Abstract
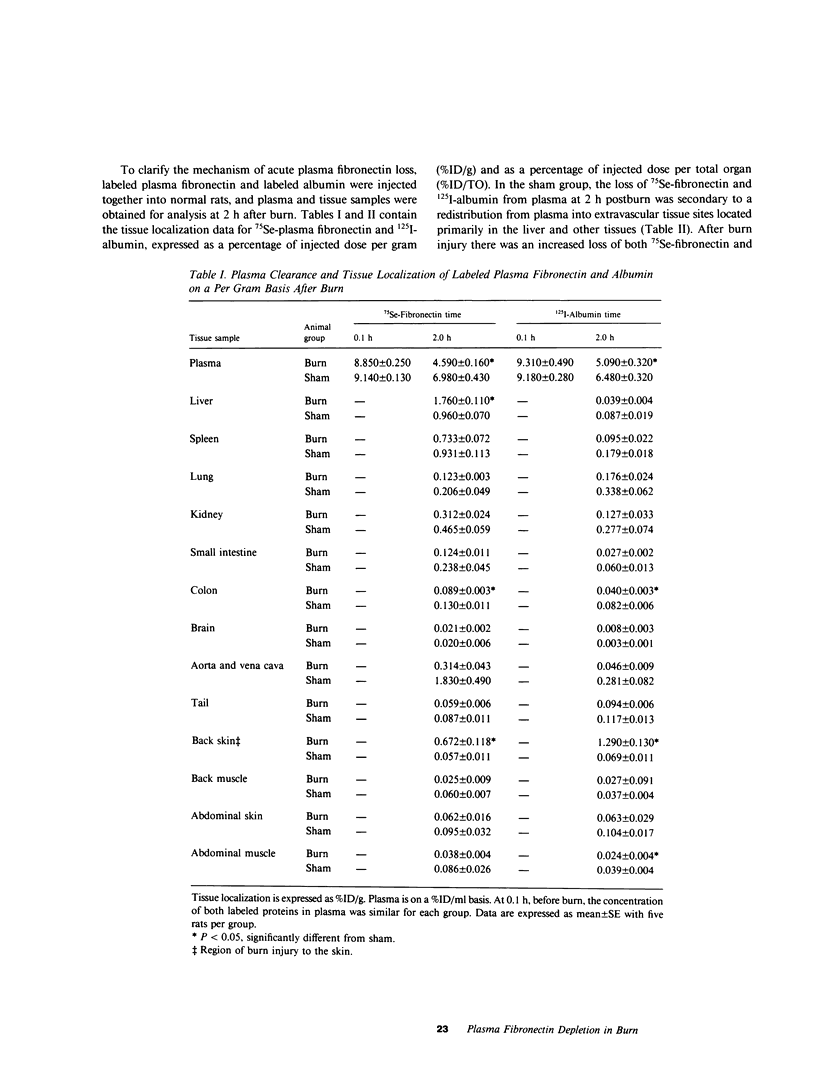
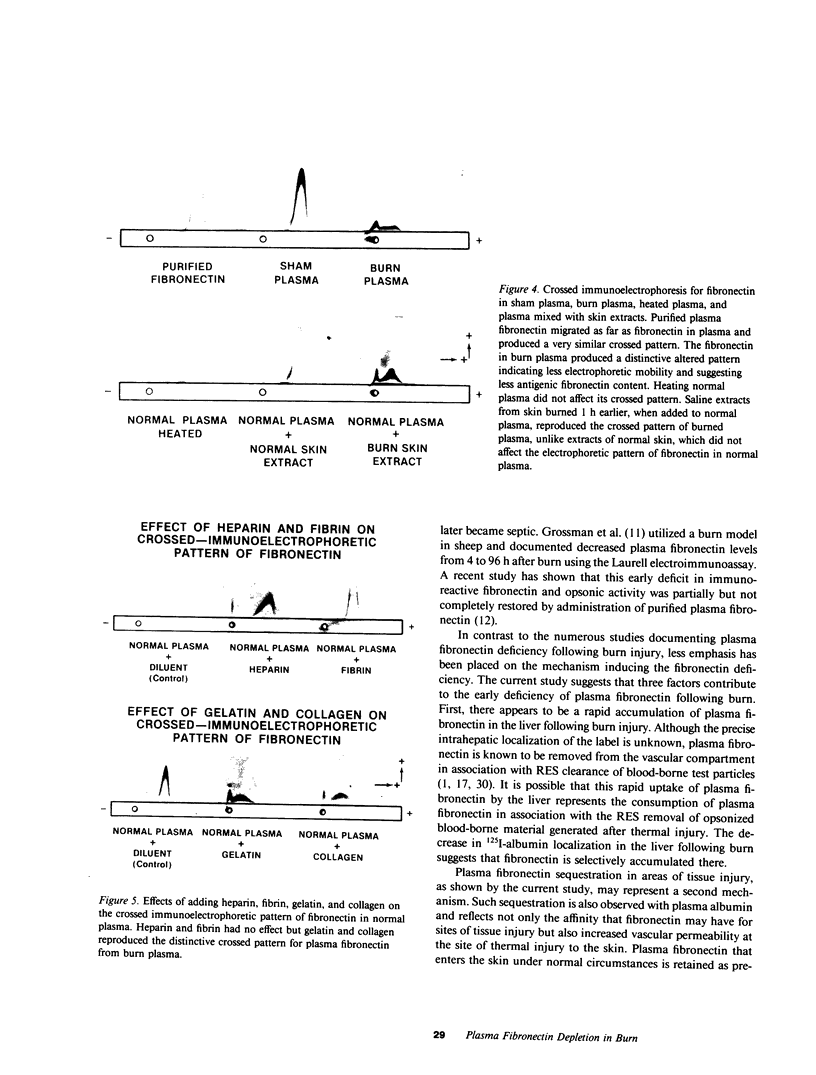
Plasma fibronectin was depleted within 15 min following sublethal burn, followed by partial recovery at 8 h and complete restoration by 24 h in anesthetized rats. Radiolabeled 75Se-plasma fibronectin, injected intravenously before burn, was rapidly sequestered in burn skin as well as the liver. Fibronectin levels at 2 h postburn as detected by immunoassay vs. 75Se-plasma fibronectin indicated that more fibronectin was in the plasma than detected by electroimmunoassay. Crossed immunoelectrophoretic analysis of fibronectin in early postburn plasma demonstrated a reduced electrophoretic mobility of the fibronectin antigen. Addition of heparin or fibrin, both of which have affinity for fibronectin, to normal plasma was unable to reproduce this altered fibronectin electrophoretic pattern. In contrast, addition of gelatin or native collagen to normal plasma reproduced the abnormal electrophoretic pattern of fibronectin seen in burn plasma. Extracts of burned skin, but not extracts of normal skin, when added to normal plasma, elicited a similar altered electrophoretic pattern for fibronectin. By gel filtration, fibronectin in burn plasma had an apparent molecular weight approximately 40% greater than that observed in normal plasma. These data suggest the release into the blood of a gelatinlike ligand from burned skin, which complexes with plasma fibronectin. Thus, fibronectin deficiency acutely postburn appears mediated by (a) its accumulation at the site of burn injury; (b) its removal from the circulation by the liver; and (c) its presence in the plasma in a form that is less detectable by immunoassay.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenstock F. A., Saba T. M., Weber P. An affinity method for the rapid purification of opsonic alpha 2 SB glycoprotein from serum. Adv Shock Res. 1979;2:55–71. [PubMed] [Google Scholar]
- Blumenstock F., Weber P., Saba T. M., Laffin R. Electroimmunoassay of alpha-2-opsonic protein during reticuloendothelial blockade. Am J Physiol. 1977 Mar;232(3):R80–R87. doi: 10.1152/ajpregu.1977.232.3.R80. [DOI] [PubMed] [Google Scholar]
- Bray J. P., Estess F., Bass J. A. Anticollagen antibodies following thermal trauma. Proc Soc Exp Biol Med. 1969 Feb;130(2):394–398. doi: 10.3181/00379727-130-33563. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Dvorak H. F., Colvin R. B. Fibronectin in delayed-type hypersensitivity skin reactions: associations with vessel permeability and endothelial cell activation. J Immunol. 1981 Feb;126(2):787–793. [PubMed] [Google Scholar]
- Clark R. A., Lanigan J. M., DellaPelle P., Manseau E., Dvorak H. F., Colvin R. B. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982 Nov;79(5):264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- Deno D. C., Saba T. M., Lewis E. P. Kinetics of endogenously labeled plasma fibronectin: incorporation into tissues. Am J Physiol. 1983 Oct;245(4):R564–R575. doi: 10.1152/ajpregu.1983.245.4.R564. [DOI] [PubMed] [Google Scholar]
- Dessau W., Jilek F., Adelmann B. C., Hörmann H. Similarity of antigelatin factor and cold insoluble globulin. Biochim Biophys Acta. 1978 Mar 28;533(1):227–237. doi: 10.1016/0005-2795(78)90566-4. [DOI] [PubMed] [Google Scholar]
- Dillon B. C., Estes J. E., Saba T. M., Blumenstock F. A., Cho E., Lee S. K., Lewis E. P. Actin-induced reticuloendothelial phagocytic depression as mediated by its interaction with fibronectin. Exp Mol Pathol. 1983 Apr;38(2):208–223. doi: 10.1016/0014-4800(83)90086-2. [DOI] [PubMed] [Google Scholar]
- Dillon B. C., Saba T. M., Cho E., Lewis E. Opsonic fibronectin deficiency in the etiology of starvation-induced reticuloendothelial phagocytic dysfunction. Exp Mol Pathol. 1982 Apr;36(2):177–192. doi: 10.1016/0014-4800(82)90092-2. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Gold L. I., Pearlstein E. Fibronectin-collagen binding and requirement during cellular adhesion. Biochem J. 1980 Feb 15;186(2):551–559. doi: 10.1042/bj1860551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F., Billingham R. E., Burgess L. Distribution of fibronectin during wound healing in vivo. J Invest Dermatol. 1981 Mar;76(3):181–189. doi: 10.1111/1523-1747.ep12525694. [DOI] [PubMed] [Google Scholar]
- Grossman J. E., Demling R. H., Duy N. D., Mosher D. F. Response of plasma fibronectin to major body burn. J Trauma. 1980 Nov;20(11):967–970. doi: 10.1097/00005373-198011000-00011. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Ruoslahti E. Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J Cell Biol. 1979 Oct;83(1):255–259. doi: 10.1083/jcb.83.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham K. C., Brew S. A., Miekka S. I. Interaction of plasma fibronectin with gelatin and complement C1q. Mol Immunol. 1983 Mar;20(3):287–295. doi: 10.1016/0161-5890(83)90068-8. [DOI] [PubMed] [Google Scholar]
- Kaplan J. E., Molnar J., Saba T. M., Allen C. Comparative disappearance and localization of isotopically labeled opsonic protein and soluble albumin following surgical trauma. J Reticuloendothel Soc. 1976 Nov;20(5):375–384. [PubMed] [Google Scholar]
- Kaplan J. E., Snedeker P. W. Maintenance of fibrin solubility by plasma fibronectin. J Lab Clin Med. 1980 Dec;96(6):1054–1061. [PubMed] [Google Scholar]
- Lanser M. E., Saba T. M. Correction of serum opsonic defects after burn and sepsis by opsonic fibronectin administration. Arch Surg. 1983 Mar;118(3):338–342. doi: 10.1001/archsurg.1983.01390030070011. [DOI] [PubMed] [Google Scholar]
- Lanser M. E., Saba T. M., Scovill W. A. Opsonic glycoprotein (plasma fibronectin) levels after burn injury. Relationship to extent of burn and development of sepsis. Ann Surg. 1980 Dec;192(6):776–782. doi: 10.1097/00000658-198012000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- Niehaus G. D., Dillon B. C., Schumacker P. T., Saba T. M. Influence of gelatin on bioassayable and immunoreactive opsonic fibronectin. Proc Soc Exp Biol Med. 1981 Oct;168(1):15–23. doi: 10.3181/00379727-168-41228. [DOI] [PubMed] [Google Scholar]
- Oh E., Pierschbacher M., Ruoslahti E. Deposition of plasma fibronectin in tissues. Proc Natl Acad Sci U S A. 1981 May;78(5):3218–3221. doi: 10.1073/pnas.78.5.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese A. C., Doran J. E., Callaway B. D., Mansberger A. R. Sequestration of fibronectin at the site of an injury. Adv Shock Res. 1982;8:119–127. [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Blumenstock F. A., Scovill W. A., Bernard H. Cryoprecipitate reversal of opsonic alpha2-surface binding glycoprotein deficiency in septic surgical and trauma patients. Science. 1978 Aug 18;201(4356):622–624. doi: 10.1126/science.675246. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Jaffe E. Plasma fibronectin (opsonic glycoprotein): its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med. 1980 Apr;68(4):577–594. doi: 10.1016/0002-9343(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Schildt B. E., Bouveng R. Effect of burning on hepatic phagocytosis as studied in vitro. Life Sci I. 1971 Apr 1;10(7):397–404. doi: 10.1016/0024-3205(71)90145-7. [DOI] [PubMed] [Google Scholar]
- Scovill W. A., Saba T. M., Blumenstock F. A., Bernard H., Powers S. R., Jr Opsonic alpha2 surface binding glycoprotein therapy during sepsis. Ann Surg. 1978 Oct;188(4):521–529. doi: 10.1097/00000658-197810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E. L., Mosher D. F., Hezzey A., Golenwsky G. Effect of blood transfusion on in vivo levels of plasma fibronectin. J Lab Clin Med. 1981 Sep;98(3):336–341. [PubMed] [Google Scholar]
- Turinsky J., Shangraw R. Biphasic alterations in glucose metabolism by soleus muscle from the burned limb. Adv Shock Res. 1979;2:23–30. [PubMed] [Google Scholar]
- Weeke B. Crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:47–56. doi: 10.1111/j.1365-3083.1973.tb03778.x. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. The major cell surface glycoprotein of chick embryo fibroblasts is an agglutinin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3158–3162. doi: 10.1073/pnas.72.8.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]




