Abstract
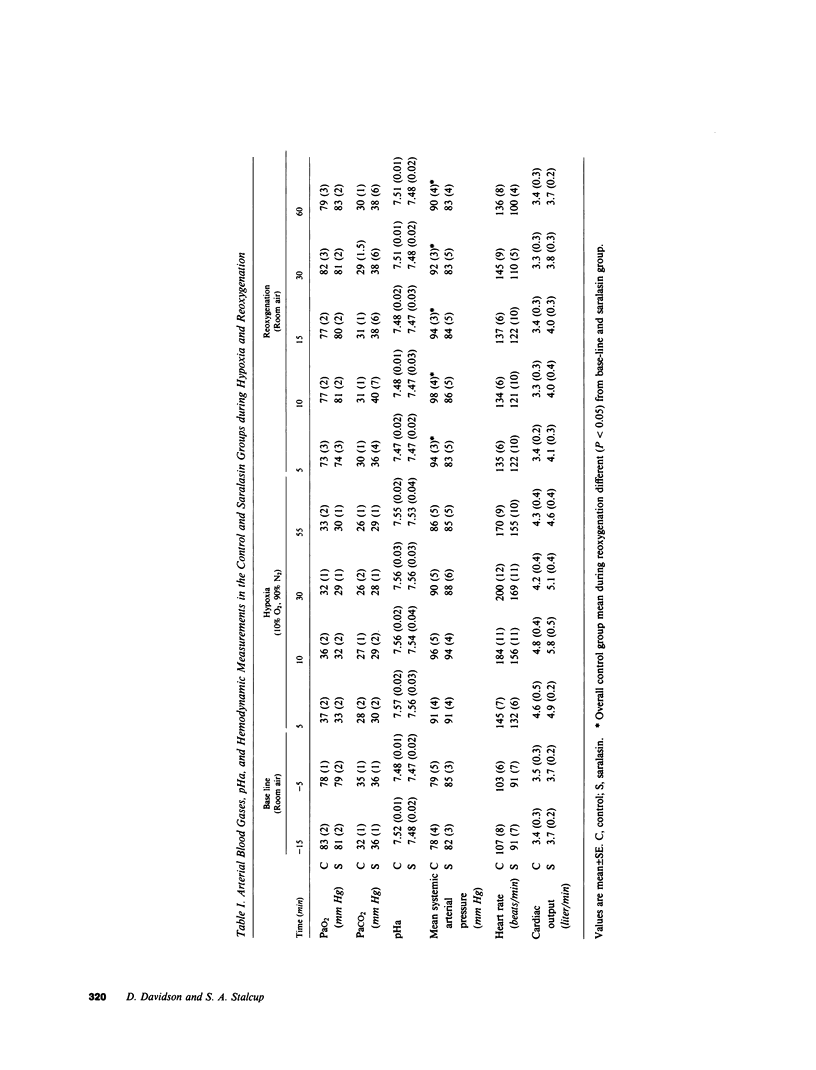
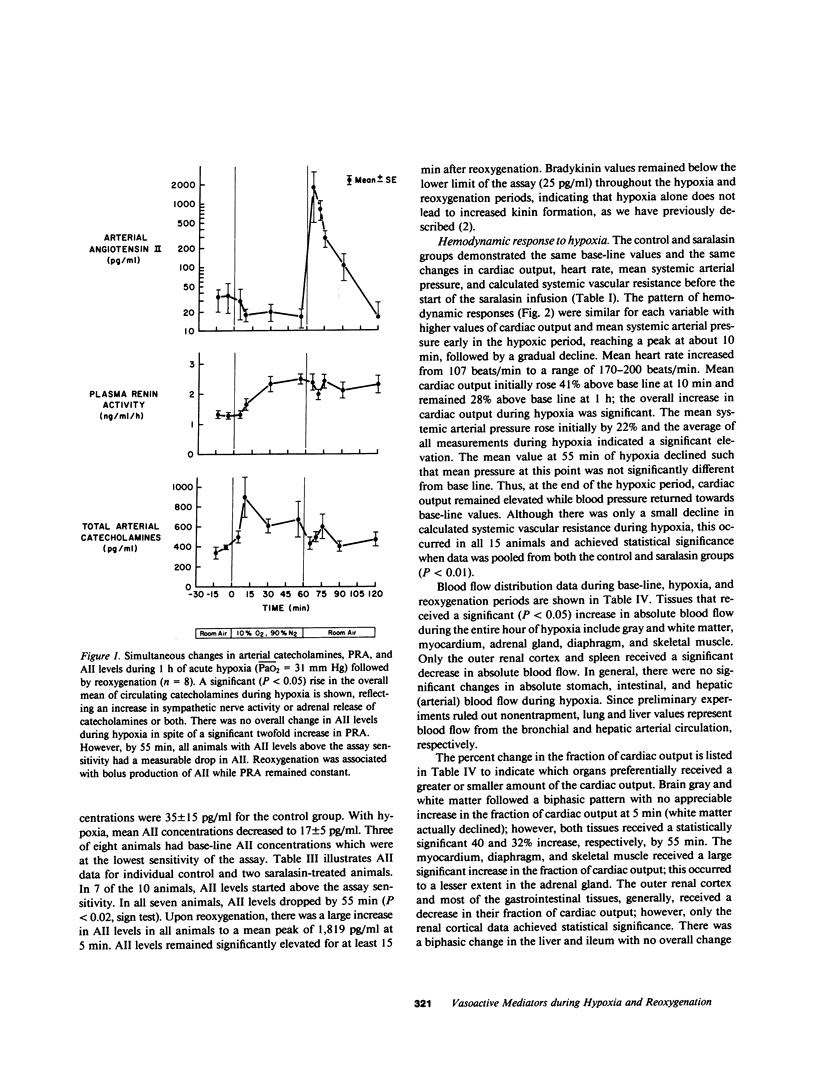
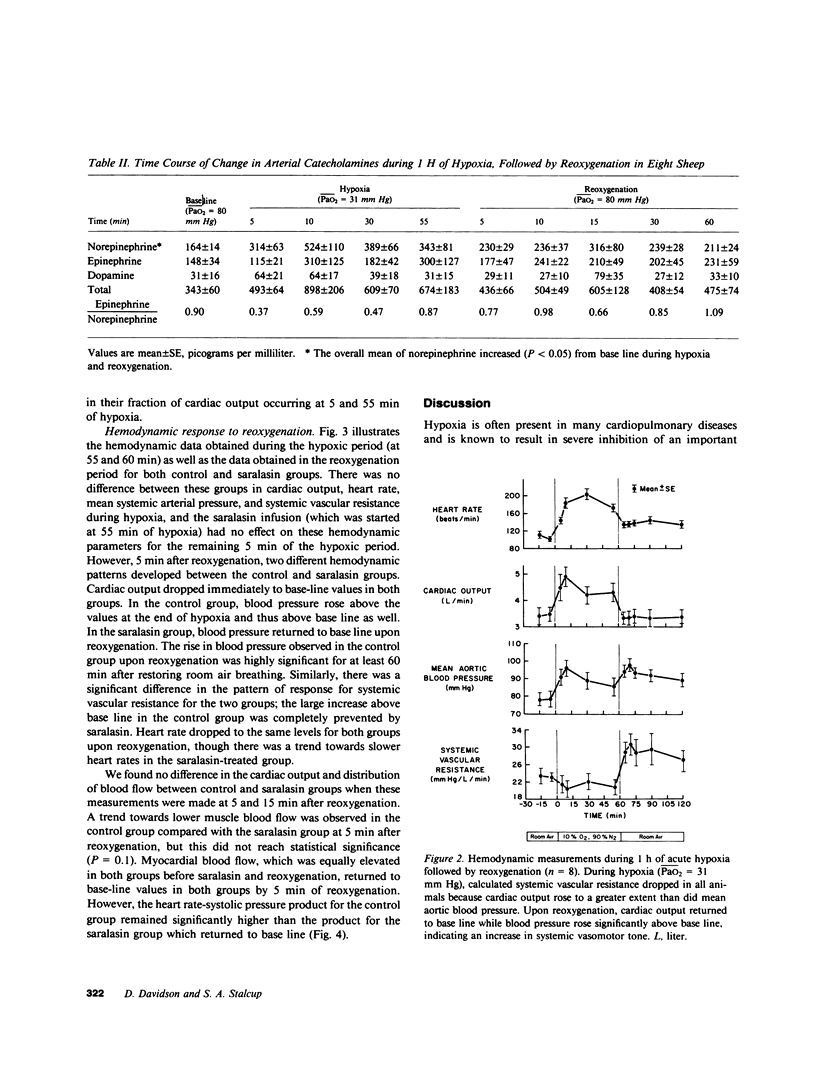
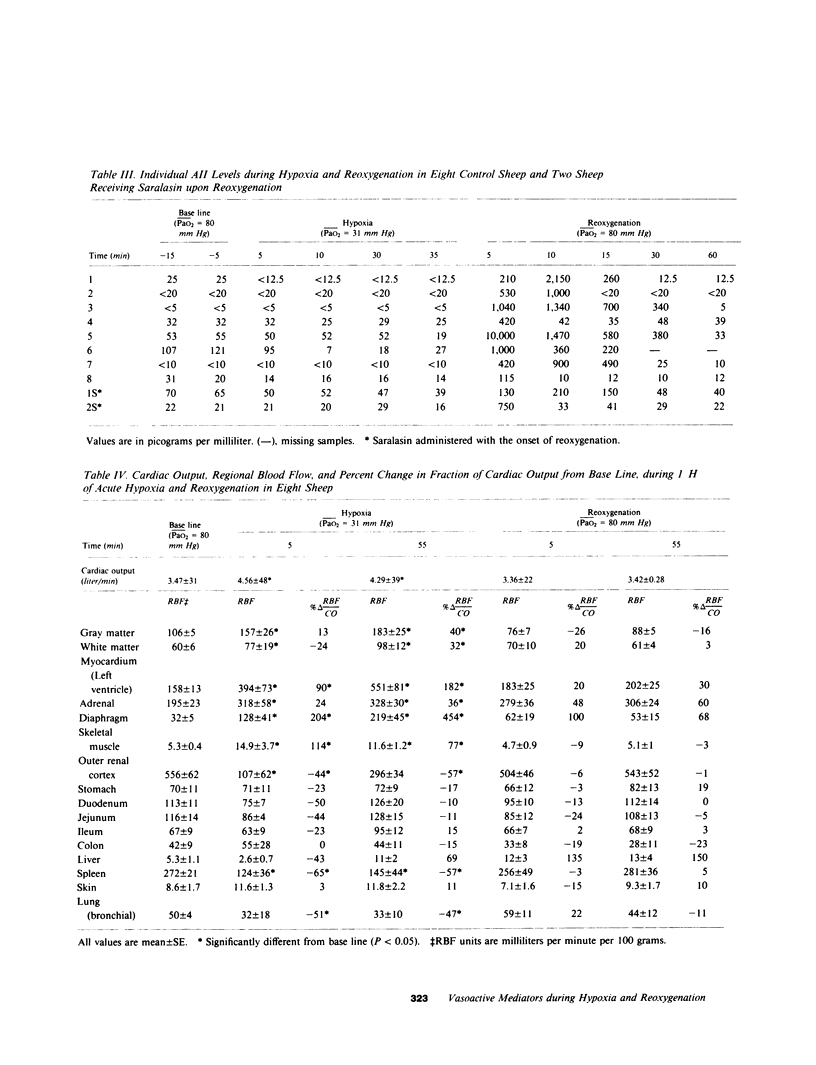
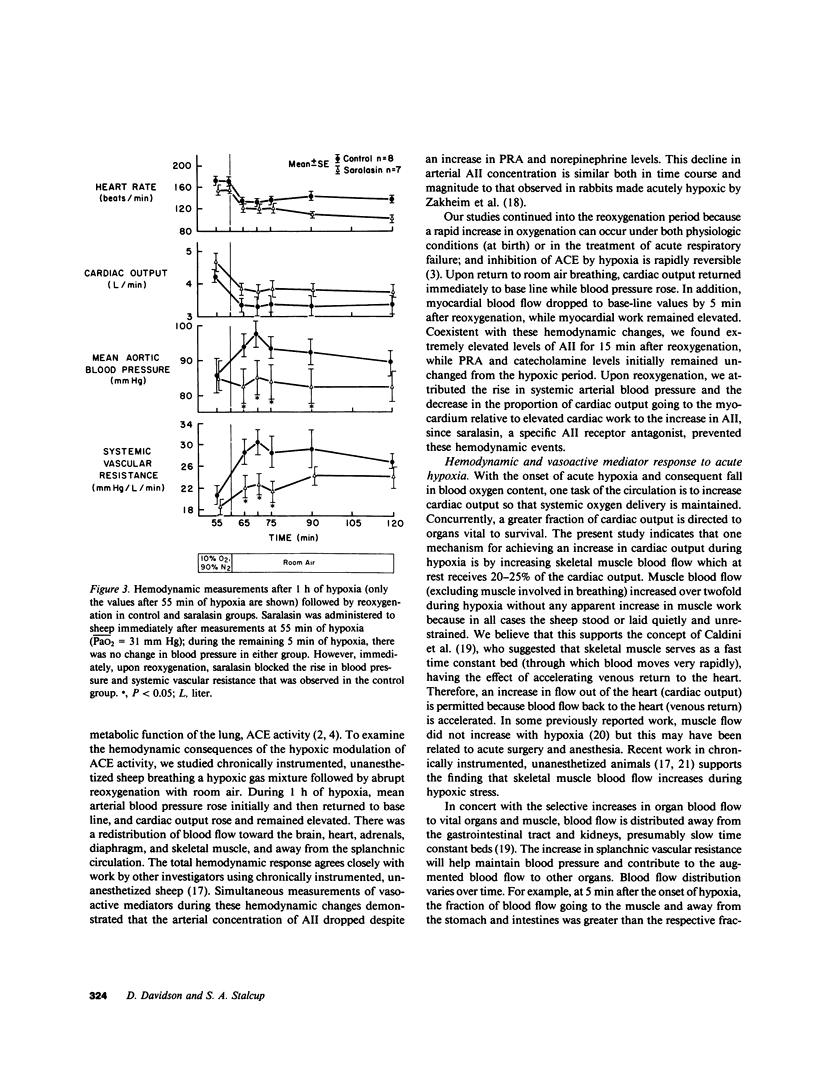
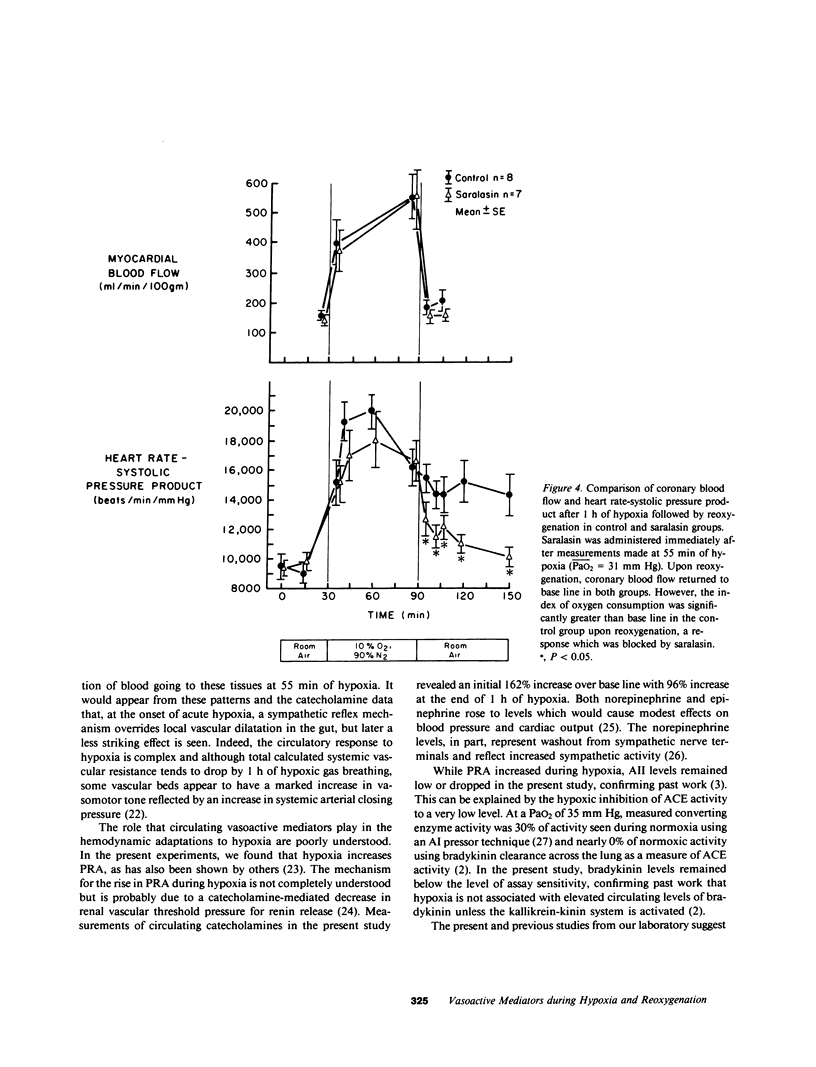
The hemodynamic consequences of the hypoxic inhibition of angiotensin-converting enzyme activity were studied in chronically instrumented unanesthetized sheep (n = 8) breathing a hypoxic gas mixture for 60 min (PaO2 = 31 mm Hg) followed by reoxygenation with room air. Changes in cardiac output, vascular pressures, blood flow distribution, arterial pH, PaCO2, PaO2, and arterial levels of plasma renin activity, angiotensin II, bradykinin, and catecholamines were measured at selected time points. Seven additional sheep underwent the same protocol but received saralasin, an angiotensin II receptor blocker beginning at 55 min of hypoxia and extending into the reoxygenation period. During hypoxia, both groups developed identical hemodynamic patterns including a rise in cardiac output (25%), blood pressure (15%), and preferential blood flow distribution to the heart, brain, adrenals, diaphragm, and skeletal muscle, as well as a decrease in the fraction of cardiac output to the kidneys and most of the gut. This was associated with a decrease in angiotensin II concentrations (from 35 to 17 pg/ml) in spite of a doubling in plasma renin activity and catecholamines. Bradykinin levels did not change. Upon reoxygenation, bolus production of angiotensin II (from 17 to 1,819 pg/ml) occurred in spite of a constant level of plasma renin activity. Concurrently, different hemodynamic patterns between control and saralasin groups emerged upon reoxygenation, including an elevation from base line in blood pressure and systemic vascular resistance in the control group. Cardiac work (heart-rate systolic pressure product) in the control group remained elevated upon reoxygenation while coronary blood flow returned to base-line values. Saralasin reduced cardiac work upon reoxygenation and restored the match between coronary blood flow and work. We conclude that plasma renin activity and oxygen tension together govern angiotensin II levels for an optimal level of systemic vasomotor tone during hypoxia. However, upon reoxygenation, bolus production of angiotensin II may result in pathophysiologic circulatory patterns, such as impairment in oxygen delivery to the myocardium proportional to persistently elevated cardiac work in the immediate postresuscitation period.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi H., Strauss W., Ochi H., Wagner H. N., Jr The effect of hypoxia on the regional distribution of cardiac output in the dog. Circ Res. 1976 Sep;39(3):314–319. doi: 10.1161/01.res.39.3.314. [DOI] [PubMed] [Google Scholar]
- Archie J. P., Jr, Fixler D. E., Ullyot D. J., Hoffman J. I., Utley J. R., Carlson E. L. Measurement of cardiac output with and organ trapping of radioactive microspheres. J Appl Physiol. 1973 Jul;35(1):148–154. doi: 10.1152/jappl.1973.35.1.148. [DOI] [PubMed] [Google Scholar]
- Broughton Pipkin F., Lumbers E. R., Mott J. C. Factors influencing plasma renin and angiotensin II in the conscious pregnant ewe and its foetus. J Physiol. 1974 Dec;243(3):619–636. doi: 10.1113/jphysiol.1974.sp010769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldini P., Permutt S., Waddell J. A., Riley R. L. Effect of epinephrine on pressure, flow, and volume relationships in the systemic circulation of dogs. Circ Res. 1974 May;34(5):606–623. doi: 10.1161/01.res.34.5.606. [DOI] [PubMed] [Google Scholar]
- Catravas J. D., Gillis C. N. Metabolism of [3H]benzoyl-phenylalanyl-alanyl-proline by pulmonary angiotensin converting enzyme in vivo: effects of bradykinin, SQ 14225 or acute hypoxia. J Pharmacol Exp Ther. 1981 May;217(2):263–270. [PubMed] [Google Scholar]
- Davidson D., Stalcup S. A., Mellins R. B. Angiotensin-converting enzyme activity and its modulation by oxygen tension in the guinea pig fetal-placental unit. Circ Res. 1981 Feb;48(2):286–291. doi: 10.1161/01.res.48.2.286. [DOI] [PubMed] [Google Scholar]
- Farhi E. R., Cant J. R., Barger A. C. Interactions between intrarenal epinephrine receptors and the renal baroreceptor in the control of PRA in conscious dogs. Circ Res. 1982 Apr;50(4):477–485. doi: 10.1161/01.res.50.4.477. [DOI] [PubMed] [Google Scholar]
- Gavras H., Kremer D., Brown J. J., Gray B., Lever A. F., MacAdam R. F., medina A., Morton J. J., Robertson J. I. Angiotensin- and norepinephrine-induced myocardial lesions: experimental and clinical studies in rabbits and man. Am Heart J. 1975 Mar;89(3):321–332. doi: 10.1016/0002-8703(75)90082-4. [DOI] [PubMed] [Google Scholar]
- Gunther S., Cannon P. J. Modulation of angiotensin II coronary vasoconstriction by cardiac prostaglandin synthesis. Am J Physiol. 1980 Jun;238(6):H895–H901. doi: 10.1152/ajpheart.1980.238.6.H895. [DOI] [PubMed] [Google Scholar]
- Heistad D. D., Abboud F. M. Dickinson W. Richards Lecture: Circulatory adjustments to hypoxia. Circulation. 1980 Mar;61(3):463–470. doi: 10.1161/01.cir.61.3.463. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P., Belfrage E., Daleskog M. Vascular and metabolic effects of circulating epinephrine and norepinephrine. Concentration-effect study in dogs. J Clin Invest. 1979 Nov;64(5):1221–1228. doi: 10.1172/JCI109576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismay M. J., Lumbers E. R., Stevens A. D. The action of angiotensin II on the baroreflex response of the conscious ewe and the conscious fetus. J Physiol. 1979 Mar;288:467–479. [PMC free article] [PubMed] [Google Scholar]
- Jakschik B. A., Marshall G. R., Kourik J. L., Needleman P. Profile of circulating vasoactive substances in hemorrhagic shock and their pharmacologic manipulation. J Clin Invest. 1974 Oct;54(4):842–852. doi: 10.1172/JCI107824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. A., Blantz R. C. Geometric error in tissue gamma-counting: methods for minimization. J Appl Physiol. 1972 Apr;32(4):533–534. doi: 10.1152/jappl.1972.32.4.533. [DOI] [PubMed] [Google Scholar]
- Leuenberger P. J., Stalcup S. A., Mellins R. B., Greenbaum L. M., Turino G. M. Decrease in angiotensin I conversion by acute hypoxia in dogs. Proc Soc Exp Biol Med. 1978 Sep;158(4):586–589. doi: 10.3181/00379727-158-40252. [DOI] [PubMed] [Google Scholar]
- Liang C. S., Gavras H. Renin-angiotensin system inhibition in conscious dogs during acute hypoxemia. Effects on systemic hemodynamics, regional blood flows, and tissue metabolism. J Clin Invest. 1978 Nov;62(5):961–970. doi: 10.1172/JCI109225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H. C., Lassen N. A., Tweed W. A., Johnson G., Jones M., Palahniuk R. J. Pressure passive cerebral blood flow and breakdown of the blood-brain barrier in experimental fetal asphyxia. Acta Paediatr Scand. 1979 Jan;68(1):57–63. doi: 10.1111/j.1651-2227.1979.tb04430.x. [DOI] [PubMed] [Google Scholar]
- Milledge J. S., Catley D. M. Renin, aldosterone, and converting enzyme during exercise and acute hypoxia in humans. J Appl Physiol Respir Environ Exerc Physiol. 1982 Feb;52(2):320–323. doi: 10.1152/jappl.1982.52.2.320. [DOI] [PubMed] [Google Scholar]
- Mott J. C. The place of the renin-angiotensin system before and after birth. Br Med Bull. 1975 Jan;31(1):44–50. doi: 10.1093/oxfordjournals.bmb.a071240. [DOI] [PubMed] [Google Scholar]
- Muldoon S. M., Tyce G. M., Moyer T. P., Rorie D. K. Measurement of endogenous norepinephrine overflow from canine saphenous veins. Am J Physiol. 1979 Feb;236(2):H263–H267. doi: 10.1152/ajpheart.1979.236.2.H263. [DOI] [PubMed] [Google Scholar]
- O'Brodovich H. M., Stalcup S. A., Pang L. M., Lipset J. S., Mellins R. B. Bradykinin production and increased pulmonary endothelial permeability during acute respiratory failure in unanesthetized sheep. J Clin Invest. 1981 Feb;67(2):514–522. doi: 10.1172/JCI110061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rooke G. A., Feigl E. O. Work as a correlate of canine left ventricular oxygen consumption, and the problem of catecholamine oxygen wasting. Circ Res. 1982 Feb;50(2):273–286. doi: 10.1161/01.res.50.2.273. [DOI] [PubMed] [Google Scholar]
- Said S. I. Metabolic functions of the pulmonary circulation. Circ Res. 1982 Mar;50(3):325–333. doi: 10.1161/01.res.50.3.325. [DOI] [PubMed] [Google Scholar]
- Stalcup S. A., Leuenberger P. J., Lipset J. S., Osman M. M., Cerreta J. M., Mellins R. B., Turino G. M. Impaired angiotensin conversion and bradykinin clearance in experimental canine pulmonary emphysema. J Clin Invest. 1981 Jan;67(1):201–209. doi: 10.1172/JCI110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalcup S. A., Lipset J. S., Legant P. M., Leuenberger P. J., Mellins R. B. Inhibition of converting enzyme activity by acute hypoxia in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1979 Feb;46(2):227–234. doi: 10.1152/jappl.1979.46.2.227. [DOI] [PubMed] [Google Scholar]
- Stalcup S. A., Lipset J. S., Mellins R. B. Modulation of converting enzyme activity by hypoxia and its physiological effects. Ciba Found Symp. 1980;78:293–311. doi: 10.1002/9780470720615.ch16. [DOI] [PubMed] [Google Scholar]
- Stalcup S. A., Lipset J. S., Woan J. M., Leuenberger P., Mellins R. B. Inhibition of angiotensin converting enzyme activity in cultured endothelial cells by hypoxia. J Clin Invest. 1979 May;63(5):966–976. doi: 10.1172/JCI109397. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sylvester J. T., Gilbert R. D., Traystman R. J., Permutt S. Effects of hypoxia on the closing pressure of the canine systemic arterial circulation. Circ Res. 1981 Oct;49(4):980–987. doi: 10.1161/01.res.49.4.980. [DOI] [PubMed] [Google Scholar]
- Szidon P., Bairey N., Oparil S. Effect of acute hypoxia on the pulmonary conversion of angiotensin I to angiotensin II in dogs. Circ Res. 1980 Feb;46(2):221–226. doi: 10.1161/01.res.46.2.221. [DOI] [PubMed] [Google Scholar]
- Zakheim R. M., Molteni A., Mattioli L., Park M. Plasma angiotensin II levels in hypoxic and hypovolemic stress in unanesthetized rabbits. J Appl Physiol. 1976 Oct;41(4):462–465. doi: 10.1152/jappl.1976.41.4.462. [DOI] [PubMed] [Google Scholar]
- Zimmerman B. G. Blockade of adrenergic potentiating effect of angiotensin by 1-Sar-8-Ala-angiotensin II. J Pharmacol Exp Ther. 1973 Jun;185(3):486–492. [PubMed] [Google Scholar]


