Significance
Ovulation in mammals requires activation of EGF receptor (EGFR) signaling within the ovarian follicle, but the mechanisms responsible for implementing the EGFR network during follicular growth remain incompletely understood. The final phase of growth is driven by FSH. Here we show that during this phase EGFR expression increases sharply in follicular granulosa cells and that this increase requires FSH; we provide evidence that the FSH-dependent increase is essential for EGFR signaling. FSH also is known to induce expression of luteinizing hormone (LH) receptors in the granulosa, permitting them to release EGFR ligands in response to preovulatory LH. By coordinating receptor expression and ligand release, FSH endows fully grown follicles with the capacity to activate EGFR signaling at ovulation.
Keywords: ovary, follicle, FSH, EGFR, granulosa
Abstract
Fertility depends on the precise coordination of multiple events within the ovarian follicle to ensure ovulation of a fertilizable egg. FSH promotes late follicular development, including expression of luteinizing hormone (LH) receptor by the granulosa cells. Expression of its receptor permits the subsequent LH surge to trigger the release of ligands that activate EGF receptors (EGFR) on the granulosa, thereby initiating the ovulatory events. Here we identify a previously unknown role for FSH in this signaling cascade. We show that follicles of Fshb−/− mice, which cannot produce FSH, have a severely impaired ability to support two essential EGFR-regulated events: expansion of the cumulus granulosa cell layer that encloses the oocyte and meiotic maturation of the oocyte. These defects are not caused by an inability of Fshb−/− oocytes to produce essential oocyte-secreted factors or of Fshb−/− cumulus cells to respond. In contrast, although expression of both Egfr and EGFR increases during late folliculogenesis in Fshb+/− females, these increases fail to occur in Fshb−/− females. Remarkably, supplying a single dose of exogenous FSH activity to Fshb−/− females is sufficient to increase Egfr and EGFR expression and to restore EGFR-dependent cumulus expansion and oocyte maturation. These studies show that FSH induces an increase in EGFR expression during late folliculogenesis and provide evidence that the FSH-dependent increase is necessary for EGFR physiological function. Our results demonstrate an unanticipated role for FSH in establishing the signaling axis that coordinates ovulatory events and may contribute to the diagnosis and treatment of some types of human infertility.
Fertility in mammals depends on the coordinated execution of multiple events within the fully grown ovarian follicle at the time of ovulation (1, 2). The oocyte undergoes meiotic maturation, during which it progresses to metaphase II of meiosis and acquires the ability to begin embryonic development (3). Concomitantly, the layer of granulosa cells (GCs) immediately surrounding the oocyte, termed the “cumulus,” undergoes a process termed “expansion,” which is required for sperm to penetrate this layer and reach the oocyte (4–7). At the perimeter of the follicle, an inflammatory response associated with rupture of the follicular wall permits the cumulus–oocyte complex (COC) to escape from the follicle and enter the oviduct where fertilization will occur. These events are triggered by the preovulatory release of luteinizing hormone (LH), which acts on LH receptors (LHCGR) on the mural GCs that line the interior wall of the fully grown follicle (8).
Recent studies have identified a key downstream effector of LH activity at ovulation. Binding of LH to LHCGR triggers the release of the EGF-related peptides amphiregulin (AREG, betacellulin (BTC), and epiregulin (EREG) (9–11). These bind to EGF receptors (EGFRs) located on both the mural and cumulus GCs (12–19) and activate MAPK3/1 as well as other signaling networks (20–28). Considerable evidence supports the view that the EGFR signaling mediates many or most ovulatory events. First, the release of the EGFR ligands follows the LH surge but precedes the LH-dependent responses (9–11). Second, EGF and the EGFR ligands can induce cumulus expansion and oocyte maturation in vitro, independently of LH (9, 10, 20, 29). Third, these events are impaired in mice bearing a hypomorphic Egfr allele that reduces EGFR activity by about one-half and in mice in which Egfr has been selectively inactivated in GCs through a targeted mutation (22, 23). Thus, the activation of EGFR signaling in GCs of mature follicles appears to be a major effector of the ovulatory response to LH.
FSH binds to receptors located on GCs and induces the expression of numerous genes, including Lhcgr (8, 30). Lhcgr expression is impaired substantially in mice that lack either FSH, because of targeted mutation of the Fshb gene that encodes its β-subunit, or the FSH receptor and in humans bearing spontaneous mutations; these individuals fail to ovulate (31–34). Thus, the ovulatory response to LH depends strictly on the prior FSH-dependent expression of Lhcgr, and in this manner FSH indirectly controls the LHCGR-regulated release of the EGFR ligands. We report here that FSH also drives an increase in EGFR expression during late folliculogenesis and provide evidence that this increase is essential to enable the ovulatory response to EGF. By coordinating the expression of EGFR and the release of its ligands, FSH endows full-grown follicles with the capacity to activate EGFR signaling at ovulation.
Results
COCs of Fshb−/− Females Do Not Expand or Up-Regulate Expansion-Related Transcripts in Response to EGF.
To examine whether FSH was required for activation of follicular EGFR signaling, we analyzed Fshb−/− mice, which cannot produce FSH (35). Follicular development in Fshb−/− females is overtly normal until the early antral stage but then becomes arrested. These females fail to ovulate and hence are infertile (35, 36). Nonetheless, there is little or no effect on the expression of GC genes not regulated by FSH (31), and the follicles retain the ability to support the development of fully grown meiotically competent oocytes and to maintain them in prophase arrest (36). Although the oocytes can mature in vitro, they develop poorly after fertilization, indicating that some aspect of their development is abnormal (31, 36). Fshb+/− females are fertile (31, 36) and served as controls in our experiments. Because the absence of FSH eventually leads to follicular atresia (37), we used prepubertal Fshb−/− females so that we could study the large cohort of follicles that initiates growth shortly after birth in the mouse, reaching full size after about 3 wk. By using prepubertal Fshb+/− and Fshb−/− animals of the same age, we were able to compare follicles that had been growing for the same period in the presence or absence of FSH, before follicular atresia occurs in Fshb−/− females.
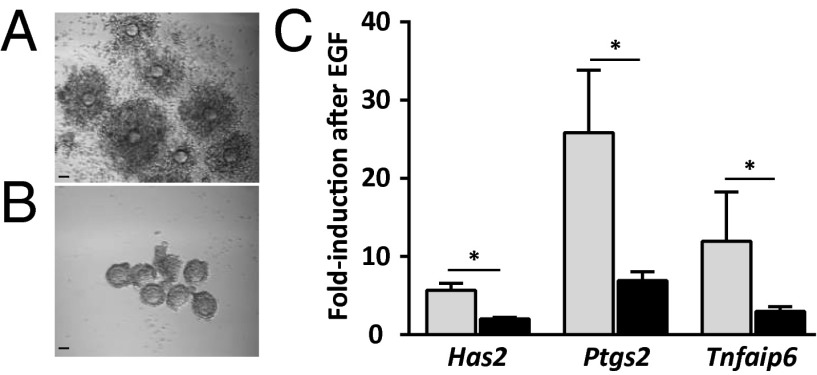
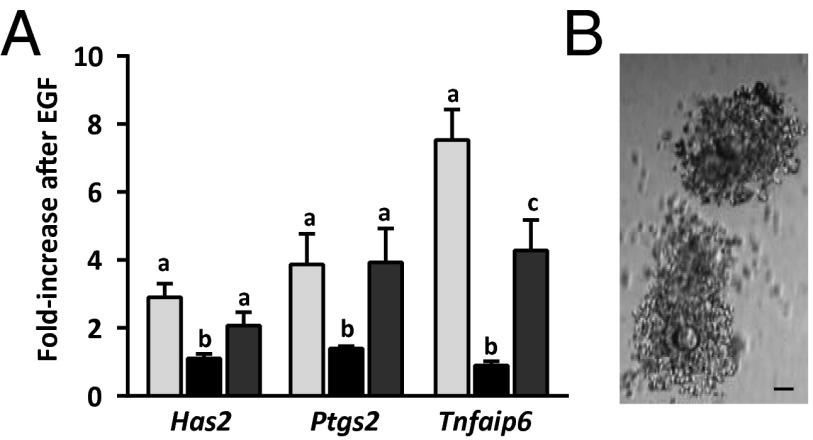
We first assessed cumulus expansion, which occurs downstream of EGFR signaling (9, 10). When we obtained COCs of Fshb+/− females at postnatal days (P) 21/23 and incubated them in the presence of EGF for 16 h, they expanded as expected (Fig. 1A). Although growing follicles of Fshb−/− females do not form large antra (35, 36), we were able to puncture the largest follicles and recover oocytes enclosed by GCs, which we provisionally term “COCs.” In contrast to the COCs of Fshb+/− females, COCs from Fshb−/− females of the same age did not expand in response to EGF (Fig. 1B). Hence, this EGFR-dependent event failed to occur in COCs from follicles that had grown in the absence of FSH. We note that COCs from hypogonadal mice lacking FSH (on a different genetic background than the mice used here) can undergo expansion in vitro (38).
Fig. 1.
COCs of Fshb−/− females do not undergo cumulus expansion or up-regulate expansion-related mRNAs in response to EGF. (A and B) COCs isolated from follicles of Fshb+/− females (A) or Fshb−/− females (B) at P21/23 and incubated for 16 h in the presence of EGF. Micrographs are representative of three independent experiments. (Scale bars: 50 µm.) (C) Fold-increase in Has2, Ptgs2, and Tnfaip6 in COCs of Fshb+/− females (gray bars) and Fshb−/− females (black bars) following EGF stimulation relative to nonstimulated follicles of the same genotype. Data were analyzed using two-sample t test. *P < 0.05.
Cumulus expansion requires EGFR-dependent up-regulation of a subset of genes, including hyaluronan synthase 2 (Has2), prostaglandin-endoperoxide synthase 2 (Ptgs2), and tumor necrosis factor, alpha-induced protein (Tnfaip6) (6, 39–43). Therefore we isolated COCs from P21/23 Fshb+/− and Fshb−/− females and examined basal and EGF-stimulated expression of these genes. Before EGF treatment, Has2, Ptgs2, and Tnfaip6 mRNA levels were lower in Fshb−/− COCs than in heterozygotes (Fig. S1). Upon EGF stimulation, the quantity of all three transcripts increased significantly more in COCs of Fshb+/− females than in those of Fshb−/− females (Fig. 1C). Several lines of evidence indicate that the impaired Fshb−/− response is unlikely to reflect incipient atresia of the follicles (37). First, histological sections of Fshb−/− ovaries at P24 showed no evidence of increased pycnotic nuclei in Fshb−/− as compared with Fshb+/− ovaries (Fig. S2A). Second, GCs isolated from Fshb+/− and Fshb−/− follicles at P21/23 formed indistinguishable monolayers in tissue culture (Fig. S2B). Third, there was no difference between the two genotypes in the follicular expression of genes associated with atresia or apoptosis (Fig. S2C) (44, 45). We conclude that COCs of Fshb−/− females express lower constitutive levels of the three EGF-regulated transcripts and show an impaired functional and transcriptional response to EGF stimulation.
Oocyte-Secreted Factors Required for EGF Responses Are Functional in COCs of Fshb−/− Females.
Oocyte-secreted factors (OSFs), including growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15), are essential for EGF-induced Has2, Ptgs2, and Tnfaip6 up-regulation and cumulus expansion (46–51). Their effects in GCs are transduced by activation of the Sma- and Mad-related proteins (SMAD) pathway. Therefore we tested whether the failure of Fshb−/− COCs to expand in response to EGF could be attributed to insufficient production of OSFs or their inability to activate SMAD signaling.
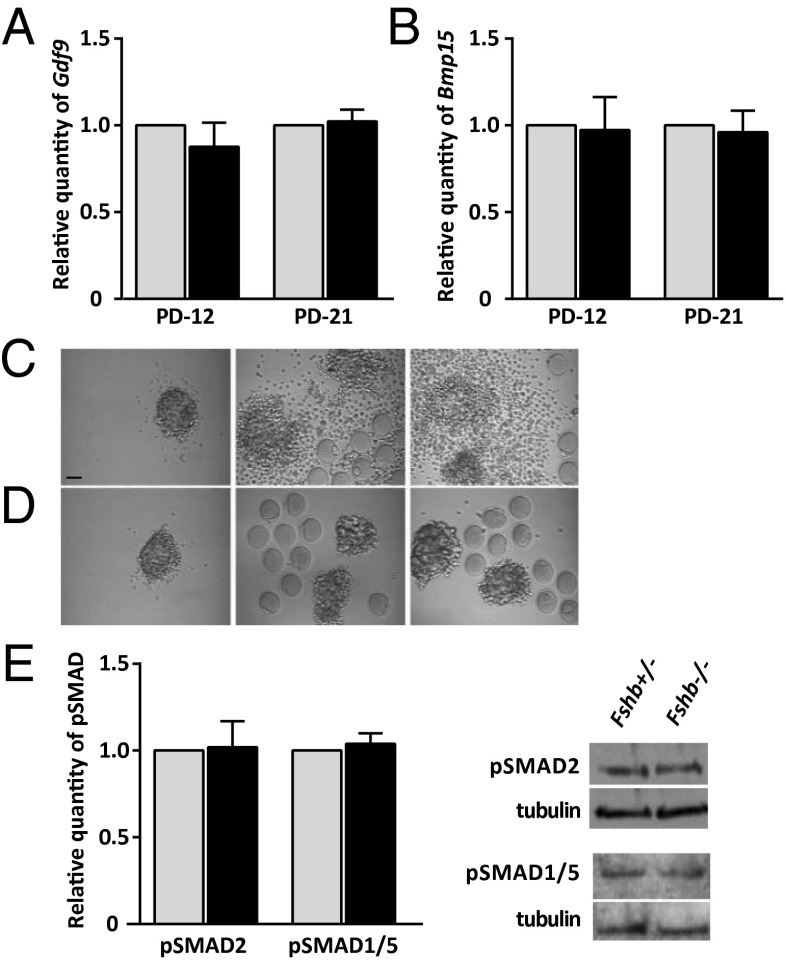
We first quantified Gdf9 and Bmp15 mRNAs and found no difference in transcript levels between Fshb+/− and Fshb−/− females in either growing (P12) or fully grown (P21/23) oocytes (Fig. 2 A and B). To test directly whether the oocytes produced OSFs, we then performed a cross-fostering experiment. We removed the oocyte from complexes of Fshb+/− and Fshb−/− females at P21/23 and incubated the oocytectomized complexes (OOXs) with oocytes derived from the other genotype. As expected, neither Fshb+/− nor and Fshb−/− OOXs expanded in response to EGF when cultured in the absence of oocytes (Fig. 2 C and D, Left). Heterozygous OOXs underwent expansion in response to EGF when incubated with oocytes of Fshb+/− females (Fig. 2C, Center) or with oocytes of Fshb−/− females (Fig. 2C, Right). Thus, the oocytes of Fshb−/− females produce sufficient OSFs to permit cumulus expansion in response to EGF. In contrast, Fshb−/− OOXs failed to expand in response to EGF when incubated with oocytes of either Fshb+/− (Fig. 2D, Center) or Fshb−/− (Fig. 2D, Right) females. However, the levels of phosphorylated SMAD2/3 (pSMAD2/3) (a GDF9 effector) and pSMAD1/5 (a BMP15 effector) in COCs were similar in P21/23 Fshb+/− and Fshb−/− females (Fig. 2E). This finding suggests that the Fshb−/− complexes activate SMAD signaling in response to OSFs. Together, these results indicate that the inability of the Fshb−/− complexes to expand in response to EGF is not caused by a failure of the oocytes to produce OSFs or by a failure of GCs to respond.
Fig. 2.
Fshb−/− follicles produce OSFs required for response to EGF. (A and B) Relative quantity of Gdf9 (A) and Bmp15 (B) in growing (P12) and fully grown (P21/23) oocytes of Fshb+/− females (gray bars) and Fshb−/− females (black bars) (value set to 1 for each mRNA). (C and D) Fshb+/− (C) or Fshb−/− (D) OOX following incubation for 16 h in the presence of EGF with no oocytes (Left) or with oocytes of Fshb+/− females (Center) or Fshb−/− females (Right). Micrographs are representative of three independent experiments. (Scale bar: 50 µm.) (E) Basal levels of pSMAD2 and pSMAD1/5 in freshly collected COCs of Fshb+/− females (gray bars) and Fshb−/− females (black bars) at P21/23. Representative immunoblots are shown. Data in A, B, and E were analyzed using single-sample t test.
Egfr and EGFR Expression and Activity Are Reduced in the GCs of Fshb−/− Females.
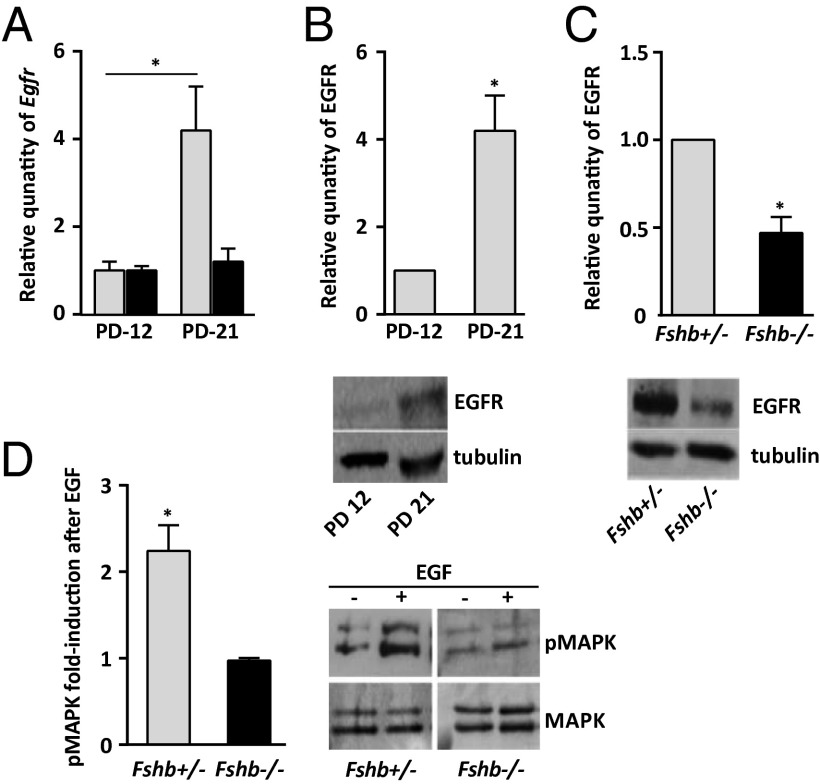
In mice that bear the hypomorphic Egfrwa/wa allele, EGFR activity in GCs is reduced by about one half, and oocyte maturation and ovulation are severely impaired (22). This impairment demonstrates that a relatively modest reduction in EGFR activity can have a significant physiological effect and prompted us to examine whether the absence of cumulus expansion in response to EGF in the Fshb−/− females was associated with a reduced expression of Egfr. At P12, when the population of growing follicles is at the secondary preantral stage, we observed no difference in the quantity of Egfr mRNA in GCs of Fshb+/− and Fshb−/− females (Fig. 3A). By P21/23, however, Egfr had increased significantly in GCs of early antral follicles of Fshb+/− females, but no increase was apparent in GCs of follicles of Fshb−/− females of the same age (Fig. 3A). Moreover, the increase in Egfr in the GCs of growing follicles of Fshb+/− females between P12 and P21/23 was accompanied by a quantitatively similar increase in EGFR protein (Fig. 3B). However, as was consistent with the reduced quantity of Egfr, GCs of follicles of Fshb−/− females at P21/23 contained only about one half as much EGFR as those of the Fshb+/− females (Fig. 3C). Thus, Egfr mRNA and protein both accumulate during late follicular growth but fail to accumulate normally in follicles that grow in the absence of FSH. In contrast, the amounts of its ligand-encoding mRNAs Areg, Btc, and Ereg did not significantly differ between GCs of P21/23 Fshb+/− and Fshb−/− females (Fig. S3).
Fig. 3.
FSH increases the expression and activity of EGFR in GCs. (A) Relative quantity of Egfr in GCs of Fshb+/− females (gray bars) and Fshb−/− females (black bars) at P12 and P21/23. (B) Relative quantity of EGFR in GCs of Fshb+/− females at P12 and P21/23. (C) Relative quantity of EGFR in GCs of Fshb+/− females (gray bars) and Fshb−/− females (black bars) at P21/23. (D) Fold-increase in pMAPK3/1 in follicles of Fshb+/− females (gray bars) and Fshb−/− females (black bars) at P21/23 following stimulation with EGF as compared with nonstimulated controls of the same genotype. Data were analyzed using a single-sample t test in B–D and a two-sample t test in A. *P < 0.05. The asterisk in D denotes a significant difference between EGF-stimulated and nonstimulated follicles of the same genotype. Representative immunoblots are shown. (Note: The lanes shown in C are from the same blot but were not adjacent as shown here.)
Because GCs of Fshb−/− follicles contained detectable EGFR (although it was reduced compared with the heterozygotes), we tested whether EGFR signaling activity was impaired. MAPK3 and 1 are the principal effectors of EGFR signaling, and their activation by phosphorylation is necessary for cumulus expansion (21, 28, 39, 50). Upon the addition of EGF, MAPK3/1 phosphorylation increased significantly in Fshb+/− follicles but did not change detectably in Fshb−/− follicles (Fig. 3D). Thus, the reduced expression of EGFR was associated with impaired EGFR signaling in Fshb−/− follicles.
Exogenous FSH Activity Rescues EGFR Expression, Signaling, and Activity in GCs of Fshb−/− Females.
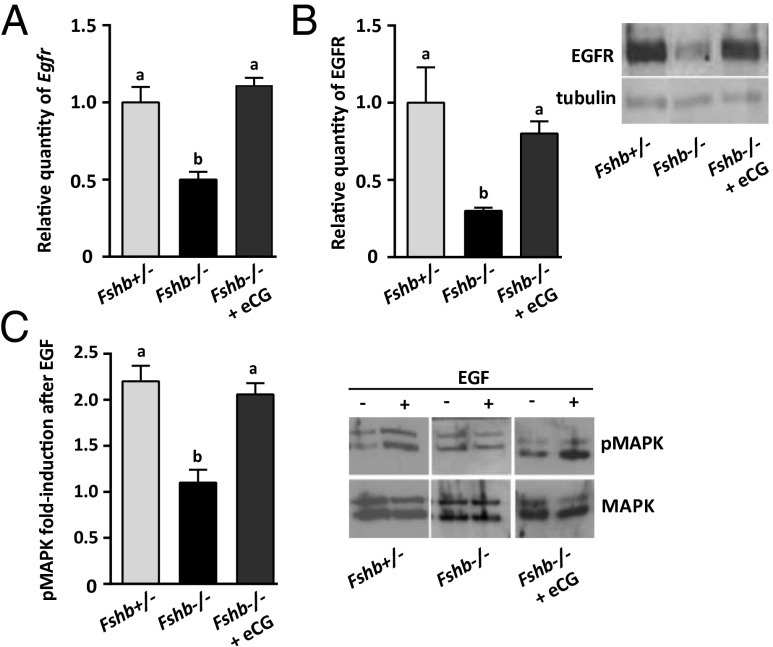
To clarify further the link between FSH and EGFR expression in GCs, we then tested whether providing a brief pulse of FSH activity could restore EGFR expression and signaling in follicles that had grown in the absence of FSH. We injected equine chorionic gonadotropin (eCG), which exhibits FSH activity and is commonly used in vivo because of its long half-life (52), into Fshb−/− females at P16, After 48 h we measured Egfr and EGFR expression in follicles in these females and in the follicles of noninjected P18 Fshb−/− and Fshb+/− females. Both Egfr (Fig. 4A) and EGFR (Fig. 4B) levels were significantly higher in eCG-injected than in noninjected Fshb−/− females and were close to the amounts in Fshb+/− females. Two known FSH targets, Cyp19a1 and Lhcgr, were reduced in Fshb−/− females, as previously reported (31), and were up-regulated after eCG priming (Fig. S4). In contrast with previous results (31), we found no change in the level of Fshr in GCs of Fshb−/− females, although it increased following eCG priming.
Fig. 4.
Supplying FSH activity to follicles of Fshb−/− females in vivo restores EGFR expression and activity. All histograms show follicles of P18 Fshb+/− females (light gray bars), P18 Fshb−/− females (black bars), and P18 Fshb−/− females injected 48 h previously with eCG (dark gray bars). (A) Relative quantity of Egfr. (B) Relative quantity of EGFR. (C) pMAPK3/1 following EGF stimulation relative to nonstimulated controls. Data were analyzed using one-way ANOVA and Tukey HSD. Different letters above bars indicate P < 0.05. Representative immunoblots are shown.
Coupled to the eCG-induced increase in Egfr expression was an increase in the phosphorylation of MAPK3/1 in response to EGF (Fig. 4C). Moreover, EGF stimulation of follicles of eCG-primed Fshb−/− females caused an increase in the quantity of the three expansion-related mRNAs to levels similar to those observed in heterozygous females (Fig. 5A) and induced cumulus expansion in primed Fshb−/− females (Fig. 5B). Further confirming the link between FSH and Egfr expression, priming P16 Fshb+/− females with eCG similarly caused a significant increase in Egfr mRNA compared with nonprimed Fshb+/− females (Fig. S5A). The up-regulation of expansion-related mRNAs in response to EGF was not augmented in the eCG-primed Fsh+/− females (Fig. S5B), possibly indicating that endogenous EGFR is sufficient for maximal activation of its signaling pathway. Hence, providing exogenous FSH activity to Fshb−/− females increased follicular EGFR expression and signaling activity and restored the physiological responses to EGF.
Fig. 5.
Supplying FSH activity to Fshb−/− females restores the ability to undergo cumulus expansion in vitro. (A) Relative quantity of Has2, Ptgs2, and Tnfaip6 in follicles of P18 Fshb+/− females (light gray bars), Fshb−/− females (black bars), and Fshb−/− females injected 48 h previously with eCG (dark gray bars) after stimulation with EGF as compared with nonstimulated follicles of the same genotype. (B) Cumulus expansion in COCs of eCG-injected Fshb−/− females following EGF stimulation. (Scale bar: 50 µm.) Data were analyzed using one-way ANOVA and Tukey HSD. Different letters above bars indicate P < 0.05.
EGF-Induced Oocyte Meiotic Maturation Is Impaired in Fshb−/− Follicles.
To test whether follicular growth in the presence of FSH was more broadly required for EGFR-dependent signaling at ovulation, we examined meiotic maturation of the oocyte. EGFR ligands do not appear to initiate maturation by acting directly on the oocyte but instead reduce the transmission of inhibitory molecules to the oocyte from the EGFR-expressing GCs (9, 10, 23, 53). We isolated follicles from Fshb+/− and Fshb−/− females at P21/23, when developing oocytes of both genotypes have acquired meiotic competence (36). After incubating the follicles overnight in the presence or absence of EGF, we removed the oocytes and recorded the fraction that had undergone germinal vesicle breakdown (GVBD) indicative of maturation initiation (54). In the absence of EGF, only a small fraction of the oocytes in both Fshb−/− and Fshb+/− follicles underwent GVBD (Fig. 6). This result demonstrates that the follicles of P21/23 Fshb−/− females retain the ability to hold oocytes in meiotic arrest. The addition of EGF triggered maturation of most oocytes within Fshb+/− follicles, but this response was attenuated significantly in Fshb−/− follicles (Fig. 6). However, when Fshb−/− females were injected with eCG 48 h before follicle isolation, the fraction of oocytes that underwent GVBD in response to EGF was restored to that of the Fshb+/− females. Hence, FSH signaling promotes EGFR-dependent ovulation-associated events in the germ line as well as in the somatic compartment of the follicle.
Fig. 6.

Prior exposure to FSH promotes EGF-stimulated oocyte meiotic maturation. Follicles of Fshb+/− females (light gray bars), Fshb−/− females (black bars), or eCG-injected Fshb−/− females (dark gray bars) at P21/23 were incubated for 16 h in the presence or absence of EGF, and the percentage of oocytes that underwent GVBD was recorded. Data were analyzed using one-way ANOVA and Tukey HSD. Different letters above bars indicate P < 0.05.
Discussion
The EGFR signaling axis is a major effector of LH-dependent ovulatory events (9, 10, 20, 22, 25). We demonstrate here that follicles of Fshb−/− females suffer a severely impaired ability to undergo two key EGFR-regulated events, expansion of the cumulus cells and oocyte meiotic maturation, in response to EGF. In other respects, however, Fshb−/− follicles resemble wild-type follicles, at least during the time period studied here. For example, they show little change in the expression of numerous genes, apart from those known to be FSH-regulated (31), are able to support growth and the acquisition of meiotic competence, and are able to hold competent oocytes in meiotic arrest (36), which requires the production of cGMP by GCs and its transfer to the oocyte (55, 56). Our results also suggest that GCs of Fshb−/− females can activate SMAD signaling in response to OSFs. In addition, transcript levels of EGF-like peptides were normal. Thus, the inability of the Fshb−/− follicles to initiate cumulus expansion or oocyte maturation in response to EGF is unlikely to reflect a nonspecific loss of follicular function in the absence of FSH but rather indicates that FSH promotes specific events that enable the ovulatory response to EGF.
Several lines of evidence suggest that FSH promotes EGF responsiveness by increasing the expression of EGFR. First, the expression of both Egfr and EGFR increased during late folliculogenesis in Fshb+/− but not in Fshb−/− females. Second, an ∼50% decrease in EGFR activity in GCs, similar to that we observed in Fshb−/− females, is sufficient to impair the ovulatory response severely (22). Third, eCG injection into Fshb−/− females increased the expression of Egfr as well as that of known FSH targets. It also restored both cumulus expansion and oocyte maturation, which are independently regulated downstream of EGFR activation. We propose that FSH stimulates an increase in EGFR expression and activity in GCs and that this increase is essential to enable its ligands to trigger cumulus expansion, oocyte maturation, and perhaps other events of ovulation. However, notwithstanding the link between the increase in EGFR expression and response to EGF, other FSH-dependent events also may prepare GCs to respond to EGFR ligands.
Egfr expression increases during antral folliculogenesis in the hamster, and this increase is abolished when FSH is depleted by hypophysectomy (13). Egfr also is expressed in antral follicles of humans (19, 57). In the goat, stimulation with exogenous FSH increases the expression of Egfr in the cumulus GCs (58). These results are consistent with ours and suggest that FSH may play a key conserved role in regulating follicular EGFR expression in mammals. Although mutations in Fshb or in the FSH receptor are relatively rare in humans (33, 34, 59), women bearing these mutations would be candidates for assisted reproduction, including in vitro maturation, because of the probable absence of LHCGR on GCs. EGF is used increasingly during in vitro maturation, where it has been shown to increase its efficiency (60–62). Our results suggest that women lacking FSH activity might require a modified therapeutic intervention.
The mechanism by which FSH regulates the expression of Egfr remains to be established. Some effects of FSH are mediated through estradiol (63). However, although cumulus expansion was impaired in some follicles of mice lacking estrogen receptor-β, it was normal in others (64). These ovaries also showed normal induction of Has2 in response to LH but reduced basal levels of Ptgs2 and Tnfaip6 (64, 65); these results suggest that EGFR signaling was partially functional in the absence of estrogen receptor-β. Alternatively, β-catenin and the transcription factor SP1, both of which are implicated in FSH-regulated gene expression (30, 66, 67), are important regulators of Egfr expression in other cell types (68–70).
Our results do not exclude a role for other factors in regulating Egfr expression, and previous work has shown that OSFs promote the expression of Egfr in the cumulus GCs (47–51, 71). However, the impaired Egfr expression we describe here is not likely to be caused by an absence of OSFs, because oocytes of Fshb−/− females express Bmp15 and Gdf9 and induce expansion of oocytectomized Fshb+/− complexes, indicating that they produce biologically active OSFs. It also is worth noting that, because OSFs typically generate differences between the cumulus and mural GCs (72), they might not be expected to regulate Egfr expression in the mural cells. FSH and OSFs may each contribute to establishing physiological levels of EGFR expression and activity.
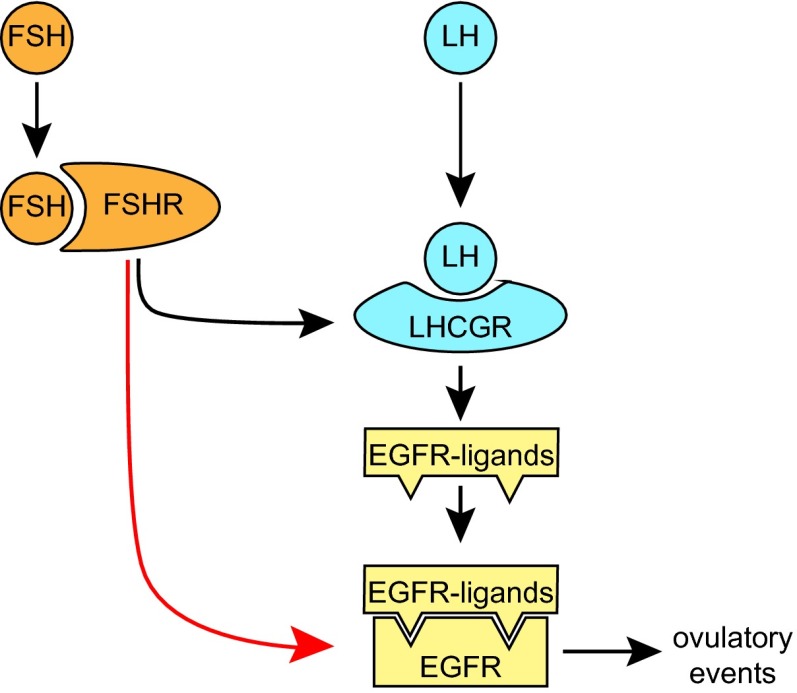
FSH has long been known to play an indispensable role in preparing the mature follicle to respond to the preovulatory LH surge (8) by stimulating the expression of LHCGR by the mural GCs. Recent work has shown that LH induces the mural GCs to release ligands that bind to EGFR on the mural and cumulus GCs and that are the proximate trigger of LH-regulated ovulatory events (9, 10, 22, 25, 27). Our results reveal that prior exposure to FSH is required for follicles to respond to EGF and that this exposure is associated with an FSH-dependent increase in EGFR expression and activity. Thus, FSH appears to play a larger role than previously thought in the ovulatory cascade (Fig. 7): It not only promotes the expression of LHCGR on the mural GCs, enabling them to release EGFR ligands in response to LH, but also promotes the expression of EGFR itself on these cells. The FSH-dependent remodeling of the late-follicular environment may ensure an efficient and coordinated response to ovulatory signals.
Fig. 7.
Dual role of FSH in establishing the EGFR signaling axis in the ovarian follicle. FSH induces GCs to express both LHCGR, which enables them to release EGFR ligands in response to the preovulatory LH surge, and EGFR, which enables them to respond to these ligands. The two-step FSH-driven remodeling of the late-follicular environment may ensure an efficient and coordinated response to ovulatory signals.
Methods
Mice.
All experiments were approved by the Animal Care Committee of the Royal Victoria Hospital and followed the regulations of the Canadian Council on Animal Care. Mice carrying a deletion in the gene encoding the β-subunit of FSH, Fshb (35), were obtained from Jackson Laboratories. Fshb+/− and Fshb−/− mice were generated by mating Fshb+/− females with Fshb−/− males, and offspring were genotyped as previously described (36). For some experiments, Fshb−/− females at P16 or P21/23 received an i.p. injection of 5 IU of eCG (Sigma) 48 h before sample collection.
Collection of Follicles, COCs, GCs, and Oocytes.
Cells were obtained from females at different ages as described (36). Briefly, intact follicles were obtained by dissecting the ovary using fine needles, and COCs were obtained by puncturing follicles that protruded from the ovarian surface. Mechanical or enzymatic methods were used to obtain purified GCs and oocytes from follicles. To examine cumulus expansion, COCs were incubated for 16 h in serum-free Eagle's minimum essential medium (MEM; Gibco) in the presence of 10 ng/mL EGF (Becton Dickinson) at 37 °C in a humidified atmosphere of 5% CO2 in air. To assess response to EGF, follicles or COCs were incubated as above for 10 min and then were processed. To assess oocyte maturation, follicles were incubated with EGF for 16 h, after which the oocytes were removed from the follicles and examined using light microscopy; the fraction that had undergone GVBD was recorded.
Oocytectomy.
Oocytectomy was performed using fine glass needles. OOX complexes were cocultured with oocytes (five oocytes per complex) in 25-µL drops of MEM under paraffin oil under the conditions described above. After 16 h, the expansion of the COCs was assessed qualitatively.
RNA Purification and Quantitative PCR.
RNA was purified, cDNA was generated, and quantitative PCR analysis performed as described (73). Primer sequences are given in Table S1. For each primer pair, a standard curve was generated using serial dilutions of cDNA prepared from ovarian RNA and used to determine the efficiency of amplification. Melting-curve analysis was used to verify product specificity. Data were analyzed using software provided by the manufacturer (Montréal Biotech). Relative quantities of amplified product were calculated according to 2-∆∆CT method, using Actb (actin) for normalization.
Immunoblotting.
Immunoblotting was performed as described (73). For EGFR and the pSMADs, each sample was loaded into one lane of a gel and was used to analyze both the protein of interest and tubulin. For MAPK3/1 analysis, each sample was suspended in loading buffer, heat-denatured, and then divided into two aliquots, one of which was used to detect total MAPK3/1 and the other to detect pMAPK3/1. Antibodies used were EGFR (sc-03; Santa Cruz Biotechnology); tubulin (T8203; Sigma); pSMAD2 (Ser465/467) (3108; Cell Signaling Technology); pSMAD1/5 (Ser463/465) (9516; Cell Signaling); MAPK3/1 (sc-94; Santa Cruz Biotechnology); and pMAPK3/1 (9106; Cell Signaling). Signals were quantified using Image J software (National Institutes of Health) and were normalized to the respective control (EGFR and pSMADs to tubulin; pMAPK3/1 to total MAPK3/1).
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism 6.0. A single-sample t test, a two-sample t test, or one-way ANOVA followed by a Tukey honestly significant difference (HSD) test was used, depending on the experiment. P < 0.05 was considered significant. All values represent the mean ± SEM of three or more independent experiments.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR) (to H.J.C.; by the Royal Victoria Hospital Foundation). S.E.-H. received support from the Centre for the Study of Reproduction at McGill and the CIHR Training Program in Reproduction, Early Development, and the Impact on Health. I.D. was supported by les Fonds de la Recherche Scientifique (Belgium).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414648111/-/DCSupplemental.
References
- 1.Richards JS. Ovulation: New factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234(1-2):75–79. doi: 10.1016/j.mce.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Richards JS, et al. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- 3.Channing CP, Hillensjo T, Schaerf FW. Hormonal control of oocyte meiosis, ovulation and luteinization in mammals. Clin Endocrinol Metab. 1978;7(3):601–624. doi: 10.1016/s0300-595x(78)80011-5. [DOI] [PubMed] [Google Scholar]
- 4.Eppig JJ. Gonadotropin stimulation of the expansion of cumulus oophori isolated from mice: General conditions for expansion in vitro. J Exp Zool. 1979;208(1):111–120. doi: 10.1002/jez.1402080112. [DOI] [PubMed] [Google Scholar]
- 5.Thibault C, Gerard M, Menezo Y. Preovulatory and ovulatory mechanisms in oocyte maturation. J Reprod Fertil. 1975;45(3):605–610. doi: 10.1530/jrf.0.0450605. [DOI] [PubMed] [Google Scholar]
- 6.Fülöp C, Salustri A, Hascall VC. Coding sequence of a hyaluronan synthase homologue expressed during expansion of the mouse cumulus-oocyte complex. Arch Biochem Biophys. 1997;337(2):261–266. doi: 10.1006/abbi.1996.9793. [DOI] [PubMed] [Google Scholar]
- 7.Salustri A, Camaioni A, Di Giacomo M, Fulop C, Hascall VC. Hyaluronan and proteoglycans in ovarian follicles. Hum Reprod Update. 1999;5(4):293–301. doi: 10.1093/humupd/5.4.293. [DOI] [PubMed] [Google Scholar]
- 8.Erickson GF, Wang C, Hsueh AJ. FSH induction of functional LH receptors in granulosa cells cultured in a chemically defined medium. Nature. 1979;279(5711):336–338. doi: 10.1038/279336a0. [DOI] [PubMed] [Google Scholar]
- 9.Ashkenazi H, et al. Epidermal growth factor family members: Endogenous mediators of the ovulatory response. Endocrinology. 2005;146(1):77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- 10.Park JY, et al. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 11.Sekiguchi T, et al. Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol. 2004;33(1):281–291. doi: 10.1677/jme.0.0330281. [DOI] [PubMed] [Google Scholar]
- 12.Gall L, Chene N, Dahirel M, Ruffini S, Boulesteix C. Expression of epidermal growth factor receptor in the goat cumulus-oocyte complex. Mol Reprod Dev. 2004;67(4):439–445. doi: 10.1002/mrd.20040. [DOI] [PubMed] [Google Scholar]
- 13.Garnett K, Wang J, Roy SK. Spatiotemporal expression of epidermal growth factor receptor messenger RNA and protein in the hamster ovary: Follicle stage-specific differential modulation by follicle-stimulating hormone, luteinizing hormone, estradiol, and progesterone. Biol Reprod. 2002;67(5):1593–1604. doi: 10.1095/biolreprod.102.005470. [DOI] [PubMed] [Google Scholar]
- 14.Hill JL, Hammar K, Smith PJ, Gross DJ. Stage-dependent effects of epidermal growth factor on Ca2+ efflux in mouse oocytes. Mol Reprod Dev. 1999;53(2):244–253. doi: 10.1002/(SICI)1098-2795(199906)53:2<244::AID-MRD13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Chabot JG, St-Arnaud R, Walker P, Pelletier G. Distribution of epidermal growth factor receptors in the rat ovary. Mol Cell Endocrinol. 1986;44(2):99–108. doi: 10.1016/0303-7207(86)90051-1. [DOI] [PubMed] [Google Scholar]
- 16.Singh B, Rutledge JM, Armstrong DT. Epidermal growth factor and its receptor gene expression and peptide localization in porcine ovarian follicles. Mol Reprod Dev. 1995;40(4):391–399. doi: 10.1002/mrd.1080400402. [DOI] [PubMed] [Google Scholar]
- 17.Göritz F, Jewgenow K, Meyer HH. Epidermal growth factor and epidermal growth factor receptor in the ovary of the domestic cat (Felis catus) J Reprod Fertil. 1996;106(1):117–124. doi: 10.1530/jrf.0.1060117. [DOI] [PubMed] [Google Scholar]
- 18.Qu J, Godin PA, Nisolle M, Donnez J. Distribution and epidermal growth factor receptor expression of primordial follicles in human ovarian tissue before and after cryopreservation. Hum Reprod. 2000;15(2):302–310. doi: 10.1093/humrep/15.2.302. [DOI] [PubMed] [Google Scholar]
- 19.el-Danasouri I, Frances A, Westphal LM. Immunocytochemical localization of transforming growth factor-alpha and epidermal growth factor receptor in human fallopian tubes and cumulus cells. Am J Reprod Immunol. 1993;30(2-3):82–87. doi: 10.1111/j.1600-0897.1993.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 20.Downs SM, Chen J. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev. 2008;75(1):105–114. doi: 10.1002/mrd.20781. [DOI] [PubMed] [Google Scholar]
- 21.Fan HY, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324(5929):938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh M, et al. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27(5):1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh M, Thao K, Conti M. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS ONE. 2011;6(6):e21574. doi: 10.1371/journal.pone.0021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keel BA, Hildebrandt JM, May JV, Davis JS. Effects of epidermal growth factor on the tyrosine phosphorylation of mitogen-activated protein kinases in monolayer cultures of porcine granulosa cells. Endocrinology. 1995;136(3):1197–1204. doi: 10.1210/endo.136.3.7867573. [DOI] [PubMed] [Google Scholar]
- 25.Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22(4):924–936. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prochazka R, Blaha M, Nemcova L. Signaling pathways regulating FSH- and amphiregulin-induced meiotic resumption and cumulus cell expansion in the pig. Reproduction. 2012;144(5):535–546. doi: 10.1530/REP-12-0191. [DOI] [PubMed] [Google Scholar]
- 27.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: Key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20(6):1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 28.Su YQ, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143(6):2221–2232. doi: 10.1210/endo.143.6.8845. [DOI] [PubMed] [Google Scholar]
- 29.Downs SM. Specificity of epidermal growth factor action on maturation of the murine oocyte and cumulus oophorus in vitro. Biol Reprod. 1989;41(2):371–379. doi: 10.1095/biolreprod41.2.371. [DOI] [PubMed] [Google Scholar]
- 30.Law NC, Weck J, Kyriss B, Nilson JH, Hunzicker-Dunn M. Lhcgr expression in granulosa cells: Roles for PKA-phosphorylated β-catenin, TCF3, and FOXO1. Mol Endocrinol. 2013;27(8):1295–1310. doi: 10.1210/me.2013-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burns KH, Yan C, Kumar TR, Matzuk MM. Analysis of ovarian gene expression in follicle-stimulating hormone beta knockout mice. Endocrinology. 2001;142(7):2742–2751. doi: 10.1210/endo.142.7.8279. [DOI] [PubMed] [Google Scholar]
- 32.Dierich A, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95(23):13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao YX, Segaloff DL. Follicle stimulating hormone receptor mutations and reproductive disorders. Prog Mol Biol Transl Sci. 2009;89:115–131. doi: 10.1016/S1877-1173(09)89005-4. [DOI] [PubMed] [Google Scholar]
- 34.Huhtaniemi IT. Themmen APN Mutations of gonadotropins and gonadotropin receptors: Elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21(5):551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- 35.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 36.Demeestere I, et al. 2012. Follicle-stimulating hormone accelerates mouse oocyte development in vivo. Biol Reprod 87(1):3, 1-11.
- 37.Chun SY, et al. Hormonal regulation of apoptosis in early antral follicles: Follicle-stimulating hormone as a major survival factor. Endocrinology. 1996;137(4):1447–1456. doi: 10.1210/endo.137.4.8625923. [DOI] [PubMed] [Google Scholar]
- 38.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol. 2007;305(1):300–311. doi: 10.1016/j.ydbio.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochsner SA, et al. Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology. 2003;144(10):4376–4384. doi: 10.1210/en.2003-0487. [DOI] [PubMed] [Google Scholar]
- 40.Fülöp C, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130(10):2253–2261. doi: 10.1242/dev.00422. [DOI] [PubMed] [Google Scholar]
- 41.Ochsner SA, Russell DL, Day AJ, Breyer RM, Richards JS. Decreased expression of tumor necrosis factor-alpha-stimulated gene 6 in cumulus cells of the cyclooxygenase-2 and EP2 null mice. Endocrinology. 2003;144(3):1008–1019. doi: 10.1210/en.2002-220435. [DOI] [PubMed] [Google Scholar]
- 42.Fülöp C, et al. Coding sequence, exon-intron structure and chromosomal localization of murine TNF-stimulated gene 6 that is specifically expressed by expanding cumulus cell-oocyte complexes. Gene. 1997;202(1-2):95–102. doi: 10.1016/s0378-1119(97)00459-9. [DOI] [PubMed] [Google Scholar]
- 43.Sugiura K, Su YQ, Eppig JJ. Targeted suppression of Has2 mRNA in mouse cumulus cell-oocyte complexes by adenovirus-mediated short-hairpin RNA expression. Mol Reprod Dev. 2009;76(6):537–547. doi: 10.1002/mrd.20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefèvre B. [Follicular atresia: Its features as predictive markers for the outcome of assisted reproduction] Gynecol Obstet Fertil. 2011;39(1):58–62. doi: 10.1016/j.gyobfe.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58(1):44–50. doi: 10.1262/jrd.2011-012. [DOI] [PubMed] [Google Scholar]
- 46.Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol. 1990;138(1):16–25. doi: 10.1016/0012-1606(90)90172-f. [DOI] [PubMed] [Google Scholar]
- 47.Diaz FJ, O’Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: Development of competence to undergo expansion. Dev Biol. 2006;299(1):91–104. doi: 10.1016/j.ydbio.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Dragovic RA, et al. Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod. 2007;76(5):848–857. doi: 10.1095/biolreprod.106.057471. [DOI] [PubMed] [Google Scholar]
- 49.Nagyova E, et al. Activation of cumulus cell SMAD2/3 and epidermal growth factor receptor pathways are involved in porcine oocyte-cumulus cell expansion and steroidogenesis. Mol Reprod Dev. 2011;78(6):391–402. doi: 10.1002/mrd.21312. [DOI] [PubMed] [Google Scholar]
- 50.Sasseville M, et al. Growth differentiation factor 9 signaling requires ERK1/2 activity in mouse granulosa and cumulus cells. J Cell Sci. 2010;123(Pt 18):3166–3176. doi: 10.1242/jcs.063834. [DOI] [PubMed] [Google Scholar]
- 51.Su YQ, et al. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol. 2010;24(6):1230–1239. doi: 10.1210/me.2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy BD, Martinuk SD. Equine chorionic gonadotropin. Endocr Rev. 1991;12(1):27–44. doi: 10.1210/edrv-12-1-27. [DOI] [PubMed] [Google Scholar]
- 53.Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA. 2005;102(45):16257–16262. doi: 10.1073/pnas.0508521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuetz AW. Role of hormones in oocyte maturation. Biol Reprod. 1974;10(2):150–178. doi: 10.1095/biolreprod10.2.150. [DOI] [PubMed] [Google Scholar]
- 55.Norris RP, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136(11):1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaccari S, Weeks JL, 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81(3):595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guzman L, et al. Human antral follicles <6 mm: A comparison between in vivo maturation and in vitro maturation in non-hCG primed cycles using cumulus cell gene expression. Mol Hum Reprod. 2013;19(1):7–16. doi: 10.1093/molehr/gas038. [DOI] [PubMed] [Google Scholar]
- 58.Almeida KC, et al. Real-time qRT-PCR analysis of EGF receptor in cumulus-oocyte complexes recovered by laparoscopy in hormonally treated goats. Zygote. 2011;19(2):127–136. doi: 10.1017/S0967199410000225. [DOI] [PubMed] [Google Scholar]
- 59.Casarini L, Pignatti E, Simoni M. Effects of polymorphisms in gonadotropin and gonadotropin receptor genes on reproductive function. Rev Endocr Metab Disord. 2011;12(4):303–321. doi: 10.1007/s11154-011-9192-2. [DOI] [PubMed] [Google Scholar]
- 60.Zamah AM, et al. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod. 2010;25(10):2569–2578. doi: 10.1093/humrep/deq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peluffo MC, et al. Amphiregulin promotes the maturation of oocytes isolated from the small antral follicles of the rhesus macaque. Hum Reprod. 2012;27(8):2430–2437. doi: 10.1093/humrep/des158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ben-Ami I, et al. In vitro maturation of human germinal vesicle-stage oocytes: Role of epidermal growth factor-like growth factors in the culture medium. Hum Reprod. 2011;26(1):76–81. doi: 10.1093/humrep/deq290. [DOI] [PubMed] [Google Scholar]
- 63.Adashi EY, Hsueh AJ. Estrogens augment the stimulation of ovarian aromatase activity by follicle-stimulating hormone in cultured rat granulosa cells. J Biol Chem. 1982;257(11):6077–6083. [PubMed] [Google Scholar]
- 64.Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146(8):3247–3262. doi: 10.1210/en.2005-0213. [DOI] [PubMed] [Google Scholar]
- 65.Binder AK, et al. The absence of ER-β results in altered gene expression in ovarian granulosa cells isolated from in vivo preovulatory follicles. Endocrinology. 2013;154(6):2174–2187. doi: 10.1210/en.2012-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alliston TN, Maiyar AC, Buse P, Firestone GL, Richards JS. Follicle stimulating hormone-regulated expression of serum/glucocorticoid-inducible kinase in rat ovarian granulosa cells: A functional role for the Sp1 family in promoter activity. Mol Endocrinol. 1997;11(13):1934–1949. doi: 10.1210/mend.11.13.0033. [DOI] [PubMed] [Google Scholar]
- 67.Parakh TN, et al. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proc Natl Acad Sci USA. 2006;103(33):12435–12440. doi: 10.1073/pnas.0603006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brandt B, Meyer-Staeckling S, Schmidt H, Agelopoulos K, Buerger H. Mechanisms of egfr gene transcription modulation: Relationship to cancer risk and therapy response. Clin Cancer Res. 2006;12(24):7252–7260. doi: 10.1158/1078-0432.CCR-06-0626. [DOI] [PubMed] [Google Scholar]
- 69.Kageyama R, Merlino GT, Pastan I. Epidermal growth factor (EGF) receptor gene transcription. Requirement for Sp1 and an EGF receptor-specific factor. J Biol Chem. 1988;263(13):6329–6336. [PubMed] [Google Scholar]
- 70.Guturi KK, et al. Mechanism of β-catenin-mediated transcriptional regulation of epidermal growth factor receptor expression in glycogen synthase kinase 3 β-inactivated prostate cancer cells. J Biol Chem. 2012;287(22):18287–18296. doi: 10.1074/jbc.M111.324798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pangas SA, Matzuk MM. The art and artifact of GDF9 activity: Cumulus expansion and the cumulus expansion-enabling factor. Biol Reprod. 2005;73(4):582–585. doi: 10.1095/biolreprod.105.042127. [DOI] [PubMed] [Google Scholar]
- 72.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14(2):159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 73.Yang Q, Allard P, Huang M, Zhang W, Clarke HJ. Proteasomal activity is required to initiate and to sustain translational activation of messenger RNA encoding the stem-loop-binding protein during meiotic maturation in mice. Biol Reprod. 2010;82(1):123–131. doi: 10.1095/biolreprod.109.076588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.