Abstract
Human ovarian cancer BG-1 cells are a valuable in vitro model that has enabled several laboratories to study the estrogenic responses of ovarian cancers. We recently discovered that there are two different BG-1 cell lines being used for experiments, denoted here as BG-1 FR and BG-1 NIEHS, which exhibit striking morphological differences. The objective of this study was to methodically analyze these two BG-1 variants and compare their characteristics. Short tandem repeat analysis revealed that the DNA profile of BG-1 FR cells was unique, yet the Short tandem repeat pattern of BG-1 NIEHS was identical with that of MCF-7 cells. From a cytogenetic analysis, it became apparent that the BG-1 FR line had the same profile as previously reported, whereas the BG-1 NIEHS and MCF-7 cells share a similar genetic display. A significant number of unique chromosomal translocations were observed between the BG-1 NIEHS and MCF-7 cells, suggesting that acquired genotypic differences resulted in the formation of two lines from a common origin. Although all cell types demonstrated a similar estrogen responsiveness in reporter gene assays, a microarray analysis revealed distinct estrogen-responsive gene expression patterns with surprisingly moderate to low overlap. We conclude that BG-1 FR is the original ovarian cancer cell line, whereas the BG-1 NIEHS is a variant from the MCF-7 cells. These findings provide much needed clarification of the identities and characteristics of key cell line models that are widely used to study estrogen action in female reproductive cancers.
Ovarian cancer is the most lethal gynecological cancer in the United States and is the fourth leading cause of cancer deaths in women (1). Surgery and chemotherapy are currently used as first-line treatments (2, 3). Hormonal therapy, a less toxic alternative to chemotherapy, also provides clinical benefits (1). However, there currently exists a need to learn more about the causes and factors involved in the progression of this disease. Thus, ovarian cancer cell lines have been derived from cancer patients, and these are used as in vitro models to characterize the molecular mechanisms underlying ovarian tumorigenesis and to facilitate the development of novel therapeutics targets (4).
Estrogens, including the endogenous ovarian hormone estradiol (E2), play an essential role in the growth, differentiation, and homeostasis of a number of target tissues (5–8). The biological effects of E2 are mediated through estrogen receptors (ERs), including ERα and ERβ, which belong to the nuclear receptor superfamily of ligand-inducible transcription factors (9). The well-known classical mechanism of receptor action involves hormone binding and association of the activated ERs with estrogen responsive elements (EREs) located in the regulatory regions of target genes (9, 10). Most ovarian cancers are epithelial in origin, and there is decreased expression of ERβ mRNA levels in epithelial ovarian cancers compared with normal ovarian tissues (11). Likewise, low or absent ERβ expression is associated with more aggressive tumors, suggesting a protective role of the receptor (11–14). There is additional evidence that the ratio of ERα to ERβ is higher in ovarian tumors than in normal tissues due to lower expression of ERβ (15).
Estrogens regulate a number of target genes through the ERs, and some of these genes have been used as biomarkers in clinical cancer research. The human FBLN1C, an isoform of the FBLN1 (fibulin-1) gene, is highly expressed in ovarian carcinomas and is estrogen-inducible in ovarian tumor cells (16, 17). The human GREB1 (gene regulated by estrogen in breast cancer 1) gene was reported as an ER-responsive gene (18, 19), and this factor appears to be a critical regulator of hormone-dependent breast cancer growth (20). The human pS2/TFF1 and PGR (progesterone receptor) genes are well-characterized ER-target genes (21, 22). Both genes are up-regulated by E2 in a subclass of ER-positive human breast cancer cells and are prognostic indicators of hormonal tumor responsiveness (23).
The human ovarian epithelial cancer cell line BG-1 was established in 1989 from a solid primary tumor of a patient with poorly differentiated stage III ovarian adenocarcinoma (24). Since that time, BG-1 cells have been used as an in vitro model to study estrogen-responsive ovarian cancers. Recently we discovered that there are two different variants of BG-1 cells being used for experiments: BG-1 FR and BG-1 NIEHS. These are names we assigned to the individual cell lines based on their uses and distribution in France (BG-1 FR) (3, 17, 25–29) and the United States (BG-1 NIEHS) (30–32), respectively.
In this study, we performed an extensive characterization of the BG-1 FR and BG-1 NIEHS cell lines and compared their features with human breast cancer MCF-7 cells, another model of estrogen responsiveness. This included cellular morphology studies, short tandem repeats (STR) analysis, also known as DNA fingerprinting (33), and molecular cytogenetic analysis. We also evaluated the basal expression levels of the ERs and ER-target genes and the cellular responses to E2 in ER/ERE-mediated activation using luciferase reporter assay in the three cell lines. Finally, we profiled whole-genome gene expression by microarray analysis.
Materials and Methods
Reagents
E2 was purchased from Sigma-Aldrich, and ICI 182780 (ICI) was purchased from Tocris Bioscience.
Plasmids
The expression vector pcDNA3 was purchased from Invitrogen. An internal control plasmid for transfection efficiency, pRL-TK, was purchased from Promega. The luciferase reporters, 3xERE Luc (synthetic vitellogenin ERE-TATA fused to a luciferase reporter gene) and pS2 Luc (endogenous human pS2 gene promoter region containing an ERE fused to a luciferase reporter gene) have been described previously (34–36).
Cell lines and tissue culture
The BG-1 FR cell line was acquired directly from Dr Charles Welander at the Wake Forest University (Winston-Salem, NC) and maintained by the laboratory of Dr Vincent Cavailles at the University of Montpellier (Montpellier, France) (3, 17, 25–29). The BG-1 NIEHS cell line was acquired from Dr Carl Barrett [National Institute of Environmental Health Sciences (NIEHS)/National Institutes of Health (NIH)] (30–32). The MCF-7 cell line was purchased from American Type Culture Collection. All three cell lines were maintained in the same culture conditions: phenol red free DMEM-F12 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Gemini Bio Products) and 4 mM L-glutamine (Invitrogen). When assessing the estrogen responsiveness, 10% charcoal/dextran stripped FBS (sFBS; HyClone, Gemini Bio Products) was substituted for the FBS in the medium for E2 treatment.
DNA isolation and STR analysis
For the BG-1 FR cell line, preparation of the DNA samples was carried out using a FTA sample collection kit (100-FTA; American Type Culture Collection), and STR analysis was performed using the Cell Authentication Testing Service at American Type Culture Collection. For the BG-1 NIEHS and MCF-7 cell lines, genomic DNA (5–10 μg) was extracted using a DNeasy blood and tissue kit (QIAGEN) according to the manufacturer's protocol. The concentration and quality of DNA were determined with an ND-1000 Nanodrop spectrophotometer. STR analysis was carried out at the Cancer Institute Cell Culture and Cytogenetics Facility Core at the University of Pittsburgh.
Cytogenetic analysis
The multicolor fluorescence in situ hybridization (M-FISH) karyotype was performed in the Comparative Molecular Cytogenetics Core Facility at the National Cancer Institute/NIH. The aim of the karyotype studies was the identification of any complex chromosomal rearrangements. Briefly, chromosome preparations were obtained from established cell cultures with the addition of colcemid (KaryoMax colcemid solution; 10 μg/mL; Invitrogen) 3 hours prior to harvest. Cells were collected and treated with a hypotonic solution (KCL 0.075 M) for 15 minutes at 37°C and fixed with methanol-acetic acid 3:1. Slides were prepared and incubated overnight for use in a Hyperspectral Karyotyping (SKY) analysis. Cell metaphases were hybridized with the 24-color human SKY paint kit (Applied Spectral Imaging Inc) according to the manufacturer's protocol (37). Hybridization was carried out in a humidity chamber at 37°C for 16 hours. The posthybridization rapid wash procedure was used with 0.4× saline sodium citrate at 72°C for 4 minutes. Detection was carried out after the manufacturer's protocol. Spectral images of the hybridized metaphases were acquired using a SD301 SpectraCube system (Applied Spectral Imaging Inc) mounted on top of an epifluorescence microscope Axioplan 2 (Zeiss). Images were analyzed using Spectral Imaging 6.0 acquisition software (Applied Spectral Imaging Inc). A minimum of 10 mitoses of comparable staining intensity and quality was examined per cell line, and each was further compared for chromosomal differences. G-banding was simulated by electronic inversion of 4′,6′-diamino-2-phenylindole counterstaining.
Cell morphological imaging
The cells were seeded in six-well plates and cultured overnight. Differential interference contrast images were taken on a Zeiss AxioObserver Z1 inverted microscope using a linkage disequilibrium Achroplan ×20/0.40 objective with a Zeiss AxioCam MRm camera.
E2 treatment and RNA extraction
Cells were cultured in phenol red-free DMEM-F12 + 10% sFBS medium for 2 days and then treated with vehicle control (EtOH) or 10 nM E2 for 18 hours. Total RNA was extracted using an RNeasy minikit (QIAGEN). First-strand cDNA synthesis was performed using Superscript reverse transcriptase according to the manufacturer's protocol (Invitrogen).
Real-time PCR analysis
The mRNA levels of ER target genes were measured using SYBR green assays (Applied Biosystems). The sequences of real-time PCR primers used in this study are shown in Supplemental Table 1. Cycle threshold values were obtained using the ABI PRISM 7900 sequence detection system and analysis software (Applied Biosystems). Each sample was normalized to β-actin expression. Experiments were repeated three times and results are presented as mean ± SEM. Fold change of basal gene expression in the three cell lines was calculated relative to the BG-1 FR cell line. Changes of endogenous gene expression by E2 were calculated as fold change relative to the vehicle control group in each cell line.
Protein extraction and Western blot analysis
Whole-cell lysates were prepared by using a BD TransFactor extraction kit (BD Biosciences). For Western blot, the samples (40 μg) were loaded on a SDS-PAGE gel and separated by electrophoresis. The proteins were electrotransferred onto nitrocellulose membranes, and membranes were subsequently blocked in PBS with 5% nonfat milk for 2 hours. The blots were incubated with primary antibody (human ERα, clone HC-20, catalog number sc-543 or human ERβ, clone H-150, catalog number sc-8974; Santa Cruz Biotechnology Inc) at 4°C overnight, washed with PBS-T and then incubated with antirabbit IRDye 800CW secondary antibody (catalog number 926-32211; LI-COR Biosciences) at room temperature for 1 hour. The immunoreactive products were detected by the Fc ODYSSEY image system (LI-COR Biosciences). Anti-α-tubulin (clone B-5-1-2, catalog number T5168; Sigma) was used as a loading control.
Transient transfection and luciferase assay
Cells were seeded in DMEM-F12 + 10% sFBS. After 24 hours, the ER-responsive reporter plasmids (3xERE Luc or pS2 Luc; 0.2 μg/well) and pRL-TK renilla luciferase plasmid (0.1 μg/well) were transiently transfected using the Effectene transfection reagent (QIAGEN) according to the manufacturer's protocol. After 8 hours, the cells were changed to fresh DMEM-F12 + 10% sFBS medium overnight and then were treated with vehicle control (EtOH) or E2 (0, 1, or 10 nM) in the absence or presence of 1 μM ICI. Luciferase assays were performed using the dual luciferase reporter activity system (Promega). Transfection efficiency was normalized to renilla luciferase. Fold changes were calculated relative to vehicle controls. All experiments were repeated at least three times. Data shown are the average of triplicate determinations in a representative experiment. Values were calculated relative to vehicle control and presented as ± SEM.
Microarray analysis
Gene expression analysis was performed at the NIEHS Microarray Core Facility using Agilent whole human genome 4 × 44 multiplex format oligo arrays (014850) (Agilent Technologies) following the Agilent 1-color microarray-based gene expression analysis protocol. Three biological replicates were examined in each treatment group. The Agilent Feature Extraction Software performed error modeling, adjusting for additive and multiplicative noise. Feature extraction data files were then imported into Partek software (Partek Genomics Suite version 6.6). A principal component analysis (PCA) was performed on all samples and all probes to identify any variability present in the data. The resulting data were then analyzed for differentially expressed genes by ANOVA. Contrasts were set at BG-1 FR vehicle control vs E2, BG-1 NIEHS vehicle control vs E2, and MCF-7 vehicle control vs E2. Gene numbers displayed in the Venn diagram were derived from the data set using a ±1.5-fold cutoff and P < .05.
Data access
Microarray expression data have been submitted to the National Center for Biotechnology Information Gene Expression Omunibus (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE58324.
Statistical analysis
One-way ANOVA with a multiple comparison test (**, P < .01; ***, P < .001 or ****, P < .0001, Figure 3) and a two-way ANOVA with a multiple comparison test (***, P < .001 or ****, P < .0001, Figures 4 and 6) were performed using Graph Pad Prism version 6.0.
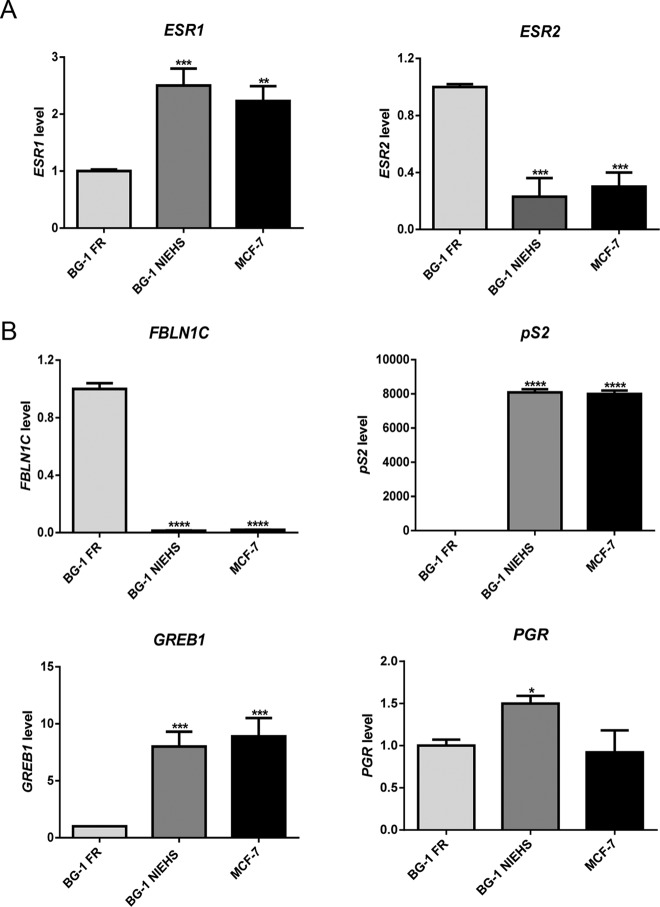
Figure 3.
Expression of ESR1 (ERα), ESR2 (ERβ), and ER-target genes in BG-1 FR, BG-1 NIEHS, and MCF-7 cell lines. A, ESR1 and ESR2 gene expression. Total RNA was extracted from the cells, and the mRNA levels were quantified by real time-PCR. Values were normalized by the expression level of β-actin. Data shown are representative of triplicates and fold change is calculated relative to the BG-1 FR cell line (set as 1) ± SEM. **, P < .01; ***, P < .001; ****, P < .0001. B, FBLN1C, pS2, GREB1, and PGR gene expression. The assay and data analysis were described as above in panel A.
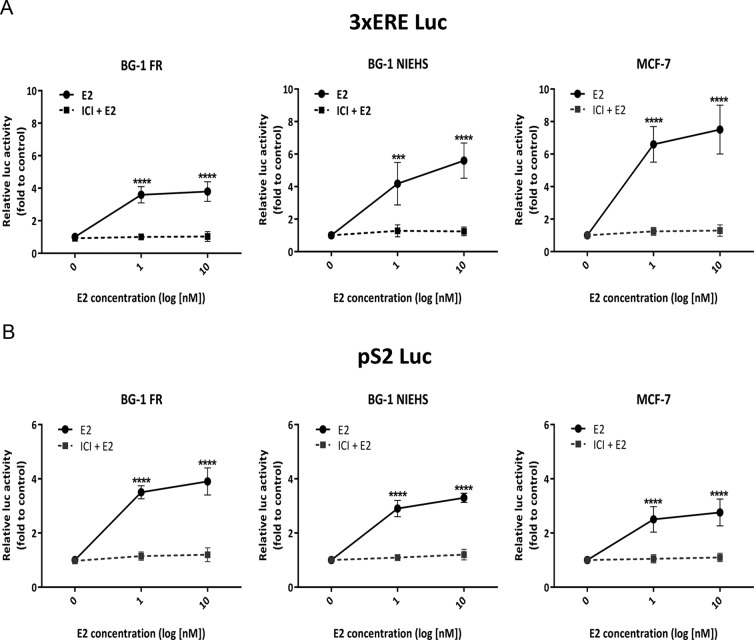
Figure 4.
ER/ERE-mediated estrogenic responses in the BG-1 FR, BG-1 NIEHS, and MCF-7 cell lines. A, Activation on the 3xERE Luc reporter. Cells were transfected with 3xERE Luc and pRL-TK (control) plasmid overnight. After changing to fresh 10% sFBS DMEM-F12 medium, cells were treated with 0, 1, or 10 nM E2 for 18 hours in the absence or presence of 1 μM ICI. E2/ERE-mediated activation was detected by luciferase reporter assays as described in Material and Methods. Data shown are the average of triplicate determinations in a representative experiment. Value were calculated relative to vehicle control and presented as ±SEM. ***, P < .001, or ****, P < .0001. B, Activation on the pS2 Luc reporter. Cells were transfected with pS2 Luc and pRL-TK (control) plasmid overnight. The treatment and luciferase reporter assay were performed as described above in panel A.
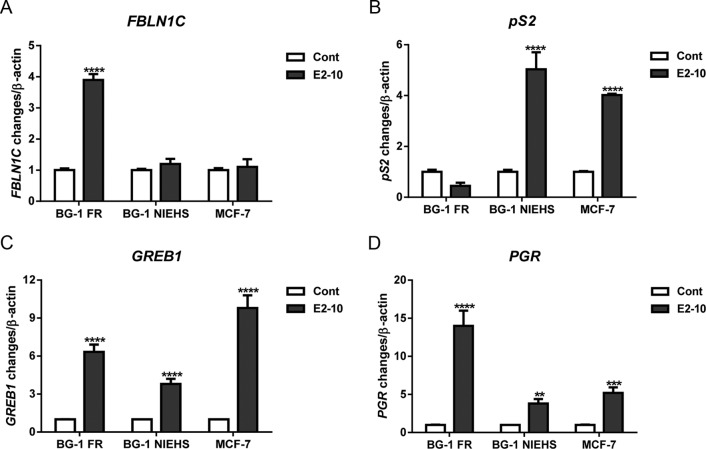
Figure 6.
The effects of E2 on expression of FBLN1C, pS2, GREB1, and PGR in BG-1 FR, BG-1 NIEHS, and MCF-7 cells. Cells were treated with vehicle control (Cont) or 10 nM E2 for 18 hours. Total RNA was extracted and used as a template for cDNA synthesis. Gene expression was quantitated by real time-PCR. Experiments were repeated three times and results are presented as mean ±SEM. **, P < .01; ***, P < .001; ****, P < .0001.
Results
Differential morphology of BG-1 FR, BG-1 NIEHS, and MCF-7 cell lines
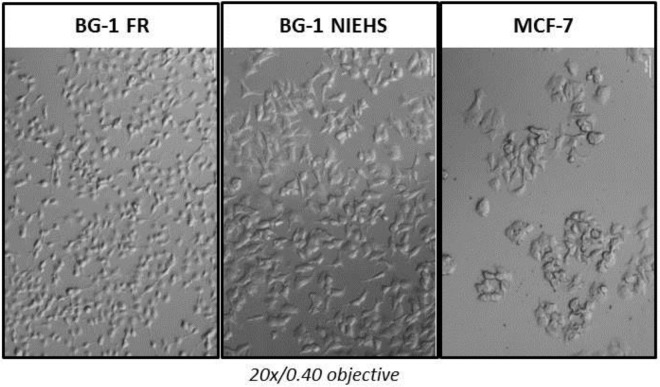
Our initial assumption that the BG-1 NIEHS cell line was an ovarian cancer cell line was due to their unique morphology compared with MCF-7 cells. The comparative morphology of the BG-1 FR, BG-1 NIEHS, and MCF-7 cells are shown in Figure 1. We found that the morphology of the BG-1 FR cells differed from the BG-1 NIEHS cells. Specifically, BG-1 FR cells were smaller and more adherent than the BG-1 NIEHS cells. The MCF-7 cells grew in clumps, were more adherent, and possessed rounded cytoplasms, whereas BG-1 NIEHS cells possessed cytoplasmic spindles and were more dispersive.
Figure 1.
Cell morphology imaging of BG-1 FR, BG-1 NIEHS, and MCF-7 cell lines. Cells were seeded in six-well plates and cultured overnight. Differential interference contrast images were taken on an inverted microscope using a ×20/0.40 objective lens with a Zeiss AxioCam MRm camera.
STR DNA and cytogenetic profiles of the BG-1 FR, BG-1 NIEHS, and MCF-7 cell lines
Noting the morphological differences between the BG-1 FR, BG-1 NIEHS, and MCF-7 cells, we next performed an STR analysis of the genetic background of those cell lines. The STR DNA profiles differed significantly between the BG-1 FR and BG-1 NIEHS cells (Table 1). Based on the STR database of the American Type Culture Collection, there was no cell line corresponding with the DNA profile of BG-1 FR cells. However, the DNA profile of BG-1 NIEHS cells matched with the profile of MCF-7 cells (Table 1).
Table 1.
Summary of STR DNA Profile of Human Cancer Cell Lines
| Cell Lines | BG-1 FR | BG-1 NIEHS | MCF-7 |
|---|---|---|---|
| Amelogenin | X | X | X |
| CF1PO | 10, 11 | 10 | 10 |
| D13S317 | 10 | 11 | 11 |
| D16S539 | 9, 11 | 11, 12 | 11, 12 |
| D18S51 | 12, 15 | 14 | 14 |
| D19S433 | 13, 15 | 13, 14 | 13, 14 |
| D21S11 | 29, 33.2 | 30 | 30 |
| D2S1338 | 22, 23 | 21, 23 | 21, 23 |
| D3S1358 | 16, 17 | 16 | 16 |
| D5S818 | 12 | 11, 12 | 11, 12 |
| D7S820 | 12 | 8, 9 | 8, 9 |
| D8S1179 | 12, 13 | 10, 14 | 10, 14 |
| TH01 | 8, 9.3 | 6 | 6 |
| TPOX | 8, 11 | 9, 12 | 9, 12 |
| vWA | 14, 17 | 14, 15 | 14, 15 |
American Type Culture Collection suggests that cell lines with 80% or greater match are considered to be related. BG-1 FR shows no match for any profile in the American Type Culture Collection STR database. BG-1 NIEHS and MCF-7 show greater than 80% value of the STR loci.
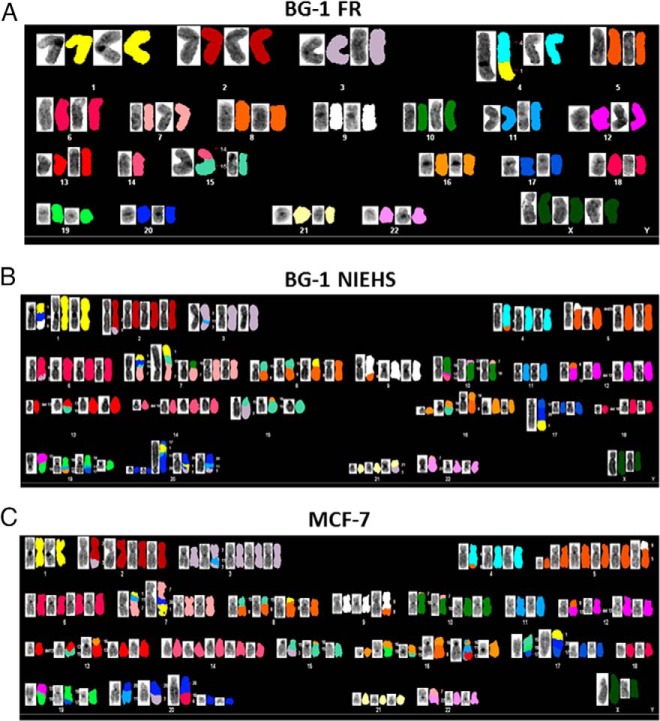
Next, we performed a molecular cytogenetic analysis in the three cell lines. The M-FISH karyotypes of the representative metaphases are shown in Figure 2. The BG-1 FR cells revealed a uniform karyotype of 46,XXX,-4,+derdic,t(1:4)(p11;q33),-14,t(14;15),-15 (Figure 2A). This karyotype was matched with the original BG-1 cells, which was reported by Geisinger et al (24). In contrast, the karyotype of the BG-1 NIEHS cells was similar to that of the MCF-7 cells (Figure 2, B and C). However, we observed a significant number of differential chromosomal translocations between the BG-1 NIEHS and MCF-7 cells. The comparison of chromosomal aberrations in the BG-1 NIEHS and the MCF-7 cells are shown in Supplemental Table 2 (red for BG-1 NIEHS and blue for MCF-7 cells).
Figure 2.
The molecular cytogenetic analysis. G-banded and M-FISH karyotype of a representative metaphase of BG-1 FR cells (A), BG-1 NIEHS cells (B), and MCF-7 cells (C).
Differential gene expression in the BG-1 FR, BG-1 NIEHS, and MCF-7 cell lines
The basal expression levels of ESR1 (ERα) and ESR2 (ERβ) genes in the BG-1 FR, BG-1 NIEHS, and MCF-7 cell lines were examined (Figure 3A). The values are represented as fold change relative to the level of BG-1 FR cells (set as 1). We found that the expression level of the ESR1 gene in the BG-1 NIEHS and the MCF-7 cells was 2-fold higher than that in the BG-1 FR cells. On the other hand, the expression level of ESR2 in the BG-1 FR cells was 3- to 4-fold higher than that of the BG-1 NIEHS and MCF-7 cells. In addition, using a Western blot analysis, the ERα protein (66 kDa) was detected in all three cell lines, but the ERβ protein (55 kDa) was seen only in BG-1 FR cells (Supplemental Figure 1).
Because all three cell lines had ER expression, we examined the basal expression levels for several ER-target genes. The real-time PCR results are shown in Figure 3B. We found that the FBLN1C gene was highly expressed in BG-1 FR cells, but this transcript was not detectable in the BG-1 NIEHS and MCF-7 cells. In contrast, the basal expression levels of the pS2 and GREB1 genes were significantly higher in the BG-1 NIEHS and MCF-7 cells than in the BG-1 FR cells. A similar expression level of the PGR gene was detected in the BG-1 FR and MCF-7 lines, and this expression was 1.5-fold higher in the BG-1 NIEHS cells. These data demonstrated that there are different basal gene expression profiles among the three cell lines.
Transcriptional activity of endogenous ER
To investigate ER-mediated transcriptional activities in the three cell lines, we examined promoter activation using two E2-responsive luciferase reporters: 3xERE Luc (synthetic vitellogenin ERE fused with luciferase reporter) and pS2 Luc (endogenous human pS2 gene promoter region containing an ERE fused to a luciferase reporter gene). The E2-mediated activities of 3xERE Luc are shown in Figure 4A. At 1 nM E2 treatment, the BG-1 FR and the BG-1 NIEHS cells displayed a 4-fold induction compared with the control, whereas a 6-fold induction was seen in the MCF-7 cells. With 10 nM of E2, the maximal response efficacies were observed in the BG-1 NIEHS cells with 6-fold or in the MCF-7 cells with 7-fold induction.
The E2-mediated activities on pS2 Luc are shown in Figure 4B. With 1 or 10 nM of E2, maximal response efficacies were observed in all cell lines with a 4-fold induction in the BG-1 FR cells or a 3-fold induction in the BG-1 NIEHS and MCF-7 cells.
In addition, E2-dependent activity was attenuated in all instances by coadministration of the pure ER antagonist ICI, thus confirming that the ERE-mediated promoter activities were ER dependent (Figure 4).
Gene expression profiles in the BG-1 FR, BG-1 NIEHS, and MCF-7 cell lines
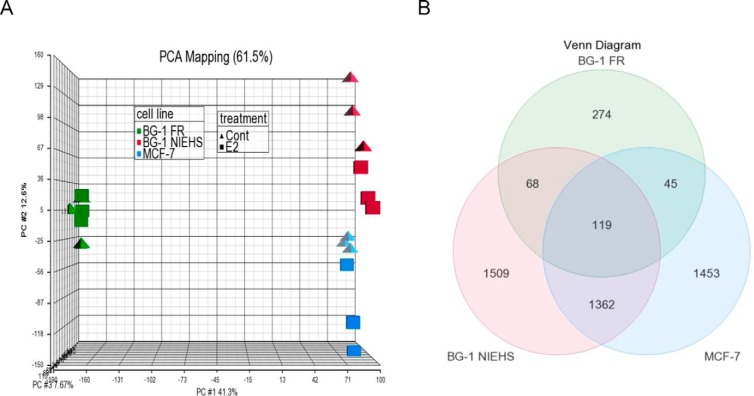
To compare the cellular responses to E2 in each cell line, we profiled whole-genome gene expression by using microarray analysis after 10 nM E2 treatment for 18 hours. PCA mapping is shown in Figure 5A. The graph shows a correlation among the triplicate samples in each treatment group (control group: triangle shape; E2 group: square shape). Notably, the PCA mapping indicates that the gene expression profile of the BG-1 FR cells (green) is clearly different from the profiles of the BG-1 NIEHS (pink) or MCF-7 (blue) cells.
Figure 5.
Microarray analysis of E2-responsive gene expression in BG-1 FR, BG-1 NIEHS, and MCF-7 cells. A, PCA mapping. The PCA was performed on all samples and all probes to characterize the variability among triplicates for each treatment group and between different treatment groups. B, Venn diagram. Contrasts were set at BG-1 FR vehicle control vs E2 (10 nM, 18 h treatment), BG-1 NIEHS vehicle control vs E2 (10 nM, 18 h treatment), and MCF-7 vehicle control vs E2 (10 nM, 18 h treatment). The gene numbers in the graph were determined with ±1.5-fold cutoff, P < .05.
Microarray data were analyzed by examining fold change between the control and E2 in each cell line. Venn diagram analysis for fold change (± 1.5-fold cutoff) and overlap between cell lines is shown in Figure 5B. We found that the BG-1 FR cells had only 506 E2-responsive genes, whereas 3058 genes were identified in the BG-1 NIEHS cells and 2979 in the MCF-7 cells. When comparing genes between the cell lines, a total of 119 genes overlapped between all three cell lines. A list of the 119 genes and their hormonal regulation attributes (up or down) is shown in Supplemental Table 3. There were 187 (68 + 119) genes overlapping between the BG-1 FR and BG-1 NIEHS cells, 164 (45 + 119) genes between the BG-1 FR and MCF-7, and 1481 (1362 + 119) genes between the BG-1 NIEHS and MCF-7 cell lines.
To confirm the microarray results, we examined the effect of E2 on several ER target genes from the microarray using real time-PCR analysis. Fold changes in gene expression, relative to the control, are shown in Figure 6. E2 induced a 4-fold increase of FBLN1C gene expression in the BG-1 FR cells, but this induction was not seen in the BG-1 NIEHS and MCF-7 cells (Figure 6A). In contrast, E2 induced the expression of the pS2 gene in the BG-1 NIEHS (5-fold) and MCF-7 cells (4-fold) but not in the BG-1 FR cell line (Figure 6B). In addition, the GREB1 and PGR genes were induced by E2 in all three cell lines (Figures 6, C and D). These results are consistent with the data from the microarray analysis.
Discussion
We and others have been using the human ovarian cancer cell line BG-1 to study ER signaling in ovarian cancer since the early 1990s when this cell line was established (24). Recently we discovered that there are two different variants of the BG-1 cells being used for experiments. We named those cell lines BG-1 FR and BG-1 NIEHS, depending on whether they were distributed from the University of Montpellier in France (3, 15, 17, 25–29) or the NIEHS/NIH in the United States (30–32, 38–40).
STR DNA profiling, also known as DNA fingerprinting, identifies variants in tetranucleotide microsatellite loci on multiple human chromosomes (33). The American Type Culture Collection developed a standard for authentication of human cell lines by comparison with established STR DNA profiling databases (4, 41, 42). In this study, the STR analysis revealed that the BG-1 FR cell line had a completely novel profile because it did not match any STR DNA profile in the American Type Culture Collection databases. When comparing our cytogenetic analysis results with the original karyotype report of the BG-1 cells (24), we discovered an identical profile in the BG-1 FR cells. Furthermore, the basal expression level of the ESR2 (ERβ) gene and its protein level were significantly higher in the BG-1 FR cell line than in the BG-1 NIEHS and MCF-7 cells. ESR2-expressing tissues in the whole body are quite restricted in comparison with ESR1-expressing tissues (43). Ovarian tissue and granulosa cells specifically are one of the highest expressing cell types for ESR2 in the body (44). The elevated expression of ESR2 in the BG-1 FR cells may be due to the cell origin, a solid primary tumor tissue in a patient with stage III ovarian adenocarcinoma (24). Regardless, our studies suggest that the BG-1 FR cell line is the original human ovarian cancer cell from the research group of Geisinger and colleagues (24). In addition, this study is the first report characterizing the STR DNA and gene expression profiles of this original BG-1 cell line.
A previous study reported that the BG-1 NIEHS cell line has a similar STR DNA profile to the MCF-7 cell line (4). We also observed identical STR DNA profiles between the BG-1 NIEHS and MCF-7 cells. Interestingly, we confirmed our earlier observations (26–28) that the BG-1 NIEHS cells were morphologically different from the MCF-7 cells, suggesting that similarities in STR profiles do not completely correlate with phenotypic features. In addition, we found that the gene expression level of ESR1 (ERα) in both the BG-1 NIEHS and the MCF-7 cells was much higher than the level in the BG-1 FR cells. This observation suggests that BG-1 NIEHS cells are a derivative of MCF-7 cells.
Using microarray analysis, we found that although BG-1 NIEHS is a variant of MCF-7 cells the E2-responsive gene expression profiles between the three cell lines are quite different. Specifically, there was an approximate 40%–50% overlap in E2-responsive genes identified between any two cell lines. One specific marker of E2 responsiveness in the breast and ovary is fibulin-1, a secreted glycoprotein that binds with extracellular matrix proteins (16, 17). Several reports suggested that the elevated expression of fibulin-1 protein is associated with mammary (45) and ovarian tumors (46). However, the E2 responsiveness of the FBLN1 gene is differentially stimulated by E2 in mammary and ovarian tumors. Namely, the expression level of a specific isoform of the FBLN1 gene, FBLN1C, is selectively induced by E2 only in human ovarian cancer but not in breast cancer cells (17, 25, 46). In this study, we found that the FBLN1C mRNA was highly expressed in BG-1 FR cells and up-regulated by E2. However, the expression of FBLN1C mRNA and hormonal responsiveness were not observed in BG-1 NIEHS and MCF-7 cell lines, thereby suggesting that the BG-1 NIEHS cell line shares more characteristics with MCF-7 cells than with ovarian cancer cells.
Several human breast cancer cell lines, including MCF-7, have a range of 76–88 chromosomes (47). Numerous common chromosomal translocations have been identified in the MCF-7 clonal variants (48). These chromosomal abnormalities may influence the biologic and pharmacological response of the cells (47). In this study, we also found different chromosomal aberrations from the cytogenetic analysis in BG-1 NIEHS and MCF-7 cells. These differences could explain the distinct gene expression profiles and morphologies observed. To evaluate the effect of distinct gene expression in BG-1 NIEHS and MCF-7 cells, we performed Ingenuity pathway analysis for the cell specific E2-regulated genes (Supplemental Table 4). Interestingly, cell morphology- and cell function-related genes were ranked in BG-1 NIEHS, whereas the cell cycle-related genes were ranked in MCF7 cells. These profiles may reflect the differential cell morphology and cell growth between BG-1 NIEHS and MCF7 cells. Although a clear correlation between chromosomal locations and gene expression profiles was not apparent, further analyses could be pursued in future studies and may need to be considered in comparing experimental results from different laboratories using MCF-7 cells.
In summary, we recently discovered that there are two different BG-1 cell lines being used for experimental studies, denoted here as BG-1 FR and BG-1 NIEHS. Based on STR DNA and gene expression profiling, we conclude that the BG-1 FR cell line is the original human ovarian cancer cells from the research group of Geisinger and colleagues (24). This study is the first report characterizing the STR DNA and gene expression profiles of the original BG-1 cell line. More importantly, we concluded that the BG-1 NIEHS variant cells were derived from MCF-7 cells and listed in the publications that used BG-1 NIEHS cells in Supplemental Table 5. This information may possibly require a reevaluation of the reported results. Altogether these findings provide much-needed clarification of the identities and characteristics of these in vitro cell models that are widely used to study estrogen responses in female ovarian and breast cancers.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Lois Annab [National Institute of Environmental Health Sciences (NIEHS)] and John Risinger and Deepak Nagendra (Michigan State University College of Human Medicine) for the collection of the BG-1 cell lines. We also thank Steffi Oesterreich and Courtney Anderson (University of Pittsburgh Cancer Institute) for help with the STR analysis. In addition, we thank Laura Wharey and Rick Fannin (NIEHS Microarray Core) and Jeff Tucker (NIEHS Fluorescence Microscopy and Imaging Center) for assistance. We also thank Sylvia Hewitt and Lois Annab for reviewing and Colin Luh for editing this manuscript.
This work was supported by the Division of Intramural Research of the National Institute of Environmental Health Sciences through Grant Z01 ES70065 (to K.S.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BG-1 FR
- BG-1 cells being used for experiments in France
- BG-1 NIEHS
- BG-1 cells being used for experiments the United States
- E2
- 17β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen-responsive element
- FBS
- fetal bovine serum
- M-FISH
- multicolor fluorescence in situ hybridization
- NIEHS
- National Institute of Environmental Health Sciences
- PCA
- principal component analysis
- sFBS
- stripped FBS
- STR
- short tandem repeats.
References
- 1. Simpkins F, Garcia-Soto A, Slingerland J. New insights on the role of hormonal therapy in ovarian cancer. Steroids. 2013;78:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGuire WP, 3rd, Markman M. Primary ovarian cancer chemotherapy: current standards of care. Br J Cancer. 2003;89(suppl 3):S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Badia E, Docquier A, Busson M, et al. Long-term treatment with the pure anti-estrogen fulvestrant durably remodels estrogen signaling in BG-1 ovarian cancer cells. J Steroid Biochem Mol Biol. 2012;132:176–185. [DOI] [PubMed] [Google Scholar]
- 4. Korch C, Spillman MA, Jackson TA, et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol Oncol. 2012;127:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–1644. [DOI] [PubMed] [Google Scholar]
- 7. Nilsson S, Makela S, Treuter E, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. [DOI] [PubMed] [Google Scholar]
- 8. Pettersson K, Gustafsson JA. Role of estrogen receptor β in estrogen action. Annu Rev Physiol. 2001;63:165–192. [DOI] [PubMed] [Google Scholar]
- 9. Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interv. 2005;5:343–357. [DOI] [PubMed] [Google Scholar]
- 10. O'Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. [DOI] [PubMed] [Google Scholar]
- 11. Drummond AE, Fuller PJ. The importance of ERβ signalling in the ovary. J Endocrinol. 2010;205:15–23. [DOI] [PubMed] [Google Scholar]
- 12. Brandenberger AW, Tee MK, Jaffe RB. Estrogen receptor α (ER-α) and β (ER-β) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-β in neoplastic tissues. J Clin Endocrinol Metab. 1998;83:1025–1028. [DOI] [PubMed] [Google Scholar]
- 13. Pujol P, Rey JM, Nirde P, et al. Differential expression of estrogen receptor-α and -β messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res. 1998;58:5367–5373. [PubMed] [Google Scholar]
- 14. Rutherford T, Brown WD, Sapi E, Aschkenazi S, Munoz A, Mor G. Absence of estrogen receptor-β expression in metastatic ovarian cancer. Obstet Gynecol. 2000;96:417–421. [DOI] [PubMed] [Google Scholar]
- 15. Docquier A, Garcia A, Savatier J, et al. Negative regulation of estrogen signaling by ERβ and RIP140 in ovarian cancer cells. Mol Endocrinol. 2013;27:1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roger P, Pujol P, Lucas A, Baldet P, Rochefort H. Increased immunostaining of fibulin-1, an estrogen-regulated protein in the stroma of human ovarian epithelial tumors. Am J Pathol. 1998;153:1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moll F, Katsaros D, Lazennec G, et al. Estrogen induction and overexpression of fibulin-1C mRNA in ovarian cancer cells. Oncogene. 2002;21:1097–1107. [DOI] [PubMed] [Google Scholar]
- 18. Henley DV, Mueller S, Korach KS. The short-chain fatty acid methoxyacetic acid disrupts endogenous estrogen receptor-α-mediated signaling. Environ Health Perspect. 2009;117:1702–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reid G, Metivier R, Lin CY, et al. Multiple mechanisms induce transcriptional silencing of a subset of genes, including oestrogen receptor α, in response to deacetylase inhibition by valproic acid and trichostatin A. Oncogene. 2005;24:4894–4907. [DOI] [PubMed] [Google Scholar]
- 20. Rae JM, Johnson MD, Scheys JO, Cordero KE, Larios JM, Lippman ME. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res Treat. 2005;92:141–149. [DOI] [PubMed] [Google Scholar]
- 21. Berry M, Nunez AM, Chambon P. Estrogen-responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc Natl Acad Sci USA. 1989;86:1218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katzenellenbogen BS. Mechanisms of action and cross-talk between estrogen receptor and progesterone receptor pathways. J Soc Gynecol Investig. 2000;7:S33–S37. [DOI] [PubMed] [Google Scholar]
- 23. Rio MC, Bellocq JP, Daniel JY, et al. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988;241:705–708. [DOI] [PubMed] [Google Scholar]
- 24. Geisinger KR, Kute TE, Pettenati MJ, et al. Characterization of a human ovarian carcinoma cell line with estrogen and progesterone receptors. Cancer. 1989;63:280–288. [DOI] [PubMed] [Google Scholar]
- 25. Bardin A, Moll F, Margueron R, et al. Transcriptional and posttranscriptional regulation of fibulin-1 by estrogens leads to differential induction of messenger ribonucleic acid variants in ovarian and breast cancer cells. Endocrinology. 2005;146:760–768. [DOI] [PubMed] [Google Scholar]
- 26. Hayashido Y, Lucas A, Rougeot C, Godyna S, Argraves WS, Rochefort H. Estradiol and fibulin-1 inhibit motility of human ovarian- and breast-cancer cells induced by fibronectin. Int J Cancer. 1998;75:654–658. [DOI] [PubMed] [Google Scholar]
- 27. Galtier-Dereure F, Capony F, Maudelonde T, Rochefort H. Estradiol stimulates cell growth and secretion of procathepsin D and a 120-kilodalton protein in the human ovarian cancer cell line BG-1. J Clin Endocrinol Metab. 1992;75:1497–1502. [DOI] [PubMed] [Google Scholar]
- 28. Moll F, Millet C, Noel D, et al. Chordin is underexpressed in ovarian tumors and reduces tumor cell motility. FASEB J. 2006;20:240–250. [DOI] [PubMed] [Google Scholar]
- 29. Cunat S, Rabenoelina F, Daures JP, et al. Aromatase expression in ovarian epithelial cancers. J Steroid Biochem Mol Biol. 2005;93:15–24. [DOI] [PubMed] [Google Scholar]
- 30. Annab LA, Hawkins RE, Solomon G, Barrett JC, Afshari CA. Increased cell survival by inhibition of BRCA1 using an antisense approach in an estrogen responsive ovarian carcinoma cell line. Breast Cancer Res. 2000;2:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hall JM, Korach KS. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003;17:792–803. [DOI] [PubMed] [Google Scholar]
- 32. Baldwin WS, Travlos GS, Risinger JI, Barrett JC. Melatonin does not inhibit estradiol-stimulated proliferation in MCF-7 and BG-1 cells. Carcinogenesis. 1998;19:1895–1900. [DOI] [PubMed] [Google Scholar]
- 33. Crawford MH, Beaty KG. DNA fingerprinting in anthropological genetics: past, present, future. Investig Genet. 2013;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hall JM, McDonnell DP, Korach KS. Allosteric regulation of estrogen receptor structure, function, and coactivator recruitment by different estrogen response elements. Mol Endocrinol. 2002;16:469–486. [DOI] [PubMed] [Google Scholar]
- 35. Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982;10:7895–7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tremblay GB, Tremblay A, Copeland NG, et al. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor β. Mol Endocrinol. 1997;11:353–365. [DOI] [PubMed] [Google Scholar]
- 37. Schrock E, du Manoir S, Veldman T, et al. Multicolor spectral karyotyping of human chromosomes. Science. 1996;273:494–497. [DOI] [PubMed] [Google Scholar]
- 38. Wang S, Aarts JM, Evers NM, Peijnenburg AA, Rietjens IM, Bovee TF. Proliferation assays for estrogenicity testing with high predictive value for the in vivo uterotrophic effect. J Steroid Biochem Mol Biol. 2012;128:98–106. [DOI] [PubMed] [Google Scholar]
- 39. Kang NH, Hwang KA, Kim TH, Hyun SH, Jeung EB, Choi KC. Induced growth of BG-1 ovarian cancer cells by 17β-estradiol or various endocrine disrupting chemicals was reversed by resveratrol via downregulation of cell cycle progression. Mol Med Rep. 2012;6:151–156. [DOI] [PubMed] [Google Scholar]
- 40. Hall JM, Korach KS. Endocrine disrupting chemicals promote the growth of ovarian cancer cells via the ER-CXCL12-CXCR4 signaling axis. Mol Carcinog. 2013;52:715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. American Type Culture Collection Standards Development Organization Work Group ASN-0002. Cell line misidentification: the beginning of the end. Nat Rev Cancer. 2010;10:441–448. [DOI] [PubMed] [Google Scholar]
- 42. Masters JR, Thomson JA, Daly-Burns B, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci USA. 2001;98:8012–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology. 1997;138:4613–4621. [DOI] [PubMed] [Google Scholar]
- 44. Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146:3247–3262. [DOI] [PubMed] [Google Scholar]
- 45. Greene LM, Twal WO, Duffy MJ, et al. Elevated expression and altered processing of fibulin-1 protein in human breast cancer. Br J Cancer. 2003;88:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clinton GM, Rougeot C, Derancourt J, et al. Estrogens increase the expression of fibulin-1, an extracellular matrix protein secreted by human ovarian cancer cells. Proc Natl Acad Sci USA. 1996;93:316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rondon-Lagos M, Verdun Di Cantogno L, Marchio C, et al. Differences and homologies of chromosomal alterations within and between breast cancer cell lines: a clustering analysis. Mol Cytogenet. 2014;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bahia H, Ashman JN, Cawkwell L, et al. Karyotypic variation between independently cultured strains of the cell line MCF-7 identified by multicolour fluorescence in situ hybridization. Int J Oncol. 2002;20:489–494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.