanol reward. sca is a fibrinogen-related secreted peptide that plays a role in neurogenesis, spacing differentiation and boundary formation via a Notch (N)-dependent pathway4,5. During development, Sca associates with N, and can stabilize N protein at the cell surface, thus sharpening proneural cluster boundaries and ensuring the establishment of single pioneer neurons5.
Notch is required for memory formation in both mice and flies. Mice heterozygous for Notch1 and the downstream cofactor RBP-J show deficits in spatial learning and memory10. Temperature-sensitive Notch mutant flies show deficits in 24 hr memory for an odor associated with shock11,12 and RNAi-mediated Notch silencing in the MB reduces this memory12. We speculate that sca may regulate Notch signaling to mediate memories of ethanol reward. This may occur in the mushroom body αβ neurons, where 5-120-GAL4 is expressed, and which are required for long-term memory41.
Mammalian microfibril-associated glycoprotein (MAGP) shows peptide homology to sca and interacts with the Notch signaling pathway via direct interactions with the Notch1 receptor, as well as the Jagged1, Jagged2, and Dll-1 Notch ligands6,7. This suggests potential conservation of sca – Notch interactions between flies and mammals. It would be intriguing to investigate whether MAGP mediates memory of reward in mammals as it does in flies.
METHODS
Strains
Flies were grown and maintained on standard cornmeal/molasses/yeast/agar media at 25°C and 70% humidity on a 12:12 L:D cycle with lights-on at 9:00 AM. Behavioral characterization was performed on wild-type Canton-S (CS) flies recently isogenized for the 2nd and 3rd chromosomes. All GAL4 and UAS lines42 were backcrossed for at least 5 generations to a w-Berlin strain, recently isogenized for the 2nd and 3rd chromosome. No significant differences were found between CS and Berlin backgrounds in conditioned preference (Wilcoxon two-sample: 30 min memory Z(1,16)=-0.05, p=0.96, 24 hr memory Z(1,16)=-0.17, p=0.86). Lines 4-59 and 5-66a were obtained from our P[GAL4GawB] screen collection (see Supplementary Fig. 5b,c for expression). UAS-shi was obtained from S. Sweeney. sca1 and scaBP2 were obtained from Bloomington Stock Center and UAS-sca from N. Baker. The screen was carried out using 160 P[GAL4] homozygous viable strains in a w- Berlin genetic background (carrying the GawB element)42 with previously characterized expression in the mushroom body. 5-120 was backcrossed for 5 generations onto a newly isogenized w- Berlin background and re-tested.
Conditioned Preference for an Odor Associated with Ethanol
Groups of 50 male flies were collected 0-1 days after eclosion and trained at 3-5 days of age. Flies were trained in vials (2.5 cm diameter and 9.5 cm height) containing 1% agar. Vials contained 64 evenly-spaced perforations and a mesh lid to allow even distribution of ethanol. Vials were placed into a holder in a 30 cm length × 15 cm height × 15 cm width training chamber. The training chamber (Aladin Enterprises, Inc., San Francisco, CA) had three nozzles to allow for air/odorants/ethanol to stream in and one nozzle for waste to stream out.
Humidified air was bubbled through 95% ethanol to vaporize ethanol with combined flow rate of 80 U vaporized ethanol and 70 U humidified air (where 100 U is equal to 1.7 L/min at room temperature). Humidified air was streamed over odors placed in a 2.5 cm diameter and 13 cm height cylinder at a flow rate of 130 U for training and 100 U for tests. Odors used were 3 mL iso-amyl alcohol (1:36 in mineral oil) and a mixture of 2 mL ethyl acetate (1:36 in mineral oil) and 1 mL acetic acid (1:400 in mineral oil). Odors were replaced daily.
Reciprocal training was performed to ensure that inherent preference for either odor did not affect conditioning. Training consisted of 3 repetitions (spaced by 50 min) of 10 min of odor 1, then 10 min odor 2 plus ethanol. A separate group of flies was simultaneously trained with 10 min odor 2, then 10 min odor 1 plus ethanol. Vials of flies from Group 1 and Group 2 were paired according to placement in the training chamber and tested simultaneously.
The testing chamber was a 6 cm cube with a mesh Y-maze in the middle (Aladin Enterprises, Inc., San Francisco, CA). Odors were streamed in through opposite arms of the Y (each 6 cm). Vials of flies were placed at the lower Y arm and flies climbed up the mesh cylinder and chose between opposing arms of the Y to 2.5 cm diameter, 9.5 cm height vials. After 2 min, vials were removed and capped. The number of flies that moved into the odor 1 and odor 2 vials were counted. A preference index for the odor paired with ethanol was calculated as (# flies in paired odor vial - # flies in unpaired odor vial) / total # flies. A performance index for conditioned odor preference or aversion (CPI) was calculated by averaging the preference indexes for reciprocally trained groups of flies.
Memory was tested 30 min or 24 hrs post-training. Immediately after training, yeast was added to the training vials to ensure flies did not become food deprived prior to test. For experiments lasting several days, flies were trained on food containing 10 g yeast, 10 g sugar and 4 g agar boiled in 200 mL water. All training and tests were performed in a dark room under red light. The temperature was controlled with an oil-filled radiator (DeLonghi TRD0715T, Dubuque, IA) and humidity controlled with a warm-mist humidifier (Vicks V745A, Proctor & Gamble, San Ramon, CA).
Odor controls
Odor controls were performed exactly as the test described above, except that instead of choosing between two different odors, flies chose between each single odor and air streamed through mineral oil. Preference index was calculated by (# flies in odor vial - # flies in air vial) / total # flies. Odor control data for all UAS-GAL4 experiments is presented in Supplementary Table 1 and for all sca-related experiments is presented in Supplementary Table 5.
Sucrose Conditioning
Groups of 50 4-5 day old males were trained using odor training boxes described in the conditioned preference protocol above, and tested in the Y-maze apparatus described above. Flies were food deprived for 16-20 hrs prior to training. Reciprocal training was performed to ensure that inherent preference for either odor did not affect conditioning scored. Training consisted of 10 min habituation to the training chamber with air (flow rate 130), 10 min presentation of odor 1 with plain filter paper pre-soaked in water and dried, then 10 min of odor 2 with filter paper pre-soaked in 2M sucrose and dried. Simultaneously, a separate group of flies was trained using odor 1 as the sucrose-paired odor. Vials of flies from Group 1 and Group 2 were paired according to placement in the training chamber and tested simultaneously 10 min following training. Preference and performance indices for conditioned sucrose preference were calculated in the same way as for conditioned ethanol preference (described above).
Electric shock
Electric shock was delivered via a Grass S88 Stimulator (Grass Technologies, West Warwick, RI) to a small cylindrical copper grid 2 cm in height and 2.5 cm in diameter placed at the opening of the test vial from which the odor previously associated with ethanol or sucrose flowed. Electric shocks were applied every 50 msec for 1 sec each throughout the test. The % flies willing to overcome shock was measured by calculating percent of flies within each vial that walked over shock to obtain the ethanol- or sucrose-associated odor. The percentage of flies from one reciprocal pair of vials was averaged for each N=1.
Ethanol Concentrations
Fly internal ethanol concentrations were determined from whole-fly homogenates of 50 flies per sample. Flies were exposed to vaporized ethanol or air as outlined in training protocol. Flies were frozen immediately in liquid nitrogen and stored at -80°C. Flies were homogenized in 500 μL of cold 50 mM Tris-HCl (pH 7.5, Sigma, St. Louise, MO) and the homogenate was centrifuged at 14 g for 20 minutes at 4°C. Ethanol concentrations in supernatants were measured using an alcohol dehydrogenase-based spectrophotometric assay (Ethanol Assay Kit#229-29, Diagnostic Chemicals Limited, P.E.I., Canada). To calculate fly internal ethanol concentration, the volume of 1 fly was estimated to be ~2 μL43. Ethanol concentration control data is presented in Supplementary Table 2.
Pharmacology
Pharmacological treatment with 3-iodo-tyrosine (3IY) (Sigma, St. Louise, MO) and alpha-methoxytryptophan ∝MTP (Sigma, St. Louis, MO) was carried out as described previously15. 10 mg/mL 3IY or 20 mM ∝MTP were dissolved in a heated aqueous 5% sucrose, 5% yeast solution. 2 mL of cooled drug solution was added to a Kimwipe in a training vial. 1 day old males were kept in these vials for 40–48 h at 25°C and 70% relative humidity prior to training.
High Performance Liquid Chromatography
HPLC was performed as published previously44 on a Jasco model PU-2080 isocratic pump (Jasco Inc., Easton, MD) with changes as outlined below. Four replicates of 10 brains/sample were run for each condition. Ten 3-day-old adult Drosophila brains were hand dissected on dry ice between 1 and 3 pm (during the middle of the light phase) with as much precision as possible to remove all trace of eye pigmentation. Brains were placed directly into 50 μL of ice-cold 50 mM citrate acetate, pH 4.5, and quickly homogenized with a Teflon pestle. Homogenates were run through a 0.22 μm spin filter (Millipore Corporation, Bedford, MA) and frozen at -80°C. The typical injection volume was 10 μL, or one brain equivalent. Standard mixes of dopamine, and serotonin (Sigma–Aldrich, St. Louis, MO) were injected at a concentration of 10 ng/mL and a calibration curve was generated based on injections containing 1, 5, 10, 50 and 100 pg.
Ethanol Hyperactivity
Male flies (n=50) were transferred to perforated vials containing 1% agar capped with a mesh lid as described previously. The flies were allowed to habituate to the environment 15 min prior to exposure to air or 53% ethanol vapor for 10 min under light conditions. Activity was filmed using a Sony HDR-SR12 Digital HD Video Camera Recorder (Sony Corporation, Japan). Recorded activity was hand scored as the percent of flies showing active locomotion during each 30-sec interval for 10 min.
Immunohistochemistry and Imaging
4-day old adult male brains were dissected in a phosphate-buffered saline solution (PBS) and fixed for 45 min at room temperature with 4% formaldehyde (Ted Pella, Inc, Redding, CA). Tissue was left overnight at 4°C with 1:200 goat rabbit anti-GFP (Invitrogen Molecular Probes, Eugene, OR) and 1:50 goat anti-mouse nc82 (Jackson Laboratories, Sacramento, CA), washed four times and left overnight in 1:500 Alexa-Fluor 594 goat anti-mouse (Invitrogen Molecular Probes, Eugene, OR) and 1:200 Cy2 goat anti-rabbit (Jackson Laboratories, Sacramento, CA). Brains were mounted in Fluoromount-G (Southern Biotech, Birmingham, AL).
Samples were imaged using a Nikon C1si Spectral Confocal (Nikon Imaging Center @ Q3B, UCSF, San Francisco, CA). Images were acquired using a 20X objective and scanned at a resolution of 1024 × 1024 pixels. Images were prepared using Nikon EZ-C1, NIS C for C1 analysis software (http://www.nikon-instruments.jp). Adobe Photoshop CS was used to tile images and to enhance contrast on whole images. Mushroom body expression was analyzed similarly to previous MB-GAL4 characterizations45.
Real-Time Quantitative PCR
RNA was extracted from 4 day-old adult flies by homogenization in Trizol (Invitrogen) and stored in -80°C. Samples were treated with DNase (Promega RQ1 RNase-free DNAse M6101) and cDNA was synthesized using Applied Biosystems TaqMan reverse transcription reagents (ABS N808-0234). Quantitative RT-PCR was performed using TaqMan Universal PCR Master Mix (no AmpErase UNG, ABS432018) on an Applied Biosystems 7900HT Fast Real-Time PCR System. Primers and probes recognizing sca (Dm01793316_g1) and Rpl32 (Dm02151827_g1) were designed by and obtained from Applied Biosystems.
Statistics
Statistics were performed using JMP 7.0.1 (2007, SAS Institute Inc.). Shapiro-Wilk tests were used to establish normality and Levene’s test to establish homogeneity of variance. Non-parametric tests were used primarily because the small sample size (N=8 per strain per condition for conditioned preference experiments) often resulted in non-normality and lack of homogeneity of variance within the data. In addition, we chose to non-parametric tests because they tend to be more conservative than their parametric equivalents. Wilcoxon tests were used to compare performance indices to zero. Kruskal-Wallis tests followed by Students-t, Tukey’s or Dunnet’s post-hoc tests were used to compare the performance indices between groups. For ethanol hyperactivity assays, repeated measures ANOVA was used to compare activity between groups. Significance was marked at p<0.05. All statistics reported are outlined in detail in Supplementary Tables 3 and 4.
Supplementary Material
Acknowledgments
We thank S. Waddell, J. Levine, M. Sokolowski, A. Barron and members of the Heberlein Lab for reagents, input and advice, and S. Birman, F. Wolf, S. Sweeney, K. Kaiser, N. Baker and the Bloomington Stock Center for flies. Funding was provided by a HSFC Research Fellowship to K.R.K. and NIH to U.H.
Footnotes
AUTHOR CONTRIBUTIONS
K.R.K. conceived, conducted and interpreted experiments, performed data analysis and co-wrote the paper; R.A. assisted in behavior experiments; Z.M. conducted control experiments, J.H. performed HPLC experiments; U.H. conceived and interpreted experiments and co-wrote the paper.
Supplementary Information accompanies the paper
The authors declare that they have no competing interests.
References
- 1.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 2.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–262. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 3.Rodan AR, Rothenfluh A. The genetics of behavioral alcohol responses in Drosophila. Int Rev Neurobiol. 2010;91:25–51. doi: 10.1016/S0074-7742(10)91002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker NE, Mlodzik M, Rubin GM. Spacing differentiation in the developing Drosophila eye: A fibrinogen-related lateral inhibitor encoded by scabrous. Science. 1990;250:1370–1377. doi: 10.1126/science.2175046. [DOI] [PubMed] [Google Scholar]
- 5.Powell PA, Wesley C, Spencer S, Cagan RL. Scabrous complexes with Notch to mediate boundary formation. Nature. 2001;409:626–630. doi: 10.1038/35054566. [DOI] [PubMed] [Google Scholar]
- 6.Nehring LC, Miyamoto A, Hein PW, Weinmaster G, Shipley JM. The extracellular matrix protein MAGP-2 interacts with Jagged1 and induces its shedding from the cell surface. J Biol Chem. 280:20349–20355. doi: 10.1074/jbc.M500273200. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto A, Lau R, Hein PW, Shipley JM, Wienmaster G. Microfibrillar proteins MAGP-1 and MAGP-2 induce Notch1 extracellular domain dissociation and receptor activation. J Biol Chem. 281:10089–10097. doi: 10.1074/jbc.M600298200. [DOI] [PubMed] [Google Scholar]
- 8.Louvi A, Artavanis-Tsakonas S. Notch signaling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, et al. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci USA. 2004;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa RM, Honjo T, Silva AJ. Learning and memory deficits in Notch mutant mice. Curr Biol. 2003;13:1348–1354. doi: 10.1016/s0960-9822(03)00492-5. [DOI] [PubMed] [Google Scholar]
- 11.Presente A, Boyles RS, Serway CN, de Belle S, Andres A. Notch is required for long-term memory in Drosophila. Proc Natl Acad Sci. 2004;101:1764–1768. doi: 10.1073/pnas.0308259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge A, Hannan F, Xie Z, Feng C, Tully T, Zhou H, Xie Z, Zhong Y. Notch signaling in Drosophila long-term memory formation. Proc Natl Acad Sci. 2004;101:10172–10176. doi: 10.1073/pnas.0403497101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips TJ, Shen EH. Neurochemical basis of locomotion and ethanol stimulant effects. Int Rev Neurobiol. 1996;39:243–282. doi: 10.1016/s0074-7742(08)60669-8. [DOI] [PubMed] [Google Scholar]
- 14.Deadwyler SA. Electrophysiological correlates of abused drugs: Relation to natural rewards. Ann N Y Acad Sci. 2010;1187:140–147. doi: 10.1111/j.1749-6632.2009.05155.x. [DOI] [PubMed] [Google Scholar]
- 15.Bainton RJ, et al. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 16.Kong EC, et al. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS One. 2010;5:e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamoto T. Targeted expression of temperature-sensitive dynamin to study neural mechanisms of complex behavior in Drosophila. J Neurogenet. 2002;16:205–228. doi: 10.1080/01677060216295. [DOI] [PubMed] [Google Scholar]
- 18.Friggi-Grelin F, et al. Targeted expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Chaney S, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 20.Dhonnchadha BAN, Cunningham KA. Serotonergic mechanisms in addiction-related memories. Behav Brain Res. 2008;195:29–52. doi: 10.1016/j.bbr.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53:103–15. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krashes MJ, et al. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claridge-Change A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaFerriere H, Guarnieri D, Sitaraman D, Diegelmannm S, Heberlein U, Zars T. Genetic Dissociation of ethanol sensitivity and memory formation in Drosophila melanogaster. Genetics. 2008;178:1895–1902. doi: 10.1534/genetics.107.084582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X, Lee EC, Baker NE. Molecular analysis of scabrous mutant alleles from Drosophila melanogaster indicates a secreted protein with two functional domains. Genetics. 1995;141:607–617. doi: 10.1093/genetics/141.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devineni A, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- 28.Pautassi RM, Molina JC, Spear N. Infant rats exhibit aversive learning mediated by ethanol’s orosensory effects but are positively reinforced by ethanol’s post-ingestive effects. Pharm Biochem Behav. 2008;88:393–402. doi: 10.1016/j.pbb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low M, Grady DK. Ethanol-conditioned place preference reduced in dopamine D2 receptor-deficient mice. Pharmacol Biochem Behav. 2000;67:693–699. doi: 10.1016/s0091-3057(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 30.Risinger FO, Freeman PA, Greengard P, Fienberg AA. Motivational effects of ethanol in DARPP-32 knock-out mice. J Neurosci. 2001;21:340–348. doi: 10.1523/JNEUROSCI.21-01-00340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker BM, Ettenberg A. Intracerebroventricular ethanol-induced conditioned place preference are prevented by fluphenazine infusions into the nucleus accumbens of rats. Behav Neurosci. 2007;121:401–410. doi: 10.1037/0735-7044.121.2.401. [DOI] [PubMed] [Google Scholar]
- 32.Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacol. 2008;34:1443–1453. doi: 10.1038/npp.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 34.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zars T, Fischer M, Schultz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- 37.Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- 38.McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1332. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- 39.King I, Tsai LT-Y, Pflanz R, Voight A, Lee S, Jackle H, Lu B, Heberlein U. Drosophila tao controls mushroom body development and ethanol-stimulated behavior through par-1. J Neurosci. 2011;31:1139–1148. doi: 10.1523/JNEUROSCI.4416-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akalal DB, Yu D, Davis RL. A late-phase, long-term memory trace forms in the γ neurons of Drosophila mushroom bodies after olfactory classical conditioning. J Neurosci. 30:16699–166708. doi: 10.1523/JNEUROSCI.1882-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- 42.Brand AH, Manuokian AS, Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- 43.Berger KH, et al. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res. 2008;32:895–908. doi: 10.1111/j.1530-0277.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardie SL, Hirsh J. An improved method for the separation and detection of biogenic amines in adult Drosophila brain extract by high performance liquid chromatography. J Neurosci Methods. 2006;153:243–249. doi: 10.1016/j.jneumeth.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Aso Y, et al. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


