Abstract
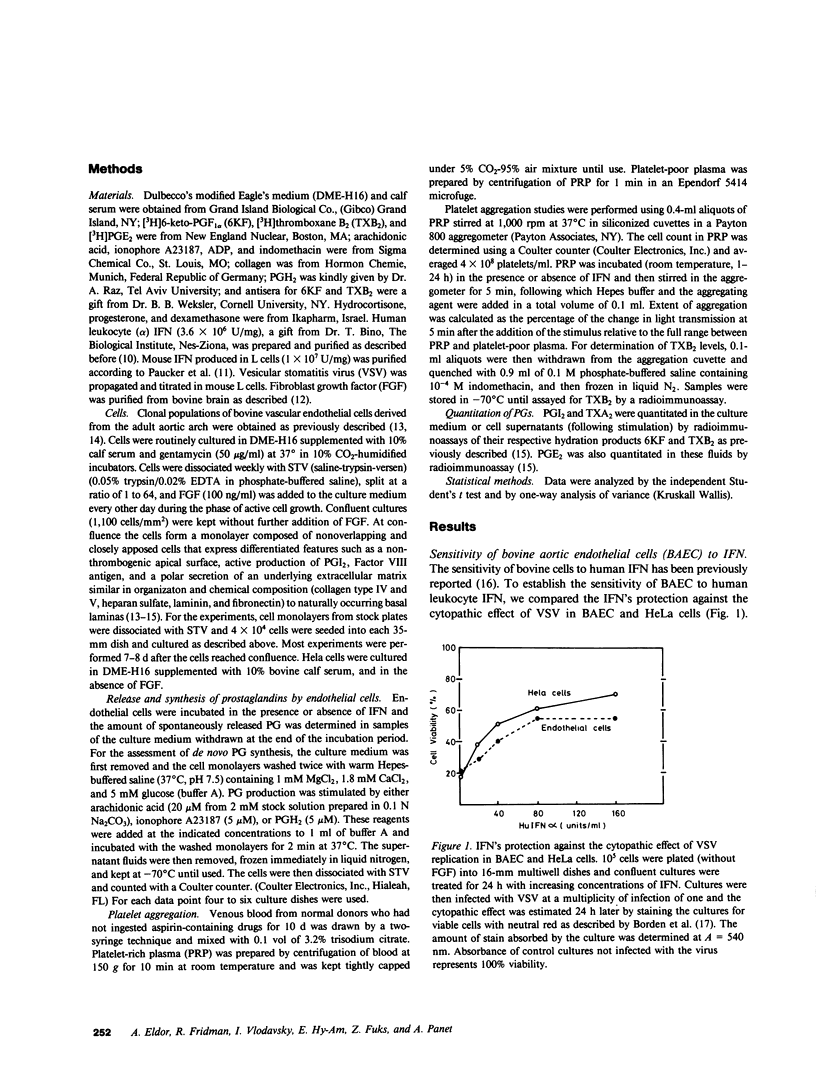
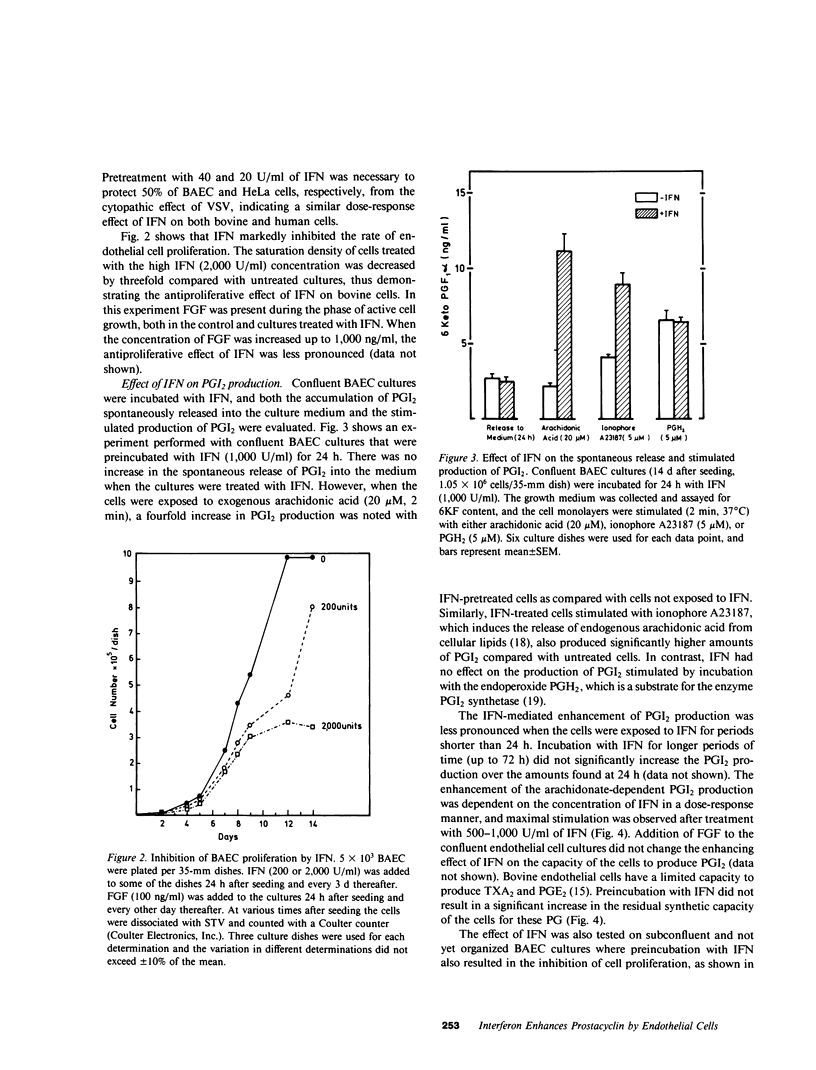
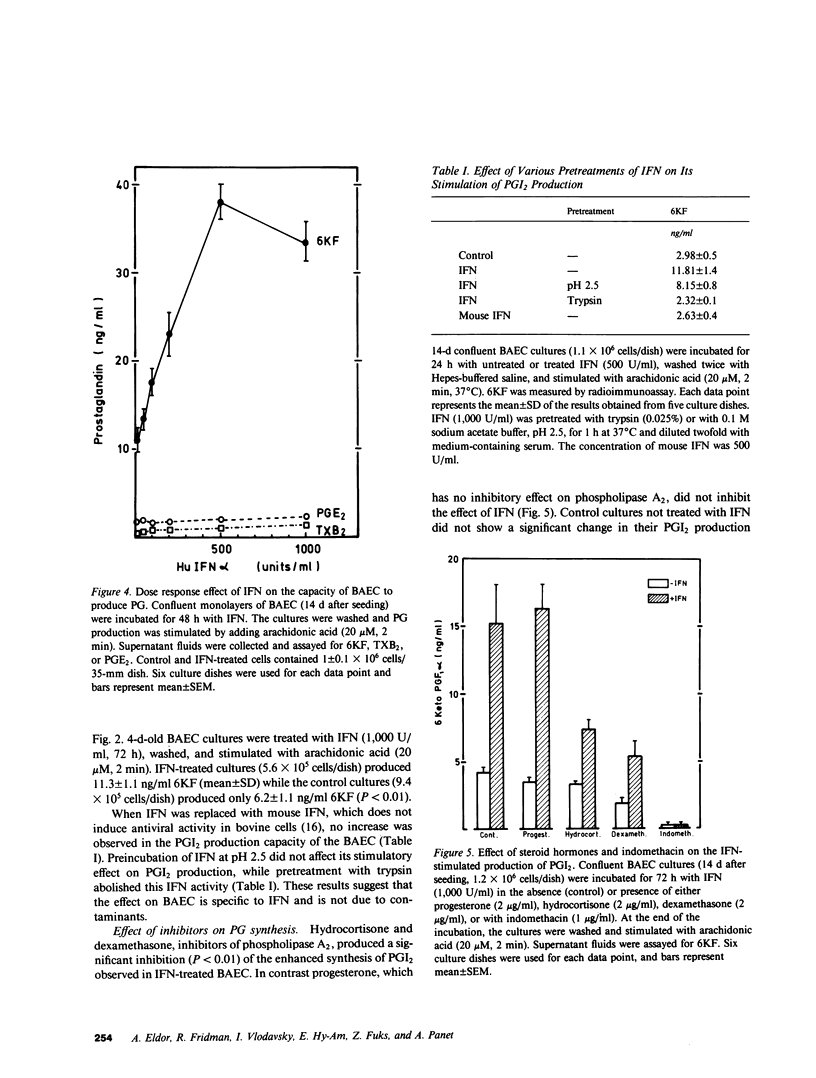
The effects of interferon (IFN) on the arachidonate metabolism and physiological functions of cultured endothelial cells and blood platelets have been examined. Cultured bovine aortic endothelial cells were found to be sensitive to the antiviral and antiproliferative activities of human leukocyte (alpha) IFN and to increase their capacity to synthesize prostacyclin (PGI2) upon exposure to IFN. Several observations indicate that IFN stimulates PGI2 synthesis at the level of the enzymes phospholipase A2 and cyclooxygenase: (a) PGI2 production was dependent upon the supply of exogenous arachidonic acid or the liberation of endogenous cellular arachidonate by ionophore A23187, but was not observed when IFN-treated cells were exposed to the endoperoxide prostaglandin H2. (b) IFN had no effect on the spontaneous release of PGI2 into the culture medium during the incubation period (24-72 h). (c) The stimulatory effect of IFN on PGI2 production was inhibited by both glucocorticoids and indomethacin. The effect of IFN on platelet prostaglandin metabolism was also investigated. Incubation of platelet-rich plasma with IFN had no effect on platelet aggregation and thromboxane A2 production. The biological significance of the findings presented in this paper may be considered in view of the protective role of PGI2 in the vessel wall and the fact that infection with certain viruses induces endothelial damage both in man and experimental animal models.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borden E. C., Leonhardt P. H. Aquanititave semimicro, semiautomated colorimetric assay for interferon. J Lab Clin Med. 1977 May;89(5):1036–1042. [PubMed] [Google Scholar]
- Bougnoux P., Bonvini E., Chang Z. L., Hoffman T. Effect of interferon on phospholipid methylation by peripheral blood mononuclear cells. J Cell Biochem. 1982;20(3):215–223. doi: 10.1002/jcb.240200302. [DOI] [PubMed] [Google Scholar]
- Cantell K., Hirvonen S. Large-scale production of human leukocyte interferon containing 10(8) units per ml. J Gen Virol. 1978 Jun;39(3):541–543. doi: 10.1099/0022-1317-39-3-541. [DOI] [PubMed] [Google Scholar]
- Chandrabose K. A., Cuatrecasas P., Pottathil R., Lang D. J. Interferon-resistant cell line lacks fatty acid cyclooxygenase activity. Science. 1981 Apr 17;212(4492):329–331. doi: 10.1126/science.6163214. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Moskowitz M. A., Zetter B. R., Antoniades H. N., Levine L. Platelet-dependent stimulation of prostacyclin synthesis by platelet-derived growth factor. Nature. 1980 Dec 11;288(5791):600–602. doi: 10.1038/288600a0. [DOI] [PubMed] [Google Scholar]
- Curwen K. D., Gimbrone M. A., Jr, Handin R. I. In vitro studies of thromboresistance: the role of prostacyclin (PGI2) in platelet adhesion to cultured normal and virally transformed human vascular endothelial cells. Lab Invest. 1980 Mar;42(3):366–374. [PubMed] [Google Scholar]
- Eldor A., Vlodavsky I., Hy-Am E., Atzmon R., Weksler B. B., Raz A., Fuks Z. Cultured endothelial cells increase their capacity to synthesize prostacyclin following the formation of a contact inhibited cell monolayer. J Cell Physiol. 1983 Feb;114(2):179–183. doi: 10.1002/jcp.1041140206. [DOI] [PubMed] [Google Scholar]
- Eldor A., Vlodavsky I., HyAm E., Atzmon R., Fuks Z. The effect of radiation on prostacyclin (PGI2) production by cultured endothelial cells. Prostaglandins. 1983 Feb;25(2):263–279. doi: 10.1016/0090-6980(83)90109-0. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Stringfellow D. A. Virus and interferon effects on cellular prostaglandin biosynthesis. J Immunol. 1980 Jul;125(1):431–437. [PubMed] [Google Scholar]
- Flower R. J., Blackwell G. J. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979 Mar 29;278(5703):456–459. doi: 10.1038/278456a0. [DOI] [PubMed] [Google Scholar]
- Fuse A., Mahmud I., Kuwata T. Mechanism of stimulation by human interferon of prostaglandin synthesis in human cell lines. Cancer Res. 1982 Aug;42(8):3209–3214. [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Greenburg G. Purification of the fibroblast growth factor activity from bovine brain. J Biol Chem. 1978 May 25;253(10):3736–3743. [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J., Braun D., Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-boye D., Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature. 1974 Oct 11;251(5475):543–545. doi: 10.1038/251543a0. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N. K., Gorman R. R. Regulation of 3T3-L1 fibroblast differentiation by prostacyclin (prostaglandin I2). Biochim Biophys Acta. 1981 Feb 23;663(2):457–466. doi: 10.1016/0005-2760(81)90174-0. [DOI] [PubMed] [Google Scholar]
- Huttner J. J., Gwebu E. T., Panganamala R. V., Milo G. E., Cornwell D. C., Sharma H. M., Geer J. C. Fatty acids and their prostaglandin derivatives: inhibitors of proliferation in aortic smooth muscle cells. Science. 1977 Jul 15;197(4300):289–291. doi: 10.1126/science.877555. [DOI] [PubMed] [Google Scholar]
- Jameson P. Induction of the interferon protein in vivo: viral inducers. Tex Rep Biol Med. 1981;41:135–143. [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A., Broekman M. J. Synthesis of prostacyclin from platelet-derived endoperoxides by cultured human endothelial cells. J Clin Invest. 1980 Nov;66(5):979–986. doi: 10.1172/JCI109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A. Enzymatic conversion of prostaglandin endoperoxide H2 and arachidonic acid to prostacyclin by cultured human endothelial cells. J Biol Chem. 1978 Oct 25;253(20):7138–7141. [PubMed] [Google Scholar]
- McKay D. G., Margaretten W. Disseminated intravascular coagulation in virus diseases. Arch Intern Med. 1967 Aug;120(2):129–152. [PubMed] [Google Scholar]
- Miller J. L., Stuart M. J., Walenga R. W. Arachidonic acid metabolism in guinea pig megakaryocytes. Biochem Biophys Res Commun. 1982 Jul 30;107(2):752–759. doi: 10.1016/0006-291x(82)91555-8. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Fabricant C. G., Fabricant J., Litrenta M. M. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol. 1979 Sep;96(3):673–706. [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A., Vane J. R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977 Jan 1;1(8001):18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- Paucker K., Berman B. J., Golgher R. R., Stancek D. Purification, characterization, and attempts at isotopic labeling of mouse interferon. J Virol. 1970 Feb;5(2):145–152. doi: 10.1128/jvi.5.2.145-152.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G., Carruba G., Garaci E., Jaffe B. M., Benedetto A. Prostaglandins of the A series inhibit Sendai virus replication in cultured cells. J Gen Virol. 1981 Mar;53(Pt 1):75–83. doi: 10.1099/0022-1317-53-1-75. [DOI] [PubMed] [Google Scholar]
- Schwartzman M., Liberman E., Raz A. Bradykinin and angiotensin II activation of arachidonic acid deacylation and prostaglandin E2 formation in rabbit kidney. Hormone-sensitive versus hormone-insensitive lipid pools of arachidonic acid. J Biol Chem. 1981 Mar 10;256(5):2329–2333. [PubMed] [Google Scholar]
- Spector A. A., Kaduce T. L., Hoak J. C., Fry G. L. Utilization of arachidonic and linoleic acids by cultured human endothelial cells. J Clin Invest. 1981 Oct;68(4):1003–1011. doi: 10.1172/JCI110322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey M. G., Rochette-Egly C., Castagna M. Effect of interferon on concentrations of cyclic nucleotides in cultured cells. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3890–3893. doi: 10.1073/pnas.76.8.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B. Prostacyclin. Prog Hemost Thromb. 1982;6:113–138. [PubMed] [Google Scholar]
- Yaron M., Yaron I., Gurari-Rotman D., Revel M., Lindner H. R., Zor U. Stimulation of prostaglandin E production in cultured human fibroblasts by poly(I)-poly(C) and human interferon. Nature. 1977 Jun 2;267(5610):457–459. doi: 10.1038/267457a0. [DOI] [PubMed] [Google Scholar]


