Introduction
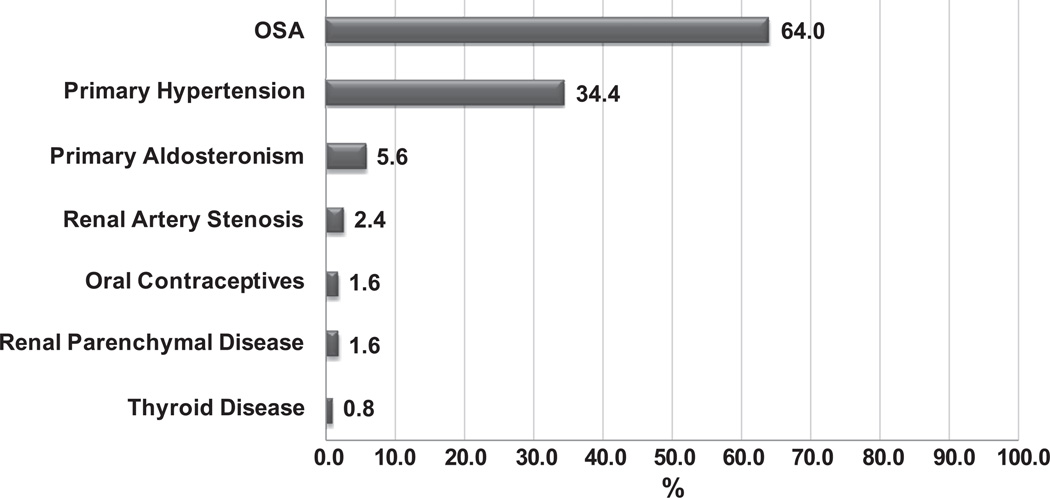
Obstructive sleep apnea (OSA) is highly relevant to patients with hypertension (HTN): These two conditions frequently co-exist (an estimated 50% of patients with HTN suffer from concomitant OSA), and recent evidence supports the notion that OSA represents the most prevalent secondary contributor to elevated blood pressure (BP) in patients with resistant HTN (Figure 1).1
Figure 1.
Prevalence of secondary causes of hypertension associated with resistant hypertension in a cohort of 125 patients from Brazil. Reproduced with permission: Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: The most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817.
Epidemiological association of OSA and HTN
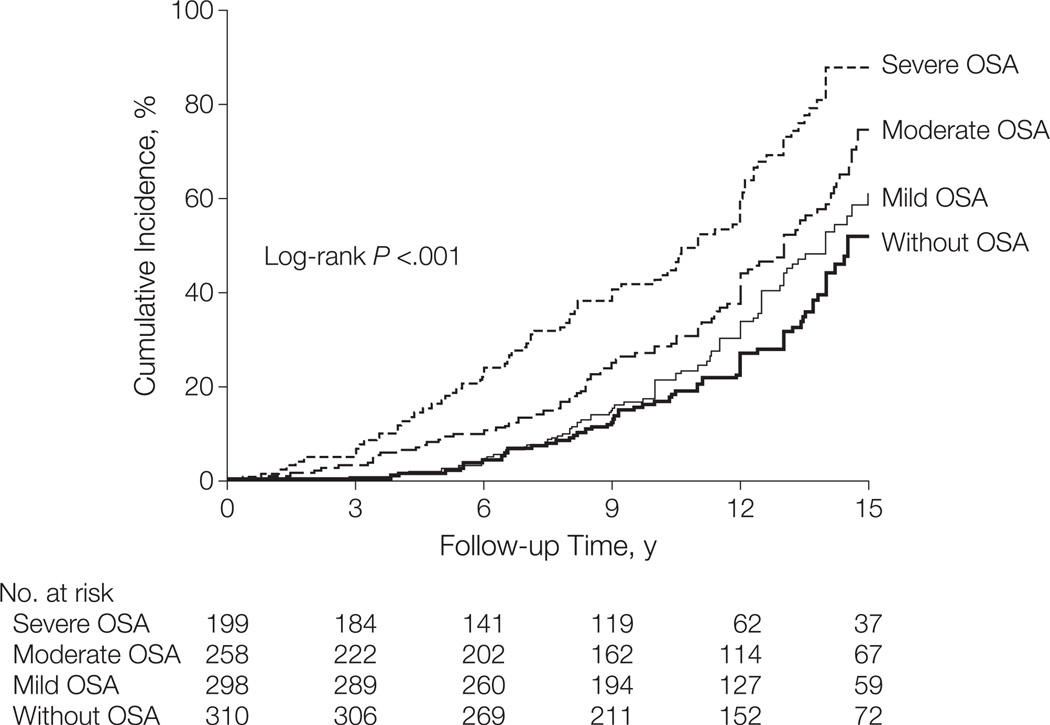
Previously published population based studies identified an independent correlation between greater apnea hypopnea index (AHI) and increasing BP, both at baseline and also when measured over long term follow up.2 On the other hand, isolated systolic HTN, which was more commonly seen in elderly patients, was not associated with OSA in any age group.3 The key challenge in deciphering the OSA-HTN connection lies in appropriately accounting for the many confounding variables, particularly obesity and age. Two recent prospective longitudinal cohort studies addressed these questions in normotensive subjects and reached opposing conclusions: The first study reported that after adjusting for relevant confounders, OSA was not associated with incident systolic HTN (1180 subjects over 7.5 year mean follow up period).4 The second study (also from Spain, 1889 participants, 12.2 years of median follow up) identified an increased hazard ratio for incident hypertension in patients with OSA compared to control subjects, and in this second study, the OSA-HTN association remained independent of confounders including age and obesity. Furthermore, follow-up of this patient cohort revealed a dose response relationship between the severity of OSA and the cumulative incidence of HTN (Figure 2).5 Given the extensive follow up period, this second study provides relatively robust epidemiological evidence implicating OSA as a factor in the development of HTN.
Figure 2.
Cumulative incidence of HTN in the participants of a prospective cohort study by Marin et al. who were not treated with CPAP. (From Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbe F, Vicente E, Wei Y, Nieto FJ, Jelic S. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA : the journal of the American Medical Association. 2012;307:2169–2176.5 Used with permission).
Focus on unique patient populations
An association between OSA and elevated BP has been recently reported in various specific patient cohorts: elderly women,6 prehypertensive subjects,7 primary care patients,8 patients after spinal cord injury,9 and in patients after stroke.10 In children, where fewer studies are available, the OSA-blood pressure relationship remains evident,11, 12 but the challenge is again in separating what portion of BP elevation can be attributable to OSA, or to obesity, or to an interaction between these. Although relatively modestly powered, these studies may be helpful in identifying specific patient groups where diagnosis and treatment of OSA would have a more pronounced effect on BP, and ultimately would be able to reduce HTN related morbidity and mortality. If such patient cohorts were identified, they would then be prime targets for cost effective OSA treatment.
Diurnal variation of BP
The physiological nocturnal BP decrease in normal individuals (“dipping pattern”) seems to be altered in patients with OSA, and more recent data confirm these findings in older adults as well.13 Night-time BP may reflect cardiovascular risk as well as day-time BP, and a nocturnal non-dipping pattern has been shown to confer an increased rate of adverse events.14 The mechanisms underlying the non-dipping pattern have received considerable attention. However, studies evaluating diurnal biomarker variation corresponding to the OSA-induced BP changes have been inconclusive.15 This line of research nevertheless remains potentially valuable, as continuous methods of 24-hour BP monitoring are becoming more affordable and readily available. Indeed, a relatively novel categorization of sleep-related HTN in OSA has been proposed: a sustained type (both nocturnal and morning HTN), and a surge type (morning HTN only without nocturnal HTN), but a validation of the variability in the effect of these two types of sleep related HTN on hard clinical outcomes is needed.16
In children, the data on OSA and nocturnal BP dipping remain conflicted, and lead us to conclude that either: 1) children with OSA may not have been exposed to the pathophysiology of OSA long enough to affect their BP, and/or 2) elevation of BP in the REM phase of sleep may not suffice to change the overall mean nocturnal BP in children.17, 18
Pathophysiologic links between OSA and HTN
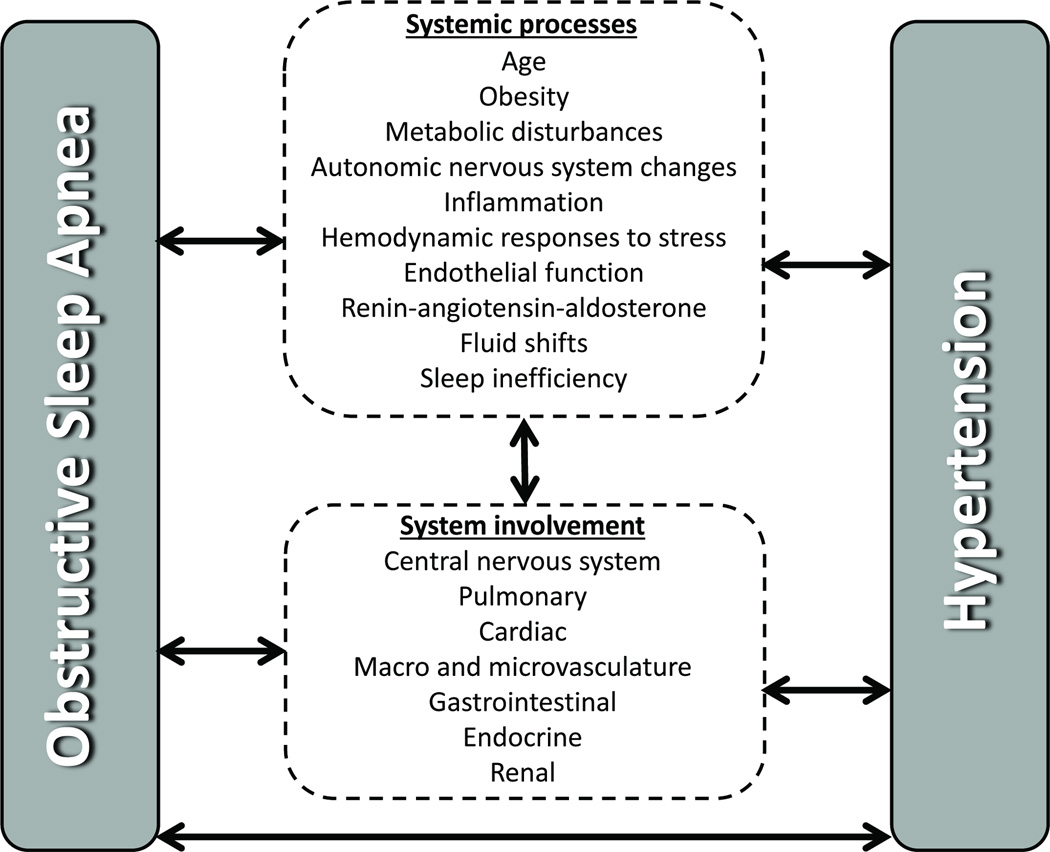
Given that HTN and OSA are complex processes with multifactorial etiologies, it is no surprise that they appear to be linked by an interplay of mechanisms, schematically shown in Figure 3.
Figure 3.
Schematic representation of the complex interactions between BP and OSA.
Neural circulatory mechanisms
Repetitive OSA-induced hypoxemia and hypercapnia elicit reflex changes in both sympathetic and parasympathetic activation.19,20 These autonomic derangements, with consequent increases in catecholamine levels, persist even into the daytime, and could contribute to the development of HTN.21 Effective OSA treatment with CPAP reduces urinary catecholamine levels,22 and this reduction seems to be especially evident in patients with more severe OSA.23 Animal studies suggest that renal denervation attenuates the BP rise associated with OSA events.24 Autonomic ganglia-mediated changes in cardiac arrhythmogenicity related to OSA are discussed in sections below.
Inflammatory and cytokine mediated effects of OSA
Preliminary data on molecular mechanisms linking OSA to cardiovascular morbidity related to HTN suggest that OSA correlates with an increased burden of systemic inflammation and higher concentrations of hs-CRP, IL-1, IL-8, IL-6, TNF-α, Rantes and sICAM.25 Whether these markers truly signify a worse prognosis for OSA patients, and to what degree a potential therapeutic intervention could alter their pathogenesis remains to be determined. Encouraging data have been suggested by studies in a murine model, where atorvastatin, which is known to reduce inflammation, prevented various adverse cardiovascular processes related to intermittent hypoxia.26
Hemodynamic effects of OSA
Two studies identified an OSA-associated impairment in the cardiovascular response to exercise, but differ as to whether this difference remains independent after adjusting for gender, body mass index, and other co-morbidities.27, 28 Decreased functional aerobic capacity in OSA patients raises particular concern given its power to predict both overall and cardiovascular mortality.
Age related modulation of OSA pathophysiology
A number of studies have reported that the effects of OSA on cardiovascular conditions such as hypertension and atrial fibrillation are more evident in younger than older subjects. The attractive hypothesis that OSA affects younger versus older patients differently was assessed in a pilot study of changes in the renal vascular resistance index but no substantial differences between the age categories were found.29
Renin-angiotensin-aldosterone system
Evidence relating OSA to markers of the renin-angiotensin-aldosterone system, clearly of high interest in patients with HTN, is unfortunately limited.30 A curious observation in patients with resistant hypertension due to hyperaldosteronism suggested that dietary salt intake was related to the severity of OSA (this was not found to be the case in hypertensive patients without hyperaldosteronism). This raises the question of whether salt intake and aldosterone levels constitute yet other variables which we need to control for when assessing the OSA-HTN relationship.31 In a broader sense, a clearer understanding of the role of renin-angiotensin-aldosterone in the OSA-BP relationship would be of considerable clinical significance, given the ready availability of medication groups affecting such targets.
Nocturnal fluid redistribution
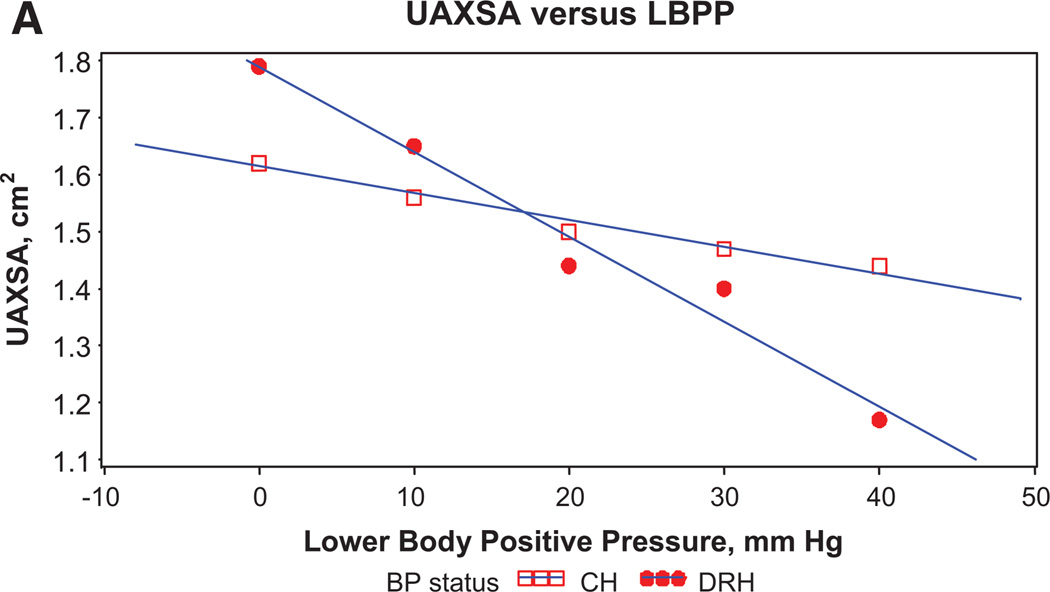
Whether nocturnal rostral fluid shift plays a significant role in patients with HTN and OSA was addressed in a recent study that examined the effects of graded lower body positive pressure on upper airway cross-sectional area (among other endpoints).32 The authors reported that the HTN subjects reduced their mean upper airway cross-sectional area in direct relationship to the amount of displaced fluid from the legs, and that this upper airway reduction was significantly more pronounced in the resistant HTN patients compared to the controlled HTN patients (Figure 4). These data could support the notion that HTN begets HTN in part via the OSA pathway: HTN patients could be prone to increased rostral fluid shift which worsens their OSA (narrowed airway) subsequently further increasing BP, leading eventually to resistant hypertension, a correlation where OSA has been shown consistently to be highly prevalent.
Figure 4.
Relationship between mean upper airway cross-sectional area (UAXSA) in response to the graded lower body positive pressure (LBPP) in patients with controlled hypertension (CH) and drug resistant hypertension (DRH). Modified with permission (Friedman O, Bradley TD, Logan AG. Influence of lower body positive pressure on upper airway cross-sectional area in drug-resistant hypertension. Hypertension. 2013;61:240–245).
Sleep inefficiency
OSA is an important cause of impaired sleep quality. Sleep inefficiency and short sleep duration have been postulated as potential contributors to elevated BP. A recent longitudinal study identified chronic insomniacs with short sleep duration as being at increased risk of incident HTN, but this effect was largely explained by controlling for obesity.33 On the other hand, several studies showed positive correlations between sleep deprivation and various adverse cardiovascular risk factors: arterial stiffness,34 endothelial dysfunction, sympathetic activity,35 non-dipping nocturnal BP pattern,36 and insulin insensitivity.37
Novel drug targets
Sudden change in altitude may adversely affect both OSA and HTN, and a recent randomized controlled trial of acetazolamide in addition to CPAP versus CPAP with placebo suggested that this carbonic anhydrase inhibitor ameliorated the BP rise seen with sleeping at high elevation in treated OSA patients.38 Even though BP was not the primary endpoint of this study, the possible mechanisms behind the BP findings were intriguing as both acid-base imbalance and partial pressures of oxygen could pose possible targets for therapeutic intervention.
System-specific pathophysiology related to OSA
The complexity of the influence of OSA on specific organ systems is underlined by the possible bi-directionality in the cause-effect relationships; for example does OSA lead to altered cerebral hemodynamics resulting in HTN, or does OSA lead to HTN directly with the alterations in the cerebral perfusion as a consequence (Figure 3).
Central nervous system
Patients with OSA had altered cerebral vasomotor reactivity when assessed by the breath-holding maneuver,39 and prospective follow-up revealed that 2 years of CPAP attenuated this impairment.40 While the mechanisms of cerebral vascular damage remain unclear, several murine studies have shed light on possible mediators. In an evaluation of the topographical effects of chronic intermittent hypoxia and the interaction with BP changes, areas of the brain which regulate sympathetic outflow were preeminently affected.41 Delta-FosB in the preoptic nucleus was necessary for hypoxia to induce hypertension, suggesting that neural adaptation may contribute to the increase in BP.42 These studies support a likely direct effect of OSA on the central neural vasculature, and have implications for understanding the link between OSA and both HTN and stroke, as well as potential avenues for targeted intervention.43
Pulmonary
OSA-related hypoxemia may have a particularly deleterious effect in patients with idiopathic pulmonary fibrosis, possibly by aggravating pulmonary hypertension.44 Similarly concerning are OSA-related nocturnal hypoxemic episodes in patients with precapillary pulmonary HTN of thromboembolic or idiopathic etiology, where the addition of OSA related stress on an already burdened pulmonary vasculature and right ventricle could theoretically be avoided by the appropriate use of CPAP.45 Whether OSA treatment provides tangible clinical benefit remains unclear.
Cardiovascular
A smaller study on cardiac morphology of OSA patients treated with 3 months of CPAP suggested that indices of both right and left ventricular function improved mildly46 which has particular relevance to the recently recognized high prevalence of OSA in patients with ischemic and hypertrophic cardiomyopathy.47, 48 Emerging evidence also links OSA to thoracic aortic dilatation, with implications for risk of aneurysm formation. One possible mechanism could be the OSA-induced increase in aortic wall dilatory transmural pressure.49
While OSA may cause structural changes in large vessels, it has also been shown to impair endothelial function. This impairment may be reversible by CPAP treatment.50 Further work is required in identifying all the pathways involved, but recent evidence implicates the lectin-like oxidized low-density lipoprotein receptor 1 (a scavenger receptor known to be also up-regulated in atherosclerosis) as playing an important role.51
Atrial fibrillation is a common endpoint of hypertensive cardiac structural changes. Provocative canine experiments suggest that atrial arrhythmogenicity is increased by simulated OSA, and that this effect is likely mediated by cardiac autonomic mechanisms.52, 53 Especially exciting are data showing that blockade of cardiac autonomic ganglia markedly attenuates the occurrence of atrial fibrillation in response to simulated OSA, suggesting a novel option for preventing atrial fibrillation in OSA patients.52
Gastrointestinal/Endocrine
In patients with metabolic syndrome in whom HTN coexists with dyslipidemia, obesity, and pre-diabetes, the treatment of OSA could theoretically improve prognosis by a multifaceted beneficial effect on BP as well as on lipid profile and glucose metabolism. Post-prandial dyslipidemia, which confers significant cardiovascular risk, was shown to improve after 2 months of effective CPAP treatment.54 Additionally, improvements in obesity, caloric intake, and body composition have been reported in successfully treated OSA patients.55 More complex mechanisms centered around altered insulin resistance may also play a role in the susceptibility of OSA patients to HTN related morbidity as this could be confounded by diabetes mellitus.56
Renal
A recent pilot study of renal sympathetic denervation in patients with resistant hypertension suggested that this procedure may also attenuate the severity of sleep apnea.57 However, much remains to be learned before renal denervation can be utilized clinically in this regard.
Effect of OSA treatment on BP
Prevention and treatment of HTN with CPAP
A possibly preventive role of CPAP in reducing incident HTN was suggested by the large prospective Zaragoza Sleep Cohort Study, which reported a lower incidence of newly diagnosed HTN in those OSA patients who tolerated CPAP.5 These findings were not confirmed in a recent randomized controlled study, also from Spain, possibly due to limited power, although the tendency to benefit from CPAP therapy was encouraging.58Acute decreases in BP and sympathetic traffic during sleep can be achieved with effective OSA treatment; however, the data regarding long term, clinically meaningful BP reduction with CPAP treatment have been less clear. Meta-analyses previously conducted on this topic spoke to the modest but statistically significant beneficial effect of CPAP,59 and congruent with these findings was the recently published meta-analysis by Montesi et al. which included 28 studies representing 1948 patients, and reported weighted mean decrease in systolic and diastolic BP of 2.58 mm Hg and 2.01mm Hg, respectively, favoring those treated with CPAP.60
Predictors of antihypertensive efficacy of CPAP
The knowledge of specific patient characteristics which may predispose to a more substantial BP reduction after initiation of CPAP would be of high value. A modestly powered study of 24 patients with OSA reported the following variables to be independent predictors of the CPAP-related fall in 24-hour mean BP: male gender, sleepiness, body mass index, smoking, alcohol use, and baseline BP.61
Studies in unique patient cohorts
A pilot study of diabetic patients (type 2) with OSA showed that systolic and diastolic BP in those receiving CPAP dropped by 9 mm Hg and 7 mm Hg, respectively.62 These BP changes could not be attributed to changes in weight, waist circumference, or glycemic control, but the authors reported significant decrease in urinary noradrenaline and dopamine. These fairly substantial decreases in BP are encouraging and raise the possibility that diabetic patients may be especially responsive to CPAP therapy as an antihypertensive strategy.
Prehypertensive patients with severe OSA were the focus of a randomized study which showed that 3 months of CPAP produced a significant decrease in daytime, nighttime, systolic, and diastolic BP, all of which translated into a reduction in the frequency of prehypertension and masked hypertension.63
In patients with coronary artery disease and OSA, CPAP treatment led to an effective reduction in diastolic BP and improvements in daytime somnolence; however, only a trend in the reduction of systolic BP was noted, possibly due to modest power of the study, timing of the BP checks, and relatively short CPAP treatment period (1 month).64 In an adequately-powered, randomized controlled trial of CPAP in patients with metabolic syndrome in India, 3 months of CPAP led to a decrease in both systolic and diastolic BP (3.9 mm Hg, 2.5 mm Hg, respectively), and improved lipid profiles and glycated hemoglobin percentages, ultimately resulting in a 13% reduction in the frequency of metabolic syndrome (compared to a 1% reduction in controls).65
Alternatives and supplements to CPAP therapy in OSA
Oral appliances may offer an important and effective alternative to CPAP therapy in patients with mild to moderate OSA, particularly in those who cannot tolerate chronic CPAP treatment.66, 67 Both OSA treatment modalities seem to result on average in similar changes in 24-hour mean BP, possibly because the greater efficacy of CPAP is offset by inferior compliance relative to oral appliances.68
Beneficial effects of bariatric surgery on OSA and BP have been reported in several observational studies,69, 70 but the recently published randomized controlled trial which compared the change in apnea-hypopnea index after a bariatric surgery versus regular weight loss program showed only a trend favoring the surgical arm.71 Nevertheless, the substantial decrease in weight in both treatment arms translated to an improvement in OSA severity (decrease in AHI by 26 for surgically treated patients versus decrease by 14 for those in the regular weight loss arm), suggesting that life style modification aimed at weight loss and increased activity should play a prominent role in managing OSA patients.
Conclusion
Key points
1) Epidemiological evidence implicates OSA as one of the modifiable and highly prevalent factors in the development of HTN. 2) A nocturnal non-dipping pattern of BP has been confirmed in older adults with OSA but not in children. 3) OSA patients have decreased exercise tolerance and higher diastolic blood pressure during exercise testing. 4) Resistant HTN patients may exhibit nocturnal rostral fluid shifts and decreased airway diameter. 5) Conflicting data exist regarding the role of CPAP in reducing incident HTN in OSA patients. 6) Results of meta-analyses speak consistently to a very modest 2 mm Hg antihypertensive effect of CPAP.
Remaining unknowns
Given the emerging importance of “individualized medicine”,72 the routine use of home BP monitoring devices could facilitate greater focus on particular subsets of patients, who would most profoundly benefit from OSA treatment in terms of BP reduction (“responders”).73 Such studies could also reveal a predictive model for the BP response to effective OSA treatment, especially because the average BP decrease obtained with OSA treatment has been modest, yet “responder” patients manifest substantial BP improvements. The increased power of longitudinal trials using well designed portable therapeutic systems could also help shed further light on the controversy regarding the degree to which age, obesity, and other co-morbidities explain the increased prevalence of HTN and HTN-related adverse outcomes among patients with OSA, and how life-style modifications fit into the complex interactions between OSA and BP. Most important, however, is to confirm that any BP reduction is accompanied by tangible improvements in cardiovascular outcomes.
Supplementary Material
Acknowledgments
Sources of Funding
Mayo Foundation; National Institutes of Health (R01 HL065176); Grants from European Regional Development Fund (CZ.1.05/1.1.00/02.0123); Internal Grant Agency, Ministry of Health, Czech Republic (NT11401-5/2011, CZ.1.07/2.3.00/20.0022).
Abbreviations
- BP
Blood pressure
- CPAP
Continuous positive airway pressure
- HTN
Hypertension
- OSA
Obstructive sleep apnea
Footnotes
Conflict(s) of Interest/Disclosure(s)
TKo: No conflict(s) of interest/disclosure(s). TKa: No conflict(s) of interest/disclosure(s). VKS: Philips Respironics Foundation (gift to Mayo Foundation), consultant for: Respicardia, ResMed, Medtronic, and NeuPro; working with Mayo Health Solutions and their industry partners on intellectual property related to sleep and cardiovascular disease.
References
- 1.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: The most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 3.Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA, Young T, Pickering TG. Age-dependent associations between sleep-disordered breathing and hypertension: Importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the sleep heart health study. Circulation. 2005;111:614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 4.Cano-Pumarega I, Duran-Cantolla J, Aizpuru F, Miranda-Serrano E, Rubio R, Martinez-Null C, de Miguel J, Egea C, Cancelo L, Alvarez A, Fernandez-Bolanos M, Barbe F. Obstructive sleep apnea and systemic hypertension: Longitudinal study in the general population: The vitoria sleep cohort. Am J Respir Crit Care Med. 2011;184:1299–1304. doi: 10.1164/rccm.201101-0130OC. [DOI] [PubMed] [Google Scholar]
- 5.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbe F, Vicente E, Wei Y, Nieto FJ, Jelic S. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sforza E, Chouchou F, Collet P, Pichot V, Barthelemy JC, Roche F. Sex differences in obstructive sleep apnoea in an elderly french population. Eur Respir J. 2011;37:1137–1143. doi: 10.1183/09031936.00043210. [DOI] [PubMed] [Google Scholar]
- 7.Roche F, Pepin JL, Achour-Crawford E, Tamisier R, Pichot V, Celle S, Maudoux D, Chouchou F, Ntougou-Assoumou HG, Levy P, Barthelemy JC. At 68 years, unrecognised sleep apnoea is associated with elevated ambulatory blood pressure. Eur Respir J. 2012;40:649–656. doi: 10.1183/09031936.00162710. [DOI] [PubMed] [Google Scholar]
- 8.Brostrom A, Sunnergren O, Johansson P, Svensson E, Ulander M, Nilsen P, Svanborg E. Symptom profile of undiagnosed obstructive sleep apnoea in hypertensive outpatients in primary care: A structural equation model analysis. Qual Prim Care. 2012;20:287–298. [PubMed] [Google Scholar]
- 9.LaVela SL, Burns SP, Goldstein B, Miskevics S, Smith B, Weaver FM. Dysfunctional sleep in persons with spinal cord injuries and disorders. Spinal Cord. 2012;50:682–685. doi: 10.1038/sc.2012.31. [DOI] [PubMed] [Google Scholar]
- 10.Cereda CW, Tamisier R, Manconi M, Andreotti J, Frangi J, Pifferini V, Bassetti CL. Endothelial dysfunction and arterial stiffness in ischemic stroke: The role of sleep-disordered breathing. Stroke. 2013;44:1175–1178. doi: 10.1161/STROKEAHA.111.000112. [DOI] [PubMed] [Google Scholar]
- 11.Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Fedok F, Vlasic V, Graff G. Blood pressure associated with sleep-disordered breathing in a population sample of children. Hypertension. 2008;52:841–846. doi: 10.1161/HYPERTENSIONAHA.108.116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archbold KH, Vasquez MM, Goodwin JL, Quan SF. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: Report from the tucson children's assessment of sleep apnea study. J Pediatr. 2012;161:26–30. doi: 10.1016/j.jpeds.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endeshaw YW, White WB, Kutner M, Ouslander JG, Bliwise DL. Sleep-disordered breathing and 24-hour blood pressure pattern among older adults. J Gerontol A Biol Sci Med Sci. 2009;64:280–285. doi: 10.1093/gerona/gln011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waeber B, Mourad JJ, O'Brien E. Nighttime blood pressure: A target for therapy? Curr Hypertens Rep. 2010;12:474–479. doi: 10.1007/s11906-010-0152-0. [DOI] [PubMed] [Google Scholar]
- 15.von Kanel R, Natarajan L, Ancoli-Israel S, Mills PJ, Loredo JS, Dimsdale JE. Day/night rhythm of hemostatic factors in obstructive sleep apnea. Sleep. 2010;33:371–377. doi: 10.1093/sleep/33.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki N, Ozono R, Yamauchi R, Teramen K, Edahiro Y, Ishii K, Seto A, Kihara Y. The relationship between morning hypertension and sleep quality in patients with obstructive sleep apnea syndrome. Clin Exp Hypertens. 2013;35:250–256. doi: 10.3109/10641963.2013.780069. [DOI] [PubMed] [Google Scholar]
- 17.Horne RS, Yang JS, Walter LM, Richardson HL, O'Driscoll DM, Foster AM, Wong S, Ng ML, Bashir F, Patterson R, Jolley D, Walker AM, Anderson V, Davey MJ, Nixon GM. Nocturnal dipping is preserved in children with sleep disordered breathing regardless of its severity. Pediatr Pulmonol. 2013;48:1127–1134. doi: 10.1002/ppul.22727. [DOI] [PubMed] [Google Scholar]
- 18.Nisbet LC, Yiallourou SR, Biggs SN, Nixon GM, Davey MJ, Trinder JA, Walter LM, Horne RS. Preschool children with obstructive sleep apnea: The beginnings of elevated blood pressure? Sleep. 2013;36:1219–1226. doi: 10.5665/sleep.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David Robertson, Italo Biaggioni, Geoffrey Burnstock, Phillip A. Low, Julian FR Paton., editors. Primer on the Autonomic Nervous System. (3rd Edition) 2012;112 Sleep Apnea. Chapter authors: Konecny T, Somers VK. Elsevier: ISBN-13: 9780123865250. [Google Scholar]
- 20.Parati G, Lombardi C, Hedner J, Bonsignore MR, Grote L, Tkacova R, Levy P, Riha R, Bassetti C, Narkiewicz K, Mancia G, McNicholas WT. Position paper on the management of patients with obstructive sleep apnea and hypertension: Joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological Research) ACTION B26 on obstructive sleep apnea. J Hypertens. 2012;30:633–646. doi: 10.1097/HJH.0b013e328350e53b. [DOI] [PubMed] [Google Scholar]
- 21.Freet CS, Stoner JF, Tang X. Baroreflex and chemoreflex controls of sympathetic activity following intermittent hypoxia. Auton Neurosci. 2013;174:8–14. doi: 10.1016/j.autneu.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Leung RS. Sleep-disordered breathing: Autonomic mechanisms and arrhythmias. Prog Cardiovasc Dis. 2009;51:324–338. doi: 10.1016/j.pcad.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Pinto P, Barbara C, Montserrat JM, Patarrao RS, Guarino MP, Carmo MM, Macedo MP, Martinho C, Dias R, Gomes MJ. Effects of CPAP on nitrate and norepinephrine levels in severe and mild-moderate sleep apnea. BMC Pulm Med. 2013;13:13. doi: 10.1186/1471-2466-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, Bohm M. Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension. 2012;60:172–178. doi: 10.1161/HYPERTENSIONAHA.112.191965. [DOI] [PubMed] [Google Scholar]
- 25.Testelmans D, Tamisier R, Barone-Rochette G, Baguet JP, Roux-Lombard P, Pepin JL, Levy P. Profile of circulating cytokines: Impact of OSA, obesity and acute cardiovascular events. Cytokine. 2013;62:210–216. doi: 10.1016/j.cyto.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Totoson P, Fhayli W, Faury G, Korichneva I, Cachot S, Baldazza M, Ribuot C, Pepin JL, Levy P, Joyeux-Faure M. Atorvastatin protects against deleterious cardiovascular consequences induced by chronic intermittent hypoxia. Exp Biol Med. (Maywood) 2013;238:223–232. doi: 10.1177/1535370212473696. [DOI] [PubMed] [Google Scholar]
- 27.Rizzi CF, Cintra F, Mello-Fujita L, Rios LF, Mendonca ET, Feres MC, Tufik S, Poyares D. Does obstructive sleep apnea impair the cardiopulmonary response to exercise? Sleep. 2013;36:547–553. doi: 10.5665/sleep.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansukhani MP, Allison TG, Lopez-Jimenez F, Somers VK, Caples SM. Functional aerobic capacity in patients with sleep-disordered breathing. Am J Cardiol. 2013;111:1650–1654. doi: 10.1016/j.amjcard.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel HM, Mast JL, Sinoway LI, Muller MD. Effect of healthy aging on renal vascular responses to local cooling and apnea. J Appl Physiol. 2013;115:90–96. doi: 10.1152/japplphysiol.00089.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–459. doi: 10.1378/chest.06-1442. [DOI] [PubMed] [Google Scholar]
- 31.Pimenta E, Stowasser M, Gordon RD, Harding SM, Batlouni M, Zhang B, Oparil S, Calhoun DA. Increased dietary sodium is related to severity of obstructive sleep apnea in patients with resistant hypertension and hyperaldosteronism. Chest. 2013;143:978–983. doi: 10.1378/chest.12-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman O, Bradley TD, Logan AG. Influence of lower body positive pressure on upper airway cross-sectional area in drug-resistant hypertension. Hypertension. 2013;61:240–245. doi: 10.1161/HYPERTENSIONAHA.112.203547. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, Bixler EO. Insomnia with objective short sleep duration and incident hypertension: The Penn State Cohort. Hypertension. 2012;60:929–935. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunbul M, Kanar BG, Durmus E, Kivrak T, Sari I. Acute sleep deprivation is associated with increased arterial stiffness in healthy young adults. Sleep Breath. 2013 doi: 10.1007/s11325-013-0873-9. In Press. [DOI] [PubMed] [Google Scholar]
- 35.Dettoni JL, Consolim-Colombo FM, Drager LF, Rubira MC, Souza SB, Irigoyen MC, Mostarda C, Borile S, Krieger EM, Moreno H, Jr, Lorenzi-Filho G. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol. 2012;113:232–236. doi: 10.1152/japplphysiol.01604.2011. [DOI] [PubMed] [Google Scholar]
- 36.Carev M, Karanovic N, Bagatin J, Matulic NB, Pecotic R, Valic M, Marinovic-Terzic I, Karanovic S, Dogas Z. Blood pressure dipping and salivary cortisol as markers of fatigue and sleep deprivation in staff anesthesiologists. Coll Antropol. 2011;35(Suppl 1):133–138. [PubMed] [Google Scholar]
- 37.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latshang TD, Nussbaumer-Ochsner Y, Henn RM, Ulrich S, Lo Cascio CM, Ledergerber B, Kohler M, Bloch KE. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: A randomized controlled trial. JAMA. 2012;308:2390–2398. doi: 10.1001/jama.2012.94847. [DOI] [PubMed] [Google Scholar]
- 39.Jimenez Caballero PE, Coloma Navarro R, Segura Martn T, Ayo Martn O. Cerebral hemodynamic changes at basilar artery in patients with obstructive sleep apnea syndrome. A case-control study. Acta Neurol Scand. 2013 doi: 10.1111/ane.12156. In Press. [DOI] [PubMed] [Google Scholar]
- 40.Jimenez Caballero PE, Coloma Navarro R, Ayo Martin O, Segura Martin T. Cerebral hemodynamic changes in obstructive sleep apnea syndrome after continuous positive airway pressure treatment. Sleep Breath. 2013;17:1103–1108. doi: 10.1007/s11325-013-0810-y. [DOI] [PubMed] [Google Scholar]
- 41.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol. 2011;301:R131–R139. doi: 10.1152/ajpregu.00830.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham JT, Knight WD, Mifflin SW, Nestler EJ. An essential role for deltafosb in the median preoptic nucleus in the sustained hypertensive effects of chronic intermittent hypoxia. Hypertension. 2012;60:179–187. doi: 10.1161/HYPERTENSIONAHA.112.193789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capone C, Faraco G, Coleman C, Young CN, Pickel VM, Anrather J, Davisson RL, Iadecola C. Endothelin 1-dependent neurovascular dysfunction in chronic intermittent hypoxia. Hypertension. 2012;60:106–113. doi: 10.1161/HYPERTENSIONAHA.112.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolilekas L, Manali E, Vlami KA, Lyberopoulos P, Triantafillidou C, Kagouridis K, Baou K, Gyftopoulos S, Vougas KN, Karakatsani A, Alchanatis M, Papiris S. Sleep oxygen desaturation predicts survival in idiopathic pulmonary fibrosis. J Clin Sleep Med. 2013;9:593–601. doi: 10.5664/jcsm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jilwan FN, Escourrou P, Garcia G, Jais X, Humbert M, Roisman G. High occurrence of hypoxemic sleep respiratory disorders in precapillary pulmonary hypertension and mechanisms. Chest. 2013;143:47–55. doi: 10.1378/chest.11-3124. [DOI] [PubMed] [Google Scholar]
- 46.Colish J, Walker JR, Elmayergi N, Almutairi S, Alharbi F, Lytwyn M, Francis A, Bohonis S, Zeglinski M, Kirkpatrick ID, Sharma S, Jassal DS. Obstructive sleep apnea: Effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–681. doi: 10.1378/chest.11-0615. [DOI] [PubMed] [Google Scholar]
- 47.Konecny T, Kuniyoshi FH, Orban M, Pressman GS, Kara T, Gami A, Caples SM, Lopez-Jimenez F, Somers VK. Under-diagnosis of sleep apnea in patients after acute myocardial infarction. J Am Coll Cardiol. 2010;56:742–743. doi: 10.1016/j.jacc.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konecny T, Brady PA, Orban M, Lin G, Pressman GS, Lehar F, Tomas K, Gersh BJ, Tajik AJ, Ommen SR, Somers VK. Interactions between sleep disordered breathing and atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2010;105:1597–1602. doi: 10.1016/j.amjcard.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 49.Clarenbach CF, Camen G, Sievi NA, Wyss C, Stradling JR, Kohler M. Effect of simulated obstructive hypopnea and apnea on thoracic aortic wall transmural pressures. J Appl Physiol. 2013;115:613–617. doi: 10.1152/japplphysiol.00439.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohler M, Craig S, Pepperell JC, Nicoll D, Bratton DJ, Nunn AJ, Leeson P, Stradling JR. CPAP improves endothelial function in patients with minimally symptomatic OSA: Results from a Subset Study of the MOSAIC Trial. Chest. 2013;144:896–902. doi: 10.1378/chest.13-0179. [DOI] [PubMed] [Google Scholar]
- 51.Akinnusi ME, Laporta R, El-Solh AA. Lectin-like oxidized low-density lipoprotein receptor-1 modulates endothelial apoptosis in obstructive sleep apnea. Chest. 2011;140:1503–1510. doi: 10.1378/chest.11-0302. [DOI] [PubMed] [Google Scholar]
- 52.Ghias M, Scherlag BJ, Lu Z, Niu G, Moers A, Jackman WM, Lazzara R, Po SS. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol. 2009;54:2075–2083. doi: 10.1016/j.jacc.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Lu Z, Nie L, He B, Yu L, Salim M, Huang B, Cui B, He W, Wu W, Jiang H. Increase in vulnerability of atrial fibrillation in an acute intermittent hypoxia model: Importance of autonomic imbalance. Auton Neurosci. 2013;177:148–153. doi: 10.1016/j.autneu.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 54.Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: A randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med. 2011;184:355–361. doi: 10.1164/rccm.201102-0316OC. [DOI] [PubMed] [Google Scholar]
- 55.Tuomilehto H, Gylling H, Peltonen M, Martikainen T, Sahlman J, Kokkarinen J, Randell J, Tukiainen H, Vanninen E, Partinen M, Tuomilehto J, Uusitupa M, Seppa J. Sustained improvement in mild obstructive sleep apnea after a diet- and physical activity-based lifestyle intervention: Postinterventional follow-up. Am J Clin Nutr. 2010;92:688–696. doi: 10.3945/ajcn.2010.29485. [DOI] [PubMed] [Google Scholar]
- 56.Lindberg E, Theorell-Haglow J, Svensson M, Gislason T, Berne C, Janson C. Sleep apnea and glucose metabolism: A long-term follow-up in a community-based sample. Chest. 2012;142:935–942. doi: 10.1378/chest.11-1844. [DOI] [PubMed] [Google Scholar]
- 57.Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinski P, Bielen P, Michalowska I, Kabat M, Warchol E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewicz A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 58.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, Martinez-Alonso M, Carmona C, Barcelo A, Chiner E, Masa JF, Gonzalez M, Marin JM, Garcia-Rio F, Diaz de Atauri J, Teran J, Mayos M, de la Pena M, Monasterio C, del Campo F, Montserrat JM. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: A randomized controlled trial. JAMA. 2012;307:2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 59.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–423. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 60.Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587–596. doi: 10.5664/jcsm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yorgun H, Kabakci G, Canpolat U, Kirmizigul E, Sahiner L, Ates AH, Sendur MA, Kaya EB, Demir AU, Aytemir K, Tokgozoglu L, Oto A. Predictors of blood pressure reduction with nocturnal continuous positive airway pressure therapy in patients with obstructive sleep apnea and prehypertension. Angiology. 2013 doi: 10.1177/0003319713477908. In Press. [DOI] [PubMed] [Google Scholar]
- 62.Myhill PC, Davis WA, Peters KE, Chubb SA, Hillman D, Davis TM. Effect of continuous positive airway pressure therapy on cardiovascular risk factors in patients with type 2 diabetes and obstructive sleep apnea. J Clin Endocrinol Metab. 2012;97:4212–4218. doi: 10.1210/jc.2012-2107. [DOI] [PubMed] [Google Scholar]
- 63.Drager LF, Pedrosa RP, Diniz PM, Diegues-Silva L, Marcondes B, Couto RB, Giorgi DM, Krieger EM, Lorenzi-Filho G. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–555. doi: 10.1161/HYPERTENSIONAHA.110.165969. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Q, Liu ZH, Luo Q, Zhao ZH, Zhang HL, Wang Y. Effects of continuous positive airway pressure on blood pressure and daytime sleepiness in obstructive sleep apnea patients with coronary heart diseases under optimal medications. Sleep Breath. 2012;16:341–347. doi: 10.1007/s11325-011-0498-9. [DOI] [PubMed] [Google Scholar]
- 65.Sharma SK, Agrawal S, Damodaran D, Sreenivas V, Kadhiravan T, Lakshmy R, Jagia P, Kumar A. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277–2286. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 66.Li W, Xiao L, Hu J. The comparison of CPAP and oral appliances in treatment of patients with OSA: A systematic review and meta-analysis. Respir Care. 2013;58:1184–1195. doi: 10.4187/respcare.02245. [DOI] [PubMed] [Google Scholar]
- 67.Iftikhar IH, Hays ER, Iverson MA, Magalang UJ, Maas AK. Effect of oral appliances on blood pressure in obstructive sleep apnea: A systematic review and meta-analysis. J Clin Sleep Med. 2013;9:165–174. doi: 10.5664/jcsm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, Marks GB, Cistulli PA. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: A randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 69.Hady HR, Dadan J, Golaszewski P. 100 obese patients after laparoscopic adjustable gastric banding - the influence on BMI, gherlin and insulin concentration, parameters of lipid balance and co-morbidities. Adv Med Sci. 2012;57:58–64. doi: 10.2478/v10039-012-0008-8. [DOI] [PubMed] [Google Scholar]
- 70.Woodman G, Cywes R, Billy H, Montgomery K, Cornell C, Okerson T. Effect of adjustable gastric banding on changes in gastroesophageal reflux disease (GERD) and quality of life. Curr Med Res Opin. 2012;28:581–589. doi: 10.1185/03007995.2012.666962. [DOI] [PubMed] [Google Scholar]
- 71.Dixon JB, Schachter LM, O'Brien PE, Jones K, Grima M, Lambert G, Brown W, Bailey M, Naughton MT. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: A randomized controlled trial. JAMA. 2012;308:1142–1149. doi: 10.1001/2012.jama.11580. [DOI] [PubMed] [Google Scholar]
- 72.Bejan-Angoulvant T, Baguet JP, Erpeldinger S, Boivin JM, Mercier A, Leftheriotis G, Gagnol JP, Fauvel JP, Giraud C, Bricca G, Gueyffier F. The IDEAL study: Towards personalized drug treatment of hypertension. Therapie. 2012;67:195–204. doi: 10.2515/therapie/2012031. [DOI] [PubMed] [Google Scholar]
- 73.Bravata DM, Ferguson J, Miech EJ, Agarwal R, McClain V, Austin C, Struve F, Foresman B, Li X, Wang Z, Williams LS, Dallas MI, Couch CD, Sico J, Fragoso C, Matthias MS, Chumbler N, Myers J, Burrus N, Dube A, French DD, Schmid AA, Concato J, Yaggi HK. Diagnosis and Treatment of Sleep Apnea in patients' homes: The rationale and methods of the "GoToSleep" randomized-controlled trial. J Clin Sleep Med. 2012;8:27–35. doi: 10.5664/jcsm.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.