Abstract
Without timely pharmacological treatment, nerve agent exposure can cause a large number of casualties, as occurred in the recent sarin attack in Syria. Nerve agent-induced seizures are initiated due to inhibition of acetylcholinesterase, but they become quickly refractory to muscarinic antagonists, and their suppression by benzodiazepines can be only temporary. Therefore, novel treatments are necessary to stop seizures and prevent brain damage and the resulting long-term behavioral deficits. We have previously shown that LY293558, a GluK1/AMPA receptor antagonist, is a very effective anticonvulsant and neuroprotectant against nerve agent exposure. In the present study, we examined whether the protection against nerve agent-induced seizures and neuropathology conferred by LY293558 translates into protection against pathophysiological alterations in the basolateral amygdala (BLA) and the development of anxiety, which is the most prevalent behavioral deficit resulting from exposure. LY293558 (15 mg/kg) was administered to rats along with atropine and the oxime HI-6, at 20 min after exposure to soman (1.2 x LD50). At 24 h, 7 days, and 30 days after exposure, soman-exposed rats that did not receive LY293558 had reduced but prolonged evoked field potentials in the BLA, as well as increased paired-pulse ratio, suggesting neuronal damage and impaired synaptic inhibition. In contrast, soman-exposed rats that received LY293558 did not differ from controls in these parameters. Similarly, long-term potentiation of synaptic transmission was impaired at 7 days after exposure in the soman-exposed rats that did not receive anticonvulsant treatment, while this impairment was not present in the LY293558-treated rats. Anxiety-like behavior assessed by the open field and acoustic startle response tests was increased in the soman-exposed rats at 30 and 90 days after exposure, while soman-exposed rats treated with LY293558 did not differ from controls. Along with our previous findings, the present data demonstrate the remarkable efficacy of LY293558 in counteracting nerve agent-induced seizures, neuropathology, pathophysiological alterations in the BLA, and anxiety-related behavioral deficits.
Keywords: Soman, LY293558, Basolateral Amygdala, Anxiety, Long-Term Potentiation, paired-pulse ratio
1.0 Introduction
The development of nerve agents started before WWII, but it was not until 1981 that these chemical weapons were used in the Iraq-Iran war (Newmark, 2004), and, in 1988, against Kurdish civilians (Dworkin et al., 2008). Subsequently, nerve agents were used again in terrorist attacks in Japan (Morita et al., 1995; Nagao et al., 1997), accidental exposures in Iraq have been reported (e.g., Loh et al., 2010), and recently the world was reminded again of the devastating effects of these agents after the sarin attack in Syria, which killed 1,429 people, including 426 children, and left many to deal with the long-term health consequences (Dolgin, 2013; Sellstrom et al., 2013). Nerve agents are organophosphates with the same primary mechanism of action as the organophosphorous pesticides, exposure to which kills about 200,000 people per year, particularly in rural areas of developing countries (Eddleston et al., 2008).
Nerve agents act by irreversibly inhibiting acetylcholinesterase (AChE) activity (Bajgar, 2005; Collombet et al., 2011; Prager et al., 2013). Without pharmacological intervention after nerve agent exposure, death may ensue due to the peripheral cholinergic crisis, and/or to intense seizure activity (status epilepticus, SE). If death is prevented but SE is not controlled in a timely manner, profound brain damage may occur (McDonough et al., 1995; McDonough and Shih, 1997; Prager et al., 2014; Shih et al., 2003), producing long-term behavioral deficits (Coubard et al., 2008; Filliat et al., 2007; Prager et al., 2014). Thus, suppression of nerve agent-induced seizures is necessary to prevent neuropathology and the subsequent development of behavioral and neurological disorders.
Although seizures are initiated by cholinergic–primarily muscarinic–overstimulation, muscarinic receptor antagonists can terminate nerve agent-induced seizures only if given very soon after exposure (Lallement et al., 1998; McDonough and Shih, 1993; Shih and McDonough, 1999; Skovira et al., 2010). Glutamatergic mechanisms are responsible for strengthening and sustaining the seizures (McDonough and Shih, 1997), and, therefore, benzodiazepines, which suppress glutamatergic excitation by enhancing GABAergic activity (Campo-Soria et al., 2006; Gielen et al., 2012) are considered the first line of treatment for nerve agent-induced SE; diazepam is currently the only FDA-approved anticonvulsant for nerve agent exposure. However, after the initial cessation of SE by diazepam, seizures can return (Apland et al., 2014; Shih et al., 1999) and neuroprotection is not achieved (Apland et al., 2014), while repeated administrations of diazepam to suppress recurring seizures may be detrimental, since this drug suppresses the cardiovascular and respiratory systems (Chen et al., 2011; Mehta et al., 2007). Therefore, the development of novel and effective pharmacological treatments is imperative.
We have previously tested the anti-seizure and neuroprotective efficacy of (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl) ethyl]decahydroisoquinoline-3-carboxylic acid (LY293558; Bleakman et al., 1996; Jane et al., 2009), an antagonist of AMPA receptors as well as the kainate receptors that contain the GluK1 subunit (formerly known as GluR5 kainate receptor subunit; Collingridge et al., 2009; Jane et al., 2009), against the nerve agent soman. We found that LY293558 is very effective in terminating soman-induced seizures and protecting against neuropathology, whether it is administered 1 h after exposure (in an animal model in which peripheral effects are controlled by oxime pretreatment and administration of atropine within the first min after soman injection; Figueiredo et al., 2011b), or in a model in which all treatments are delayed to 20 min after exposure (resembling a real-life situation where pretreatment is not feasible and medical assistance may not be available immediately; Apland et al., 2013). However, we have also found that in addition to neuronal damage, soman exposure causes pathophysiological alterations in the basolateral amygdala (BLA), which are accompanied by an increase in anxiety-like behavior (Prager et al., 2014). Increased anxiety is the most well-documented behavioral deficit resulting from nerve agent-induced brain damage in animals (Coubard et al., 2008; Filliat et al., 2007; Hoffman et al., 2007; Langston et al., 2012; Moffett et al., 2011), but also in humans, as we know from the enduring psychiatric symptoms presented by the victims of the sarin attack in Japan (Hoffman et al., 2007; Ohtani et al., 2004). Therefore, in the present study, we examined whether the efficacy of LY293558 against seizures and neuropathology translates into protection against the pathophysiological changes seen in the BLA and the development of anxiety-like behavior.
2.0 Materials and Methods
2.1 Animals
Experiments were performed using 6-week old (150–200 g) male, Sprague-Dawley rats (Taconic Farms, Derwood, MD). Animals were individually housed in an environmentally controlled room (20–23°C, ~44% humidity, 12 light/12-h dark cycle [350–400 lux], lights on at 6:00 am), with food (Harlan Teklad Global Diet 2018, 18% protein rodent diet; Harlan Laboratories; Indianapolis, IN) and water available ad libitum. Cages were cleaned weekly and animal handling was minimized to reduce animal stress (Prager et al., 2011). All animal experiments were conducted following the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council), and were approved by the U.S. Army Medical Research Institute of Chemical Defense and the Uniformed Services University of the Health Sciences Institutional Animal Care and Use Committees, who are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
2.2 Soman Administration and Drug Treatment
Soman (pinacolyl methylphosphonofluoridate; obtained from the U.S. Army Edgewood Chemical Biological Center, Aberdeen Proving Ground, MD, USA) was diluted in cold saline and administered via a single subcutaneous injection (132 μg/kg, which is approximately 1.2 x LD50; Apland et al., 2013; Figueiredo et al., 2011a; Figueiredo et al., 2011b; Jimmerson et al., 1989) to 111 rats. Following exposure to soman, rats were monitored for signs of seizure onset, and continuously rated for seizure severity according to the modified Racine scale (Apland et al., 2010; Racine et al., 1977; Racine, 1972): Stage 0, no behavioral response; Stage 1, behavioral arrest, orofacial movements, chewing; Stage 2, head nodding/myoclonus; Stage 3, unilateral/bilateral forelimb clonus without rearing, straub tail, extended body posture; Stage 4, bilateral forelimb clonus plus rearing; Stage 5, rearing and falling; Stage 6, full tonic seizures. To control the peripheral effects of soman and prevent death from cardiorespiratory suppression, rats received an intramuscular injection of 2 mg/kg atropine sulfate (Sigma-Aldrich, St. Louis, MO), a muscarinic receptor antagonist, as well as an intraperitoneal injection of 125 mg/kg HI-6 (Starks Associates, Buffalo, NY), a bispyridinium oxime that reactivates inhibited AChE primarily in the periphery (Mercey et al., 2012), at 20 min after soman exposure. We followed this drug administration protocol (Apland et al., 2013), in order to mimic more closely a real-life situation where pharmacological treatment may not be available within the first 5 minutes after exposure. Rats that were treated only with atropine and HI-6 comprised the soman group. Other rats were additionally treated with the GluK1/AMPA receptor antagonist LY293558 (15 mg/kg, intramuscularly; kindly provided by Raptor Pharmaceutical Corp., Novato, CA), immediately after the atropine + HI-6 injection, as previously described (Apland et al., 2013); these rats comprised the soman+LY293558 group. After the cessation of SE, surviving rats from the soman group (35 rats from the soman group died, while all rats in the soman+LY293558 group survived) were administered Lactated Ringer’s solution (5 mL) every 8 hours, until body weight started increasing.
2.3 Electrophysiological Experiments
At 24 h, 7 days, and 30 days after soman exposure, coronal brain slices (400 μm-thick) containing the amygdala (−2.64 to 3.36 from Bregma) were prepared as described previously (Prager et al., 2014). The recording solution (artificial cerebrospinal fluid; ACSF) consisted of (in mM): 125 NaCl, 3 KCl, 1.25 NaH2PO4, 21 NaHCO3, 2 CaCl2, 1.5 MgCl2, and 11-D-glucose (all purchased from Sigma-Aldrich). All solutions were saturated with 95% O2, 5% CO2 to achieve a pH near 7.4. Following a 1.5 h recovery, field potential recordings were obtained in an interface-type chamber (Harvard Apparatus, Holliston, MA), maintained at 32–33°C, with a flow rate of the ACSF at ~1.5 mL/min. Field potentials were evoked by stimulating the external capsule at 0.05 Hz with a bipolar concentric stimulating electrode made of tungsten (World Precision Instruments, Sarasota, FL). For LTP experiments, a 20 min baseline was recorded; stimulus intensity was adjusted to elicit a response of about 70–80% of the maximum amplitude. High-frequency stimulation (HFS) consisted of 3 trains of pulses at 100 Hz, each train lasting 1 s, and the interval between trains was 20 s. Following HFS, field potential recordings resumed with stimulation at 0.05 Hz, for 60 min. For paired-pulse stimulation experiments, inter-stimulus interval was 60 ms. Recording glass pipettes were filled with ACSF and had a resistance of approximately 5 MΩ. Signals were digitized using the pClamp 10.4 software (Molecular Devices, Union City, CA), analyzed using AxoGraph (AxoGraph X, Berkley, CA), and final presentation was prepared using GraphPad Prism (GraphPad Software, La Jolla, CA).
2.4 Behavioral Experiments
We used two different tests of anxiety-like behavior. The open field test, which is very commonly used to test anxiety-like behavior in animals (Prut and Belzung, 2003) and the acoustic startle response. The latter test was chosen because the most prevalent psychiatric disorder found in victims of nerve agent exposure is posttraumatic stress disorder (PTSD), and a characteristic feature of PTSD patients is exaggerated responses to startle stimuli (Morgan et al., 1996). Our aim therefore was to determine if the soman-exposed rats exhibited an exaggerated startle response, and if this was prevented by treatment with LY293558.
2.4.1 Open Field Test
Anxiety-like behavior was assessed using an open field apparatus (40 × 40 × 20 cm clear Plexiglas arena), as described previously (Aroniadou-Anderjaska et al., 2012; Faraday et al., 2001; Prager et al., 2014). Animals were not handled prior to testing in order to minimize stress, especially after soman exposure when the rats are already stressed and hypersensitive to handling. As it has been pointed out in Prager et al., (2011), handling animals prior to testing increases mean heart rate, blood pressure, and serum corticosterone concentrations, and rats show minimal habituation to these physiological markers of stress (Balcombe et al., 2004). Moreover, the persisting corticosterone response after the initial handling may affect performance in subsequent behavioral testing (Brown and Martin, 1974).
One day prior to test day, rats were acclimated to the apparatus for 20 min. On the test day (30 or 90 days after exposure) rats were placed in the center of the open field and activity was measured and recorded for 20 min using an Accuscan Electronics infrared photocell system (Accuscan Instruments Inc., Columbus, OH). Data were automatically collected and transmitted to a computer equipped with “Fusion” software (Accuscan Electronics); “center” was a 25×25 cm area in the center of the apparatus. Locomotion (distance traveled in cm), total movement time, and time spent in the center of the open field were analyzed. Anxiety was measured as the ratio of the time spent in the center over the total movement time and expressed as a percentage of the total movement time (Aroniadou-Anderjaska et al., 2012; Prager et al., 2014).
2.4.2 Acoustic Startle Response Test
Acoustic startle response (ASR) testing was conducted with the use of the Med Associates Acoustic Response Test System (Med Associates, Georgia, VT), which consists of a weight-sensitive platform inside an individual sound-attenuating chamber, which contained a ventilated fan to provide background noise (Prager et al., 2014). Rats were individually placed in a ventilated holding cage small enough to restrict extensive locomotion, but large enough to allow the subject to turn around and make other small movements (Aroniadou-Anderjaska et al., 2012; Prager et al., 2014). The chamber was placed on a weight-sensitive platform; movements in response to stimuli were measured as a voltage change by a strain gauge inside each platform. Animals were acclimated to the apparatus in two sessions; on days 28 and 29 for the groups tested 30 days after the exposure, and on days 88 and 89 for the groups tested 90 days after the exposure. A 3-min adaptation period was allowed, during which no startle stimuli were presented. Startle stimuli consisted of 110 or 120 dB sound pressure level noise bursts of 20-ms duration. Each stimulus had a 2 ms rise and decay time, such that the onset and offset were abrupt, which is a primary requirement for startle (Faraday and Grunberg, 2000). Each trial type was presented eight times. Trial types were presented at random to avoid order effects and habituation; inter-trial intervals ranged randomly from 15 to 25 s. Responses were recorded by an interfaced Pentium computer as the maximum response occurring during the no-stimulus periods, and during the startle period, and were assigned a value based on an arbitrary scale used by the software of the Test System (Aroniadou-Anderjaska et al., 2012; Prager et al., 2014).
2.5 Statistical Analysis
All data are expressed as mean ± standard error. One-way ANOVA with a Dunnett t post hoc test was used to analyze the results from the behavioral tests. Results from the electrophysiological experiments were analyzed using either one-way ANOVA followed by Tukey post hoc test, or independent samples t-test. Statistical analyses were made using the software package PAWS SPSS 22 (IBM, Armonk, NY, USA). Differences were considered significant with p < 0.05. Sample size “n” refers to the number of animals in the behavioral experiments and the number of slices in the electrophysiological experiments.
3.0 Results
3.1 LY293558 Protects against Pathophysiological Alterations in the BLA After Soman Exposure
We have previously found that after soman exposure, the extensive neuronal damage in the BLA is accompanied by pathophysiological alterations, namely, reduced amplitude and increased duration of evoked field potentials, increased paired-pulse ratios, and impaired LTP of synaptic transmission (Prager et al., 2014). To determine whether LY293558 protects against these impairments, we recorded BLA field potentials evoked by stimulation of the external capsule in brain slices from soman-exposed rats treated with LY293558 (soman+LY293558 rats), and compared with soman-exposed rats that did not receive the anticonvulsant treatment (soman rats), at 24 h, 7 days, and 30 days after the exposure.
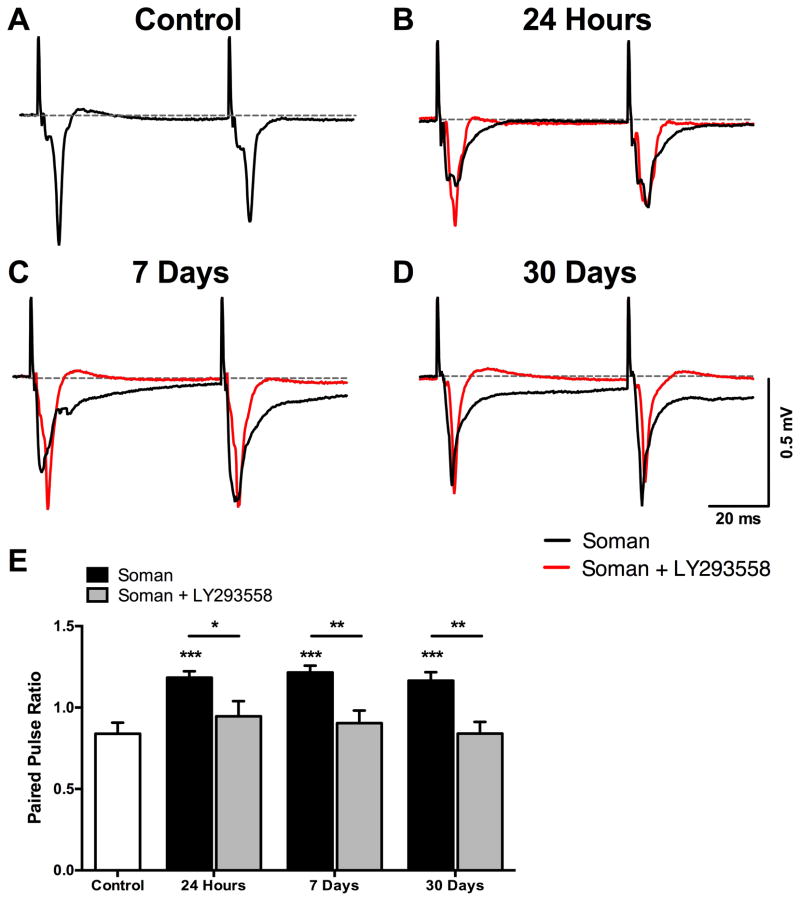
At 24 h after exposure, only field potentials of a very small amplitude and prolonged decay could be evoked in the soman group, while these alterations were not observed in the soman+LY293558 group (Fig. 1B). The increased duration of the evoked field potentials persisted in the soman group at 7 and 30 days after exposure (Fig. 1C, D), and probably reflected impaired inhibition. Impaired synaptic inhibition can affect the paired-pulse ratio (PPR), which is the ratio of the amplitude of the second response over that of the first response, when paired-pulses are delivered at small inter-pulse intervals at which monosynaptic or polysynaptic inhibition is still in effect. Therefore, to strengthen the evidence that synaptic inhibition may be impaired in the soman rats but not in the soman+LY293558 rats, we compared the PPR between the groups at the 3 time points after exposure. In the soman group, the PPR was significantly higher compared to the controls (0.84 ± 0.07, n = 11; Fig. 1A), at 24 h (1.18 ± 0.04, n = 11; p < 0.001; Fig. 1B), 7 days (1.22 ± 0.04; n = 11; p < 0.001; Fig. 1C), and 30 days (1.16 ± 0.05, n = 10; p < 0.001; Fig. 1D) post-exposure, suggesting that the inhibition that normally suppresses the amplitude of the synaptic response to the second stimulus pulse and limits contribution of spiking activity to the field potentials was reduced. In contrast, the PPR in the soman+LY293558 animals did not differ significantly from that of the controls, at 24 h (0.95 ± 0.09; n = 9), 7 days (0.90 ± 0.08; n = 8 slices from 5 rats), or 30 days (0.84 ± 0.07; n = 9; all p’s > 0.05) after exposure (Fig. 1). When comparing the soman group with the soman+LY293558 group, the PPR of the latter was significantly smaller, at 24 h (p = 0.021), 7 days (p = 0.001), and 30 days (p = 0.002) after exposure (Fig. 1E).
Figure 1.
Alterations in the BLA field potentials after soman-induced SE and protection by LY293558 treatment. A–D. Representative field potentials evoked in the BLA by paired-pulse stimulation of the external capsule, from control rats (n = 11, A), soman-exposed rats (black waveforms) at 24 hours (n = 11, B), 7 days (n = 11, C), and 30 days (n = 10, D) after exposure, and soman+LY293558 treated rats (red waveforms) at 24 hours (n = 9, B), 7 days (n = 8, C), and 30 days (n = 9, D) after exposure. Each trace is an average of 30 sweeps. In soman-exposed rats, the duration of the field potentials was prolonged (notice the slow decay of the waveforms); this was not observed in soman+LY293558 treated rats. E. Paired-pulse ratio (PPR) was significantly increased in soman-exposed rats, but not in soman+LY293558 treated rats. The PPR was significantly lower in the soman+LY293558 treated animals compared to the soman-exposed animals. *p < 0.05, **p < 0.01, ***p < 0.001 (One-Way ANOVA and Independent T-tests).
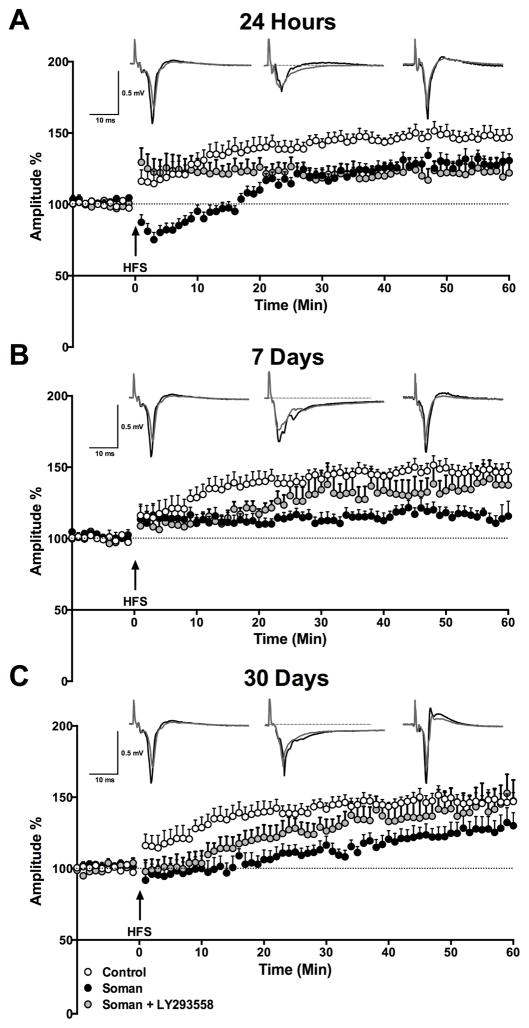
We previously found that the capacity of neuronal synapses in the BLA to express LTP was reduced 24 hours, 7 days, and 14 days after SE (Prager et al., 2014). Here, we examined whether LY293558 protects against this impairment. Potentiation of the evoked field potentials was measured by averaging the amplitude of the response from 50 to 60 min after HFS, and expressing it as a percentage of the baseline response. Compared to the percent change in the amplitude of the response in control animals (150.3 ± 6.3%, from 0.49 ± 0.02 mV at baseline to 0.73 ± 0.03 mV at 50–60 min after HFS, n = 12), the percent increase at 24 h after exposure was lower in both the soman group (119.2 ± 7.4%, from 0.30 ± 0.03 mV to 0.34 ± 0.03 mV, n = 11, p = 0.004) and the soman+LY293558 group (125.8 ± 8.4%, from 0.45 ± 0.02 mV to 0.57 ± 0.05 mV, n = 9; p = 0.032), while only the soman group displayed a prolonged post-tetanic depression (Fig. 2A). Seven days after soman exposure, the increase of the response at 50 to 60 min after HFS remained significantly lower in the soman group (120.4% ± 6.1, from 0.32 ± 0.04 mV to 0.39 ± 0.05 mV, n = 13; p = 0.007 compared to controls), but recovered to control values in the soman+LY293558 group (136.3 ± 11.7%, from 0.45 ± 0.02 mV to 0.61 ± 0.06 mV, n = 8; p = 0.290; Fig. 2B). Thirty days after soman exposure, there were no differences between any pair of groups; the potentiation of the response did not differ significantly from the controls in either the soman group (126.7 ± 9.7%, from 0.4 ± 0.03 mV to 0.50 ± 0.05 mV, n = 11; p = 0.103) or the soman+LY293558 group (143.1 ± 13.1%, from 0.46 ± 0.03 mV to 0.67 ± 0.09 mV, n = 9; p = 0.733; Fig. 2C).
Figure 2.
Effects of soman and LY293558 treatment on long-term potentiation in the BLA. The plots show the time course of the changes in the amplitudes of the field potentials after high- frequency stimulation (HFS), in control rats (open circles), soman-exposed rats (black circles), and soman-exposed rats treated with LY293558 (gray circles). The amplitudes of 3 responses recorded in each min (stimulation at 0.05 Hz) were averaged, and each data point on the plot is the mean and standard error of these averages, from 7–12 slices (see sample sizes in the text). Traces over the plots are examples from a control rat (left), soman-exposed rats (middle), and soman+LY293558 treated rats (right); the superimposed field potentials are a baseline response (gray) and a response at 50–60 min after HFS (black; each trace is the average of 20 sweeps;). A. At 24 h after soman exposure, potentiation of the responses, measured at 50–60 min after HFS, was significantly lower–compared to the control group–in both the soman and the soman+LY293558 groups. B. Seven days post-exposure, potentiation of the response in the soman+LY293558 treated animals was not significantly lower than in controls, while potentiation in the soman group remained significantly reduced. C. Thirty days after exposure, there was no significant difference between the groups in the potentiation of the responses.
3.2 LY293558 Prevents the Development of Anxiety-like Behavior After Soman Exposure
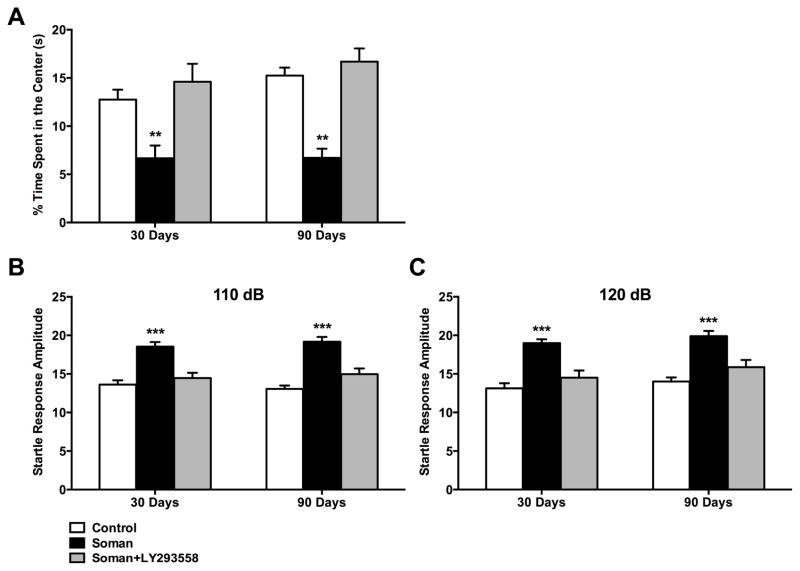
To determine whether LY293558 treatment prevents the development of anxiety-like behavior, we tested soman and soman+LY293558 rats in the open field, 30 and 90 days after exposure. In the open field test, the more anxious an animal is, the less time it spends in the center of the open field (Prut and Belzung, 2003). Thirty days after exposure, the soman rats spent significantly less time in the center of the open field (6.7 ± 1.3% of the total movement time; n = 10; p = 0.01) compared to controls (12.8 ± 1.0% of the total movement time, n = 10), while the time spent in the center of the open field by the soman+LY293558 treated animals (14.6 ± 1.9% of the total movement time, n = 10; p = 0.57) did not differ from the controls (Fig. 3A). Both soman-exposed groups did not differ from control animals in distance traveled (2,969 ± 382 cm for control animals, 3,973 ± 415 cm for the soman group, and 3,371 ± 380 cm for soman+LY293558 group), or movement time (801 ± 23 s for control animals, 838 ± 28 s for the soman group, and 861 ± 20 s for the soman+LY293558 group), 30 days after soman exposure.
Figure 3.
LY293558 protects against soman-induced, long-term increases in anxiety-like behavior, as measured by the open field and acoustic startle response (ASR) tests. A. At 30 and 90 days after soman exposure, soman-exposed rats that did not receive anticonvulsant treatment spent significantly less time in the center of the open field, compared to control rats, while soman+LY293558 treated rats did not differ from the controls. B and C. At 30 and 90 days after soman exposure, the amplitude of the startle response to 110 dB (B) and 120 dB (C) startle stimuli was significantly increased in the soman rats, but not in the soman+LY293558 rats, compared to the control group. **p < 0.01, ***p < 0.001; n = 10 per group (One-Way ANOVA with Dunnett’s T post hoc).
Ninety days after soman exposure, the soman group still spent significantly less time in the center of the open field (6.7 ± 1.0% of the total movement time; n = 10; p = 0.003) compared to controls (15.3 ± 0.8% of the total movement time; n = 10), while the soman+LY293558 group (16.7 ± 1.4% of the total movement time; n = 10) did not differ from the controls (p = 0.28; Fig. 3A). Both soman-exposed groups did not differ from control animals in distance traveled (2,126 ± 173 cm for controls, 2,045 ± 473 cm for the soman group, and 2,079 ± 227 cm for the soman+LY293558 group), or movement time (729.2 ± 38.9 s for controls, 601.9 ± 63.5 s for the soman group, and 729.4 ± 43.7 s for the soman+LY293558 group).
The ASR test for anxiety and fear measures the amplitude of the startle in response to an acoustic stimulus (Hitchcock and Davis, 1987; Li et al., 2009; Li et al., 1999). We measured the amplitude of the startle response by averaging the amplitudes of 8 trials, using two levels of acoustic stimuli, 110 dB and 120 dB noise bursts. The 110 dB level was used for comparison to ensure that the primary stimulus level of 120 dB not only met a response threshold, but also did not elicit responses that exceeded the maximum response the equipment can measure. We report the results from both acoustic stimuli because, in response to the 120 dB, some of the rats in the soman group, at the 90 days post-exposure time-point, reached the maximum amplitude the equipment can measure. Compared to the amplitude of the ASR in the control group (13.6 ± 0.6 and 13.1 ± 0.7 in response to the 110 and 120 dB noise burst, respectively; n = 10), the ASR amplitude was increased in the soman group (18.5 ± 0.6 in response to the 110 dB, p < 0.001, and 19.0 ± 0.5 in response to the 120 dB noise burst, p < 0.001; n = 10), but not in the soman+LY293558 group (14.5 ± 0.7 in response to the 110 dB, p = 0.52, and 14.5 ± 0.9 in response to the 120 dB, p = 0.31, n = 10), at 30 days after exposure (Fig. 3B, C). Similarly, compared to the amplitude of the ASR in the control group (13.1 ± 0.5 and 14.0 ± 0.5 in response to the 110 and 120 dB, respectively; n = 10), the ASR amplitude was increased in the soman group (19.2 ± 0.6 in response to the 110 dB, p < 0.001, and 19.9 ± 0.7 in response to the 120 dB, p < 0.001; n = 10), but not in the soman+LY293558 group (15.0 ± 0.7 in response to the 110 dB, p = 0.67, and 15.9 ± 0.9 in response to the 120 dB; p = 0.49; n = 10), at 90 days after exposure (Fig. 3B, C).
4.0 Discussion
The BLA appears to play a pivotal role in the mechanisms by which nerve agents exert their devastating effects. First, it is a key brain area for the initiation and propagation of seizures after exposure; seizures are not induced unless AChE decreases significantly in the BLA (Prager et al., 2013), and microinjection of nerve agents into different brain regions can elicit convulsions only when the nerve agent is injected into the BLA (McDonough et al., 1987). Second, it is one of the most severely damaged brain region after nerve agent exposure (Apland et al., 2010; Aroniadou-Anderjaska et al., 2009; Shih et al., 2003), suggesting more intense seizure activity in this area (Apland et al., 2009), and/or high susceptibility to seizure-induced damage. Finally, the most prevalent behavioral deficit resulting from nerve agent exposure is anxiety (Coubard et al., 2008; Filliat et al., 2007; Hoffman et al., 2007; Langston et al., 2012; Ohtani et al., 2004; Prager et al., 2014), and a characteristic feature of anxiety is a derangement in the excitability of the BLA (Koenigs and Grafman, 2009; Mahan and Ressler, 2012; Rauch et al., 2000; Sajdyk and Shekhar, 1997; Zhou et al., 2010). In the present study, we found that treatment with LY293558 after exposure to soman, in addition to its previously reported efficacy in stopping seizures and reducing neuropathology (Apland et al., 2013; Figueiredo et al., 2011b), also protects against pathophysiological alterations in the BLA related to the excitability of the network, and prevents the development of anxiety.
At 24 h after soman exposure that produces prolonged SE, BLA slices obtained from the exposed rats display weak field potentials (large stimulus intensities are required to elicit even small population responses), which recover gradually over a 30-day period (Prager et al., 2014 and present study). This is probably due to neuronal damage (neuronal loss and degeneration). In addition to neuronal death and irreversible degeneration that are already present at 24 h post-exposure (Figueiredo et al., 2011b; Prager et al., 2014), reversible synaptic impairments probably also occur, such as presynaptic changes associated with neurotransmitter release and postsynaptic responsiveness. Recovery of such temporary synaptic dysfunction may explain why the field potentials recover with time, despite that reduction in neuronal number persists (Prager et al., 2014). In the present study, we found that treatment with LY293558 prevents the transient impairment (reduction) in the evoked population responses. This cannot be due to possible presence of LY293558 in the brain slices of the LY293558-treated rats, for the following reasons: 1) LY293558, at 15 mg/kg, as used in the present study, falls below the Lower Limit Of Quantitation in the brain in about 1.5 h after intramuscular injection (Apland et al., 2013); 2) LY293558 washes out (see for example in Braga et al., 2003), and therefore even if it were present in the slices initially, it would wash out by the constant flow of ACSF; and 3) if LY293558 were present in the brain slices of the LY293558-treated rats, its effect should have been to suppress excitatory/glutamatergic activity and, therefore, it should reduce the amplitude of the evoked field potentials. In contrast, we find that in comparison to the untreated soman-exposed rats, the rats treated with LY293558 had field potentials of larger amplitudes, similar to those in the control rats. This can be attributed to the cessation of SE by LY293558 and the resulting protection against neuronal loss and degeneration, as we described previously (Apland et al., 2013; Figueiredo et al., 2011b).
Despite the small population responses after soman exposure, their duration is increased, and PPR also increases. Both of these observations suggest impairment in synaptic inhibition, which persists throughout the 30-day period we have examined (Prager et al., 2014 and present study; Fig. 1). Reduced inhibition in the BLA can be expected since 46% of GAD67 immuno-stained neurons are lost by 7 days after exposure (Figueiredo et al., 2011b; Prager et al., 2014), and by day 14 the ratio of GABAergic interneurons over the total number of neurons is significantly reduced (Prager et al., 2014). Interestingly, at 24 h after exposure there is no GABAergic interneuronal loss, despite the overall significant loss of neurons (Figueiredo et al., 2011b; Prager et al., 2014). Yet, the physiological recordings suggest impaired inhibition at 24 h post-exposure (before the loss of GABAergic neurons). This is also supported by the observation that the frequency and amplitude of spontaneous inhibitory postsynaptic currents, at 24 h after exposure, are dramatically reduced (Prager et al., unpublished data). Therefore, synaptic mechanisms associated with GABAergic inhibitory transmission are already defective at 24 h after soman exposure. LY293558 treatment protected against these alterations, since in the soman-exposed, LY293558-treated rats, the field potentials did not have a prolonged component and the PPR was not increased.
There is ample evidence associating anxiety with hyperexcitability in the BLA (Davis et al., 1994; Sajdyk and Shekhar, 1997; Sanders and Shekhar, 1995; Zhou et al., 2010). Therefore, impaired inhibition in the BLA may lead to the development of anxiety (Truitt et al., 2009). Along with the synaptic alterations suggestive of impaired inhibition after soman exposure, there is an increase in anxiety-like behavior, as seen in our studies (Prager et al., 2014 and present study), and previous animal studies (Coubard et al., 2008; Filliat et al., 2007; Langston et al., 2012). Similarly, the most common psychiatric disorder still present in victims of the sarin attack in Tokyo more than a decade after the exposure was anxiety and particularly PTSD (Hoffman et al., 2007; Ohtani et al., 2004), a disorder characterized by a hyperexcitable amygdala (Koenigs and Grafman, 2009); indeed, pathological alterations in the amygdala were also present in sarin-exposed individuals (Rogers et al., 2009). The present study showed that treatment with LY293558 prevents the development of anxiety-like behavior, both in relatively short-term, and 90 days after exposure to soman, which corresponds, approximately, to 9 years of human life (Sengupta, 2011). It should be noted at this point that administration of diazepam for terminating nerve agent-induced SE, does not prevent the development of anxiety-like behavior (Apland et al., 2014; Langston et al., 2012).
The BLA mediates learning and memory formation associated with fear (Davis et al., 1994; Fanselow and Gale, 2003; LeDoux, 2003) and may be the site where fear memories are stored (Gale et al., 2004). LTP of synaptic transmission is considered to be the cellular mechanism underlying learning and memory (Malenka and Nicoll, 1999; Shors and Matzel, 1997). We showed previously that LTP in the BLA is impaired at 24 h, 7 days, and 14 days after soman exposure, while at day 30 it is not significantly smaller in comparison to the controls (Prager et al., 2014). As a synaptic property, induction of LTP would require intact, “healthy” synapses; the transient impairment may imply synaptic damage and dysfunctions-after the prolonged SE-that have recovered by day 30 after the exposure. It is notable that at 24 h after exposure, only the soman-exposed rats that did not receive anticonvulsant treatment displayed prolonged post-tetanic depression (Fig. 2A), which may reflect deficits in neurotransmitter release and/or other presynaptic impairments (Fioravante and Regehr, 2011). An impairment in synaptic plasticity in the BLA for two weeks or longer in the rat would correspond to about 1–2 years in humans. During this time, in addition to hyperexcitability in the amygdala, which may impair the processing of emotionally significant sensory information, the “registration” of fear-related events may also be impaired. Indeed, in rats, fear conditioning is impaired 8 days after soman exposure (Moffett et al., 2011). Treatment with LY293558 facilitated faster recovery of synaptic plasticity, probably by limiting synaptic and neuronal damage. Thus, targeting the glutamatergic system by administration of LY293558 in order to terminate seizures induced by exposure to soman, not only produces 100% survival and provides protection against brain damage (Apland et al., 2013; Figueiredo et al., 2011b), but also limits pathophysiological alterations in the BLA, and prevents the development of anxiety-like behavior.
Soman exposure causes pathophysiological alterations in the basolateral amygdala
Soman exposure produces long-term anxiety
Post-exposure treatment with LY293558 prevents increases in paired-pulse ratio
Post-exposure treatment with LY293558 minimizes impairments in synaptic plasticity
Post-exposure treatment with LY293558 prevents the development of anxiety
Acknowledgments
This work was supported by the CounterACT program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant Number 5U01NS058162-07].
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Eric M. Prager, Email: Eric.prager683@gmail.com.
Taiza H. Figueiredo, Email: taiza.figueiredo.ctr@usuhs.edu.
Robert P. Long, II, Email: Robert.Long2@usuhs.edu.
Vassiliki Aroniadou-Anderjaska, Email: vanderjaska@usuhs.edu.
James P. Apland, Email: James.p.apland@us.army.mil.
References
- Apland JP, Aroniadou-Anderjaska V, Braga MF. Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro. Neuroscience. 2009;159:380–389. doi: 10.1016/j.neuroscience.2008.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Green CE, Swezey R, Yang C, Qashu F, Braga MF. Efficacy of the GluK1/AMPA receptor antagonist LY293558 against seizures and neuropathology in a soman-exposure model without pretreatment and its pharmacokinetics after intramuscular administration. J Pharmacol Exp Ther. 2013;344:133–140. doi: 10.1124/jpet.112.198689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Rossetti F, Miller SL, Braga MF. The case against diazepam as a treatment for nerve agent-induced seizures and neuropathology; comparison with UBP302. 19th Biennial Medical Defense Bioscience Review Program Abstract Book; Hunt Valley, MD. May 11–15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Figueiredo TH, Qashu F, Aroniadou-Anderjaska V, Souza AP, Braga MF. Higher susceptibility of the ventral versus the dorsal hippocampus and the posteroventral versus anterodorsal amygdala to soman-induced neuropathology. Neurotoxicology. 2010;31:485–492. doi: 10.1016/j.neuro.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Figueiredo TH, Apland JP, Qashu F, Braga MF. Primary brain targets of nerve agents: the role of the amygdala in comparison to the hippocampus. Neurotoxicology. 2009;30:772–776. doi: 10.1016/j.neuro.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Pidoplichko VI, Figueiredo TH, Almeida-Suhett CP, Prager EM, Braga MF. Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors. Neuroscience. 2012;221:157–169. doi: 10.1016/j.neuroscience.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar J. Complex view on poisoning with nerve agents and organophosphates. Acta medica. 2005;48:3–21. [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- Bleakman R, Schoepp DD, Ballyk B, Bufton H, Sharpe EF, Thomas K, Ornstein PL, Kamboj RK. Pharmacological discrimination of GluR5 and GluR6 kainate receptor subtypes by (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahyd roisdoquinoline-3 carboxylic-acid. Mol Pharmacol. 1996;49:581–585. [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci. 2003;23:442–452. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GM, Martin JB. Corticosterone, prolactin, and growth hormone responses to handling and new environment in the rat. Psychosom Med. 1974;36:241–247. doi: 10.1097/00006842-197405000-00007. [DOI] [PubMed] [Google Scholar]
- Campo-Soria C, Chang Y, Weiss DS. Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol. 2006;148:984–990. doi: 10.1038/sj.bjp.0706796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WB, Gao R, Su YY, Zhao JW, Zhang YZ, Wang L, Ren Y, Fan CQ. Valproate versus diazepam for generalized convulsive status epilepticus: A pilot study. Eur J Neurol. 2011;18:1391–1396. doi: 10.1111/j.1468-1331.2011.03420.x. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombet JM, Beracochea D, Liscia P, Pierard C, Lallement G, Filliat P. Long-term effects of cytokine treatment on cognitive behavioral recovery and neuronal regeneration in soman-poisoned mice. Behav Brain Res. 2011;221:261–270. doi: 10.1016/j.bbr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Coubard S, Beracochea D, Collombet JM, Philippin JN, Krazem A, Liscia P, Lallement G, Pierard C. Long-term consequences of soman poisoning in mice: part 2. Emotional behavior. Behav Brain Res. 2008;191:95–103. doi: 10.1016/j.bbr.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Dolgin E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat Med. 2013;19:1194–1195. doi: 10.1038/nm1013-1194. [DOI] [PubMed] [Google Scholar]
- Dworkin J, Prescott M, Jamal R, Hardawan SA, Abdullah A, Galea S. The long-term psychosocial impact of a surprise chemical weapons attack on civilians in Halabja, Iraqi Kurdistan. J Nerv Ment Dis. 2008;196:772–775. doi: 10.1097/NMD.0b013e3181878b69. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav. 2001;70:475–489. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Grunberg NE. The importance of acclimation in acoustic startle amplitude and pre-pulse inhibition testing of male and female rats. Pharmacol Biochem Behav. 2000;66:375–381. doi: 10.1016/s0091-3057(00)00212-4. [DOI] [PubMed] [Google Scholar]
- Figueiredo TH, Aroniadou-Anderjaska V, Qashu F, Apland JP, Pidoplichko V, Stevens D, Ferrara TM, Braga MF. Neuroprotective efficacy of caramiphen against soman and mechanisms of its action. Br J Pharmacol. 2011a;164:1495–1505. doi: 10.1111/j.1476-5381.2011.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo TH, Qashu F, Apland JP, Aroniadou-Anderjaska V, Souza AP, Braga MF. The GluK1 (GluR5) Kainate/{alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist LY293558 reduces soman-induced seizures and neuropathology. J Pharmacol Exp Ther. 2011b;336:303–312. doi: 10.1124/jpet.110.171835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliat P, Coubard S, Pierard C, Liscia P, Beracochea D, Four E, Baubichon D, Masqueliez C, Lallement G, Collombet JM. Long-term behavioral consequences of soman poisoning in mice. Neurotoxicology. 2007;28:508–519. doi: 10.1016/j.neuro.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol. 2011;21:269–274. doi: 10.1016/j.conb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen MC, Lumb MJ, Smart TG. Benzodiazepines modulate GABAA receptors by regulating the preactivation step after GABA binding. J Neurosci. 2012;32:5707–5715. doi: 10.1523/JNEUROSCI.5663-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Fear-potentiated startle using an auditory conditioned stimulus: effect of lesions of the amygdala. Physiol Behav. 1987;39:403–408. doi: 10.1016/0031-9384(87)90242-3. [DOI] [PubMed] [Google Scholar]
- Hoffman A, Eisenkraft A, Finkelstein A, Schein O, Rotman E, Dushnitsky T. A decade after the Tokyo sarin attack: a review of neurological follow-up of the victims. Mil Med. 2007;172:607–610. doi: 10.7205/milmed.172.6.607. [DOI] [PubMed] [Google Scholar]
- Jane DE, Lodge D, Collingridge GL. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology. 2009;56:90–113. doi: 10.1016/j.neuropharm.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Jimmerson VR, Shih TM, Mailman RB. Variability in soman toxicity in the rat: correlation with biochemical and behavioral measures. Toxicology. 1989;57:241–254. doi: 10.1016/0300-483x(89)90114-5. [DOI] [PubMed] [Google Scholar]
- Kellinghaus C, Stogbauer F. Treatment of status epilepticus in a large community hospital. Epilepsy Behav. 2012;23:235–240. doi: 10.1016/j.yebeh.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallement G, Dorandeu F, Filliat P, Carpentier P, Baille V, Blanchet G. Medical management of organophosphate-induced seizures. J Physiol Paris. 1998;92:369–373. doi: 10.1016/S0928-4257(99)80007-2. [DOI] [PubMed] [Google Scholar]
- Langston JL, Wright LK, Connis N, Lumley LA. Characterizing the behavioral effects of nerve agent-induced seizure activity in rats: increased startle reactivity and perseverative behavior. Pharmacol Biochem Behav. 2012;100:382–391. doi: 10.1016/j.pbb.2011.09.011. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Du Y, Li N, Wu X, Wu Y. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci Biobehav Rev. 2009;33:1157–1167. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Li L, Fulton JD, Yeomans JS. Effects of bilateral electrical stimulation of the ventral pallidum on acoustic startle. Brain Res. 1999;836:164–172. doi: 10.1016/s0006-8993(99)01651-0. [DOI] [PubMed] [Google Scholar]
- Loh Y, Swanberg MM, Ingram MV, Newmark J. Case report: Long-term cognitive sequelae of sarin exposure. Neurotoxicology. 2010;31:244–246. doi: 10.1016/j.neuro.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Dochterman LW, Smith CD, Shih TM. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology. 1995;16:123–132. [PubMed] [Google Scholar]
- McDonough JH, Jr, McLeod CG, Jr, Nipwoda MT. Direct microinjection of soman or VX into the amygdala produces repetitive limbic convulsions and neuropathology. Brain Res. 1987;435:123–137. doi: 10.1016/0006-8993(87)91593-9. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. Pharmacological modulation of soman-induced seizures. Neurosci Biobehav Rev. 1993;17:203–215. doi: 10.1016/s0149-7634(05)80151-4. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Mehta V, Singhi P, Singhi S. Intravenous sodium valproate versus diazepam infusion for the control of refractory status epilepticus in children: a randomized controlled trial. J Child Neurol. 2007;22:1191–1197. doi: 10.1177/0883073807306248. [DOI] [PubMed] [Google Scholar]
- Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc Chem Res. 2012;45:756–766. doi: 10.1021/ar2002864. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Schultz MK, Schwartz JE, Stone MF, Lumley LA. Impaired auditory and contextual fear conditioning in soman-exposed rats. Pharmacol Biochem Behav. 2011;98:120–129. doi: 10.1016/j.pbb.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Grillon C, Southwick SM, Davis M, Charney DS. Exaggerated acoustic startle reflex in Gulf War veterans with posttraumatic stress disorder. Am J Psychiatry. 1996;153:64–68. doi: 10.1176/ajp.153.1.64. [DOI] [PubMed] [Google Scholar]
- Morita H, Yanagisawa N, Nakajima T, Shimizu M, Hirabayashi H, Okudera H, Nohara M, Midorikawa Y, Mimura S. Sarin poisoning in Matsumoto, Japan. Lancet. 1995;346:290–293. doi: 10.1016/s0140-6736(95)92170-2. [DOI] [PubMed] [Google Scholar]
- Nagao M, Takatori T, Matsuda Y, Nakajima M, Iwase H, Iwadate K. Definitive evidence for the acute sarin poisoning diagnosis in the Tokyo subway. Toxicol Appl Pharmacol. 1997;144:198–203. doi: 10.1006/taap.1997.8110. [DOI] [PubMed] [Google Scholar]
- Newmark J. The birth of nerve agent warfare: lessons from Syed Abbas Foroutan. Neurology. 2004;62:1590–1596. doi: 10.1212/01.wnl.0000124519.85516.50. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Iwanami A, Kasai K, Yamasue H, Kato T, Sasaki T, Kato N. Post-traumatic stress disorder symptoms in victims of Tokyo subway attack: a 5-year follow-up study. Psychiatry Clin Neurosci. 2004;58:624–629. doi: 10.1111/j.1440-1819.2004.01313.x. [DOI] [PubMed] [Google Scholar]
- Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, Figueiredo TH, Apland JP, Braga MF. Acetylcholinesterase inhibition in the basolateral amygdala plays a key role in the induction of status epilepticus after soman exposure. Neurotoxicology. 2013;38:84–90. doi: 10.1016/j.neuro.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, Figueiredo TH, Apland JP, Rossetti F, Olsen CH, Braga MF. The recovery of acetylcholinesterase activity and the progression of neuropathological and pathophysiological alterations in the rat basolateral amygdala after soman-induced status epilepticus: Relation to anxiety-like behavior. Neuropharmacology. 2014;81C:64–74. doi: 10.1016/j.neuropharm.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Bergstrom HC, Grunberg NE, Johnson LR. The importance of reporting housing and husbandry in rat research. Front Behav Neurosci. 2011;5:38. doi: 10.3389/fnbeh.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Racine R, Rose PA, Burnham WM. Afterdischarge thresholds and kindling rates in dorsal and ventral hippocampus and dentate gyrus. Can J Neurol Sci. 1977;4:273–278. doi: 10.1017/s0317167100025117. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, Aoki S, Kato N, Kasai K. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Res. 2009;174:210–216. doi: 10.1016/j.pscychresns.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res. 1997;764:262–264. doi: 10.1016/s0006-8993(97)00594-5. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Sellstrom A, Cairns S, Barbeschi M. Report on the alleged use of chemical weapons in the Ghouta area of Damascus on 21 August 2013. United Nations: 2013. United Nations mission to investigate allegations of the use of chemcial weapons in the Syrian Arab Republic. http://www.un.org/disarmament/content/slideshow/Secretary_General_Report_of_CW_Investigation.pdf Retrieved 24 June 2014. [Google Scholar]
- Sengupta P. A scientific review of age determination for a laboratory rat: How old is it in comparison with human age? Biomedicine International. 2011;2:81–89. [Google Scholar]
- Shih T, McDonough JH, Jr, Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci. 1999;6:86–96. doi: 10.1007/BF02256439. [DOI] [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH., Jr Organophosphorus nerve agents-induced seizures and efficacy of atropine sulfate as anticonvulsant treatment. Pharmacol Biochem Behav. 1999;64:147–153. doi: 10.1016/s0091-3057(99)00114-8. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Matzel LD. Long-term potentiation: what’s learning got to do with it? Behav Brain Sci. 1997;20:597–614. doi: 10.1017/s0140525x97001593. discussion 614–555. [DOI] [PubMed] [Google Scholar]
- Skovira JW, McDonough JH, Shih TM. Protection against sarin-induced seizures in rats by direct brain microinjection of scopolamine, midazolam or MK-801. J Mol Neurosci. 2010;40:56–62. doi: 10.1007/s12031-009-9253-0. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160:284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Wang S, Zhu X. Prenatal ethanol exposure attenuates GABAergic inhibition in basolateral amygdala leading to neuronal hyperexcitability and anxiety-like behavior of adult rat offspring. Neuroscience. 2010;170:749–757. doi: 10.1016/j.neuroscience.2010.07.055. [DOI] [PubMed] [Google Scholar]