Abstract
MicroRNAs (miRNAs) have been shown to influence erythroid lineage commitment and differentiation. However, our knowledge of miRNA function in terminal erythropoiesis remains limited. To address this issue, we generated a novel animal model, where the miRNA-processing enzyme, Dicer, is selectively inactivated in erythropoietin receptor positive erythroid cells beginning with CFU-e/proerythroblast cells. This results in significant depletion of all miRNAs from the proerythroblast stage onwards, with one exception, miR-451, which is processed by Ago2 in a Dicer-independent manner. We observed that mature Dicer-dependent miRNAs, like miR-451, are dispensable under steady-state conditions, but these mutants have an impaired response to stress erythropoiesis, as demonstrated by a delay in recovery from anemia. This defect was specific to later maturing erythroid cells, as progenitor numbers were unaffected. In addition to generating a novel mouse model to study miRNA function in late erythroid cells, we conclude that miRNAs (both Dicer-dependent and independent) act primarily to regulate the optimal response to stress among late erythroid cells.
Keywords: microRNA, stress erythropoiesis, Dicer, hematopoiesis, erythropoiesis
INTRODUCTION
MicroRNAs (miRNAs) are short (~19–25nt), endogenous, non-coding RNA molecules that regulate gene expression. Like coding genes, miRNAs are transcribed in an RNA Pol II dependent fashion. The primary miRNA molecule is processed in the nucleus by an enzyme complex, which contains Drosha and DGCR8. The nascent precursor miRNA form is exported out of the nucleus and further processed in the cytoplasm by the RNAse III enzyme, Dicer to ultimately form the mature miRNA. The mature miRNA is then loaded into the RNA-induced silencing complex (RISC), which contains Argonaute 2 (Ago2). miRNAs regulate gene expression by binding to specific recognition sites in the 3’-UTRs of target genes. Based on complementarity, miRNA binding causes transcript degradation or translational inhibition. There may be several binding sites for a particular miRNA per gene, and each miRNA can target multiple genes. Furthermore, ablation of Dicer leads to the loss of all Dicer-dependent mature miRNAs, thereby allowing one to study the collective requirement of miRNAs in a specific cellular context.
Deletion of Dicer in hematopoietic stem cells leads to loss of stem cell numbers [1] and preferential impairment in the formation of erythroid precursors [2]. Overexpression of miR-150 causes a skewing of lineage commitment in megakaryocyte-erythroid progenitors (MEPs) towards megakaryocytic differentiation at the expense of erythroid cells [3]. Therefore, in the early stages of hematopoiesis miRNAs appear to regulate erythroid fate. However, our understanding of miRNA function in terminal erythropoiesis is limited. Expression of the miR-144–451 cluster increases with progressive erythroid maturation [4–6], is highly specific for the erythroid lineage [6] and processing is Dicer-independent [7–9]. Several groups have generated miR-144/451 knockout mice [10–12] with variable phenotypes at steady-state (ranging from mild anemia to reticulocytosis without anemia). However, upon treatment with phenylhydrazine (PHZ), these mice showed impaired recovery from anemia, suggesting that miR-144/451 primarily regulates stress erythropoiesis. The phosphoserine/threonine-binding protein, 14-3-3ζ, was identified as a major miR-451 target [10, 12]. 14-3-3ζ negatively regulates FoxO3, a master regulator of the cellular anti-oxidant response. As miR-144/451 knock-out mice recover, it is unclear whether other miRNAs participate in modulating compensatory mechanisms.
Very little is known about the function of other miRNAs in late erythropoiesis, especially in vivo. To address this question, we have generated a novel mouse model to study the global role of miRNAs in the erythroid differentiation.
MATERIALS AND METHODS
Generation of EpoR-Cre-Dcr-del Mice
These studies were performed with Institutional Animal Care and Use Committee approval at the University of Washington. EpoR-Cre mice [13] and Dcr-fl/fl mice [14] have been previously described and were bred to generate EpoR-Cre-Dcr-del mice. Genomic DNA from tail snips was obtained using DirectPCR (Viagen, CA). Cre positive mice were identified by PCR using the following primers - GTGGCTGGACCAATGTGAAC, CAGGAATTCAAGCTCAACCTCA. Dcr-fl/fl mice were identified by PCR using the following primers - CCTGACAGTGACGGTCCAAAG, CATGACTCTTCAACTCAAACT. Cre negative littermates were used as controls.
Stress Erythropoiesis Experiments
Mice were injected with IP phenylhydrazine (100mg/kg) once. For experiments with 5-fluorouracil (5-FU), animals were injected with 100mg/kg IP, weekly for 6 consecutive weeks.
Flow Cytometry
Single cell suspensions of spleen or freshly isolated bone marrow were stained and analyzed as previously described [15, 16].
Colony Forming Assay
Peripheral blood, spleen and bone marrow mononuclear cells were plated in methylcellulose containing hematopoietic growth factors (ReachBio, WA) as previously described [17].
Real-Time PCR
Total RNA was isolated using Trizol (Invitrogen, CA). cDNA was generated using the High-Capacity Reverse Transcription Kit (Applied Biosystems, CA). Dicer expression was measured by TaqMan Assay (Applied Biosystems, CA), using a Step One Plus instrument (Applied Biosystems, CA). Expression was normalized to β-actin using the ΔΔCt method.
RESULTS AND DISCUSSION
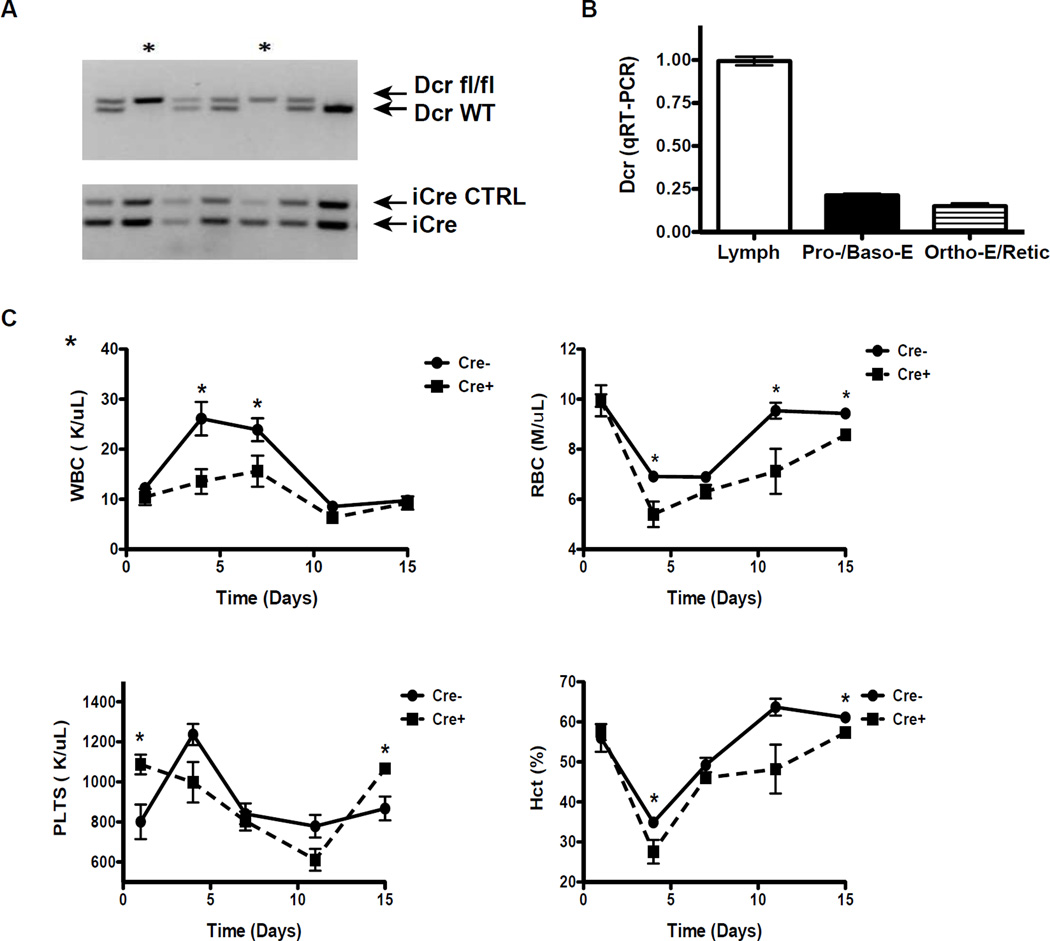
In order to deplete mature miRNA levels in the later stages of erythroid development, EpoR-Cre (Cre driven by the erythropoietin receptor promoter) mice [13] were bred with Dcr-flox/flox animals [14] to generate EpoR-Cre-Dcr-deleted (hereafter referred to as Dcr-del) mutants. In this model, Dicer is deleted downstream from the CFU-e/proerythroblast stage, when erythropoietin receptor expression begins [13, 16]. Dcr-del mutants were viable, fertile and born in the expected Mendelian ratio. PCR was used to verify Dicer deletion and Cre expression (Fig. 1A). There was no evidence of anemia or reticulocytosis, suggesting that loss of mature microRNAs in late erythroid development does not adversely affect maturation or survival red cells at steady-state (Table 1). The only notable difference in blood counts between Dcr-del and Cre-negative controls was a mildly elevated platelet count in the Dcr-del animals. Although, statistically significant, the platelet counts of Dcr-del animals are not outside the normal range. Comparison of Dicer mRNA expression between non-deleted hematopoietic cells (lymphocytes) and mature erythroid subsets (Sup. Fig. 1) reveals ~80% efficiency of Dicer ablation (Fig. 1B). The lack of an effect of late erythroid Dicer ablation on maturation at homeostasis is not entirely unexpected, since it has been reported that the expression of most miRNAs normally decline during erythroid maturation [18]. Similar findings have been reported in C. elegans, where the majority of single miRNA-deletion mutants did not have an appreciable phenotype [19]. Additionally, deletion of the miR-144/451 cluster or miR-451 alone, has a minimal effect on steady-state erythropoiesis and these miRNAs are the most highly expressed miRNA’s in late erythroid cells. However, it is unclear whether the low level of residual Dicer-dependent miRNA expression has any regulatory influence on terminal erythroid maturation.
Fig. 1.
Characterization of EpoR-Cre-Dcr-del mice. A) Genomic DNA was isolated from tail snips and presence of the Dicer flox/flox (Dcr-fl/fl), Dicer wild-type (Dcr-WT), iCre control (iCre CTRL), and iCre alleles were determined by PCR. Asterisks represent mice homozygous for the Dcr-fl/fl and positive for iCre. B) Real-time PCR of Dicer expression in lymphocytes isolated from lymph nodes and maturing erythroid cells. Erythroid subsets were isolated from PHZ-treated spleens by cell sorting using cell size and the markers CD44, Ter119, as previously described [24] (Sup. Fig. 1). Dicer expression was determined using TaqMan assays and expression was normalized to β-Actin. C) Dcr-del and Cre-negavie littermate controls were treated with PHZ and peripheral blood counts were monitored.
Table 1.
Complete Blood Counts of wild-type and EpoR-Cre-Dcr-del mice
| Cre− | Cre+ | Ref Range | p-Value | |
|---|---|---|---|---|
| WBC | 11.35 ± 0.22 | 10.3 ± 0.76 | 1.8–10.7 | 0.22 |
| RBC | 9.93 ± 0.27 | 9.67 ± 0.15 | 6.36–9.42 | 0.42 |
| HGB | 14.39 ± 0.46 | 13.95 ± 0.12 | 11–15.1 | 0.39 |
| HCT | 52.96 ± 1.82 | 53.14 ± 1.44 | 35.1–45.4 | 0.94 |
| MCV | 49.66 ± 3.26 | 54.86 ± 0.99 | 45.4–60.3 | 0.16 |
| MCH | 15.36 ± 0.91 | 14.45 ± 0.18 | 14.1–19.3 | 0.36 |
| MCHC | 26.6 ± 1 | 26.43 ± 0.65 | 30.2–34.2 | 0.89 |
| RDW | 15.68 ± 1.27 | 17.94 ± 0.12 | 12.4–27 | 0.11 |
| PLTS | 872.58 ± 47.23 | 1100.09 ± 30.24 | 592–2972 | 0.0007 |
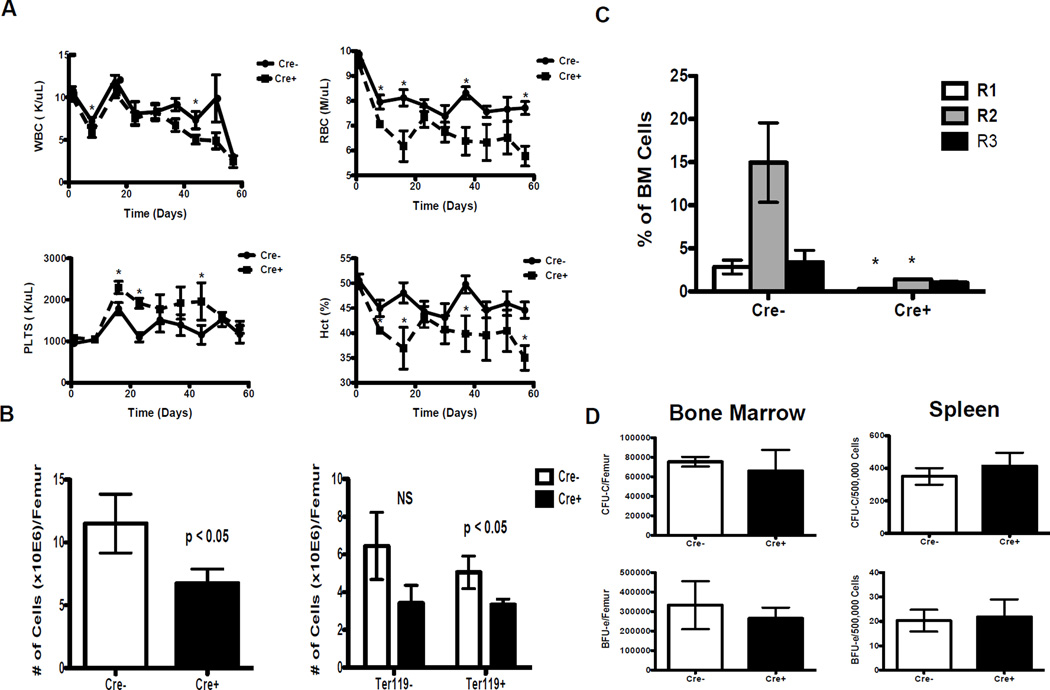
Despite the lack of a clear phenotype at steady-state, to test whether miRNAs primarily act to regulate stress responses, we treated mice with PHZ (100mg/kg IP, single injection) to induce hemolytic anemia. Dcr-del animals exhibited delayed recovery from anemia compared to Cre-negative controls (Fig. 1C). We also observed a significantly decreased total WBC count in Dcr-del animals compared to controls early after PHZ treatment (13.55 ± 2.48 vs. 26.1 ± 3.35, p = 0.017, Fig. 1C). The increased WBC counts in normal mice post-PHZ is primarily accounted for by increased lymphocytosis (9.54 ± 0.34 before PHZ and 18.42 ± 2.58 post-PHZ) and includes many nucleated red blood cells (RBCs), as we have previously documented [16]. We attribute the difference in WBC between normal and Dcr-del mice to the decreased output of nucleated erythroid cells. This finding together with the curtailed circulating red cell output suggests an impaired compensatory response in Dcr-del animals post-stress and supports the concept that in maturing red blood cells miRNAs function to fine tune the stress response. To explore the effect of Dicer deletion in late erythroid cells during chronic stress, we utilized a second model of stress hematopoiesis, repeated treatment with 5-FU (100mg/kg IP, single injection weekly, repeated for 6 weeks) at non-myeloablative doses. A more pronounced delay in erythroid recovery was seen (Fig. 2A) in Dcr-del mice. Unlike PHZ-treated animals, 5-FU treated Dcr-del remained significantly anemic at the end of treatment and did not recover to baseline levels (Fig. 2A), consistent with a selective, cumulative impairment in erythropoiesis. Dcr-del mice had a decrease in total bone marrow cellularity compared to Cre-negative controls (Fig. 2C). This was due to preferential depletion of the mature, Ter119+ erythroid pool (Fig. 2C). Detailed analysis of erythroid subsets in the bone marrow by flow cytometry and staining for CD71 and Ter119 [20], reveals a decrease in CD71hiTer119lo proerythroblasts (0.3 ± 0.1% vs. 2.4 ± 0.79%) and CD71hiTer119hi basophilic erythroblasts (1.62 ± 0.37 vs. 12.64 ± 4.41%) in Dcr-del 5-FU treated animals compared to Cre-negative controls. These specific stages of erythroid development may be the ones most susceptible to stress. It is of interest that the frequency of progenitors (BFU-E or total CFU-C) in either the bone marrow or spleen of Dcr-del mice were similar to controls after repeated 5-FU treatment (Fig. 2D), confirming that the functional consequences of selective Dicer ablation in our model is restricted specifically to terminally differentiating erythroid cells. Thus, our findings are in contrast to mice with Dicer deletion in hematopoietic stem cells (HSCs), which have significantly reduced BFU-e formation at homeostasis [2]. These data suggest that in differentiating erythroid cells, miRNAs function primarily to regulate stress responses. Identification of the specific miRNA regulated pathways during stress erythropoiesis remains to be determined.
Fig. 2.
Impaired stress erythropoiesis in Dcr-del mice. A) Blood counts in Dcr-del and Cre-negative littermate controls after weekly 5-FU injection. B) Total bone marrow cellularity and the total number of bone marrow erythroid cells per femur. C) Percentage of erythroid subsets in the bone marrow of Dcr-del and Cre-negative control animals after repeated 5-FU treatment. R1 = CD71hi/Ter119lo, R2 = CD71hi/Ter119hi, R3 = CD71int/Ter119hi. Asterisks represent p < 0.05 as compared to equivalent erythroid subset in control Cre-negative mice. D) Total CFU-C and BFU-e in bone marrow and spleen.
It is important to emphasize, that in our model, miR-451 is not deleted, since it is processed in a Dicer-independent manner [7, 8]. The primary defect in miR-451 knockout animals is an impaired response to oxidative stress [10, 12]. This is a common theme among single miRNA mutants, where significant phenotypes are only uncovered during stress conditions [21–23]. However, despite the presence of mature miR-451, we do see a phenotype similar to miR-451 loss, suggesting that other miRNAs besides miR-451 play a role in stress erythropoiesis. It appears that the primary function of miRNAs in mature erythroid cells is to regulate responses to stress by restoring homeostasis. The specific miRNAs that are involved and their targets may be identifiable using RNA-seq. This novel mouse model can be exploited to delineate miRNA function in late erythropoiesis, specifically under stress conditions either by combining our model with other genetic models of stress erythropoiesis or performing miRNA/shRNA rescue screens to identify specific miRNAs/mRNAs that restore the normal response to stress erythropoiesis.
Supplementary Material
Acknowlegements
We would like to thank Dr. J. Doulgas Engel (University of Michigan) for providing EpoR-Cre mice and Dr. Thomas Reh (University of Washington) for providing Dcr-fl/fl mice. Funding for this work was supported by NIH DK94702.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contribution. JCHB designed and performed experiments, analyzed the data and wrote and edited the manuscript. SP performed experiments. TP designed experiments, analyzed data and edited the manuscript.
The authors declare no competing financial interests.
REFERENCES
- 1.Guo S, et al. MicroRNA miR-125a controls hematopoietic stem cell number. Proc Natl Acad Sci U S A. 2010;107(32):14229–14234. doi: 10.1073/pnas.0913574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buza-Vidas N, et al. Dicer is selectively important for the earliest stages of erythroid development. Blood. 2012;120(12):2412–2416. doi: 10.1182/blood-2011-10-383653. [DOI] [PubMed] [Google Scholar]
- 3.Lu J, et al. MicroRNA-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Dev Cell. 2008;14(6):843–853. doi: 10.1016/j.devcel.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dore LC, et al. A GATA-1-regulated microRNA locus essential for erythropoiesis. Proc Natl Acad Sci U S A. 2008;105(9):3333–3338. doi: 10.1073/pnas.0712312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan M, et al. MicroRNA expression dynamics during murine and human erythroid differentiation. Exp Hematol. 2007;35(7):1015–1025. doi: 10.1016/j.exphem.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landgraf P, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheloufi S, et al. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465(7298):584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cifuentes D, et al. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science. 2010;328(5986):1694–1698. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang JS, et al. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc Natl Acad Sci U S A. 2010;107(34):15163–15168. doi: 10.1073/pnas.1006432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patrick DM, et al. Defective erythroid differentiation in miR-451 mutant mice mediated by 14-3-3zeta. Genes Dev. 2010;24(15):1614–1619. doi: 10.1101/gad.1942810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen KD, et al. The miR-144/451 locus is required for erythroid homeostasis. J Exp Med. 2010;207(7):1351–1358. doi: 10.1084/jem.20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu D, et al. miR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes Dev. 2010;24(15):1620–1633. doi: 10.1101/gad.1942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinrich AC, Pelanda R, Klingmuller U. A mouse model for visualization and conditional mutations in the erythroid lineage. Blood. 2004;104(3):659–666. doi: 10.1182/blood-2003-05-1442. [DOI] [PubMed] [Google Scholar]
- 14.Harfe BD, et al. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102(31):10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulyanova T, et al. Erythroid cells generated in the absence of specific beta1-integrin heterodimers accumulate reactive oxygen species at homeostasis and are unable to mount effective antioxidant defenses. Haematologica. 2013;98(11):1769–1777. doi: 10.3324/haematol.2013.087577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulyanova T, Padilla SM, Papayannopoulou T. Stage specific functional roles of integrins in erythropoiesis. Exp Hematol. 2014 doi: 10.1016/j.exphem.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulyanova T, et al. Combinatorial and distinct roles of {alpha}5 and {alpha}4 integrins in stress erythropoiesis in mice. Blood. 2011;117(3):975–985. doi: 10.1182/blood-2010-05-283218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, et al. miR-191 regulates mouse erythroblast enucleation by down-regulating Riok3 and Mxi1. Genes Dev. 2011;25(2):119–124. doi: 10.1101/gad.1998711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miska EA, et al. Most Caenorhabditis elegans microRNAs are individually not essential for development or viability. PLoS Genet. 2007;3(12):e215. doi: 10.1371/journal.pgen.0030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Socolovsky M, et al. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98(12):3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 21.van Rooij E, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;5824;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 22.Xu P, et al. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13(9):790–795. doi: 10.1016/s0960-9822(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 23.Li X, et al. A microRNA imparts robustness against environmental fluctuation during development. Cell. 2009;137(2):273–282. doi: 10.1016/j.cell.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, et al. Quantitative analysis of murine terminal erythroid differentiation in vivo: novel method to study normal and disordered erythropoiesis. Blood. 2013;121(8):e43–e49. doi: 10.1182/blood-2012-09-456079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.