Significance
The effect of glacial cycles on Southeast Asian (SEA) rainforest during the Quaternary is unresolved. Some historical evidence suggests rainforests were confined to small refugia during glacial maxima, but dynamic vegetation models suggest evergreen rainforests were widespread. Because Dipterocarpaceae dominate current SEA rainforests, their distributions closely reflect general rainforest extent. Here, we use an extensive georeferenced database of collection records for 317 Dipterocarpaceae species to model their climatic niches, based on current climatic conditions. These distribution models were then hindcast onto historical climatic conditions of the last glacial maximum. The results indicate that central Sundaland, exposed because of lower sea levels at glacial maxima, harbored suitable environmental conditions for Dipterocarpaceae and was probably covered by rainforest.
Keywords: Quaternary, last glacial maximum, Sundaland, Dipterocarpaceae, species distribution model
Abstract
The extent of Dipterocarp rainforests on the emergent Sundaland landmass in Southeast Asia during Quaternary glaciations remains a key question. A better understanding of the biogeographic history of Sundaland could help explain current patterns of biodiversity and support the development of effective forest conservation strategies. Dipterocarpaceae trees dominate the rainforests of Sundaland, and their distributions serve as a proxy for rainforest extent. We used species distribution models (SDMs) of 317 Dipterocarp species to estimate the geographic extent of appropriate climatic conditions for rainforest on Sundaland at the last glacial maximum (LGM). The SDMs suggest that the climate of central Sundaland at the LGM was suitable to sustain Dipterocarp rainforest, and that the presence of a previously suggested transequatorial savannah corridor at that time is unlikely. Our findings are supported by palynologic evidence, dynamic vegetation models, extant mammal and termite communities, vascular plant fatty acid stable isotopic compositions, and stable carbon isotopic compositions of cave guano profiles. Although Dipterocarp species richness was generally lower at the LGM, areas of high species richness were mostly found off the current islands and on the emergent Sunda Shelf, indicating substantial species migration and mixing during the transitions between the Quaternary glacial maxima and warm periods such as the present.
During the Quaternary glacial maxima, the forests at high northern latitudes were forced into fairly well delimited and understood refugia (1–3), and the tropical rainforests of the Neotropics and Africa were fragmented into smaller pockets (4, 5). The response of Southeast Asian (SEA) tropical forests is considerably more difficult to understand, given the interaction between climate and land area change (6–8). With sea levels lowered by 120 m at the last glacial maximum (LGM; 21 kya), a large peninsula known as Sundaland was exposed. This landmass joined the present-day islands of Borneo, Sumatra, Java, Bali, and the Malay Peninsula and was twice the size of the current land area in the region (Fig. 1). The cycle of rising and falling sea levels has repeated ∼50 times during the last 2.7 million years (9), and substantially lowered sea levels resulting from glacial conditions existed for 90% of the time (7, 10). Reconstructions of lowland rainforest throughout the Quaternary from combined vegetation and paleoclimatic models suggest their geographic extent was far greater at glacial maxima than at present (7). This led to the conclusion that SEA rainforests are currently in a refugial state, and not at the LGM, as is the case for the northern temperate forests and most other tropical regions.
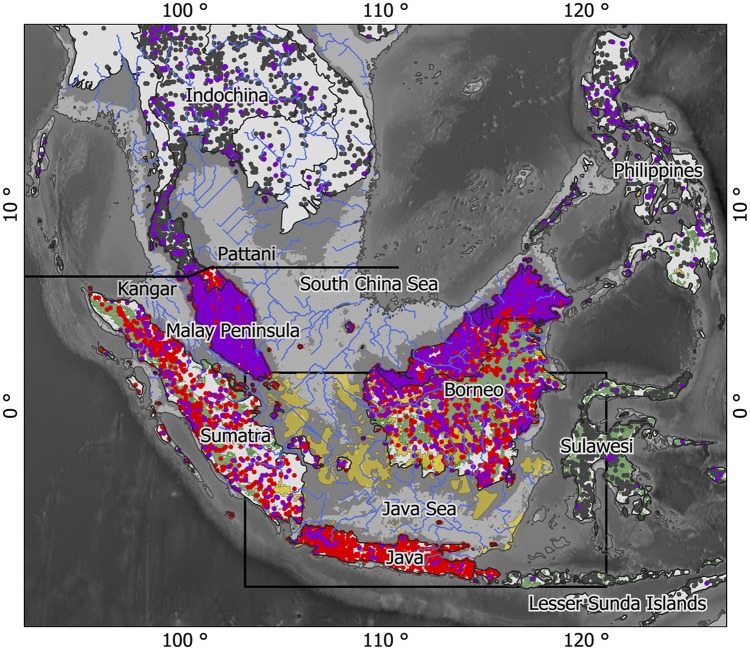
Fig. 1.
The extent of the study area between latitudes 11°S and 19°N and longitudes 92° and 127°W. NWO-ALW is defined as the islands of Borneo, Sumatra, and Java, and the area on the Malay Peninsula south of the Kangar-Pattani line. Blue lines represent paleo drainage derived from ETOPO1 1 Arc-Minute Global Relief Model bathymetric data; gray lines indicate sea level of −120 m below the present level; light gray areas indicate depths between 40 and 120 m below current sea level; dark gray areas indicate depths between 0 and 40 m below current sea level; yellow shading in the Java Sea indicates sandy soils from Emery (22), and the square box indicates the Emery’s map extent; yellow shading on land indicates >70% sand (mass %) from the International Soil Reference and Information Centre (ISRIC) (80); and green shading indicates remaining natural forest cover anno 2010 (from ref. 81; maximum latitude, 10°N). Purple dots (2,315) indicate collection sites of modeled Dipterocarpaceae species, red dots (5,481) indicate all collection sites on Sundaland, and dark gray dots indicate all collection sites within the study area but outside Sundaland. The study area contained 8,118 collection sites in total.
The presence of rainforest at the LGM in the South China Sea region (northern Sundaland) is supported by palynologic data from several deep-sea sediment cores (11–14). However, Sundaland was at least partly covered by savannah vegetation that possibly formed a corridor stretching from Thailand, through the Malay Peninsula and central Sundaland via Java, to the Lesser Sunda Islands (8, 15–18). Savannah can only occur where rainforest is absent (19). The existence of a transequatorial savannah corridor was first suggested by Morley and Flenley in 1987 (16), on the basis of one pollen core from the Malay Peninsula and six cores from southern Borneo (20) that were indicative of drier climatic conditions. On the basis of stable carbon isotope compositions of four ancient cave guano profiles (all outside of the central Sundaland area), Wurster and colleagues (15) concluded there was substantial forest contraction at the LGM on both the Malay Peninsula and Palawan, whereas rainforest was maintained at the Niah caves in northwestern Borneo (Fig. 2). The presence of savanna-like vegetation at the LGM in the region now submerged by the Java Sea is supported by the composition of extant mammal communities on small islands in this region (18). However, off the east coast of Sumatra (the Riau and Lingga Archipelagos), where Cannon and colleagues’ (7) vegetation model predicted a continuous rainforest belt crossing central Sundaland, the extant mammal community data were ambiguous (18). Here, most islands have mammal communities that are indicative for rainforest at the LGM, and few islands support mammal communities indicative of a drier vegetation type. These conflicting results might be caused by the effect of soil types on central Sundaland vegetation. The region is partly covered by sandy soils (Fig. 1) (21, 22) that may have resulted in vegetation types such as heath forest and peat and kerapah swamp forests. These forests have characteristics of both open and closed forest, even under ever-wet conditions (14, 21).
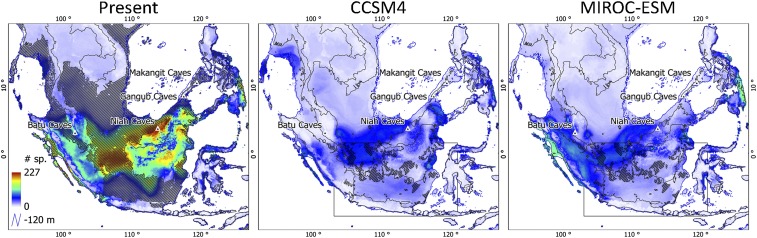
Fig. 2.
Dipterocarpaceae species richness for the present and the LGM for two global climate models: CCSM4 and MIROC-ESM. Maps were obtained by stacking SDM predictions for 317 Dipterocarp species. Hatched areas (present) indicate hypothetical richness if the sea level were 120 m below the present level. Black triangles indicate the cave locations from Wurster and colleagues (15). Hatched areas for CCSM4 and MIROC-ESM indicate the location of sandy soils on the emerged Sunda shelf (22) and on land (80); the black box indicates Emery’s (22) map extent for the sandy soils on the emerged Sunda Shelf.
At this time, the rainforests of Sundaland are characterized and dominated by species from the Dipterocarpaceae family (23–25), distinguishing the SEA rainforests from their Neotropical and African counterparts (26). Dipterocarps have their center of diversity on Sundaland (26). The distributions of Dipterocarp species are largely restricted to rainforest, with a few exceptional species occurring in the deciduous forests of Indochina (27). This makes Dipterocarps good indicators for Sundaland rainforest extent. Digitization of Dipterocarp collection records, the development of spatial databases for current and paleoclimatic conditions (28–30), and the use of species distribution models (SDMs) now allow us to examine whether Dipterocarp species, and thereby Dipterocarp rainforest, likely occurred on central Sundaland at the LGM. A SDM establishes the relationships between known occurrences of a species and abiotic environmental conditions at those sites to predict areas that are environmentally suitable to sustain viable populations (31). This prediction is made using current climate data, but the model can also be “transferred” in time by using past or future climate data simulated by global climate models (GCMs). The reliability of hindcasted SDMs to LGM climatic conditions (2, 32) is founded in the well-supported assumption of niche conservatism (33). Niche conservatism suggests the major ecological traits of species do not change very rapidly over time, which is supported by empirical tests of SDMs for this purpose (2, 34). Although SDMs do not account for biological mechanisms such as dispersal, establishment, and biotic interactions (31), we aim to determine whether appropriate Dipterocarp niche conditions, and thereby for Dipterocarp rainforest, were widespread or not on central Sundaland at the LGM. Suitable abiotic conditions to sustain viable populations of Dipterocarpaceae at the LGM would strongly challenge the transequatorial savannah corridor hypothesis.
We modeled Dipterocarp distributions using SDMs under present environmental conditions for Sundaland (including areas that are presently submerged) and hindcasted these models to LGM climatic conditions according to two GCMs: Community Climate System Model Version 4 (CCSM4) (29) and the Model for Interdisciplinary Research on Climate–Earth System Model (MIROC-ESM) (30). We used the SDM results to assess whether climatic conditions on central Sundaland at the LGM likely supported Dipterocarp rainforest, or possibly a transequatorial savannah corridor, and to assess Dipterocarp species turnover through time.
Results and Discussion
The hindcasted SDMs of 317 Dipterocarp species show that central Sundaland climate was suitable for many Dipterocarp species at the LGM (Fig. 2), suggesting central Sundaland was covered by Dipterocarp rainforest (7, 14). It is therefore unlikely that central Sundaland was covered by a transequatorial savannah corridor at the glacial maxima of the Quaternary (8, 15, 16, 18). Nevertheless, it is evident that both total area covered by rainforest and most individual species’ ranges were reduced at the LGM (for both scenarios) compared with present climatic conditions, and to present climatic conditions if Sundaland was exposed if the sea level were 120 m below the present level (Figs. 2–4). At the LGM, different species dominated, and the Dipterocarp species richness of these communities was lower, indicating that the compositions of Dipterocarp communities of SEA rainforests have been highly dynamic through the recent past.
The results of hindcasted SDMs are supported by different lines of evidence. First, the high suitability of Dipterocarp habitat in the South China Sea region (off the west coast of Borneo) at the LGM (Fig. 2) is supported by palynologic data (12, 13, 35), dynamic vegetation models (7, 36), stable isotopic composition of vascular plant fatty acids (37), and stable carbon isotope compositions of an ancient cave guano profile at the Niah Caves on Borneo (15) (Fig. 2). Second, the low suitability of Dipterocarp habitat for large sections of the southern Java Sea region (Fig. 2) is supported by palynologic evidence indicative of a dry vegetation type (38), vascular plant fatty acid stable isotopic compositions (37), and extant mammal (18) and termite communities (39) on the small islands in the Java Sea, indicating the absence of rainforest at the LGM. Third, Wurster and colleagues (15) and Dubois and colleagues (37) indicated drier vegetation at the Batu Caves locality on the Malay Peninsula (Fig. 2). Both GCMs predict low Dipterocarp richness for this locality at the LGM. Notably, the Batu Caves are in close proximity to Subang, from where palynologic data originated that led to the first suggestion of a savannah corridor at the LGM in 1987 (16). Finally, the majority of mammals on Borneo, with ancestors from continental SEA, are associated with woodland habitats: “they could only have reached Borneo provided that central Sundaland was covered by forest” (17). This raises the question of how the large megafauna, now largely extinct, would have reached Java in the absence of a transequatorial savannah corridor (40). We argue that much of the extant megafauna can persist in forested habitats (i.e., Javan and Sumatran rhino and tiger) and are capable of crossing a rainforest barrier (17, 40). In addition, the Mentawai islands (off the west coast of Sumatra) harbor a rich endemic fauna (41), which suggests the persistence of rainforest during glacial cycles. This region also coincides with high predicted Dipterocarp habitat suitability at the LGM by both GCMs.
Although climatic conditions on central Sundaland at the LGM were suitable to sustain Dipterocarps, it is uncertain whether the exposed soils on Sundaland could support Dipterocarp rainforest. Slik and colleagues (21) showed that genera with distributions spanning Sundaland from Borneo to Sumatra had a higher tolerance for sandy soils. The few existing maps of the seafloor of the Java Sea indeed indicate the presence of sections with sand (Fig. 1, yellow shading) (22). We were not able to include edaphic factors in our models because these are not known for the entire exposed Sunda Shelf. Nonetheless, plant distributions are primarily dictated by temperature and precipitation (31, 42, 43), within the climatic bounds modified by edaphic conditions. Even if the exposed region was fully covered by sandy soils, this still is no reason to assume that Dipterocarp rainforest was absent from central Sundaland. Ashton (44) showed that as many as 38 different Dipterocarp species are found to co-occur on leached sandy clay and sandy soils. Furthermore, it is likely that mature Dipterocarp rainforests have developed during the last glacial period on the exposed sandy soils, comparable to the primary succession and forest development on sand dunes elsewhere, such as the coastal lake Michigan sand dunes (45).
Another factor we cannot incorporate into our hindcasts of Dipterocarp SDMs is the effect of a lower atmospheric CO2 concentration at the LGM (189 ppm) compared with the preindustrial CO2 concentration (280 ppm) (46). The comparative advantage of plants with C4 metabolism (a large number of tropical grasses) over plants with C3 metabolism (such as the Dipterocarps) increases with lower CO2 concentrations because of higher water use efficiency. However, lower temperatures at the LGM resulted in lower water needs for transpiration, which is more advantageous to C3 plants than C4 plants. The presence of C3 trees is supported by LGM pollen assemblages from the South China Sea indicative of lowland rainforest and lower montane forest (12). Pollen in these assemblages originated from vegetation on central Sundaland and was transported by rivers into the South China Sea. Pollen cores from the Java Sea region, where maximum temperatures were higher at the LGM than present (SI Appendix, Fig. S4) and C4 grasses likely had a competitive advantage, indeed indicated an open vegetation type lacking Dipterocarps (38, 47). These findings are supported by the stable isotope composition of vascular plant fatty acids indicating a dominance of C4 vegetation (37). Cores from northeast Borneo, where the hindcasted SDMs do predict suitable Dipterocarp habitat at the LGM (Fig. 2), indicated persistent predominance of C3 vegetation.
Finally, stronger El Niño–Southern Oscillation events, the most potent source of interannual climate variability, might have promoted the presence of savannah at the LGM through increased and extended droughts. However, Tudhope and colleagues (48) concluded that during the 20th century, El Niño–Southern Oscillation has been strong compared with in previous cool (glacial) and warm (interglacial) times.
The combined ranges of the 317 Dipterocarp species indicate current areas of high richness in northwestern Borneo, in smaller pockets in northeastern Borneo, in the lowlands of east Borneo, and on Bangka island (off the east coast of Sumatra; Fig. 2). Most areas with high predicted Dipterocarp richness correspond with the localities that were characterized as Dipterocarp forest by Slik and colleagues (21), with the exception of Bangka island, which is known for tin mining and is characterized by large areas of ultramafic (toxic to many species) and nutrient-poor sandy soils that, in reality, support low levels of Dipterocarp richness. SDM predictions for all of Sundaland show that central Sundaland could harbor another area of high Dipterocarp richness, if it were completely exposed (Fig. 2). Where this hypothetical area of high species richness reaches the shores of southwestern Borneo, our findings are supported by Dipterocarp-dominated rainforest at Gunung Palung National Park (49).
Hindcasted Dipterocarp distributions suggest lower levels of local richness at the LGM than at present (Fig. 2). The pattern of lower richness is the result of marked range contractions under LGM climatic conditions (Figs. 3 and 4). SDMs tend to overestimate species richness (50), but our estimates for Dipterocarp richness are probably conservative for both present and LGM climatic conditions because of the thresholds set for the SDMs. In converting the continuous probability of presence SDM values into discrete presence/absence values, we forced 10% of the collection localities at the niche margins outside the predicted presence area. This was done to account for potential identification and georeferencing errors. Furthermore, 12% of the species showed no significant niche preference (generalists) and were omitted from the richness patterns. These species may have played an important role in the past, and future efforts to incorporate these species could further improve our understanding of past richness patterns.
Fig. 3.
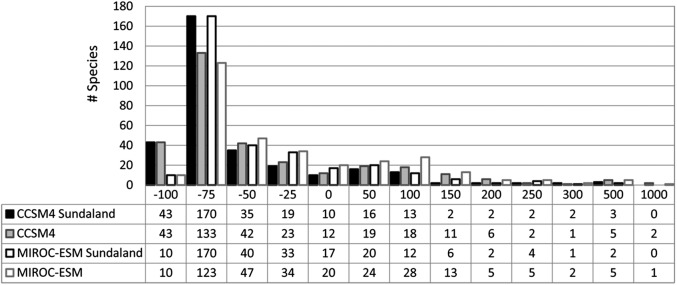
LGM range contraction and expansion (in percentages) for 317 Dipterocarpaceae species compared with the current distribution for Sundaland (as if it were completely exposed, as it was at the LGM) and for the current land area. LGM ranges were hindcasted with SDMs for the CCSM4 and MIROC-ESM GCMs. The horizontal axis labels indicate the upper bin values.
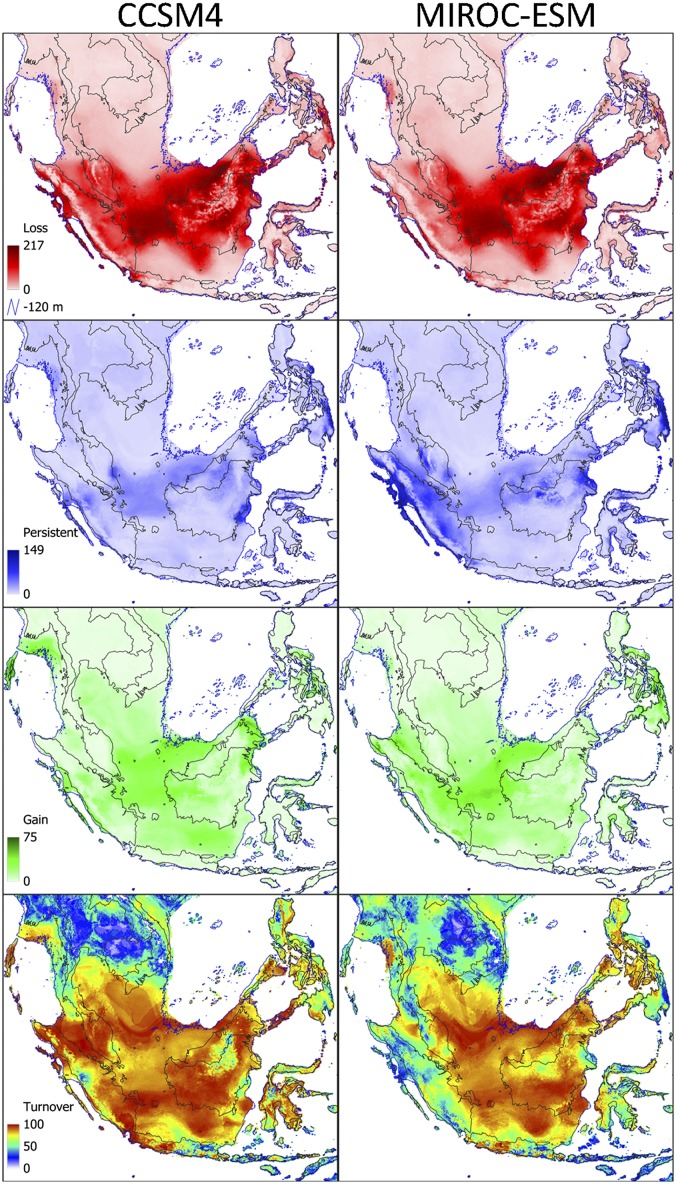
Fig. 4.
Dipterocarpaceae species loss, persistence, gain, and turnover for the LGM (∼21 kya) obtained by stacking SDM predictions for 317 Dipterocarp species, based on climate simulations from two GCMs: CCSM4 (Left) and MIROC-ESM (Right).
The projections for the two GCMs gave very similar results. The largest difference in richness patterns is found on east Sumatra, where CCSM4 hindcasts suggest lower numbers of Dipterocarp species compared with MIROC-ESM predictions (Fig. 2). Under MIROC-ESM climatic conditions, a larger number of species is predicted to have persisted on east Sumatra through the glacial cycles (Fig. 4). Quaternary pollen cores from central Sumatra suggest conditions were cooler, but not drier, as indicated by low percentages of grass pollen (51–53) and in support of forested conditions on central Sumatra, as well as southwestern Sumatra (54). It should be noted, however, that 43 (14%) and 10 (3%) species were predicted to have gone extinct under CCSM4 and MIROC-ESM, respectively (Fig. 3). This is obviously not the case, which suggests these species were possibly retracted in microrefugia (55, 56) that were not captured by the 10-km spatial resolution of the climate data we used, or that the SDMs for these species were overfitted, which is something to which SDMs for species with a small range size are prone (57). Given the evidence for forested conditions on lowland Sumatra and a lower percentage of wrongly predicted extinct species by MIROC-ESM, we consider our hindcasts with MIROC-ESM the more informative of the two.
In conclusion, SDMs applied on the largest database of collections from extant Dipterocarpaceae species available today show that abiotic conditions on the exposed Sunda Shelf at the LGM could support Dipterocarp rainforest, and that the presence of a transequatorial savannah corridor at the LGM is therefore unlikely.
Materials and Methods
Sundaland and Study Area.
The region of interest is Sundaland, which includes the SEA islands of Borneo, Sumatra, Java, Bali, and the Malay Peninsula, delineated by the Kangar-Pattani line in the northwest and by the Merrill-Dickerson/Huxley line in the east (58). However, many Dipterocarp species also occur outside Sundaland, particularly on the Philippines, Sulawesi, Lesser Sunda Islands, and Indochina. To model the distribution of species, we considered their entire ecological niche (59). We therefore considered their occurrences in the larger study area, defined by latitudes 11°S–19°N and longitudes 92°–127°E (Fig. 1).
Dipterocarpaceae Collections.
We compiled a database of Dipterocarpaceae collection records from SEA by merging the databases of the Naturalis Biodiversity Center, the Forest Research Institute Malaysia, and the Singapore Botanical Gardens. This database contains 44,257 records representing 1,478 unique names. After removing typos and synonyms (SI Appendix, Table S1), records outside the study area, unresolved taxonomic identifications, identifications from botanical gardens and cultivated areas, and geographical duplicate records at ∼10 km (5 arc-minute) spatial resolution, the dataset contained 14,602 unique collection records of 440 Dipterocarp species. A 10-km spatial resolution best matched the precision at which collections can be georeferenced. Finally, we removed all species with fewer than five records. Five was chosen as lower limit to infer any relationship between species occurrence and abiotic climatic conditions. The final dataset contained 14,432 records of 362 Dipterocarp species (SI Appendix, Table S3 and Fig. S1).
Climate Data: Present and LGM (CCSM4 and MIROC-ESM).
To represent the current climatic conditions, we used the 19 bioclimatic variables plus altitude from WorldClim (28). For climate at the LGM, we used downscaled data from two GCMs: CCSM4 (29) and MIROC-ESM (30). Both datasets were at 5 arc-minute spatial resolution. LGM data included values for the entire land area above sea level at the LGM (sea level, −120 m below present; Fig. 1). We estimated through interpolation the current climate conditions for these oceanic areas, had they not been submerged (cf. 28). To these datasets, we added a data layer reflecting dry season length (number of consecutive months with <100 mm precipitation) and a proxy for potential evapotranspiration, estimated as the ratio between the mean annual temperature (°C) and total annual precipitation (mm), multiplied by an empirically derived constant of 60 (after ref. 60, from ref. 61).
Variable Selection.
We obtained all 8,118 botanical collection localities in the study area, including all collection sites of non-Dipterocarp species from the three previously mentioned databases (Fig. 1). We assessed whether the 5,481 collection sites of Sundaland covered the full ranges of abiotic conditions by two exploratory analyses. First, we plotted kernel density plots of the 5,481 collection sites versus all 19,631 raster cells covering Sundaland with the function histniche of the adehabitatHS package (62) in R (SI Appendix, Fig. S2; 63). This indicated that the full ranges of abiotic conditions on Sundaland were covered by collection sites (SI Appendix, Fig. S2). Second, we performed a principal component analysis (PCA) on standardized and centered data from 19 current bioclimatic variables, altitude, dry season length, and potential evapotranspiration. PCA-axes 1–3 explained 84.3% of the variance in the dataset. We identified the collection sites for the four major biogeographic units [Borneo, Java (plus Bali), Sumatra, and the Malay Peninsula] on Sundaland separately (SI Appendix, Fig. S3). This analysis indicated that the entire “climatic space” of Sundaland is covered by collection sites, and that no severe biases were detected.
Multicollinearity can result in model overfitting. Therefore, we tested the climate dataset of present climatic conditions for the 5,481 raster cells with known botanical collections on Sundaland for rank correlations. We only retained uncorrelated variables with |Spearman ρ| ≤ 0.7 (64). This resulted in the selection of the following eight variables: isothermality, maximum temperature of the warmest month, minimum temperature of the coldest month, temperature annual range, precipitation of the wettest month, precipitation of the driest month, precipitation of the coldest quarter, and potential evapotranspiration (see SI Appendix, Table S2 for all correlation coefficients). Anomalies for both GCMs for the eight selected variables are shown in SI Appendix, Fig. S4.
Nonanalog LGM Climatic Conditions?
At the LGM, the climate was drier and 3–6 °C cooler than at present (SI Appendix, Fig. S4; 14, 65–66). This raises the question of whether SDMs are not unreliably extrapolated to nonanalog climate conditions at the LGM. To test whether this is the case, we developed multivariate environmental similarity surfaces (MESS) that represent how similar each site in the study area is compared with a reference set of sites (the 8,118 collection localities used for SDM calibration), with respect to the eight independent predictor variables (67). Negative values indicate sites at which at least one variable has a value outside the range of conditions of the reference set. This was done for the present, CCSM4, and MIROC-ESM. None of the datasets showed nonanalog climatic conditions within the Sundaland region (SI Appendix, Fig. S5).
SDMs, Significance Testing, and Hindcasting.
SDMs are used to predict areas that are environmentally suitable for a species from the sites where it is known to occur (31). The following steps are taken: locations of the known current distribution of a species are compiled; values for climatic predictor variables at these locations, and for a large set of random (background) locations, are extracted from spatial databases; and the climatic values are used to fit a SDM that estimates the similarity of the climate at any location to climatic conditions at known occurrence locations. The SDM is then used to predict the climatic suitability for a species across an area of interest. This prediction can be made using current climate data, but the SDM can also be transferred in time by using past or future climate data simulated by GCMs.
To model the Dipterocarp distributions, we selected the maximum entropy modeling algorithm, MaxEnt (68, 69), because it was developed to deal with presence-only data, has shown to outperform other algorithms (70, 71), can be used to reliably hindcast to LGM climatic conditions (34), performs well when few presence records are available (72), and is relatively robust against georeference errors (73). We applied a target-group background sample methodology (74) by adding a mask layer that represents all 8,118 collection sites of the study area. We applied default MaxEnt settings but excluded product and threshold rules to avoid model overfitting (75). We used all records for model training and tested the SDMs for significant deviation from random expectation, using a bias-corrected null-model (76). More specifically, we tested for each SDM the area under the receiver operating characteristic (ROC) plot (AUC) value (77), with a Monte Carlo randomization procedure. For the randomization procedure, we included a spatial filter that represented the eight biogeographic units in the study area: Malay Peninsula and Indochina, Sumatra, Borneo, Java and Bali, Philippines (including Palawan and the Bayan islands), Sulawesi, Moluccas, and the Lesser Sunda Islands (78). For each species, we assessed the number of collections per biogeographic unit, and with the same distribution, we drew random points from collection sites in the biogeographic units. These random points were then modeled with MaxEnt, using identical settings and climate variables as those used to develop the real species’ SDM, and we obtained the AUC value for a set of random points. This procedure was replicated 99 times, resulting in 99 AUC values. A SDM with an AUC value that ranks in the top 5% AUC values derived from random points performs significantly better than random expectations (P < 0.05). 317 Dipterocarp species (88%) fulfilled this requirement and were retained for subsequent analyses.
All 317 significant SDMs were projected to a dataset of the present climatic conditions for the LGM Sundaland land area and were hindcast to LGM climate datasets CCSM4 and MIROC-ESM. Finally, we converted the continuous habitat suitability maps to discrete presence/absence maps using the 10 percentile training presence threshold value. This is a conservative threshold to prevent commission errors (false-positive predictions).
Richness Patterns and Community Turnover.
To obtain patterns of richness for present and LGM climatic conditions, we stacked all 317 presence-absence predictions for each of the three projections. This resulted in three presence-absence matrices for 317 Dipterocarp species and all raster cells in the study area. The sums of species presences per raster cell were mapped for the present and for LGM models CCSM4 and MIROC-ESM (Fig. 2). To quantify range shifts, we calculated the absolute (SI Appendix, Table S3) and relative (in percentage) (Fig. 3) difference in species ranges between present for the actual land area; for the entire Sundaland region, including currently submerged land; and for both LGM conditions.
To estimate the quantitative community turnover, we calculated the percentage species turnover per raster cell, under the assumption of universal migration. Turnover (T) is defined as T = 100 × (L + G)/(SR + G), where L is the number of species lost per raster cell, G is the number of species gained per raster cell, and SR is current species richness at a raster cell (79). To assess whether the percentage community turnover could be attributed to species losses or gains, we plotted the number of species that were lost, persisted, and gained for both LGM models (Fig. 4).
Supplementary Material
Acknowledgments
We thank the editor and three anonymous reviewers for helpful comments on the manuscript. Singapore Botanical Gardens is acknowledged for their contribution of Dipterocarpaceae collection data. N.R. was supported by Nederlandse Organisatie voor Wetenschappelijk Onderzoek-Aard-en Levenswetenschappen (NWO-ALW) Grant 819.01.014.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403053111/-/DCSupplemental.
References
- 1.Svenning J-C, Normand S, Kageyama M. Glacial refugia of temperate trees in Europe: Insights from species distribution modelling. J Ecol. 2008;96(6):1117–1127. [Google Scholar]
- 2.Waltari E, et al. Locating pleistocene refugia: Comparing phylogeographic and ecological niche model predictions. PLoS ONE. 2007;2(6):e563. doi: 10.1371/journal.pone.0000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petit RJ, Hu FS, Dick CW. Forests of the past: A window to future changes. Science. 2008;320(5882):1450–1452. doi: 10.1126/science.1155457. [DOI] [PubMed] [Google Scholar]
- 4.Anhuf D, et al. Paleo-environmental change in Amazonian and African rainforest during the LGM. Palaeogeogr Palaeocl. 2006;239(3-4):510–527. [Google Scholar]
- 5.Morley RJ. Origin and evolution of tropical rain forests. John Wiley & Sons Ltd.; Chichester, England: 2000. pp 362. [Google Scholar]
- 6.Hope G, et al. History of vegetation and habitat change in the Austral-Asian region. Quat Int. 2004;118-119:103–126. [Google Scholar]
- 7.Cannon CH, Morley RJ, Bush ABG. The current refugial rainforests of Sundaland are unrepresentative of their biogeographic past and highly vulnerable to disturbance. Proc Natl Acad Sci USA. 2009;106(27):11188–11193. doi: 10.1073/pnas.0809865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird MI, Taylor D, Hunt C. Palaeoenvironments of insular Southeast Asia during the last glacial period: A savanna corridor in Sundaland? Quat Sci Rev. 2005;24(20-21):2228–2242. [Google Scholar]
- 9.Woodruff D. Biogeography and conservation in Southeast Asia: How 2.7 million years of repeated environmental fluctuations affect today’s patterns and the future of the remaining refugial-phase biodiversity. Biodivers Conserv. 2010;19(4):919–941. [Google Scholar]
- 10.Lohman DJ, et al. Biogeography of the Indo-Australian Archipelago. Annu Rev Ecol Evol Syst. 2011;42(1):205–226. [Google Scholar]
- 11.Sun XJ, Li X, Luo YL. Vegetation and climate on the Sunda Shelf of the South China Sea during the last glaciation - pollen results from Station 17962. Acta Bot Sin. 2002;44(6):746–752. [Google Scholar]
- 12.Wang X, Sun X, Wang P, Stattegger K. Vegetation on the Sunda Shelf, South China Sea, during the Last Glacial Maximum. Palaeogeogr Palaeocl. 2009;278(1-4):88–97. [Google Scholar]
- 13.Wang X, Sun X, Wang P, Stattegger K. A high-resolution history of vegetation and climate history on Sunda Shelf since the last glaciation. Sci China Ser D. 2007;50(1):75–80. [Google Scholar]
- 14.Morley RJ. A review of the Cenozoic palaeoclimate history of Southeast Asia. In: Gower DJ, et al., editors. Biotic Evolution and environmental change in southeast Asia. Cambridge University Press; Cambridge, UK: 2012. pp. 79–114. [Google Scholar]
- 15.Wurster CM, et al. Forest contraction in north equatorial Southeast Asia during the Last Glacial Period. Proc Natl Acad Sci USA. 2010;107(35):15508–15511. doi: 10.1073/pnas.1005507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morley RJ, Flenley JR. Late Cainozoic vegetational and environmental changes in the Malay archipelago. In: Whitmore TC, editor. Biogeographical evolution of the Malay archipelago. Clarendon Press; Oxford: 1987. pp. 50–59. [Google Scholar]
- 17.Earl of Cranbrook Late quaternary turnover of mammals in Borneo: The zooarchaeological record. Biodivers Conserv. 2010;19(2):373–391. [Google Scholar]
- 18.Meijaard E. Mammals of south-east Asian islands and their Late Pleistocene environments. J Biogeogr. 2003;30(8):1245–1257. [Google Scholar]
- 19.Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci Discuss. 2007;4(2):439–473. [Google Scholar]
- 20.Morley RJ. Development and vegetation dynamics of a lowland ombrogenous peat swamp in Kalimantan Tengah, Indonesia. J Biogeogr. 1981;8:383–404. [Google Scholar]
- 21.Slik JWF, et al. Soils on exposed Sunda shelf shaped biogeographic patterns in the equatorial forests of Southeast Asia. Proc Natl Acad Sci USA. 2011;108(30):12343–12347. doi: 10.1073/pnas.1103353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emery KO. Distribution pattern of sediments on the continental shelves of western Indonesia. CCOP Tech Bull. 1969;2:79–82. [Google Scholar]
- 23.Slik JWF, et al. Large trees drive forest aboveground biomass variation in moist lowland forests across the tropics. Glob Ecol Biogeogr. 2013;22(12):1261–1271. [Google Scholar]
- 24.Corlett RT. What's so special about Asian tropical forests? Curr Sci. 2007;93(11):1551–1557. [Google Scholar]
- 25.Ashton PS. 1983. Dipterocarpaceae. Flora Malesiana I, ed van Steenis CGGJ (Martinus Nijhoff, The Hague), Vol 9, part 2, pp 237–552.
- 26.Corlett RT, Primack RB. 2011. Tropical rain forest: An ecological and biogeographical comparison (Wiley-Blackwell, Hoboken, NJ), 2nd ed.
- 27.Smitinand T, Santisuk T. Dipterocarpaceae of Thailand with special reference to silvicultural ecology. Malaysian Forester. 1981;44(2-3):377–385. [Google Scholar]
- 28.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25(15):1965–1978. [Google Scholar]
- 29.Brady EC, Otto-Bliesner BL, Kay JE, Rosenbloom N. Sensitivity to Glacial Forcing in the CCSM4. J Clim. 2013;26(6):1901–1925. [Google Scholar]
- 30.Sueyoshi T, et al. Set-up of the PMIP3 paleoclimate experiments conducted using an Earth system model, MIROC-ESM. Geosci. Model Dev. 2013;6(3):819–836. [Google Scholar]
- 31.Araújo MB, Peterson AT. Uses and misuses of bioclimatic envelope modeling. Ecology. 2012;93(7):1527–1539. doi: 10.1890/11-1930.1. [DOI] [PubMed] [Google Scholar]
- 32.Svenning J-C, Fløjgaard C, Marske KA, Nógues-Bravo D, Normand S. Applications of species distribution modeling to paleobiology. Quat Sci Rev. 2011;30(21-22):2930–2947. [Google Scholar]
- 33.Wiens JJ, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett. 2010;13(10):1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 34.Hijmans RJ, Graham CH. The ability of climate envelope models to predict the effect of climate change on species distributions. Glob Change Biol. 2006;12(12):2272–2281. [Google Scholar]
- 35.Sun X, Li X, Luo Y, Chen X. The vegetation and climate at the last glaciation on the emerged continental shelf of the South China Sea. Palaeogeogr Palaeocl. 2000;160(3-4):301–316. [Google Scholar]
- 36.Prentice IC, Harrison SP, Bartlein PJ. Global vegetation and terrestrial carbon cycle changes after the last ice age. New Phytol. 2011;189(4):988–998. doi: 10.1111/j.1469-8137.2010.03620.x. [DOI] [PubMed] [Google Scholar]
- 37.Dubois N, et al. Indonesian vegetation response to changes in rainfall seasonality over the past 25,000 years. Nat Geosci. 2014;7:513–517. [Google Scholar]
- 38.van der Kaars S, et al. Late Quaternary palaeoecology, palynology and palaeolimnology of a tropical lowland swamp: Rawa Danau, West-Java, Indonesia. Palaeogeogr Palaeocl. 2001;171(3-4):185–212. [Google Scholar]
- 39.Gathorne-Hardy FJ, Syaukani, Davies RG, Eggleton P, Jones DT. Quaternary rainforest refugia in south-east Asia: Using termites (Isoptera) as indicators. Biol J Linn Soc Lond. 2002;75(4):453–466. [Google Scholar]
- 40.van den Bergh GD, de Vos J, Sondaar PY. The Late Quaternary palaeogeography of mammal evolution in the Indonesian Archipelago. Palaeogeogr Palaeocl. 2001;171(3-4):385–408. [Google Scholar]
- 41.Wilting A, Sollmann R, Meijaard E, Helgen KM, Fickel J. Mentawai’s endemic, relictual fauna: Is it evidence for Pleistocene extinctions on Sumatra? J Biogeogr. 2012;39(9):1608–1620. [Google Scholar]
- 42.Araújo MB, et al. Heat freezes niche evolution. Ecol Lett. 2013;16(9):1206–1219. doi: 10.1111/ele.12155. [DOI] [PubMed] [Google Scholar]
- 43.Boucher-Lalonde V, Morin A, Currie DJ. How are tree species distributed in climatic space? A simple and general pattern. Glob Ecol Biogeogr. 2012;21(12):1157–1166. [Google Scholar]
- 44.Ashton PS. In: Lambir’s forest: The world’s most diverse known tree assemblage? Pollination Ecology and the Rain Forest: Sarawak Studies, Ecological Studies. Roubik DW, Sakai S, Hamid Karim AA, editors. Vol 174. Springer; New York: 2005. pp. 191–216. [Google Scholar]
- 45.Lichter J. Primary Succession and Forest Development on Coastal Lake Michigan Sand Dunes. Ecol Monogr. 1998;68(4):487–510. [Google Scholar]
- 46.Bragg FJ, et al. Stable isotope and modelling evidence for CO2 as a driver of glacial–interglacial vegetation shifts in southern Africa. Biogeosciences. 2013;10(3):2001–2010. [Google Scholar]
- 47.van der Kaars WA, Dam MAC. A 135,000-year record of vegetational and climatic change from the Bandung area, West-Java, Indonesia. Palaeogeogr Palaeocl. 1995;117(1-2):55–72. [Google Scholar]
- 48.Tudhope AW, et al. Variability in the El Niño-Southern Oscillation through a glacial-interglacial cycle. Science. 2001;291(5508):1511–1517. doi: 10.1126/science.1057969. [DOI] [PubMed] [Google Scholar]
- 49.Cannon CH, Leighton M. Tree species distributions across five habitats in a Bornean rain forest. J Veg Sci. 2004;15:257–266. [Google Scholar]
- 50.Guisan A, Rahbek C. SESAM – a new framework integrating macroecological and species distribution models for predicting spatio-temporal patterns of species assemblages. J Biogeogr. 2011;38(8):1433–1444. [Google Scholar]
- 51.Stuijts I, Newsome JC, Flenley JR. Evidence for late Quaternary vegetational change in the Sumatran and Javan highlands. Rev Palaeobot Palynol. 1988;55(1-3):207–216. [Google Scholar]
- 52.Maloney BK, McCormac FG. 1996. Palaeoenvironments of North Sumatra: A 30,000 year old pollen record from Pea Bullok. Indo-Pacific Prehistory Assoc Bull 14(1):73–82.
- 53.Newsome J, Flenley JR. Late Quarternary vegetational history of the Central Highlands of Sumatra. II. Palaeopalynology and vegetational history. J Biogeogr. 1988;15:555–578. [Google Scholar]
- 54.van der Kaars S, Bassinot F, De Deckker P, Guichard F. Changes in monsoon and ocean circulation and the vegetation cover of southwest Sumatra through the last 83,000 years: The record from marine core BAR94-42. Palaeogeogr Palaeocl. 2010;296(1–2):52–78. [Google Scholar]
- 55.Dobrowski SZ. A climatic basis for microrefugia: The influence of terrain on climate. Glob Change Biol. 2011;17(2):1022–1035. [Google Scholar]
- 56.Ashcroft MB, Gollan JR, Warton DI, Ramp D. A novel approach to quantify and locate potential microrefugia using topoclimate, climate stability, and isolation from the matrix. Glob Change Biol. 2012;18(6):1866–1879. [Google Scholar]
- 57.Thorne JH, et al. Alternative biological assumptions strongly influence models of climate change effects on mountain gorillas. Ecosphere. 2013;4(9):art108. [Google Scholar]
- 58.Raes N, van Welzen PC. The demarcation and internal division of Flora Malesiana: 1857 - present. Blumea. 2009;54(1-3):6–8. [Google Scholar]
- 59.Raes N. Partial versus Full Species Distribution Models. Nat Conserv. 2012;10(2):127–138. [Google Scholar]
- 60.Loiselle BA, et al. Predicting species distributions from herbarium collections: Does climate bias in collection sampling influence model outcomes? J Biogeogr. 2008;35(1):105–116. [Google Scholar]
- 61.Holdridge LR, Grenke WC, Hatheway WH, Liang T, Tosi JA., Jr . Forest environments in tropical life zones: A pilot study. Pergamon Press; New York: 1971. [Google Scholar]
- 62.Calenge C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol Modell. 2006;197(3–4):516–519. [Google Scholar]
- 63.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- 64.Dormann CF, et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36(1):27–46. [Google Scholar]
- 65.Kershaw A, Kaars S, Flenley J. The Quaternary history of far eastern rainforests. In: Bush MB, Flenley JR, editors. Tropical Rainforest Responses to Climatic Change. Springer; Chichester, UK: 2007. pp. 77–115. [Google Scholar]
- 66.Harrison SP, Prentice CI. Climate and CO2 controls on global vegetation distribution at the last glacial maximum: Analysis based on palaeovegetation data, biome modelling and palaeoclimate simulations. Glob Change Biol. 2003;9(7):983–1004. [Google Scholar]
- 67.Elith J, Kearney M, Phillips S. The art of modelling range-shifting species. Meth Ecol Evol. 2010;1(4):330–342. [Google Scholar]
- 68.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190(3-4):231–259. [Google Scholar]
- 69.Elith J, et al. A statistical explanation of MaxEnt for ecologists. Divers Distrib. 2011;17(1):43–57. [Google Scholar]
- 70.Elith J, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006;29(2):129–151. [Google Scholar]
- 71.Aguirre-Gutiérrez J, et al. Fit-for-purpose: Species distribution model performance depends on evaluation criteria - Dutch Hoverflies as a case study. PLoS ONE. 2013;8(5):e63708. doi: 10.1371/journal.pone.0063708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wisz MS, et al. Effects of sample size on the performance of species distribution models. Divers Distrib. 2008;14(5):763–773. [Google Scholar]
- 73.Graham CH, et al. The influence of spatial errors in species occurrence data used in distribution models. J Appl Ecol. 2008;45(1):239–247. [Google Scholar]
- 74.Phillips SJ, et al. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol Appl. 2009;19(1):181–197. doi: 10.1890/07-2153.1. [DOI] [PubMed] [Google Scholar]
- 75.Merow C, Smith MJ, Silander JA. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography. 2013;36(10):1058–1069. [Google Scholar]
- 76.Raes N, ter Steege H. A null-model for significance testing of presence-only species distribution models. Ecography. 2007;30(5):727–736. [Google Scholar]
- 77.Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv. 1997;24(1):38–49. [Google Scholar]
- 78.van Welzen PC, Slik JWF. Patterns in species richness and composition of plant families in the Malay Archipelago. Blumea. 2009;54(1-3):166–171. [Google Scholar]
- 79.Thuiller W, Lavorel S, Araújo MB, Sykes MT, Prentice IC. Climate change threats to plant diversity in Europe. Proc Natl Acad Sci USA. 2005;102(23):8245–8250. doi: 10.1073/pnas.0409902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Batjes NH. 2012. ISRIC-WISE derived soil properties on a 5 by 5 arc-minutes global grid (ver. 1.2). Report 2012/01. Available at www.isric.org/sites/default/files/isric_report_2012_01.pdf. Accessed September 5, 2014.
- 81.Miettinen J, Shi CH, Tan WJ, Liew SC. 2010 land cover map of insular Southeast Asia in 250-m spatial resolution. Remote Sens Lett. 2012;3(1):11–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.