Abstract
The successful establishment and maintenance of a bacterial infection depends on the pathogen’s ability to subvert the host cell’s defense response and successfully survive, proliferate, or persist within the infected cell. To circumvent host defense systems, bacterial pathogens produce a variety of virulence factors that potentiate bacterial adherence and invasion and usurp host cell signaling cascades that regulate intracellular microbial survival and trafficking. Mycobacterium tuberculosis, probably one of the most successful pathogens on earth, has coexisted with humanity for centuries, and this intimate and persistent connection between these two organisms suggests that the pathogen has evolved extensive mechanisms to evade the human immune system at multiple levels. While some of these mechanisms are mediated by factors released by M. tuberculosis, others rely on host components that are hijacked to prevent the generation of an effective immune response thus benefiting the survival of M. tuberculosis within the host cell. Here, we describe several of these mechanisms, with an emphasis on the cyclic nucleotide signaling and subversion of host responses that occur at the intracellular level when tubercle bacilli encounter macrophages, a cell that becomes a safe-house for M. tuberculosis although it is specialized to kill most microbes.
Keywords: Mycobacterium tuberculosis, Macrophage, Immunity, Interferon, Cyclic AMP, Cyclic di-AMP
1. Introduction
The unique crosstalk between microbial pathogens and their hosts reflects the co-evolutionary balance that the host and pathogen must reach in order to secure their survival. Mycobacterium tuberculosis- an intracellular and primarily vacuolar pathogen-has evolved a plethora of virulence factors which subvert a range of host physiological responses to allow propagation of the bacilli in one of the most inhospitable cells in the body, the macrophage. However, this interaction of M. tuberculosis with macrophages is by no means unidirectional; they engage in a true two-way biochemical interaction pivoting on dedicated proteins, small molecules and secretion systems which export bacterial molecules into the host cell cytoplasm. Recognition of these pathogen-associated molecular patterns (PAMPs), during bacterial internalization as well as when M. tuberculosis either resides inside a membrane bound phagosomal compartment or translocates into the cytoplasm, by host pattern recognition receptors (PRRs) that activate myriad of signaling cascades. Signaling events initiated by PAMP and PRR interaction are critical components of the host defense arsenal and allows the host to mount immunoresponses against M. tuberculosis [1–4].
While much remains to be learned, research over the past few decades is beginning to reveal how M. tuberculosis manages to withstand the hostile environment inside the macrophages or manipulate host responses in order to replicate and persist. Many of these strategies are uniquely employed by pathogenic mycobacteria compared to other intracellular pathogens (recently reviewed by Jayachandran et al. [3]). While some overlap exists, most pathogens have evolved specific ways to interfere with and circumvent host immune responses; this may be due to either the discrete intracellular niches that different pathogens occupy or the exclusivity of various pathogens with regard to their physiological necessities.
2. Subversion of host responses from beginning to the end: phagocytosis, phagosomal trafficking and maturation
Tuberculosis (TB) is primarily an airborne respiratory infection which is transmitted by aerosolized M. tuberculosis from patients with active TB. The establishment of a primary focus of infection depends on the activation status of the resident alveolar macrophages (AM) that phagocytose the inhaled bacilli as well as the virulence of the bacilli [5–7]. As the first line of cellular defense against inhaled bacilli, AMs express a broad range of immune receptors, including Fcγ receptors (FcγRs), complement receptors (CRs), toll-like receptors (TLRs), PRRs such as C-type lectin mannose receptors (MR), dectin-1, and scavenger receptors (SRs) that mediate phagocytosis (recently reviewed by Kleinnijenhuis et al. [8]). In addition, the dendritic cell (DC)-specific intracellular adhesion molecule (ICAM)-3-grabbing nonintegrin (DC-SIGN) receptor also plays a critical role in M. tuberculosis internalization by the DCs [9–10]. An array of biosynthetically related mannosylated lipoglycoconjugates within the mycobacterial cell envelope (e.g., phosphatidyl-myo-inositol mannosides (PIMs), lipomannan (LM) and mannose-capped lipoarabinomannan (ManLAM)) [11], glycoproteins (e.g. the 38-kDa and 19-kDa protein antigens), and mycobacterial heat shock proteins 60/65 have been shown to bind to the receptors on macrophages, DCs and other phagocytic cells to facilitate receptor-mediated internalization of M. tuberculosis and bacterial entry to the phagosome. Details on receptor mediated internalization of M. tuberculosis have recently been reviewed [8, 12].
Recognition and internalization of the bacteria by specific receptors trigger diverse host intracellular signaling pathways, which initiate the development of the unique phagosome biochemistry, characteristic of M. tuberculosis infection, and determine the nature of the early inflammatory response. For example, Fc receptor-mediated internalization results in a respiratory burst [13], whereas CR3-mediated uptake of M. tuberculosis prevents the activation of the macrophages and leads to the cholesterol-dependent prevention of phagosome-lysosome fusion [14–15]. Further, activation through TLRs and dectin-1 initiates what is essentially a pro-inflammatory response by signaling through the DC-SIGN. The primary function of MRs and CRs is to modulate internalization, without necessarily triggering a pro-inflammatory response. Interestingly, although MRs and DC-SIGN are both C-type lectins that recognize M. tuberculosis ManLAM, they regulate phagosomal trafficking differently. While the former is involved in efficient phagocytosis, endocytosis and endosomal sorting, the latter targets M. tuberculosis to lysosomes. This may explain why M. tuberculosis prefers macrophages, which possess high surface MR levels, for its major intracellular niche over DCs, which possess high surface levels of DC-SIGN and rapidly shuttle bacteria to the destructive environment of the lysosomes. Employing several proteins and lipid molecules, M. tuberculosis interferes with the phagosomal maturation pathway thereby blocking its transfer to lysosomes [12]. The M. tuberculosis lipid phosphatase SapM dephosphorylates phosphatidylinositol 3-phosphate (PI3P) [16–17]; the tyrosine phosphatase PtpA dephosphorylates and inactivates the host vacuolar protein sorting- VPS33B which in turn regulates membrane fusion and arrests phagosome maturation [18]. Additionally, ManLAM inhibits the Ca/Calmodulin and Rab5-dependent recruitment of PI3K resulting in reduced PI3P formation on the phagosomal membrane [19–20]. Phagosomes containing M. tuberculosis also fail to acidify due to bacterial interference with the recruitment of the vesicular proton ATPase pump and failure to acquire late endocytic markers such as Rab7 [12].
Another host pathway that M. tuberculosis regulates coronin-1-dependent cytosolic calcium influx and activation of calcineurin via its lipoamide dehydrogenase [21–22]. During phagocytosis, coronin-1 (a phagosomal coat protein) is released from the cytosolic surface of the maturing phagosome resulting in fusion of the phagosome with the lysosome and transfer of its internal contents for degradation. M. tuberculosis is known to prevent or delay coronin-1 release, thereby blocking phagosomal fusion with lysosomes [22–23]. M. tuberculosis also possesses at least 11 eukaryotic-like protein kinases which have been shown to regulate mycobacterial signal transduction pathways, morphology, and cell division [24–25]. An example is PknD which has been shown to play an active role in the invasion of the central nervous system by M. tuberculosis [26] in addition to its role in regulation of gene expression by phosphorylation of alternative sigma factor regulators [27]. Another kinase, PknG, has been shown to be released from pathogenic mycobacteria inside the macrophage cytosol where it prevents lysosomal delivery and degradation [28–29]. It has also been proposed that M. tuberculosis interferes with phagolysosome biogenesis by a putative Zn2+-dependent metalloproteinase (Zmp1) that interferes with caspase-1 dependent activation and secretion of IL-1β [30]. Recently, several groups have reported that mycobacterial ‘enhanced intracellular survival protein’ (Eis) may inhibit JNK-dependent ROI production thereby inhibiting TNF-α production and preventing macrophage activation, inflammation, and autophagy [31–33].
There is considerable evidence that M. tuberculosis cell wall lipids act as virulence factors during infection [4, 34–37]. While there is a scarcity of information regarding the nature of molecular interactions of mycobacterial lipids with the host cells, M. tuberculosis lipids have been observed to intercalate into host membranes leading to decreased membrane fluidity and increased passive permeability [38]. The ability of mycobacterial phthiocerol dimycocerosates (PDIMs) and trehalose-6, 6′-dimycolates (TDMs) to alter host membrane fluidity may influence the process of phagocytosis as well as subsequent trafficking. M. tuberculosis lipids are abundantly produced during macrophage infection and have been shown to be actively trafficked out of the phagosome [39–42] and finally exocytosed from infected macrophages where they are taken up by neighboring macrophages [43–44]. This process could potentially influence CD1-mediated lipid antigen presentation by resident DCs and subsequent immune responses [42, 45]. Consequently, host lipids are important regulators of inflammatory signaling pathways in bacteria as well, since several host lipids are the primary building blocks of the mycobacterial lipid load [46]. Recent reports of nuclear receptor-peroxisome proliferator-activated receptor (PPAR)-mediated sensing of mycobacterial fatty acid derived eicosanoids and modulation of cytokine responses and its effect on virulence and pathogenicity of M. tuberculosis further highlights the complexity and importance of lipids in host-pathogen crosstalk [47–48].
While M. tuberculosis employs several strategies to prevent phagolysosome-mediated early killing by the host, how the bacilli manipulate other macrophage functions while residing within the phagosome remains an intriguing question. Several studies have demonstrated that M. tuberculosis successfully accesses and/or translocates into the macrophage cytoplasm from the phagosome [49–51]. ESAT-6, a member of the region of difference- 1 (RD-1) gene cluster, plays a key role in this process [51–52]. The M. bovis-derived vaccine strain Bacille Calmette-Guérin (BCG), which lacks the RD-1 region, is unable to translocate to the cytosol and is avirulent [52]. Therefore, cytosolic escape could be a potential mechanism of virulence exerted by the proteins encoded within the RD-1 region. The ESAT-6 is a member of the ESX-1 specialized secretion system that not only allows bacterial proteins to be secreted, but also damages the phagosomal membrane thereby permitting mixing of luminal contents with the cytoplasm of the host cell [1, 53–54]. The mixing of phagosomal and cytoplasmic contents allows for recognition of mycobacterial components, including bacterial chromosomal DNA, CpG motifs, peptidoglycan fragments, dsRNA, and nucleotides by a range of host cytosolic receptors such as nucleotide-binding oligomerization domain (NOD) proteins [55–56], nucleic acid receptors [1, 57–64]. Once engaged, these cystosolic receptors activate inflammatory response pathways including Type-1 IFN, the inflammasome, and autophagy [1–2, 65–67]. Interestingly, a recent study demonstrated that an ESAT-6-deficient M. tuberculosis laboratory strain of H37Rv and M. bovis BCG could translocate into the cytosol in the absence of TLR signaling, indicating that host TLR signaling plays a decisive role in preventing mycobacterial translocation into the host cytosol [68]. Since several mycobacterial components are known to regulate TLR signaling, this study suggests that M. tuberculosis may regulate its translocation into the cytosol by modulating TLR signaling. Thus, it is apparent that M. tuberculosis occupies several intracellular niches based on host cell immunity and activation status coupled with temporal requirements at particular phases of infection. Table 1 lists some of the prominent host- receptors, which are involved in recognition of M. tuberculosis and its components and initiation of immune responses. For details of receptor mediated signaling and cytokine responses refer to the chapter ‘Pro- and anti-inflammatory cytokines in TB’ by Eliana Coccia.
Table 1.
Host immune receptors involved in recognition of M. tuberculosis components
| Host receptorsa | M. tuberculosis component | Type of immune response/function | Reference |
|---|---|---|---|
| Cell surface receptors | |||
| TLRs | LM, lipoprotein, PIMs | Pro-inflammatory | 8, 12 |
| CRs | C3-opsonized/non-opsonized bacteria, PIMs | Low levels of inflammation | 12, 14, 15 |
| MR | ManLAM, PIMs | Anti-inflammatory | 8, 11, 12 |
| DC-SIGN | ManLAM, LM, PIMs | Anti-inflammatory | 8, 9, 10 |
| Dectin, Mincle | Glycolipids, TDM | Anti-/Pro-inflammation | 8, 12 |
| Fc γRs | IgG-opsonzed bacilli | Phagolysosome fusion | 8, 13 |
| SR-A, CD14 | non-opsonized bacteria | Anti-/Pro-inflammation | 8, 12 |
| Cytosolic receptors | |||
| NOD | Peptidoglycan | Pro-inflammatory | 55, 56 |
| TLR9 | CpG DNA | Pro-inflammatory | 57, 58 |
| DAI, cGAS | dsDNA | Type 1 IFN, pro- inflammatory | 59, 64 |
| AIM2, NLRP3 | dsDNA | Inflammasome activation | 65, 66 |
| IFI16 | ssDNA, dsDNA | Type 1 IFN, pro- inflammatory | 60 |
| STING | c-di-GMP, c-di-AMP, cGAMP | Type 1 IFN, pro- inflammatory | 1, 2, 62, 67 |
| DDX41 | dsDNA, c-di-GMP, c-di-AMP | Type 1 IFN, pro- inflammatory | 62, 63 |
| RNA Pol III | AT rich dsDNA | Type 1 IFN, pro- inflammatory | 61 |
DAI, DNA-dependent activator of IFN-regulatory factors; AIM2, Absent in melanoma 2; IFI16, Interferon-inducible protein-16. See Abbreviations for other definitions.
3. Host-pathogen crosstalk via nucleotide second messengers
While there is no doubt that the role of ESX-1-based specialized secretion, phagosomal permeabilization, and cytosolic translocation during M. tuberculosis infection are important determinants of host-pathogen molecular exchange and crosstalk, there is a vast array of small, soluble, signaling molecules from the bacteria that enter the host cell by known or unknown mechanisms [69–71]. These small molecules have been shown to control host gene expression or relay information to effector molecules within the host cell [72–74]. Classical second messengers include diverse molecules such as cyclic nucleotides (cAMP and cGMP), guanosine pentaphosphate or tetraphosphate [(p)ppGpp], Ca2+, inositol trisphosphate, and diacylglycerol (DAG) [75–78]. Recent additions to this list include cyclic-di-nucleotide molecules such as cyclic di-guanylate (c-di-GMP) [79–80], cyclic di-adenylate (c-di-AMP) [81], and cyclic-GMP-AMP (c-GAMP) [82–85]. Nucleotide polymers are perhaps the oldest molecules of life, and the cyclized form of the energy building block ATP, 3′, 5′-cyclic adenosine monophosphate (cAMP), is a carrier of sensory information in all domains of life While the roles of cAMP and its sister molecule 3′, 5′-cyclic guanosine monophosphate (cGMP) have been studied for over five decades [69–70], the cyclic dinucleotides c-di-GMP [79–80], c-di-AMP [81], and c-GAMP [82–85] were more recently identified as important signaling molecules in both prokaryotes and eukaryotes. In this review, we aim to provide a perspective on how cAMP and c-di-nucleotides participate in the crosstalk between M. tuberculosis and the macrophage, and how these molecules facilitate M. tuberculosis survival and pathogenesis.
3.1 Modulation of cAMP homeostasis and subversion of host immunity
3.1.1 cAMP signaling in bacteria and the host: an overview
Although the basic module of cyclic nucleotide signaling is conserved from bacteria to complex eukaryotes, the molecular players that are involved in the signaling pathways are often markedly different [69, 86–87]. The initiation of signaling cascades is triggered by the first messenger (external stimuli or stress such as alterations in levels of ambient biochemicals including nutrients, hormones, or neurotransmitters) leading to activation of adenylyl (ACs) and guanylyl cyclases (GCs) which catalyze the formation of cyclic nucleotides (the second messenger) from either ATP or GTP. This is followed by binding of the cyclic nucleotides to corresponding receptors leading to downstream regulatory effector functions and, finally, culminating in the hydrolysis of the cyclic nucleotide by corresponding phosphodiesterases (PDEs) [88]. In prokaryotes and eukaryotes the primary effector function of cyclic nucleotide signaling is regulation of gene expression. As cyclic nucleotide signaling governs functions unique to both the host and the bacterial pathogen, there are numerous bacterial virulence strategies that interfere with host cyclic nucleotide signaling. Similarly, there are host defense strategies for counter-regulation.
In bacteria, glucose depletion induces AC activition in some but not all species [69, 89–90]. The use of the cAMP receptor protein (CRP) family of transcription factors, which bind upstream of specific promoters to stimulate transcription, is a common means of cAMP message relay. The effects of cAMP are further amplified in some cases by cAMP-mediated co-regulation of other global regulators [74, 91–92]. In Escherichia coli alone, CRP is known to activate transcription from more than 100 different promoters, suggesting a wide range of cAMP-mediated regulatory effects in bacteria [93]. In eukaryotes, binding of the first messenger to G-protein coupled receptors (GPCRs) leads to activation of cellular ACs [86, 94–96]. The resulting elevated cAMP levels lead to protein kinase A (PKA) activation by promoting release of its catalytic subunit. PKA-mediated phosphorylation of target proteins, including the cAMP response element binding protein (CREB), finally results in differential expression of target genes. AC activity is also regulated by an inhibitory G-protein α-subunit (Gαi) that reduces AC activity following binding to ligands which include several chemokines and leukotrienes [94]. To date, among the existing six classes of ACs, four are restricted to prokaryotes [71]. The most widely distributed ACs are the class III enzymes which are metal-dependent [71]. Class III ACs are found in mycobacteria and are also represented by the eukaryotic, GPCR-activated ACs. The activity of all three classes of cAMP-hydrolyzing PDEs is also metal dependent. Class I PDE enzymes are present exclusively in eukaryotes; while low affinity, class II PDEs have been identified in yeast, Vibrio species, and Dictyostelium. Class III PDEs were first described in E. coli. In eukaryotes, 10 AC isoforms and 11 PDE families are known to be expressed in a tissue-specific manner [71]. The structural and functional classifications of the cAMP ACs and PDEs are described in detail by a recent review [71].
Literature spanning several decades has described a multitude of bacterial signaling pathways that are regulated by cAMP. These include catabolite repression in enteric bacteria, regulation of competence, chromosomal replication by binding to DnaA, phototaxis and heterocyst formation in cyanobacteria, secondary metabolite production and germination in Streptomycetes, regulation of virulence regulons in Pseudomonas, and biofilm formation in Vibrio cholerae [69, 90, 97–98]. In higher eukaryotes, cAMP and cGMP are important second messengers that mediate effects of light, nitric oxide, hormones, and other signals to regulate vision, muscle contraction, vasodilatory effects, sleep, memory, and various other functions [99]. cAMP mediates the regulation of ion channels such as the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, which have also recently been reported to be co-regulated by c-di-AMP [100]. PKA-independent functions of cAMP include activation of calcium channels and binding of cAMP to exchange protein activated (Epac) family proteins. These proteins activate small Ras-like GTPases such as Ras-proximate-1 (Rap1) which is predominantly involved in cell adhesion and cell junction formation during cell proliferation [101–103]. cAMP is also known to enhance the phosphorylation of extracellular-signal-regulated kinases-1/2 (ERK-1/2) via a mitochondria-derived, reactive oxygen species (ROS)-dependent activation of Ras [104].
It is evident from the above discussion that cAMP plays important regulatory roles in many cellular processes in both bacteria and host cells. Not surprisingly, there are also highly efficient, bacterial systems that target host ACs and PDEs to subvert host cAMP-governed processes. Elevated levels of cAMP can suppress innate immune functions by modulating the expression of inflammatory mediators, inhibiting phagocytic responses, and reducing intracellular killing of ingested pathogens [69–70]. Increased intracellular cAMP is also known to interfere with the phagosomal actin assembly cascade and phagosome maturation in macrophages [105]. Bacterial pathogens are known to elevate host intracellular cAMP levels via several distinct mechanisms: (i) by direct injection of bacterial ACs into the host cell (ii) by regulating the activity of host ACs through GPCR, (iii) by secretion of cAMP directly into the host cell, and (iv) by exploiting alternative host signaling pathways to indirectly trigger cAMP production (see reviews by Ahuja et al. [106] Agarwal and Bishai. [70], McDonough and Rodriguez. [69]). A rapid increase of cAMP levels in the host cell following infection with pathogenic bacteria results in impaired chemotaxis, phagocytosis, ROI and RNI responses, induction of apoptosis, and reduced bactericidal activity of macrophages and neutrophils [107–114].
3.1.2. Subversion of cAMP signaling during M. tuberculosis infection
Adenylate cyclase activity was first reported in mycobacterial cell-free extracts close to four decades ago [115–116]. To date, 16 AC-like proteins, ten of which possess confirmed AC activity, have been reported in M. tuberculosis [71, 117]. In contrast, only a single protein (Rv0805) with cAMP PDE activity has been identified [118]. In addition to the AC catalytic core region, many M. tuberculosis ACs contain other domains such as receptor binding domains, DNA-binding elements, and HAMP (histidine kinases, ACs, methyl-binding proteins, and phosphatases) domains suggesting that their AC activity is linked to a regulatory function [71]. Furthermore, sub-cellular localization studies of mycobacterial ACs reveals multiple cellular locations suggesting the presence of soluble, membrane-bound, and membrane-integral subclasses [71]. The presence of multiple AC genes in the M. tuberculosis genome is also intriguing as the mammalian genome encodes multiple ACs with tissue-specific expression. Since M. tuberculosis can infect a range of cell types, whether mycobacterial ACs have host cell-specific expression remains an interesting puzzle.
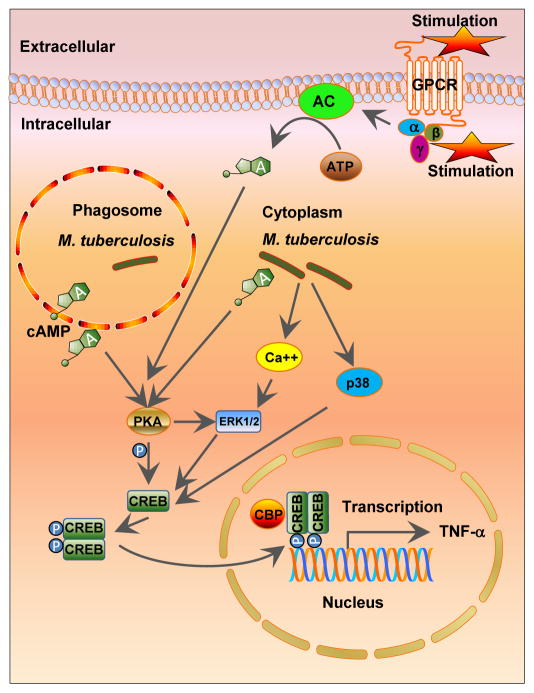
Unlike E. coli, exogenous glucose does not have a prominent effect on M. tuberculosis cAMP levels [116, 119]. However, a range of in vitro conditions that mimic infection conditions, such as pH, fatty acids, carbon dioxide (CO2), hypoxia and starvation, directly alter expression of M. tuberculosis ACs [74]. Several studies have reported an increase in cAMP level in the macrophage cystosol following mycobacterial infection [119–120]. Agarwal et al. reported the key of role of an M. tuberculosis AC, Rv0386, in delivering bacterial-derived cAMP into the host cytoplasm [120]. In addition, that study highlighted the role of the bacterial cAMP-specific PDE, Rv0805, which, when over-expressed in M. tuberculosis, significantly reduced intra-bacterial and intra-macrophage cAMP concentrations with consequent reductions in CREB phosphorylation and TNF-α production in murine macrophages (Figure 1). Agarwal et al. also demonstrated that a dysregulated host inflammatory response following the M. tuberculosis-mediated, cAMP intoxication of the macrophage cytoplasm favors bacterial survival. This confirms cAMP as a mycobacterial virulence mediator and suggests that there may be therapeutic value in manipulating mycobacterial cAMP or PDE activity [121].
Figure 1. cAMP-mediated signaling in M. tuberculosis infection.
Macrophages produce intracellular cAMP through G-protein-coupled receptor (GPCR)-adenylate cyclases (ACs). Increased cAMP stimulates protein kinase A (PKA) leading to the phosphorylation of cAMP response-element-binding protein (CREB), and subsequent transcriptional changes including modulation of cytokine expression. M. tuberculosis secretes cAMP directly into host macrophages leading to increased intracellular cAMP levels following infection. The M. tuberculosis infection-induced cAMP burst activates the PKA–CREB pathway leading to production of TNF-α, one of the key cytokines for TB granuloma formation. P = phosphate, TNF-a = tumor necrosis factor-a, CBP = CREB-binding protein, ERK1/2 = extracellular-signal-regulated kinase-1/2, p38 = p38 mitogen-activated protein (MAP) kinase.
Because of the functional redundancies of multiple ACs, several investigators targeted downstream signaling molecules to study the effect of modulation of cAMP in M. tuberculosis. M. tuberculosis possesses ten putative cAMP-binding proteins [71, 74, 122], two bona fide CRP-family transcription factors (Crp and Cmr), and a cAMP-responsive protein lysine acetylase [123–125]. Of the two transcription factors, Crp, controls a regulon of >100 genes in M. tuberculosis [124], while Cmr, has been shown to control the expression of a different set of genes in response to cAMP levels and macrophage passage [126]. Deletion of the crp gene (Rv3676) results in a mutant strain with impaired in vitro and in vivo growth and attenuated virulence in mice [123]. The functions of the cAMP-responsive lysine acetylase and the remaining putative cAMP-binding proteins remain unknown.
Recently, another exciting novel function of cAMP in M. tuberculosis has recently been reported by Pelly et al., wherein they have identified a small, non-coding RNA (sRNA) ncrMT1302 in a locus involved in cAMP metabolism that is responsive to changes in pH and cAMP concentration [127]. The differential expression of ncrMT1302 observed in wild-type M. tuberculosis during growth is abolished in a strain lacking MT1302 (Rv1264), an AC-encoding gene. They also report that ncrMT1302 is expressed in M. tuberculosis residing in the lungs of mice during active infection. As cAMP contributes to virulence and a pH stress response is vital for the survival of the bacillus, this study demonstrates a key link between cAMP-mediated responses and sRNA-regulated transcriptional regulation.
3. 2. Modulation of cyclic di-nucleotide signaling and subversion of host immunity
3.2.1. Cyclic di-nucleotide signaling: an overview
With the exception of c-di-GMP, which was discovered more than two and a half decades ago as an allosteric activator of cellulose synthase in the fruit-degrading bacterium Gluconacetobacter xylinus [128], both c-di-AMP and the cyclic AMP-GMP hybrid dinucleotide c-GAMP (both bacterial and eukaryotic) are the newest additions to the growing list of second messenger molecules that are involved in host-pathogen crosstalk (see [77, 79, 129–130] for recent reviews). c-di-GMP and c-di-AMP synthesizing and degrading enzymes are found in a number of bacterial species [130–131], but to date such enzymes have not been found in mammalian cells. These two signaling, cyclic dinucleotides (CDNs) share several common features. Both c-di-AMP and c-di-GMP are synthesized by domains that are usually part of multi-domain proteins, such that more than one input signal may affect their enzymatic activity. Both molecules regulate a variety of similar physiological processes, including cell wall metabolism, antibiotic resistance, biofilm formation, cell differentiation, morphology, and motility [130–131]. And several of the regulatory functions exerted by these two CDNs have direct effects on bacterial virulence mechanisms [130–131]. For example, c-di-GMP plays a central role in the switch from the motile to sessile state within multicellular biofilms in several bacterial pathogens [132]. Likewise, deletion mutants for the cyclase gene or gene domain for either of these CDNs exhibit compromised virulence in these pathogens [130, 132–133].
While the precise mechanism of c-di-AMP-mediated regulation in bacteria remains unknown, the basic signaling modules are similar for both c-di-AMP and c-di-GMP. Following an external or internal signal, condensation of two nucleotide triphosphates (GTP or ATP) by CDN cyclases generates either c-di-GMP or c-di-AMP. The CDNs then bind to target proteins and elicit allosteric changes which alter effector protein function thereby regulating specific cellular pathways [78, 131]. Finally, CDNs are degraded by specific CDN-PDEs. Both of these CDNs have also been shown to bind specific riboswitches that are known to regulate transcription and translation of downstream sequences [134–136].
The hybrid CDN of bacterial origin, 3′-5′-c-GMP-AMP (cGAMP), is a canonical nucleotide formed by 3′-5′ linkages between the guanosine and the adenosine residues, as is also the case with c-di-AMP and c-di-GMP [64, 83]. To date, 3′-5′-cGAMP has only been reported in Vibrio cholerae where it is required for efficient intestinal colonization [137]. The mammalian cGAMP is a 2′3′-CDN produced in mammalian cells by cGAMP synthase (cGAS) in response to double-stranded DNA detected in the cytoplasm [64, 85, 129]. 2′3′-cGAMP is also referred to as ‘non-canonical’ cGAMP due to the presence of the atypical 2′-3′ phosphodiester linkages between the nucleotide residues. A fascinating phenomenon is that despite structural and source differences between the bacterial and host-derived CDNs, both the 3′5′-cGAMP of bacterial origin and 2′3′-cGAMP of mammalian origin (as well as c-di-AMP and c-di-GMP) are detected by a common detector protein in the host cell cytoplasm - the stimulator of interferon gene signaling (STING) protein. STING activates the TBK1-IRF3-dependent Type-I IFN signaling pathway leading to IFN-β production [82–84]. However, recent studies have revealed mechanisms that may differentiate these two sets of CDNs. Certain variants of STING are able to distinguish between the non-canonical and canonical cGAMP [138–139]. Further, cGAMP is more potent in activating the Type I IFN response than c-di-AMP or c-di-GMP, bacterial-derived cyclic dinucleotides that also bind STING [140]. Moreover, while physiologically relevant levels of c-di-AMP and c-di-GMP trigger robust secretion of IL-1β in an NLRP3 inflammasome-dependent manner [141], the host-derived cGAMP does not stimulate IL-1β release [142]. Thus, host cells appear capable of recognizing the presence of bacterial CDNs in the cytoplasm and launching an immune response that is qualitatively different from the response generated by self-derived cGAMP, though there is a certain degree of overlap with respect to the Type-I IFN responses. Given the presence of cytosolic DNA receptors, such as DDX41, which recognize both bacterial DNA (bDNA) and bacterial CDNs and the fact that some host receptors are regulated by bacterial CDNs (e.g., IFI16 and p202) [143], it may be difficult to assess the relative contribution of bDNA versus CDNs to simulating IFN-β induction during bacterial infection. Further evidence of overlap between the bDNA- and CDN-based responses is in the induction and regulation of autophagy- a key mechanism by which macrophages kill intracellular bacteria [1–2, 144]. Induction of autophagy has been reported in murine macrophage cells following exogenous stimulation with synthetic, bacterial CDNs [2]. Further, direct interaction between cGAS and the autophagy protein Beclin-1 not only suppresses cGAMP synthesis to halt IFN-β production upon dsDNA stimulation, but also enhances autophagy-mediated degradation of cytosolic pathogen DNA to prevent excessive cGAS activation and persistent immune stimulation [145]. Thus, the cGAS-Beclin-1 interaction governs innate host defense strategies by regulating both cGAMP production and autophagy induction.
The Type-I IFN response is a well-characterized and critical antiviral host response. In the case of bacterial infections, Type-I IFNs appear to exert both beneficial and detrimental effects on the host [146–148]. An enhanced Type I IFN response as a consequence of elevated c-di-AMP levels has been observed in several studies with laboratory as well as clinical isolates of pathogenic bacteria [149–151]. A c-di-AMP over-secreting Listeria monocytogenes strain which induces a host IFN-β response was found to be attenuated in a mouse model of infection [149]. Similarly, the deletion of a c-di-GMP cyclase gene, cgsB, in Brucella melitensis produced hypervirulence, while deletion of the PDE genes, bpdA and bpdB, resulted in attenuation of virulence [152]. Likewise, deletion of CDN PDEs from other pathogenic bacteria has resulted in attenuation of virulence in animal models of infection [133, 153]. In contrast, mutation of the c-di-AMP cyclase, dacA, reduces fitness in some strains of Staphylococcus aureus and L. monocytogenes [154–155].
3.2.2. Cyclic dinucleotides and modulation of host responses during M. tuberculosis infection
While most of the bacterial species possess multiple CDN-cyclases and CDN-PDEs, the M. tuberculosis genome encodes only one cyclase and one PDE for c-di-GMP and similarly one cyclase and one PDE for c-di-AMP (Table 2) [156–158]. However, the diguanylate cyclase (DGC) domain-containing protein in M. tuberculosis (Rv1354c) is a bi-functional protein possessing both GGDEF and EAL domains which possess cyclase and phosphodiesterase activities, respectively [157]. Studies with M. smegmatis c-di-GMP null and overexpression mutants demonstrate that while neither of these defects affect growth or biofilm formation, they do affect long-term survival under conditions of nutritional starvation [158]. Furthermore, Hong et al. reported that while a diguanylate cyclase (DGC) deletion mutant of the M. tuberculosis Rv1354c gene exhibited an increased dormancy phenotype, the c-di-GMP PDE (Rv1357c) deletion strain exhibited a reduced dormancy phenotype [159]. They also reported that the c-di-GMP PDE deletion strain was attenuated for virulence and pathogenicity in both human THP-1 derived macrophages as well as in a mouse model. However, none of these studies convincingly demonstrated whether the attenuation phenotype is due to the effects of altered c-di-GMP levels on the bacteria alone, the host responses, or both.
Table 2.
Cyclic di-nucleotide cyclases and phosphodiesterase of M. tuberculosis
| Cyclic di-GMP | ||
|---|---|---|
| Gene number/name | Function | Domain composition |
| Rv1354c | Cyclase- phosphodiesterase (bi- functional protein) |
|
| Rv1357 | Phosphodiesterase |
|
| Cyclic di-AMP | ||
| Rv3586 disA dacA |
Cyclase |
|
| Rv2837c NrnA cnpB |
Phosphodiesterase |
|
Domain composition of cyclic di-nucleotide cyclases and phosphodiesterases are shown as predicted using the SSDB Motif (http://www.kegg.jp/kegg/ssdb/) and conserved domain (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi?db+cdd) databases. Gene nomenclature is according to the M. tuberculosis H37Rv genome annotation. Major functional domains: GGDEF, c-di-GMP cyclase; EAL, c-di-GMP phosphodiesterase; DisA-N, c-di-AMP cyclase; DHH, c-di-AMP phosphodiesterase.
In M. tuberculosis, Rv3586 (disA, also referred as dacA) encodes a diadenylate cyclase [156]. Orthologues of dacA exist in all mycobacterial genomes with the exception of M. leprae. It has been reported that synthesis of c-di-AMP by a DisA homologue in M. smegmatis is inhibited by RadA (Rv3585), encoded by the adjacent gene, through a physical interaction with the cyclase [160]. Furthermore, a c-di-AMP binding transcription factor, DarR, was identified in M. smegmatis, and this transcription factor represses the expression of several genes associated with fatty acid metabolism and transportation [161]. However, a DarR orthologue has not been identified in M. tuberculosis.
Dey et al. recently showed that c-di-AMP is produced by M. tuberculosis and is secreted into the host cytosol during infection. This leads to STING-dependent induction of the cytoplasmic surveillance pathway (CSP) and consequently, induction of the Type I IFN pathway, autophagy, and increased secretion of a number of pro-inflammatory cytokines such as IL-1α, IL-6 and TNF-α (Dey, B et al., 2014, unpublished data). They further found that a di-adenylate cyclase (dacA) over-expressing M. tuberculosis strain that secretes excess c-di-AMP into the macrophage cytoplasm displayed attenuated virulence in mice compared to the dacA deficient and wild type strains. c-di-AMP-mediated IFN-β induction during M. tuberculosis infection was also found to be dependent on STING-signaling with contributions from DDX41 (Dey, B et al., 2014, unpublished data). In addition, cGAMP synthase (cGAS) [82, 84, 162], while contributing to the overall Type I IFN response, was not strictly required for c-di-AMP mediated responses since c-di-AMP could activate the Type I IFN pathway even in the absence of this cytosolic DNA receptor (Dey, B et al., 2014, unpublished data).
These observations by Dey et al. expand upon earlier studies which suggested that mycobacterial DNA is the exclusive ligand for inducing the host Type I IFN response, and that the bacterial Esx-1 secretion apparatus is required for signaling [1]. By employing multiple bacterial strains (the M. tuberculosis CDC1551 and Erdman strains, and M. bovis BCG) each modified to overexpress c-di-AMP and a variety of host phagocytic cells, including those defective in important mediators of the CSP (STING, DDX41, and cGAS), Dey et al. consistently demonstrated that c-di-AMP, not bacterial DNA alone, is a key mediator of Type I IFN responses (Figure 2) (Dey, B et al., 2014, unpublished data). They also showed that bacterial-derived c-di-AMP activates Type I IFN in the absence of an Esx-1 secretion system and, while contributory, Esx-1 is not required for c-di-AMP-triggered IRF pathway activation. Along similar lines, Bishai and colleagues and others also found that a c-di-AMP-PDE deletion strain (Rv2837c) of M. tuberculosis induced a heightened Type-I IFN response and that this mutant was also attenuated in the murine model of TB (Bishai and colleagues, unpublished data)[150].
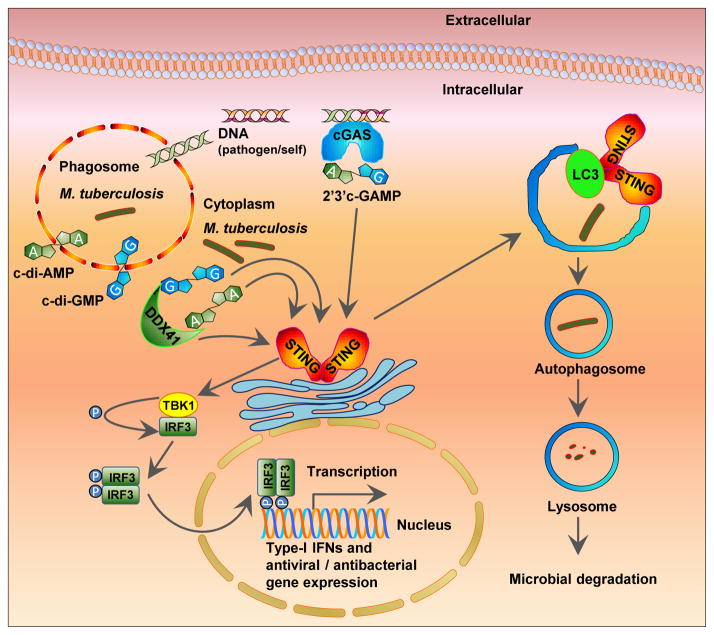
Figure 2. Cyclic dinucleotide signaling in M. tuberculosis infection.
Cyclic dinucleotides (c-di-AMP and c-di-GMP) secreted by either phagosomal or cytoplasmic M. tuberculosis are detected by host cytoplasmic receptors DDX41 and STING. Receptor-bound DDX41 also interacts with and activates STING. Activated STING subsequently interacts with and activates kinase TBK1 leading to phosphorylation and dimerization of IRF3 which translocates into the nucleus and stimulates transcription of Type-1 IFN response genes. Activated STING also co-localizes with LC3, an autophagosome membrane component, and initiates autophagosome formation that ultimately leads to bacterial degradation. Correspondingly, bacterial DNA gains access to the cytosolic compartment and binds to the dsDNA receptor cyclic-GAMP synthase (cGAS) stimulating the synthesis of 2′,3′-cGAMP. Host-produced cGAMP binds to STING and stimulates a signaling cascade similar to that of the bacterial cyclic dinucleotides. P = phosphate, IFN = interferon, STING = stimulator of interferon genes, TBK1 = TANK-binding kinase 1, IRF3 = interferon regulatory factor 3.
Cyclic di-AMP mediated enhanced Type I IFN response during M. tuberculosis infection observed by Dey et al. extends correlative observations by several studies with laboratory as well as clinical isolates of pathogenic bacteria [149–151]. While Type I IFNs are critical for resistance to viruses, there are reports as to whether the IFN-α/β response is beneficial or detrimental to the host during TB [146–148]. For example, loss of the IFN-α/β receptor knockout mice confers resistance to M. tuberculosis infection, suggesting that Type I IFN responses are counterproductive in TB [163]. In contrast, IFNα/β promote antigen cross-presentation in DCs and activation of cytolytic CD8 T cells, which are crucial for M. tuberculosis clearance [164–165]. Furthermore, in Type II IFN deleted mutant mice, the Type I IFN response has also been shown to limit lung infectivity of M. tuberculosis [166]. Importantly, a human transcriptome analysis of peripheral blood in patients with TB also revealed high levels of Type I and Type II IFN inducible genes, suggesting an overlapping and dynamic role of both types of IFN in TB pathogenesis [167].
It is evident from the above discussion that CDNs play an important regulatory role in many cellular processes in both the virulence and pathogenicity of M. tuberculosis and host-pathogen crosstalk during infection. Thus, it is not surprising that both the host and pathogen employ strategies to regulate intracellular CDN levels to establish supremacy over the other. Future research in these areas is warranted to uncover the exquisite virulence mechanism based on CDNs that M. tuberculosis utilizes to manipulate host defense machinery.
4. Summary and future perspectives
The enduring co-evolution of M. tuberculosis with its hosts has enabled the pathogen to develop a number of strategies to thwart the host defense for its survival, especially within macrophages. These tactics range from interfering with phagosomal acidification and trafficking, blocking autophagy and apoptosis-mediated killing, perturbing calcium signaling, and inhibiting inflammasome activation in order to modulate the host cytokine responses and quench the reactive oxygen and nitrogen species produced by activated macrophages. Manipulation of these host pathways is achieved by employing a plethora of bacterial components including cell wall lipids, serine threonine kinases, phosphatases and proteases, and actively using specialized secretion systems. In this review, we have focused on a relatively recently described virulence strategy involving perturbation of nucleotide-based second messenger signaling in the host.
Subversion of host signaling molecules, particularly nucleotide second messengers, has emerged as a common evolutionary strategy of pathogens to counteract the host’s innate responses [168–171]. For instance, a number of pathogenic viruses such as Murine hepatitis virus (MHV) and Group A rotavirus (RVA) efficiently counteract innate immunity by degrading host derived oligoadenylates employing specific phosphodiesterase thus inhibiting activation of ribonuclease L (RNase L), which constitute important components of the host antiviral pathway [172–173]. Moreover, recent research has demonstrated that cGAS is an important innate sensor of retroviral DNA such that infection with human immunodeficiency viruses (HIV), simian immunodeficiency viruses (SIV), or murine leukemia virus (MLV) activates cGAS to produce cGAMP, thus stimulating the cGAMP/STING/IFN axis [82]. These viruses are well-known to obviate CSP responses by concealing viral nucleic acids within capsid structures and/or limiting the accumulation of cytosolic viral DNA by co-opting host factors such as TREX1 and SAMHD1 [174]. TREX1 is a cytosolic exonuclease, which inhibits the host CSP or Interferon stimulatory DNA response by degrading the cytosolic DNA derived from HIV. TREX1 has also been shown to play an important role in M. tuberculosis-induced Type I IFN response. Knocking out the TREX1 gene substantially increased the cellular innate response while its over-expression resulted in a reduced host response to M. tuberculosis infection [1]. Such observations may establish important links in the co-pathogenesis of HIV and M. tuberculosis, each of which is known to exacerbate the other in co-infected humans. It is interesting to speculate presence of specific bacterial and viral mechanisms to manipulate CDN second messenger signaling to facilitate TB-HIV co-infection. Box 1 summarizes some of the key issues related to cyclic nucleotide signaling in M. tuberculosis infection.
Box 1. Key issues on cyclic nucleotide signaling in M. tuberculosis infection.
How does M. tuberculosis secrete cyclic mono- and di-nucleotides?
Do mycobacteria secrete cyclic nucleotide cyclases and phosphodiesterases into the host cell to utilize host substrate molecule as well as to subvert host signaling pathways?
How do both the bacilli and the host regulate the balance and interconnected functions of multiple cyclic nucleotides?
How does the host differentiate seemingly similar self and non-self cyclic nucleotides to initiate specific signaling cascades?
Like the host, does M. tuberculosis expresses different cyclic nucleotide cyclases and phosphodiesterases in a tissue- or organ-specific manner during infection?
Does cyclic-nucleotide signaling plays any role in TB-HIV co-infection?
Does small molecule targeting of the cyclic nucleotide signaling pathways have potential for developing therapeutics or immunotherapeutics against TB?
The past decade has revealed numerous mechanisms which contribute to the virulence of M. tuberculosis. Similarly there are host mechanisms in place to counteract these M. tuberculosis virulence strategies. Considering the overlapping and interconnected nature of the host-microbe crosstalk in tuberculosis, future research to characterize this interplay promises to unravel new vistas of therapeutic and prophylactic possibilities against this stubborn pathogen.
Highlights.
The macrophage cytosolic surveillance pathway detects foreign DNA and nucleotides.
M.tb delivers pathogen-derived cAMP into the host cell eliciting hyperinflammation.
c-di-AMP and c-di-GMP are bacterial derived nucleotides detected by host receptors.
Bacterial DNA and M.tb- derived c-di-AMP trigger IFN-β secretion and autophagy.
Manipulation of the cytosolic surveillance pathway may enable therapeutics for TB.
Acknowledgments
Research in the laboratory of William R. Bishai is funded by the NIH grants AI36973, AI37856, and AI097138 and the Howard Hughes Medical Institute (HHMI). Postdoctoral fellowship support to Bappaditya Dey is funded by HHMI. We thank Ruchi Jain Dey and Shaaretha Pelly for critical reading of the manuscript and useful suggestions. We acknowledge Connie William for proofreading of the manuscript.
Abbreviations
- AC
Adenylyl Cyclase
- AKAP7
A-Kinase Anchor Protein 7 isoform gamma
- AM
Alveolar Macrophage
- ATP
Adenosine Triphosphate
- BCG
Bacille Calmette–Guérin
- cAMP
Cyclic Adenosine Monophosphate
- CD1
Cluster of Differentiation 1
- c-di-AMP
Cyclic-di-Adenosine Monophosphate
- c-di-GMP
Cyclic-di-Guanosine Monophosphate
- CDN
Cyclic Dinucleotide
- cGAMP
Cyclic- Guanosine Monophosphate - Adenosine Monophosphate
- cGAS
Cyclic GMP-AMP synthase
- cGMP
Cyclic Guanosine Monophosphate
- CR
Complement Receptor
- CREB
cAMP Response Element-Binding Protein
- CSP
Cytoplasmic Surveillance Pathway
- DAG
Diacyl-Glycerol
- DC
Dendritic Cell
- DC-SIGN
Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-integrin
- DGC
Diguanylate Cyclase
- disA
DNA Integrity Scanning Protein-A
- Eis
Enhanced Intracellular Survival protein
- ESAT-6
Early Secretory Antigenic Target - 6
- ESX-1
ESAT-6 secretion system
- FcγR
Complement Factor- γ Receptor
- GC
Guanylyl Cyclase
- GTP
Guanosine Triphosphate
- HCN
Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels
- HIV
Human Immunodeficiency Virus
- ICAM
Intercellular Adhesion Molecule 1
- IFN
interferon
- IL
interleukin
- LM
Lipomannan
- M. tuberculosis
Mycobacterium tuberculosis
- ManLAM
Mannose-Capped Lipoarabinomannan
- MHV
Murine Hepatitis Virus
- RNasL
Ribonuclease L
- MLV
Murine Leukemia Virus
- MR
Mannose Receptors
- MyD88
Myeloid Differentiation; Primary Response Gene 88
- NLRP3
NLR Family Pyrin Domain Containing 3
- NOD
Nucleotide-Binding Oligomerization Domain
- PAMP
Pathogen Associated Molecular Pattern
- PDE
Phosphodiesterase
- PDIM
Phthiocerol Dimycocerosates
- PI3P
Phosphatidylinositol 3-Phosphate
- PIM
phosphatidyl-myo-inositol mannosides
- PKA
Protein Kinase A
- (p)ppGpp
Guanosine Pentaphosphate or Tetraphosphate
- PRR
Pattern Recognition Receptor
- SAMHD1
SAM Domain and HD Domain-Containing Protein 1
- SR
Scavenger Receptors
- STING
Stimulator of Interferon Gene
- TB
Tuberculosis
- TDM
Trehalose Dimycolates
- TLR
Toll-Like Receptor
- TNF
Tumor Necrosis Factor
- TREX1
Three Prime Repair Exonuclease 1
- VPS33B
Vacuolar Protein Sorting-Associated Protein 33b
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manzanillo PS, et al. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe. 2012;11(5):469–80. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150(4):803–15. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayachandran R, BoseDasgupta S, Pieters J. Surviving the macrophage: tools and tricks employed by Mycobacterium tuberculosis. Curr Top Microbiol Immunol. 2013;374:189–209. doi: 10.1007/82_2012_273. [DOI] [PubMed] [Google Scholar]
- 4.Stanley SA, Cox JS. Host-pathogen interactions during Mycobacterium tuberculosis infections. Curr Top Microbiol Immunol. 2013;374:211–41. doi: 10.1007/82_2013_332. [DOI] [PubMed] [Google Scholar]
- 5.Stokes RW, Thorson LM, Speert DP. Nonopsonic and opsonic association of Mycobacterium tuberculosis with resident alveolar macrophages is inefficient. J Immunol. 1998;160(11):5514–21. [PubMed] [Google Scholar]
- 6.Johnston RB, Jr, Godzik CA, Cohn ZA. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978;148(1):115–27. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dannenberg AM., Jr Perspectives on clinical and preclinical testing of new tuberculosis vaccines. Clin Microbiol Rev. 2010;23(4):781–94. doi: 10.1128/CMR.00005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinnijenhuis J, et al. Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol. 2011;2011:405310. doi: 10.1155/2011/405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehlers S. DC-SIGN and mannosylated surface structures of Mycobacterium tuberculosis: a deceptive liaison. Eur J Cell Biol. 2010;89(1):95–101. doi: 10.1016/j.ejcb.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Schafer G, et al. Non-opsonic recognition of Mycobacterium tuberculosis by phagocytes. J Innate Immun. 2009;1(3):231–43. doi: 10.1159/000173703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177(3):1805–16. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- 12.Schlesinger LSA, AK, Torelles JB, Roberts E, Vergne I, Deretic V, Kaufmann SHE, Briton WJ, editors. Handbook of Tuberculosis: Immunology and Cell Biology. Wiley-VCH; Weinheim: 2008. Determinants of Phagocytosis, Phagosome Biogenesis and Autophagy for Mycobacterium tuberculosis. [Google Scholar]
- 13.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282(5394):1717–21. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 14.Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288(5471):1647–50. doi: 10.1126/science.288.5471.1647. [DOI] [PubMed] [Google Scholar]
- 15.Peyron P, et al. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J Immunol. 2000;165(9):5186–91. doi: 10.4049/jimmunol.165.9.5186. [DOI] [PubMed] [Google Scholar]
- 16.Vergne I, et al. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2005;102(11):4033–8. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleh MT, Belisle JT. Secretion of an acid phosphatase (SapM) by Mycobacterium tuberculosis that is similar to eukaryotic acid phosphatases. J Bacteriol. 2000;182(23):6850–3. doi: 10.1128/jb.182.23.6850-6853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bach H, et al. Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell Host Microbe. 2008;3(5):316–22. doi: 10.1016/j.chom.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med. 2003;198(4):653–9. doi: 10.1084/jem.20030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergne I, et al. Cell biology of mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol. 2004;20:367–94. doi: 10.1146/annurev.cellbio.20.010403.114015. [DOI] [PubMed] [Google Scholar]
- 21.Deghmane AE, et al. Lipoamide dehydrogenase mediates retention of coronin-1 on BCG vacuoles, leading to arrest in phagosome maturation. J Cell Sci. 2007;120(Pt 16):2796–806. doi: 10.1242/jcs.006221. [DOI] [PubMed] [Google Scholar]
- 22.Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. 2008;3(6):399–407. doi: 10.1016/j.chom.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Jayachandran R, et al. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell. 2007;130(1):37–50. doi: 10.1016/j.cell.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 24.Han G, Zhang CC. On the origin of Ser/Thr kinases in a prokaryote. FEMS Microbiol Lett. 2001;200(1):79–84. doi: 10.1111/j.1574-6968.2001.tb10696.x. [DOI] [PubMed] [Google Scholar]
- 25.Ponting CP, et al. Eukaryotic signalling domain homologues in archaea and bacteria. Ancient ancestry and horizontal gene transfer. J Mol Biol. 1999;289(4):729–45. doi: 10.1006/jmbi.1999.2827. [DOI] [PubMed] [Google Scholar]
- 26.Be NA, Bishai WR, Jain SK. Role of Mycobacterium tuberculosis pknD in the pathogenesis of central nervous system tuberculosis. BMC Microbiol. 2012;12:7. doi: 10.1186/1471-2180-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenstein AE, et al. M. tuberculosis Ser/Thr protein kinase D phosphorylates an anti-anti-sigma factor homolog. PLoS Pathog. 2007;3(4):e49. doi: 10.1371/journal.ppat.0030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walburger A, et al. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science. 2004;304(5678):1800–4. doi: 10.1126/science.1099384. [DOI] [PubMed] [Google Scholar]
- 29.Scherr N, et al. Survival of pathogenic mycobacteria in macrophages is mediated through autophosphorylation of protein kinase G. J Bacteriol. 2009;191(14):4546–54. doi: 10.1128/JB.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Master SS, et al. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3(4):224–32. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei J, et al. Identification of a Mycobacterium tuberculosis gene that enhances mycobacterial survival in macrophages. J Bacteriol. 2000;182(2):377–84. doi: 10.1128/jb.182.2.377-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin DM, et al. Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog. 2010;6(12):e1001230. doi: 10.1371/journal.ppat.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim KH, et al. Mycobacterium tuberculosis Eis protein initiates suppression of host immune responses by acetylation of DUSP16/MKP-7. Proc Natl Acad Sci U S A. 2012;109(20):7729–34. doi: 10.1073/pnas.1120251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goren MB, Brokl O, Schaefer WB. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: phthiocerol dimycocerosate and the attenuation indicator lipid. Infect Immun. 1974;9(1):150–8. doi: 10.1128/iai.9.1.150-158.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goren MB, Brokl O, Schaefer WB. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: correlation of virulence with elaboration of sulfatides and strongly acidic lipids. Infect Immun. 1974;9(1):142–9. doi: 10.1128/iai.9.1.142-149.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox JS, et al. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999;402(6757):79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- 37.Camacho LR, et al. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol. 1999;34(2):257–67. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 38.Rhoades ER, Ullrich HJ. How to establish a lasting relationship with your host: lessons learned from Mycobacterium spp. Immunol Cell Biol. 2000;78(4):301–10. doi: 10.1046/j.1440-1711.2000.00938.x. [DOI] [PubMed] [Google Scholar]
- 39.Astarie-Dequeker C, et al. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog. 2009;5(2):e1000289. doi: 10.1371/journal.ppat.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beatty WL, et al. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1(3):235–47. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 41.Beatty WL, Ullrich HJ, Russell DG. Mycobacterial surface moieties are released from infected macrophages by a constitutive exocytic event. Eur J Cell Biol. 2001;80(1):31–40. doi: 10.1078/0171-9335-00131. [DOI] [PubMed] [Google Scholar]
- 42.van den Elzen P, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437(7060):906–10. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 43.Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240(1):252–68. doi: 10.1111/j.1600-065X.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell DG, et al. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10(9):943–8. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckman EM, et al. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372(6507):691–4. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 46.Kim MJ, et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010;2(7):258–74. doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahajan S, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. J Immunol. 2012;188(11):5593–603. doi: 10.4049/jimmunol.1103038. [DOI] [PubMed] [Google Scholar]
- 48.Rajaram MV, et al. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185(2):929–42. doi: 10.4049/jimmunol.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Wel N, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129(7):1287–98. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 50.Stamm LM, et al. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J Exp Med. 2003;198(9):1361–8. doi: 10.1084/jem.20031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith J, et al. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun. 2008;76(12):5478–87. doi: 10.1128/IAI.00614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis KN, et al. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J Infect Dis. 2003;187(1):117–23. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao LY, et al. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol. 2004;53(6):1677–93. doi: 10.1111/j.1365-2958.2004.04261.x. [DOI] [PubMed] [Google Scholar]
- 54.Simeone R, et al. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 2012;8(2):e1002507. doi: 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandey AK, et al. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009;5(7):e1000500. doi: 10.1371/journal.ppat.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Austin CM, Ma X, Graviss EA. Common nonsynonymous polymorphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. J Infect Dis. 2008;197(12):1713–6. doi: 10.1086/588384. [DOI] [PubMed] [Google Scholar]
- 57.Bauer S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98(16):9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 59.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448(7152):501–5. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 60.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138(3):576–91. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parvatiyar K, et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13(12):1155–61. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z, et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12(10):959–65. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao P, et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153(5):1094–107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong KW, Jacobs WR., Jr Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell Microbiol. 2011;13(9):1371–84. doi: 10.1111/j.1462-5822.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10 (3):266–72. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 67.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–92. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rahman A, et al. Mycobacterium tuberculosis subverts the TLR-2-MyD88 pathway to facilitate its translocation into the cytosol. PLoS One. 2014;9(1):e86886. doi: 10.1371/journal.pone.0086886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonough KA, Rodriguez A. The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol. 2012;10(1):27–38. doi: 10.1038/nrmicro2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agarwal N, Bishai WR. cAMP signaling in Mycobacterium tuberculosis. Indian J Exp Biol. 2009;47(6):393–400. [PubMed] [Google Scholar]
- 71.Shenoy AR, Visweswariah SS. New messages from old messengers: cAMP and mycobacteria. Trends Microbiol. 2006;14(12):543–50. doi: 10.1016/j.tim.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 72.Pilz RB, Casteel DE. Regulation of gene expression by cyclic GMP. Circ Res. 2003;93(11):1034–46. doi: 10.1161/01.RES.0000103311.52853.48. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, et al. Synergism between calcium and cyclic GMP in cyclic AMP response element-dependent transcriptional regulation requires cooperation between CREB and C/EBP-beta. Mol Cell Biol. 2003;23(12):4066–82. doi: 10.1128/MCB.23.12.4066-4082.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bai G, Knapp GS, McDonough KA. Cyclic AMP signalling in mycobacteria: redirecting the conversation with a common currency. Cell Microbiol. 2011;13(3):349–58. doi: 10.1111/j.1462-5822.2010.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Springett GM, Kawasaki H, Spriggs DR. Non-kinase second-messenger signaling: new pathways with new promise. Bioessays. 2004;26(7):730–8. doi: 10.1002/bies.20057. [DOI] [PubMed] [Google Scholar]
- 76.Dalebroux ZD, et al. ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev. 2010;74(2):171–99. doi: 10.1128/MMBR.00046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romling U. Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci Signal. 2008;1(33):pe39. doi: 10.1126/scisignal.133pe39. [DOI] [PubMed] [Google Scholar]
- 78.Gomelsky M. cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all! Mol Microbiol. 2011;79(3):562–5. doi: 10.1111/j.1365-2958.2010.07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77(1):1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boyd CD, O’Toole GA. Second messenger regulation of biofilm formation: breakthroughs in understanding c-di-GMP effector systems. Annu Rev Cell Dev Biol. 2012;28:439–62. doi: 10.1146/annurev-cellbio-101011-155705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328(5986):1703–5. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao D, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341(6148):903–6. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X, et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51(2):226–35. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun L, et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–30. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolb A, et al. Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem. 1993;62:749–95. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- 87.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12(3):141–51. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antoni FA. Molecular diversity of cyclic AMP signalling. Front Neuroendocrinol. 2000;21(2):103–32. doi: 10.1006/frne.1999.0193. [DOI] [PubMed] [Google Scholar]
- 89.Perlman RL, De Crombrugghe B, Pastan I. Cyclic AMP regulates catabolite and transient repression in E. coli. Nature. 1969;223(5208):810–2. doi: 10.1038/223810a0. [DOI] [PubMed] [Google Scholar]
- 90.Botsford JL. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981;45(4):620–42. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev. 2002;66(3):373–95. doi: 10.1128/MMBR.66.3.373-395.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee HJ, et al. Vibrio vulnificus rpoS expression is repressed by direct binding of cAMP-cAMP receptor protein complex to its two promoter regions. J Biol Chem. 2008;283(45):30438–50. doi: 10.1074/jbc.M802219200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng D, et al. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 2004;32(19):5874–93. doi: 10.1093/nar/gkh908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Landry Y, et al. Heptahelical and other G-protein-coupled receptors (GPCRs) signaling. Curr Med Chem. 2006;13(1):51–63. [PubMed] [Google Scholar]
- 95.Livigni A, et al. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol Biol Cell. 2006;17(1):263–71. doi: 10.1091/mbc.E05-09-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feliciello A, Gottesman ME, Avvedimento EV. cAMP-PKA signaling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 2005;17 (3):279–87. doi: 10.1016/j.cellsig.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 97.Fong JC, Yildiz FH. Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation. J Bacteriol. 2008;190(20):6646–59. doi: 10.1128/JB.00466-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith RS, Wolfgang MC, Lory S. An adenylate cyclase-controlled signaling network regulates Pseudomonas aeruginosa virulence in a mouse model of acute pneumonia. Infect Immun. 2004;72(3):1677–84. doi: 10.1128/IAI.72.3.1677-1684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3(9):710–8. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 100.Lolicato M, et al. Cyclic dinucleotides bind the C-linker of HCN4 to control channel cAMP responsiveness. Nat Chem Biol. 2014;10(6):457–62. doi: 10.1038/nchembio.1521. [DOI] [PubMed] [Google Scholar]
- 101.Menter DG, Dubois RN. Prostaglandins in cancer cell adhesion, migration, and invasion. Int J Cell Biol. 2012;2012:723419. doi: 10.1155/2012/723419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stork PJ, Dillon TJ. Multiple roles of Rap1 in hematopoietic cells: complementary versus antagonistic functions. Blood. 2005;106(9):2952–61. doi: 10.1182/blood-2005-03-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dillon TJ, et al. Regulation of the small GTPase Rap1 and extracellular signal-regulated kinases by the costimulatory molecule CTLA-4. Mol Cell Biol. 2005;25(10):4117–28. doi: 10.1128/MCB.25.10.4117-4128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tai P, Ascoli M. Reactive oxygen species (ROS) play a critical role in the cAMP-induced activation of Ras and the phosphorylation of ERK1/2 in Leydig cells. Mol Endocrinol. 2011;25(5):885–93. doi: 10.1210/me.2010-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kalamidas SA, et al. cAMP synthesis and degradation by phagosomes regulate actin assembly and fusion events: consequences for mycobacteria. J Cell Sci. 2006;119(Pt 17):3686–94. doi: 10.1242/jcs.03091. [DOI] [PubMed] [Google Scholar]
- 106.Ahuja N, Kumar P, Bhatnagar R. The adenylate cyclase toxins. Crit Rev Microbiol. 2004;30(3):187–96. doi: 10.1080/10408410490468795. [DOI] [PubMed] [Google Scholar]
- 107.Lowrie DB, Jackett PS, Ratcliffe NA. Mycobacterium microti may protect itself from intracellular destruction by releasing cyclic AMP into phagosomes. Nature. 1975;254(5501):600–2. doi: 10.1038/254600a0. [DOI] [PubMed] [Google Scholar]
- 108.Bourne HR, et al. Modulation of inflammation and immunity by cyclic AMP. Science. 1974;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- 109.Colic M, et al. 8-Chloro-cAMP modulates apoptosis of thymocytes and thymocyte hybridoma. Transplant Proc. 2001;33(3):2347–9. doi: 10.1016/s0041-1345(01)02017-6. [DOI] [PubMed] [Google Scholar]
- 110.Alexeyev OA, et al. Impaired neutrophil function in the cutaneous form of anthrax. Infection. 1994;22(4):281–2. doi: 10.1007/BF01739917. [DOI] [PubMed] [Google Scholar]
- 111.Hoover DL, et al. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor necrosis factor alpha and interleukin-6 by increasing intracellular cyclic AMP. Infect Immun. 1994;62(10):4432–9. doi: 10.1128/iai.62.10.4432-4439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61(10):4064–71. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pearson RD, et al. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J Immunol. 1987;139(8):2749–54. [PubMed] [Google Scholar]
- 114.Confer DL, Eaton JW. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982;217(4563):948–50. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 115.Padh H, Venkitasubramanian TA. Cyclic adenosine 3′, 5′-monophosphate in mycobacteria. Indian J Biochem Biophys. 1976;13(4):413–4. [PubMed] [Google Scholar]
- 116.Padh H, Venkitasubramanian TA. Adenosine 3′,5′-monophosphate in Mycobacterium phlei and Mycobacterium tuberculosis H37Ra. Microbios. 1976;16(65–66):183–9. [PubMed] [Google Scholar]
- 117.Spreadbury CL, et al. Point mutations in the DNA- and cNMP-binding domains of the homologue of the cAMP receptor protein (CRP) in Mycobacterium bovis BCG: implications for the inactivation of a global regulator and strain attenuation. Microbiology. 2005;151(Pt 2):547–56. doi: 10.1099/mic.0.27444-0. [DOI] [PubMed] [Google Scholar]
- 118.Shenoy AR, et al. The Rv0805 gene from Mycobacterium tuberculosis encodes a 3′,5′-cyclic nucleotide phosphodiesterase: biochemical and mutational analysis. Biochemistry. 2005;44(48):15695–704. doi: 10.1021/bi0512391. [DOI] [PubMed] [Google Scholar]
- 119.Bai G, Schaak DD, McDonough KA. cAMP levels within Mycobacterium tuberculosis and Mycobacterium bovis BCG increase upon infection of macrophages. FEMS Immunol Med Microbiol. 2009;55(1):68–73. doi: 10.1111/j.1574-695X.2008.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Agarwal N, et al. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature. 2009;460(7251):98–102. doi: 10.1038/nature08123. [DOI] [PubMed] [Google Scholar]
- 121.Maiga M, et al. Adjuvant host-directed therapy with types 3 and 5 but not type 4 phosphodiesterase inhibitors shortens the duration of tuberculosis treatment. J Infect Dis. 2013;208(3):512–9. doi: 10.1093/infdis/jit187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCue LA, McDonough KA, Lawrence CE. Functional classification of cNMP-binding proteins and nucleotide cyclases with implications for novel regulatory pathways in Mycobacterium tuberculosis. Genome Res. 2000;10(2):204–19. doi: 10.1101/gr.10.2.204. [DOI] [PubMed] [Google Scholar]
- 123.Rickman L, et al. A member of the cAMP receptor protein family of transcription regulators in Mycobacterium tuberculosis is required for virulence in mice and controls transcription of the rpfA gene coding for a resuscitation promoting factor. Mol Microbiol. 2005;56(5):1274–86. doi: 10.1111/j.1365-2958.2005.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bai G, McCue LA, McDonough KA. Characterization of Mycobacterium tuberculosis Rv3676 (CRPMt), a cyclic AMP receptor protein-like DNA binding protein. J Bacteriol. 2005;187(22):7795–804. doi: 10.1128/JB.187.22.7795-7804.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nambi S, Basu N, Visweswariah SS. cAMP-regulated protein lysine acetylases in mycobacteria. J Biol Chem. 2010;285(32):24313–23. doi: 10.1074/jbc.M110.118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gazdik MA, et al. Rv1675c (cmr) regulates intramacrophage and cyclic AMP-induced gene expression in Mycobacterium tuberculosis-complex mycobacteria. Mol Microbiol. 2009;71(2):434–48. doi: 10.1111/j.1365-2958.2008.06541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pelly S, Bishai WR, Lamichhane G. A screen for non-coding RNA in Mycobacterium tuberculosis reveals a cAMP-responsive RNA that is expressed during infection. Gene. 2012;500(1):85–92. doi: 10.1016/j.gene.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ross P, et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature. 1987;325(6101):279–81. doi: 10.1038/325279a0. [DOI] [PubMed] [Google Scholar]
- 129.Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. 2014;54(2):289–96. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 130.Corrigan RM, Grundling A. Cyclic di-AMP: another second messenger enters the fray. Nat Rev Microbiol. 2013;11(8):513–24. doi: 10.1038/nrmicro3069. [DOI] [PubMed] [Google Scholar]
- 131.Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7(4):263–73. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 132.Ryan RP. Cyclic di-GMP signalling and the regulation of bacterial virulence. Microbiology. 2013;159(Pt 7):1286–97. doi: 10.1099/mic.0.068189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Corrigan RM, et al. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 2011;7(9):e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith KD, Strobel SA. Interactions of the c-di-GMP riboswitch with its second messenger ligand. Biochem Soc Trans. 2011;39(2):647–51. doi: 10.1042/BST0390647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith KD, et al. Structural basis of ligand binding by a c-di-GMP riboswitch. Nat Struct Mol Biol. 2009;16(12):1218–23. doi: 10.1038/nsmb.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nelson JW, et al. Riboswitches in eubacteria sense the second messenger c-di-AMP. Nat Chem Biol. 2013;9(12):834–9. doi: 10.1038/nchembio.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Davies BW, et al. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell. 2012;149(2):358–70. doi: 10.1016/j.cell.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Diner EJ, et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 2013;3(5):1355–61. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yi G, et al. Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic dinucleotides. PLoS One. 2013;8(10):e77846. doi: 10.1371/journal.pone.0077846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14(1):19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- 141.Abdul-Sater AA, et al. Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome. EMBO Rep. 2013;14(10):900–6. doi: 10.1038/embor.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shu C, Li X, Li P. The mechanism of double-stranded DNA sensing through the cGAS-STING pathway. Cytokine Growth Factor Rev. 2014 doi: 10.1016/j.cytogfr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Panchanathan R, et al. Identification of a negative feedback loop between cyclic di-GMP-induced levels of IFI16 and p202 cytosolic DNA sensors and STING. Innate Immun. 2013 doi: 10.1177/1753425913507097. [DOI] [PubMed] [Google Scholar]
- 144.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13(10):722–37. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liang Q, et al. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe. 2014;15(2):228–38. doi: 10.1016/j.chom.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207(10):2053–63. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rayamajhi M, et al. Antagonistic crosstalk between type I and II interferons and increased host susceptibility to bacterial infections. Virulence. 2010;1(5):418–22. doi: 10.4161/viru.1.5.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Manca C, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci U S A. 2001;98(10):5752–7. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schwartz KT, et al. Hyperinduction of host beta interferon by a Listeria monocytogenes strain naturally overexpressing the multidrug efflux pump MdrT. Infect Immun. 2012;80(4):1537–45. doi: 10.1128/IAI.06286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang J, et al. Deletion of the cyclic di-AMP phosphodiesterase gene (cnpB) in Mycobacterium tuberculosis leads to reduced virulence in a mouse model of infection. Mol Microbiol. 2014 doi: 10.1111/mmi.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barker JR, et al. STING-dependent recognition of cyclic di-AMP mediates type I interferon responses during Chlamydia trachomatis infection. MBio. 2013;4(3):e00018–13. doi: 10.1128/mBio.00018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Petersen E, et al. Brucella melitensis cyclic di-GMP phosphodiesterase BpdA controls expression of flagellar genes. J Bacteriol. 2011;193(20):5683–91. doi: 10.1128/JB.00428-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang J, et al. Deletion of the cyclic di-AMP phosphodiesterase gene (cnpB) in Mycobacterium tuberculosis leads to reduced virulence in a mouse model of infection. Mol Microbiol. 2014;93(1):65–79. doi: 10.1111/mmi.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dengler V, et al. Mutation in the C-di-AMP cyclase dacA affects fitness and resistance of methicillin resistant Staphylococcus aureus. PLoS One. 2013;8(8):e73512. doi: 10.1371/journal.pone.0073512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Witte CE, et al. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. MBio. 2013;4(3):e00282–13. doi: 10.1128/mBio.00282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bai Y, et al. Mycobacterium tuberculosis Rv3586 (DacA) is a diadenylate cyclase that converts ATP or ADP into c-di-AMP. PLoS One. 2012;7(4):e35206. doi: 10.1371/journal.pone.0035206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gupta K, Kumar P, Chatterji D. Identification, activity and disulfide connectivity of C-di-GMP regulating proteins in Mycobacterium tuberculosis. PLoS One. 2010;5(11):e15072. doi: 10.1371/journal.pone.0015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kumar M, Chatterji D. Cyclic di-GMP: a second messenger required for long-term survival, but not for biofilm formation, in Mycobacterium smegmatis. Microbiology. 2008;154(Pt 10):2942–55. doi: 10.1099/mic.0.2008/017806-0. [DOI] [PubMed] [Google Scholar]
- 159.Hong Y, et al. Cyclic di-GMP mediates Mycobacterium tuberculosis dormancy and pathogenecity. Tuberculosis (Edinb) 2013;93(6):625–34. doi: 10.1016/j.tube.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 160.Zhang L, He ZG. Radiation-sensitive gene A (RadA) targets DisA, DNA integrity scanning protein A, to negatively affect cyclic Di-AMP synthesis activity in Mycobacterium smegmatis. J Biol Chem. 2013;288(31):22426–36. doi: 10.1074/jbc.M113.464883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang L, Li W, He ZG. DarR, a TetR-like transcriptional factor, is a cyclic di-AMP-responsive repressor in Mycobacterium smegmatis. J Biol Chem. 2013;288(5):3085–96. doi: 10.1074/jbc.M112.428110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Civril F, et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498(7454):332–7. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dorhoi A, et al. Type I IFN signaling triggers immunopathology in tuberculosis-susceptible mice by modulating lung phagocyte dynamics. Eur J Immunol. 2014 doi: 10.1002/eji.201344219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kuchtey J, et al. Enhancement of dendritic cell antigen cross-presentation by CpG DNA involves type I IFN and stabilization of class I MHC mRNA. J Immunol. 2005;175(4):2244–51. doi: 10.4049/jimmunol.175.4.2244. [DOI] [PubMed] [Google Scholar]
- 165.Thornley TB, et al. Type 1 IFN mediates cross-talk between innate and adaptive immunity that abrogates transplantation tolerance. J Immunol. 2007;179(10):6620–9. doi: 10.4049/jimmunol.179.10.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Desvignes L, Wolf AJ, Ernst JD. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J Immunol. 2012;188(12):6205–15. doi: 10.4049/jimmunol.1200255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Berry MP, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reddick LE, Alto NM. Bacteria fighting back: how pathogens target and subvert the host innate immune system. Mol Cell. 2014;54(2):321–8. doi: 10.1016/j.molcel.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nitta S, et al. Hepatitis C virus NS4B protein targets STING and abrogates RIG-I-mediated type I interferon-dependent innate immunity. Hepatology. 2013;57(1):46–58. doi: 10.1002/hep.26017. [DOI] [PubMed] [Google Scholar]
- 170.Finlay BB, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276(5313):718–25. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 171.Brodsky IE, Medzhitov R. Targeting of immune signalling networks by bacterial pathogens. Nat Cell Biol. 2009;11(5):521–6. doi: 10.1038/ncb0509-521. [DOI] [PubMed] [Google Scholar]
- 172.Silverman RH, Weiss SR. Viral Phosphodiesterases That Antagonize Double-Stranded RNA Signaling to RNase L by Degrading 2-5A. J Interferon Cytokine Res. 2014;34(6):455–63. doi: 10.1089/jir.2014.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang R, et al. Homologous 2′,5′-phosphodiesterases from disparate RNA viruses antagonize antiviral innate immunity. Proc Natl Acad Sci U S A. 2013;110(32):13114–9. doi: 10.1073/pnas.1306917110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hasan M, et al. Trex1 regulates lysosomal biogenesis and interferon-independent activation of antiviral genes. Nat Immunol. 2013;14(1):61–71. doi: 10.1038/ni.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]