Abstract
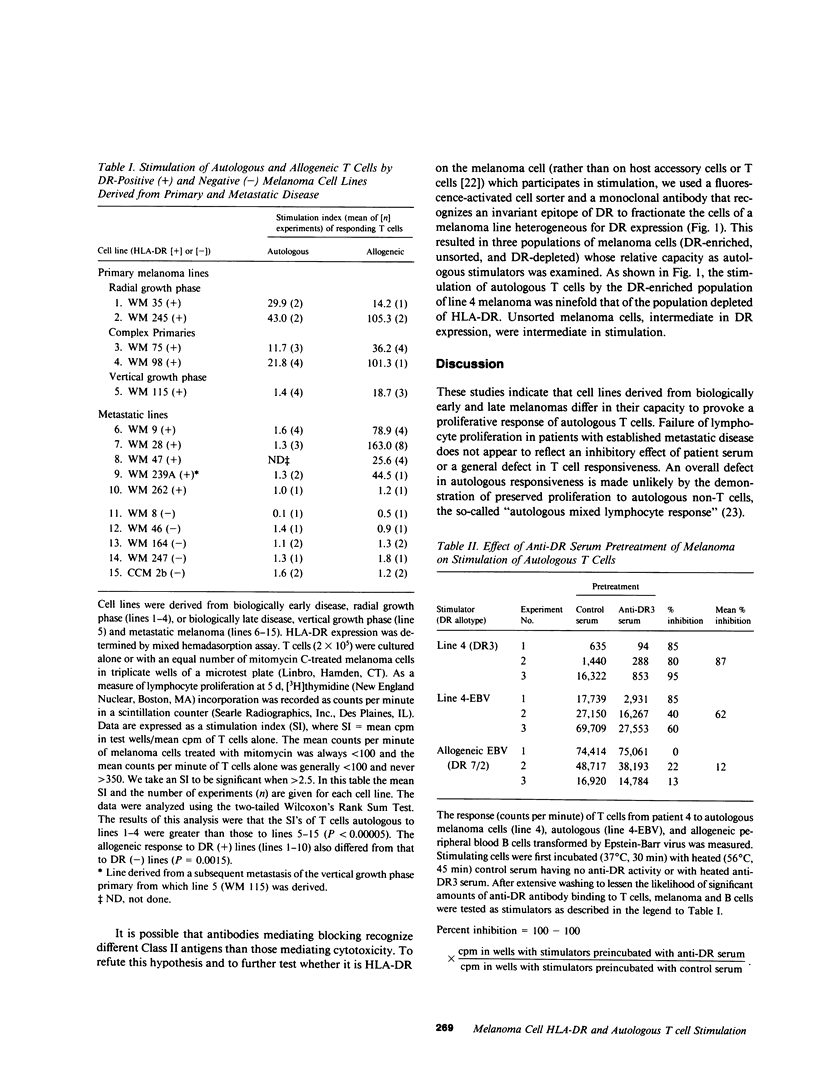
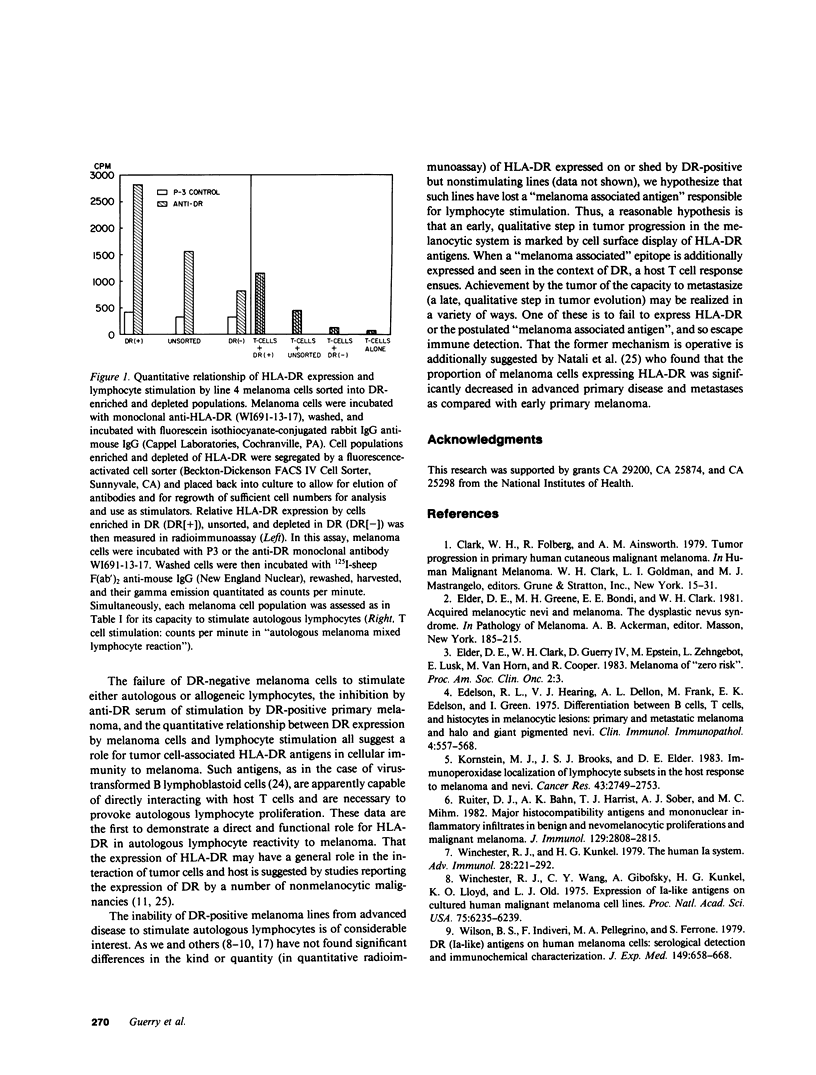
HLA-DR histocompatibility antigens are commonly expressed by the melanocytes of melanoma and its precursors, but not by the melanocyte of normal skin. Further, the primary lesion of biologically early melanoma is commonly infiltrated with host T cells. Advanced disease is characterized by a paucity of such cells. To investigate the interaction of melanoma cells and autologous lymphocytes and its dependence on HLA-DR expression, we have established cell lines from biologically early (4 lines) and advanced disease (11 lines) and examined their capacity to stimulate blastogenesis of autologous T cells in vitro. Melanocytes from early disease expressed HLA-DR antigens and stimulated autologous T cells. Those from advanced disease, irrespective of DR expression, were nonstimulatory. To determine whether expression of DR was required for melanoma cells to be stimulatory, we first treated a stimulating cell line of DR3 allospecificity with anti-DR3-specific serum and demonstrated marked inhibition of its capacity to provoke blastogenesis. Next we used fluorescence-activated flow cytometry to sort a stimulating line heterogeneous for DR expression into DR-enriched and -depleted populations. When such cells were examined in the lymphocyte proliferation assay, their stimulatory capacity was proportional to their quantitative expression of HLA-DR. These studies indicate that cell lines may reflect important biological differences between early and advanced melanoma. HLA-DR expression may be an early event in neoplasia of melanocytes. These antigens are able to interact directly with autologous T cells; and their expression is necessary, but not sufficient, for melanoma cells to induce lymphocyte proliferation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edelson R. L., Hearing V. J., Dellon A. L., Frank M., Edelson E. K., Green I. Differentiation between B cells, T cells, and histiocytes in melanocytic lesions: primary and metastatic melanoma and halo and giant pigmented nevi. Clin Immunol Immunopathol. 1975 Nov;4(4):557–568. doi: 10.1016/0090-1229(75)90097-5. [DOI] [PubMed] [Google Scholar]
- Goldstein L. T., Klinman N. R., Manson L. A. A microtest radioimmunoassay for noncytotoxic tumor-specific antibody to cell-surface antigens. J Natl Cancer Inst. 1973 Nov;51(5):1713–1715. doi: 10.1093/jnci/51.5.1713. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Clark W. H., Jr, Mastrangelo M. J., Guerry D. P., 4th, Elder D. E., LaRossa D., Hamilton R., Bondi E., Tuthill R., Steplewski Z. Specific immunoreactivity of hybridoma-secreted monoclonal anti-melanoma antibodies to cultured cells and freshly derived human cells. Cancer Res. 1980 Oct;40(10):3602–3609. [PubMed] [Google Scholar]
- Houghton A. N., Eisinger M., Albino A. P., Cairncross J. G., Old L. J. Surface antigens of melanocytes and melanomas. Markers of melanocyte differentiation and melanoma subsets. J Exp Med. 1982 Dec 1;156(6):1755–1766. doi: 10.1084/jem.156.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz T., Chu E., Geha R. S. Antigen presentation by human B cells: T cell proliferation induced by Epstein Barr virus B lymphoblastoid cells. J Immunol. 1982 Oct;129(4):1446–1450. [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Herlyn D., Herlyn M. Study of antibodies against human melanoma produced by somatic cell hybrids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3405–3409. doi: 10.1073/pnas.75.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein M. J., Brooks J. S., Elder D. E. Immunoperoxidase localization of lymphocyte subsets in the host response to melanoma and nevi. Cancer Res. 1983 Jun;43(6):2749–2753. [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. Lymphocyte transformation induced by autologous cells. IV. Human T-lymphocyte proliferation induced by autologous or allogeneic non-T lymphocytes. J Exp Med. 1976 May 1;143(5):1042–1054. doi: 10.1084/jem.143.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd K. O., Ng J., Dippold W. G. Analysis of the biosynthesis of HLA-DR glycoproteins in human malignant melanoma cell lines. J Immunol. 1981 Jun;126(6):2408–2413. [PubMed] [Google Scholar]
- Mitchell K. F., Fuhrer J. P., Steplewski Z., Koprowski H. Biochemical characterization of human melanoma cell surfaces: dissection with monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7287–7291. doi: 10.1073/pnas.77.12.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K. F., Ward F. E., Koprowski H. DR antigens on melanoma cells: analysis with monoclonal antibodies. Hum Immunol. 1982 Feb;4(1):15–26. doi: 10.1016/0198-8859(82)90046-5. [DOI] [PubMed] [Google Scholar]
- Mittal K. K., Mickey M. R., Singal D. P., Terasaki P. I. Serotyping for homotransplantation. 18. Refinement of microdroplet lymphocyte cytotoxicity test. Transplantation. 1968 Nov;6(8):913–927. doi: 10.1097/00007890-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M., Mann D. L. Evidence that antisera that react with products of the human HLA-DR locus may block in vitro antigen-induced proliferation by inducing suppression. J Immunol. 1982 May;128(5):2063–2066. [PubMed] [Google Scholar]
- Natali P. G., Cordiali-Fei P., Cavaliere R., Di Filippo F., Quaranta V., Pellegrino M. A., Ferrone S. Ia-like antigens on freshly explanted human melanoma. Clin Immunol Immunopathol. 1981 May;19(2):250–259. doi: 10.1016/0090-1229(81)90067-2. [DOI] [PubMed] [Google Scholar]
- Natali P. G., De Martino C., Quaranta V., Bigotti A., Pellegrino M. A., Ferrone S. Changes in Ia-like antigen expression on malignant human cells. Immunogenetics. 1981;12(3-4):409–413. doi: 10.1007/BF01561680. [DOI] [PubMed] [Google Scholar]
- Pollack M. S. The detection of weak allogeneic stimulation by DR-positive tumor cell lines. Transplant Proc. 1981 Dec;13(4):1947–1951. [PubMed] [Google Scholar]
- Ruiter D. J., Bhan A. K., Harrist T. J., Sober A. J., Mihm M. C., Jr Major histocompatibility antigens and mononuclear inflammatory infiltrate in benign nevomelanocytic proliferations and malignant melanoma. J Immunol. 1982 Dec;129(6):2808–2815. [PubMed] [Google Scholar]
- Sugden B., Mark W. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J Virol. 1977 Sep;23(3):503–508. doi: 10.1128/jvi.23.3.503-508.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. J., Herlyn M. F., Elder D. E., Clark W. H., Steplewski Z., Koprowski H. Expression of DR antigens in freshly frozen human tumors. Hybridoma. 1982;1(2):161–168. doi: 10.1089/hyb.1.1982.1.161. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Indiveri F., Pellegrino M. A., Ferrone S. DR (Ia-like) antigens on human melanoma cells. Serological detection and immunochemical characterization. J Exp Med. 1979 Mar 1;149(3):658–668. doi: 10.1084/jem.149.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Kunkel H. G. The human Ia system. Adv Immunol. 1979;28:221–292. [PubMed] [Google Scholar]
- Winchester R. J., Wang C. Y., Gibofsky A., Kunkel H. G., Lloyd K. O., Old L. J. Expression of Ia-like antigens on cultured human malignant melanoma cell lines. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6235–6239. doi: 10.1073/pnas.75.12.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]


